Abstract
Purpose
To assess volumetric proton MR spectroscopic imaging of the human brain on multi-vendor MRI instruments.
Methods
Echo-planar spectroscopic imaging (EPSI) was developed on instruments from three manufacturers, with matched specifications and acquisition protocols that accounted for differences in sampling performance, RF power, and data formats. Inter-site reproducibility was evaluated for signal-normalized maps of N-acetylaspartate (NAA), Creatine (Cre) and Choline using phantom and human subject measurements. Comparative analyses included metrics for spectral quality, spatial coverage, and mean values in atlas-registered brain regions.
Results
Inter-site differences for phantom measurements were under 1.7% for individual metabolites and 0.2% for ratio measurements. Spatial uniformity ranged from 79% to 91%. The human studies found differences of mean values in the temporal lobe, but good agreement in other white-matter regions, with maximum differences relative to their mean of under 3.2%. For NAA/Cre, the maximum difference was 1.8%. In grey-matter a significant difference was observed for frontal lobe NAA. Primary causes of inter-site differences were attributed to shim quality, B0 drift, and accuracy of RF excitation. Correlation coefficients for measurements at each site were over 0.60, indicating good reliability.
Conclusion
A volumetric intensity-normalized MRSI acquisition can be implemented in a comparable manner across multi-vendor MR instruments.
Keywords: MR spectroscopic imaging, quantitative mapping, multi-center studies, MR standardization, clinical equivalency
INTRODUCTION
MR spectroscopic imaging (MRSI) of the brain offers the ability to map a number of metabolites that provide information on tissue viability and disease processes, making this technique a promising tool for both research and clinical studies (1). Currently, the commercially available implementations of MRSI vary between sites and vendors and remain limited in terms of restrictive spatial sampling and relative complexity of the data processing and analysis, all of which limit its use for multi-center trials. In vivo MRS studies are also limited by sensitivity considerations and changes of metabolite distributions can be diffuse, with the result that metabolite images may not be visually interpretable in the sense of anatomical MRI, but require quantitative assessments and comparisons against reference values. Therefore, implementations of MRSI would greatly benefit from a quantitative approach, which is independent from the instrument used or the site where data are acquired, and the availability of normative metabolite maps.
Signal amplitudes in in vivo MRS are typically dependent on the acquisition method used and for comparisons between subjects or across subject groups it is essential that all datasets be acquired in an identical manner. Similarly, reconstruction methods for MRSI include multiple steps that may impact the resultant signal intensity and therefore between-study comparisons must maintain identical processing methods. It is also necessary that normal variations in metabolite concentrations be taken into account; for example with tissue type, brain region, age, and gender (2-5), and these factors are usually accounted for by comparison against data obtained on the same MR instrument, at the same location, in a group of control subjects matched to the study group under investigation. Although many studies have reported metabolite values from normal control subjects, these values can rarely be used as the reference information for other investigations and for multi-center trials. Because multiple measurements must be acquired to account for the normal variability between subjects and measurement error, this requirement to obtain the normative metabolite information represents a considerable cost.
To address the above-mentioned limitations of multi-center MRSI studies and reduce burdens associated with redundant normative data collection, this study has evaluated a standardized 1H echo planar spectroscopic imaging (EPSI) sequence implemented on MR instruments from three different manufacturers at three sites. This work extends a previous development of this acquisition method (5,6) on a single-vendor platform that was of value for clinical research studies (7-12). The EPSI method is an efficient acquisition approach that can be used to map metabolite distributions over a large volume of the brain within clinically-acceptable acquisition times (13). The sequence requirements are similar to many MRI methods, indicating that it could be readily implemented on different MRI platforms. Additional benefits of this implementation include that the scan setup requirements are comparable to standard MRI sequences, and the processing methods are automated, making this approach well-suited to routine studies. To ensure consistency of the data processing this project has used the MIDAS package (Metabolite Imaging and Data Analysis System) (6), which provides automated processing and includes features as signal intensity normalization, calculation of tissue content maps, and non-linear registration to a standardized spatial reference, which facilitates statistical comparisons between studies.
MRS analysis methods have commonly used metabolite ratios to address concerns for signal calibration; however, this results in loss of information and an analysis based on individual metabolite concentrations may be more desirable, for which a calibration procedure is required. Although absolute quantitation methods for MRSI have been implemented (14,15), their implementation requires that relaxation rates be accounted for, which becomes impractical for routine clinical studies. Therefore, this study implements signal normalization to “institutional units” (5,16) using tissue water as an internal reference, with the reference measurement being tightly integrated with the metabolite acquisition.
Previous studies that have examined standardized MRS methods for studies of human brain showed high reproducibility of a specialized MRSI method in a dual-center setting with the identical MR instrument (17) or used single-voxel MRS (18). The INTERPRET project (19) also established some standardization of MRS sequence parameters for brain cancer diagnosis, using single-voxel and limited-coverage MRSI protocols at 1.5T; however, differences between sequence implementations on each instrument remained. Because the analysis used a “self-scaling” of each spectrum and a pattern recognition analysis approach (20), these differences were not important; however, this also meant that potentially valuable information on signal intensities was ignored. For MRS analysis based on normalized results greater attention must be paid to the consistency of all sequence parameters.
In this report, technical differences and limitations on cross-platform MRSI implementation are described, and results obtained from mapping proton MRS observed metabolite distributions throughout a large portion of the brain are presented. In addition to providing information on spatial variations, reproducibility, and spectral quality differences of metabolite distributions, numerical results are compared using a spectroscopic phantom and normal subjects.
METHODS
Data Acquisition
The volumetric MRSI pulse sequence has been presented previously (21) and is shown in Supporting Figure S1. This was developed on MR instruments from three different manufacturers at three locations (Site 1: Discovery MR750, General Electric Healthcare; Site 2: Magnetom Trio/TIM, Siemens Healthcare; and Site 3: Achieva, Philips Healthcare). The sequence used chemical shift-selective (CHESS) water suppression, lipid inversion nulling (22) with TI=198 ms, 73° excitation angle, TR=1710 ms, spin-echo acquisition with TE=70 ms, a 135 mm-thick oblique axial excitation slab to cover the cerebrum and most of the cerebellum, continuous sampling during an echo-planar readout, and two-dimensional phase encoding. For Sites 1 and 2 the echo-planar readout used sinusoidal gradient ramps of 130 μs, a 140 μs flat-top region, and sampling of 100 points at a 4 μs interval. The total readout used 1000 lobes, and the amplitude was 12.5 mT/m for a 280 mm field of view. The final resolution was 50x50x18 k-space and 500 spectral sample points with 1250 Hz spectral width after removal of x2 oversampling in the spatial readout dimension and combination of odd and even echoes prior to Fourier reconstruction. Due to technical limitations at Site 3 in the size of the acquisition buffer and number of pulse sequence components, data was acquired only during the flat-top gradient of 402 μs with 50 sample points. The waveform used linear ramps of 100 μs, amplitude 10 mT/m, 512 gradient lobes, and resulted in a final sweep width of 830 Hz and 256 spectral points after echo combination.
The TE of 70 ms was chosen based on previous studies that found this to provide good quality spectra, with minimal baseline variations from macromolecules and lipids and better signal-to-noise ratio (SNR) relative to implementations at longer TEs (5,23). The TR of 1710 ms was set to accommodate the interleaved water reference acquisition and the flip angle of 73° set according to the Ernst angle calculation for optimal sensitivity with typical metabolites T1 values. The water reference acquisition used a gradient-echo acquisition with 20° excitation angle and TE=6.3 ms with identical spatial and spectral parameters as the metabolite MRSI (5,24). For studies in humans a single outer volume saturation band was positioned over the eyeballs, sinuses, and oral cavity. The scan time for the EPSI sequence was 25 min 39s.
Pulse shapes, sequence timing, and gradient waveforms were identically implemented at each site with the exception of the following differences due to scanner hardware and software limitations: 1) restrictions on the sampling for Site 3 as previously described; 2) a limitation of body excitation coil maximum transmit B1 of 15 μT on one instrument (Site 3) compared to 25 μT on two other instruments that resulted in slice selective pulses with lower bandwidth; and 3) restrictions on continuous sampling on one instrument (Site 1) that skipped 8 samples every 16K points of the echo-planar readouts. The missing 8 samples were substituted by corresponding samples at a previous readout cycle.
The MR protocol also included a manufacturer-provided T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) sequence, TR/TE/TI = 2150/4.4/1100 ms, and 256 × 256 × 144 points at 1 mm isotropic resolution. This MRI data was incorporated into the MRSI processing and used for a high-resolution segmentation of grey matter, white matter, and cerebral spinal fluid (CSF), so while different structural MRI sequences could be used this would potentially impact the MRSI quantitation. The acquired multichannel MRSI raw data and the reconstructed MRIs were transferred to local computers for further processing.
A 20 cm-diameter spherical spectroscopic phantom was constructed that contained 50 mM Potassium Phosphate, 12.5 mM N-Acetylaspartate, 10 mM Creatine, 3 mM Choline chloride, 7.5 mM myo-Inositol, 12.5 mM Glutamate, 1.5 mM Taurine, 6.25 mM Glutamine, 5 mM Lithium Lactate, and 0.1% Sodium Azide solved in distilled-water with pH adjusted to 1.0 using NaOH and HCl solutions. This was shipped between the participant sites and scanned with the lipid inversion pulse off at five separate occasions at approximately weekly intervals at each site. A total of 30 healthy subjects, age 22 to 30, were recruited as follows: Site 1, 6 male, 4 female, age 26.5 3.1 y.o.; Site 2, 6 male, 4 female, age 27.9 2.6 y.o.; and Site 3, 6 male, 4 female, age 26.4 2.9 y.o. There was no significant differences between age and gender of these subject groups (p = 0.26). Subjects were screened to exclude any history of brain disease or injury, substance abuse, or psychiatric illness. The study was approved for human subject’s research by the Institutional Review Boards at each site and written informed consent was obtained before participation. All measurements were performed at 3.0 Tesla using the whole-body transmit coil of each MR instrument and an 8-channel phased-array receive-only head coil (InVivo Corp). The resultant MRSI data covered a spatial region of 280 × 280 × 180 mm3 with a nominal spatial resolution of 5.6 × 5.6 × 10 mm3. Standard manufacturer-provided RF power adjustment and B0 shimming up to 2nd order were used at all sites. For Site 1 a single-shot spiral pulse sequence was used for rapid field map acquisition, and a least-squares calculation of the shim currents was performed to minimize the root-mean-square (RMS) value of the B0 inhomogeneity over a manually delineated brain region on the 3D field map (25). At Site 2 shimming was performed automatically, but in two subjects this was followed by manual optimization of shim currents. At Site 3, shimming was performed up to 2nd order using a field-map-based approach (26) that optimized B0 over a manually defined outline of the brain.
Data Processing
All data were imported into the MIDAS package and processed using automated procedures (6). Software was written to read the EPSI raw data in DICOM format from Site 2 and custom data formats from Sites 1 and 3, and to define sequence parameters that were not recorded in the data headers. The MRSI reconstruction included resampling and echo combination using the interlaced Fourier transform (27) and multi-channel combination using sum-of-squares (28) with phase and magnitude maps derived from the water-reference images. MRSI processing included lipid k-space extrapolation (29), and spectral lineshape and B0 correction using time-dependent phase correction functions derived from the water-reference. Automated parametric spectral fitting (30) was used to map the relative concentration of N-acetylaspartate (NAA), total creatine (Cre), and total choline (Cho), with an estimation of the quality of the fit indicated by the Cramer-Rao lower bounds (CRLB). This procedure incorporated prior spectral information obtained by spectral simulation (31). Voxels were excluded from the spectral fitting if the linewidth of the water SI signal exceeded 18 Hz. The metabolite images were interpolated to 64 × 64 × 32 and the effective voxel volume was 1.5 ml following spatial smoothing. Signal normalization used the water reference SI as an internal reference (32,33). For this, the water SI was converted to a 100% water-equivalent image using the tissue contribution maps and literature values for the water content of grey- and white-matter and CSF, and the resultant map used to correct for the receiver coil sensitivity and normalize the metabolite images (5). The resultant individual metabolite maps therefore represent the metabolite signal relative to a reference signal equivalent to 100% water. The signal normalization procedure included an estimate of the water T1 (34,35) but did not account for metabolite relaxation and the results are therefore in institutional units. Tissue maps were obtained by segmentation of the T1-weighted MRI using FSL/FAST (36,37), and converted to the MRSI spatial resolution and point-spread function.
For the human studies a nonlinear spatial transform to a brain atlas (38,39) was applied to all metabolite maps, SI-resolution tissue maps, and maps indicating quality of the spectral analysis, to enable between-group voxel-based image analyses. The BrainWeb simulated MRI from the Montreal Neurological Institute (40) was used as the spatial reference, which was associated with an atlas that identified nine anatomical regions defining the left and right frontal lobes (LFL/RFL), temporal lobes (LTL/RTL), parietal lobes (LPL/RPL), occipital lobes (LOL/ROL), and the cerebellum (Cbl). This spatial normalization included interpolation to 2-mm isotropic voxels.
Data Analysis
For the phantom studies the mean and standard deviation values of individual metabolites and their ratios were calculated from a region in the center of the object. Inter-site comparisons were carried out using maximum difference between values obtained at all three Sites for each individual metabolite values and their ratios. The intra-site coefficients of variation (COV) were also measured from the five repetitive phantom scans. To assess uniformity the normalized absolute average deviation (NAAD) measure was used (41), as:
| [1] |
where Xi is the individual pixel value, N is the total number of pixels within the region used for the measure of mean intensity, and x̄ is the mean pixel intensity within the region. The NAAD indicates the percent image uniformity, where 100% is a perfectly uniform image. For each map, the NAAD was calculated at a central slice and at a central volume-of-interest (VOI), corresponding to 75% of the phantom volume. Finally, mean SNR values of the NAA peak at all voxels of the middle slice were calculated from all scans.
Statistical analyses for the human subject studies were carried out following spatial normalization using MIDAS. Voxels were excluded from analysis with a linewidth greater than 12 Hz and a fractional content of total grey-matter (GM) and white-matter (WM) of less than 70%. This value was chosen to minimize bias arising from low SNR regions and errors in CSF volume estimation (42). Outlying values were also excluded based on a threshold of three times the standard deviation away from the mean, as calculated from all voxels within the selection. A correction for signal loss due to cerebral spinal fluid (CSF) partial volume was applied to the individual metabolite images as Met’ = Met/(1-fcsf) for 0 <fcsf< 0.3, where Met is the uncorrected metabolite value and fcsf is the fraction of CSF in the MRSI voxel. Average metabolite values corresponding to 100% GM and 100% WM within each brain region were obtained by regression of each parameter against the tissue content determined from the MRSI-resolution tissue segmentation images, for all voxels over each of the atlas-defined regions.
Inter-site comparisons of the human MRSI results were conducted via visual inspection and quantitatively via statistical analyses. Mean and standard deviation values of regional metabolite distributions over the atlas-defined lobar regions were derived, and results averaged over each 10 (intra-site) scans and all 30 (inter-site) scans. Homogeneity of each group was measured by using intra-site COV, defined as standard deviation within the site’s group divided by the mean values from that group. Measurement of reliability at each site for each metabolite was examined by intra-class correlation coefficient (ICC), which is the ratio of the variance of inter-site metabolite distributions to the sum of the variance of inter-site metabolite distributions, the variance of within site metabolite distributions and the contribution of noise. Because subjects were selected randomly at each site and the groups were age- and gender-matched, it was assumed that the variability over all inter-site results to be greater than the intra-site group variability. In other words, without loss of generality the groups are exchangeable between sites.
Mean SNR values of the NAA peak and mean CRLB of all MRSI voxels within each brain region were calculated. One-way analysis-of-variance (ANOVA), with 5% significance level, was performed for the measurements from the atlas-defined brain regions to assess differences between sites. Additional tests were carried out to examine the significance of differences between left and right hemispheres for each brain region at each Site using paired t-tests (α = 0.05). All statistical analyses were made using Excel (Microsoft).
Lastly, image-based analyses were carried out to determine mean values of the fitted spectral linewidth and B0 inhomogeneity over each subject group at each voxel location across the whole-brain; which were then compared between the sites.
RESULTS
The quantitation of individual metabolite values and their ratios obtained from the phantom studies for each Site demonstrated a strong quantitative agreement, with the largest differences between the values at each site relative to their mean value of 1.4%, 1.6%, and 1.7% for Cho, Cre, and NAA, respectively, and 0.06% and 0.2% for NAA/Cre and Cho/Cre. The intra-site variability was higher for Site 3 with, for example, a mean COV of 5.2% compared to 1.3% (Site 1) and 0.2% (Site 2) for NAA. The one-way ANOVA analysis on the individual metabolites and their ratios indicated statistically non-significant differences between sites (p-values ranged from 0.11 to 0.85). The mean metabolite and ratio values are plotted in Supporting Figure S2.
The fitted-volume measures (i.e., the percentage of fitted voxels that met the line-width and the outlier exclusion criteria) for the MRSI phantom data obtained at each Site are listed in Table S1, together with the NAAD values and the mean SNR values calculated from all voxels at a center slice of the NAA maps. Site 3 exhibited the largest fitted volume of 84%, whereas volumetric NAAD values were largest for Site 2, with values over 87%, followed by Site 3 and Site 1, although all produced acceptably uniform maps (volumetric and center-slice NAAD of > 79% and > 87%, respectively). For the water reference images, high uniformity (i.e., > 97%) was obtained at all sites indicative of consistent water excitation profiles.
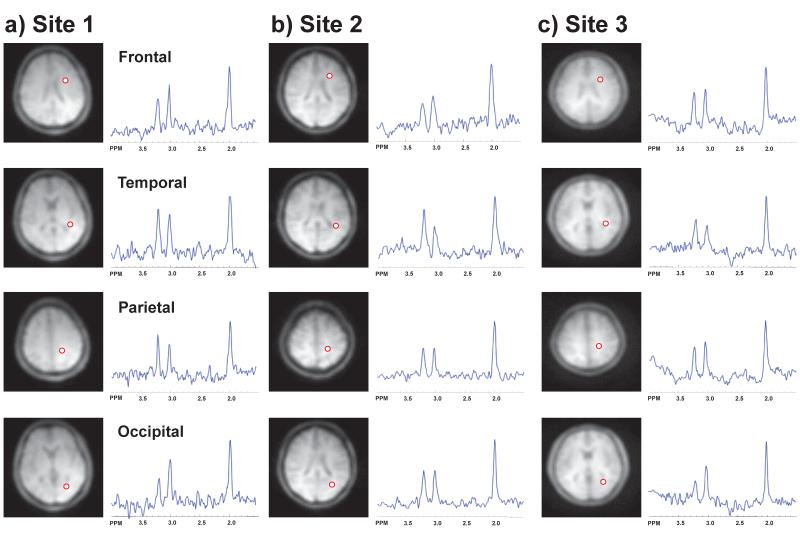
Representative spectra from each of the lobes analyzed for one subject at each site are shown in Figure 1. Representative maps for NAA and Cho from 16 contiguous axial slices obtained from a different subject at each site, prior to atlas registration and exclusion of spectrally suboptimal voxels, are shown in Figures 2 and 3. These results demonstrate comparable quality and spatial extent of the metabolite maps, although with differences of SNR and regional coverage. The bright spots, primarily located at the edges of the brain, typically indicate voxels with errors in the spectral fitting, commonly caused by residual water or lipid signals, and these results will be excluded by the outlier removal filter applied in the quantitative analysis procedure. In Figures 4 and 5 are shown group mean metabolite distributions for NAA and Cho at each site, and the corresponding standard deviation maps. For these maps, voxels were excluded based on the previously-described criteria that have resulted in the absence of signal in regions with large CSF volume fraction, such as the ventricles and inter-hemispheric fissure, and larger B0 inhomogeneity, such as the inferior frontal regions. These results illustrate the larger volume over which the linewidth criterion was achieved for Site 3, and a lower standard deviation over the whole brain for Site 2.
Figure 1.
Representative human brain spectra from a) Site 1, b) Site 2, and c) Site 3, for single voxels in the frontal, temporal, parietal, and occipital regions. The voxel locations are indicated by the circle superimposed on the water reference SI images.
Figure 2.
Representative individual-subject NAA metabolite images are shown at 16 contiguous slices from studies performed at Sites 1 (a), 2 (b), and 3 (c).
Figure 3.
Representative individual-subject Cho metabolite images from studies performed at Sites 1 (a), 2 (b), and 3 (c).
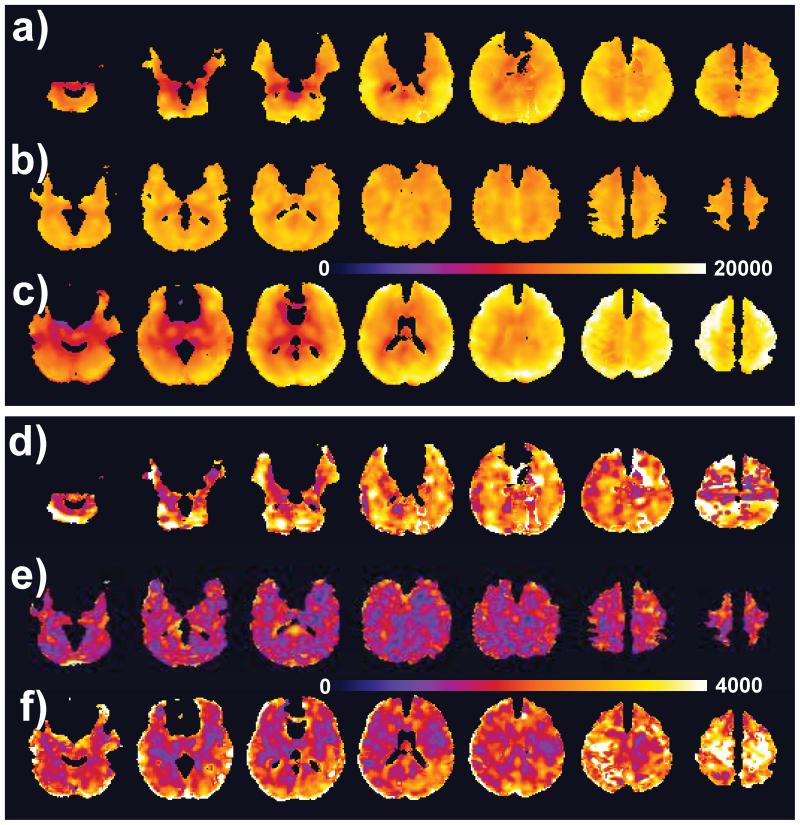
Figure 4.
Group mean (a,b,c) and standard deviation (d,e,f) maps for NAA from Site 1 (a and d), Site 2 (b and e), and Site 3 (c and f). Each group of images is shown with the same color scale, in institutional units. Images were generated for all voxels that had a linewidth of ≤12 Hz and a fractional content of GM plus WM of >70%. The images were also corrected for CSF volume contribution.
Figure 5.
Group mean (a,b,c) and standard deviation (d,e,f) maps for Choline from Site 1 (a and d), Site 2 (b and e), and Site 3 (c and f). Each group of images is shown with the same color scale, in institutional units. Voxel selection criteria are the same as for Figure 4.
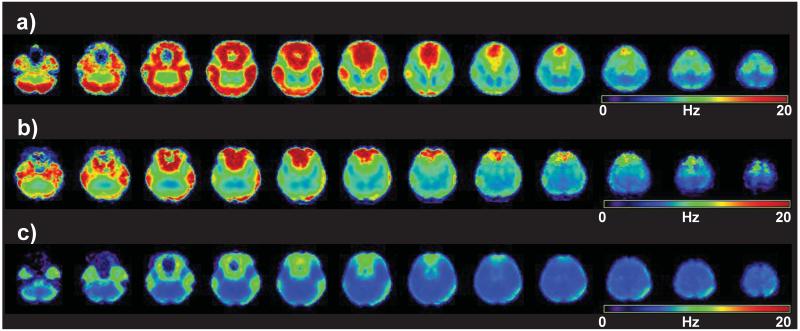
The differences in image quality and the extent of coverage of the brain seen in both the individual-subject and group mean metabolite maps are largely attributed to differences in B0 inhomogeneity. This is indicated in maps of the mean value of the fitted spectral linewidth for each subject group, shown in Figure 6. The average fitted volume, with respect to the whole brain, for a linewidth criterion of <12 Hz, were 52% (range 37% to 61%), 62% (range 56% to 70%), and 76% (range 70% to 78%) for Sites 1 through 3, respectively. The average linewidth images include contributions from long-term frequency drifts (21) and eddy currents during the echo-planar readout that remain incompletely accounted for during processing. In this regard, Site 3 showed the smallest frequency drift over the EPSI acquisition, at <6 Hz, relative to ~8 Hz and ~9 Hz at Sites 2 and 1, respectively. It is worth noting the strong correlation between the spectral linewidth distributions displayed in Figure 6 and the quality of metabolite maps shown in Figures 4 and 5.
Figure 6.
Maps of the mean values of the fitted spectral linewidth (in standard space) across each group of subjects, shown for Sites 1, 2, and 3 in a), b) and c), respectively. Data are only calculated for voxels where the water linewidth was less than 18 Hz.
Table 1 lists mean metabolite values (in institutional units) of each brain region averaged over 10 subjects at each site, and the ICCs. Corresponding values for pure GM and pure WM, generated using linear regression against the tissue fraction, and the regional mean and GM and WM values for the metabolite ratios are presented in the supplemental material. Table 2 summarizes the regional mean CRLB values for Cho, the SNR for NAA, and the ICC at each site for Cre. The CRLB indicates relatively decreased performance (larger CRLB values) at Site 3 with a larger variation, particularly in the temporal lobes. Throughout the whole-brain, Cho CRLB for the GM tissue was larger than that for WM tissue; however, the mean CRLB values still largely fell within acceptable ranges (<10%) for most brain regions at all sites. Similar results were obtained for the CRLB of NAA and Cre. The SNR was significantly greater at Site 2, with no significant differences between Site 1 and Site 3.
Table 1.
Mean metabolite concentrations of each brain region with associated standard deviation (SD) averaged over the 10 normal subjects and ICC calculated for each metabolite at each site. Results are reported in institutional units and have been scaled by 10−3 relative to the values shown in Figure 6. The groups marked with an asterisk are statistically different (p < 0.05; one-way ANOVA), and the underlined numbers indicate statistically significant difference between right and left lobes for that brain region.
| Brain regions | RFL | LFL | RTL | LTL | RPL | LPL | ROL | LOL | Cbl | ICC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| NAA | * | * | * | ||||||||||||||||
| Site 1 | 14.12 | 0.98 | 13.44 | 1.11 | 11.03 | 1.24 | 13.38 | 1.56 | 13.97 | 0.95 | 14.17 | 1.39 | 13.54 | 0.84 | 14.92 | 1.76 | 12.29 | 1.08 | 0.69 |
| Site 2 | 13.76 | 0.63 | 13.58 | 0.73 | 14.30 | 0.81 | 14.20 | 0.66 | 14.27 | 0.36 | 13.85 | 0.61 | 14.86 | 0.47 | 14.46 | 0.53 | 14.25 | 0.46 | 0.90 |
| Site 3 | 14.44 | 1.04 | 14.57 | 1.07 | 11.12 | 1.07 | 12.42 | 1.29 | 14.97 | 1.16 | 15.64 | 1.21 | 14.24 | 1.34 | 15.31 | 1.67 | 10.68 | 1.68 | 0.70 |
| Cho | * | * | * | ||||||||||||||||
| Site 1 | 2.42 | 0.31 | 2.26 | 0.26 | 2.04 | 0.38 | 2.37 | 0.55 | 2.09 | 0.31 | 2.14 | 0.38 | 1.64 | 0.29 | 1.94 | 0.52 | 3.06 | 0.42 | 0.71 |
| Site 2 | 2.24 | 0.21 | 2.18 | 0.21 | 2.24 | 0.18 | 2.21 | 0.15 | 2.02 | 0.16 | 1.94 | 0.18 | 1.67 | 0.16 | 1.65 | 0.16 | 2.88 | 0.21 | 0.90 |
| Site 3 | 2.16 | 0.34 | 2.09 | 0.38 | 1.43 | 0.32 | 1.55 | 0.39 | 2.07 | 0.31 | 2.12 | 0.32 | 1.50 | 0.33 | 1.57 | 0.33 | 1.03 | 0.51 | 0.68 |
| Cre | * | * | * | ||||||||||||||||
| Site 1 | 9.76 | 0.89 | 9.32 | 0.82 | 8.59 | 1.01 | 10.32 | 1.57 | 9.60 | 0.75 | 9.74 | 1.08 | 10.01 | 0.99 | 11.03 | 1.57 | 12.97 | 1.80 | 0.73 |
| Site 2 | 8.76 | 0.50 | 8.60 | 0.59 | 9.38 | 0.53 | 9.29 | 0.59 | 9.02 | 0.56 | 8.75 | 0.50 | 9.50 | 0.71 | 9.33 | 0.67 | 13.23 | 0.92 | 0.88 |
| Site 3 | 8.96 | 0.84 | 8.91 | 1.00 | 6.51 | 0.93 | 7.36 | 1.37 | 9.51 | 0.85 | 9.92 | 1.02 | 8.93 | 1.25 | 9.84 | 1.36 | 5.68 | 2.60 | 0.67 |
Table 2.
Mean CRLB for Cho and SNR for each brain region with associated standard deviation (SD), averaged over the 10 normal subjects at each site; and the ICC calculated for Cre metabolite for each site.
| Brain regions | RFL | LFL | RTL | LTL | RPL | LPL | ROL | LOL | Cerebrum | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Cho CRLB | ||||||||||||||||||
| Site 1 | 5.6 | 1.6 | 5.3 | 1.4 | 5.2 | 1.6 | 5.2 | 1.3 | 5.4 | 1.4 | 5.4 | 1.4 | 6.1 | 1.9 | 6.6 | 2.2 | 5.6 | 1.6 |
| Site 2 | 6.2 | 2.2 | 5.6 | 1.8 | 5.1 | 1.2 | 4.6 | 1.2 | 5.9 | 1.4 | 5.4 | 1.3 | 5.5 | 1.6 | 5.4 | 2.1 | 5.5 | 1.6 |
| Site 3 | 5.1 | 2.2 | 4.9 | 2.2 | 7.2 | 5.5 | 6.1 | 4.0 | 4.9 | 1.8 | 4.4 | 1.3 | 6.9 | 4.9 | 5.7 | 2.9 | 5.7 | 3.1 |
| SNR | ||||||||||||||||||
| Site 1 | 14.9 | 3.6 | 15.2 | 3.8 | 12.8 | 3.3 | 15.2 | 4.0 | 15.5 | 3.9 | 16.5 | 3.9 | 13.6 | 3.3 | 15.8 | 3.7 | 14.9 | 3.7 |
| Site 2 | 17.0 | 4.0 | 18.3 | 4.5 | 20.3 | 4.9 | 20.6 | 5.0 | 20.7 | 4.3 | 22.1 | 4.5 | 22.7 | 4.7 | 23.8 | 5.2 | 20.7 | 4.6 |
| Site 3 | 13.6 | 6.6 | 12.9 | 6.6 | 11.7 | 5.5 | 13.5 | 5.8 | 14.9 | 6.5 | 16.6 | 6.8 | 13.4 | 6.7 | 14.9 | 6.2 | 13.9 | 6.3 |
| Cre ICC | ||||||||||||||||||
| Site 1 | 0.71 | 0.76 | 0.73 | 0.73 | 0.75 | 0.68 | 0.78 | 0.69 | 0.73 | |||||||||
| Site 2 | 0.86 | 0.84 | 0.92 | 0.94 | 0.82 | 0.89 | 0.86 | 0.90 | 0.88 | |||||||||
| Site 3 | 0.70 | 0.67 | 0.65 | 0.64 | 0.69 | 0.71 | 0.64 | 0.70 | 0.67 | |||||||||
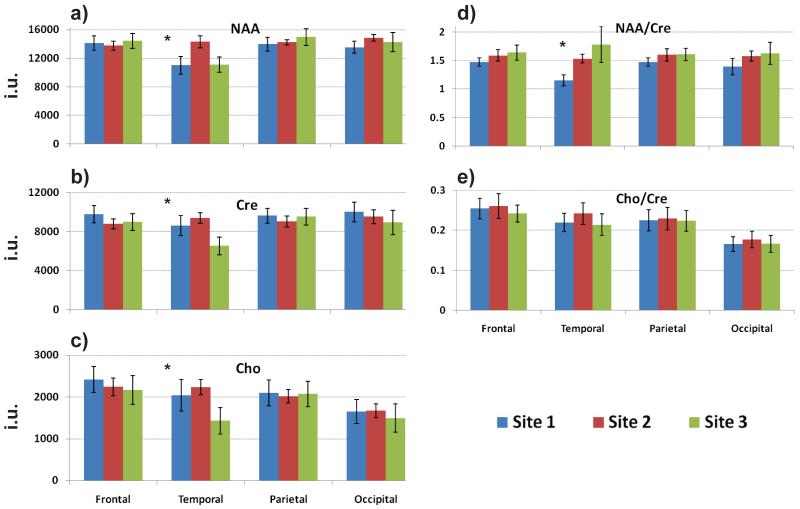
In Figure 7 are shown average metabolite values and their ratios from the brain lobar analysis. Results are shown for the right hemisphere, with similar findings obtained from the left hemisphere. The one-way ANOVA analysis on the individual metabolites and their ratios indicated statistically non-significant differences (p-values > 0.11) in all regions except for the temporal lobes, although the Cho/Cre ratio showed no significant difference in this region. Maps of the mean and standard deviation for NAA and Cho from each site are presented in the supplemental material. These results illustrate the wider extent of voxels meeting the quality selection criteria for Site 3 and a slightly larger standard deviation across the group for Site 1.
Figure 7.
Comparison of the regional mean values for the human MRSI results. Average (a) NAA, (b) Cre, and (c) Cho metabolite values and (d, e) their ratios obtained from the brain lobar analysis at the three Sites. Only results for the right brain hemisphere are shown. The asterisks indicate statistically significant inter-site differences, for p < 0.05.
DISCUSSION
A volumetric EPSI acquisition has been implemented on MR instruments from three different vendors at different sites and demonstrated to map regional metabolite distributions with results that were consistent and comparable between sites. Significant differences were found mainly in the temporal lobe and cerebellum, which are regions that are most strongly impacted by magnetic susceptibility effects from the adjacent skull base and air spaces and by differences in the slab selection profiles. Similar spectral quality results were obtained at all sites for the phantom experiments with acceptable uniformity at all sites as confirmed by NAAD values. The in vivo measurements also indicated some differences of SNR, which did not have a strong impact on the relative quality of spectral fitting results, and the differences in image quality and the extent of coverage of the brain were attributed to inter-site differences in RF excitation profiles and B0 homogeneity.
One objective of this study was to examine the feasibility of achieving greater standardization of the MRSI measurement, with the aim of increasing acceptance as a clinical modality and making available normative values that can be used to improve diagnostic sensitivity. The present study targeted a volumetric MRSI approach with a self-calibrated processing procedure, which has been shown to be of value for a number of clinical applications (7-12). Features of the acquisition method included the use of lipid nulling (22) to sample a wide volume of the brain, thereby avoiding the volume localization requirement of the vendor-provided implementations of MRSI and providing a simpler scan prescription. In this and previous studies (5,9), this method of lipid suppression has been found to be easy to implement and to enable sampling close to the cortical surface. An additional feature of this implementation is the use of spatial oversampling, which represents a compromise with some loss of SNR for the purpose of reducing the intra-voxel B0 inhomogeneity (43) to minimize line broadening and lipid bleeding and to sample a wider volume of the brain.
Two primary differences between MR instruments were found that are likely to have contributed to differences in the resultant metabolite image quality. The first is differences in B0 shimming, which may be the cause of the poorer between-site comparisons seen in the temporal lobe. It is likely that these differences reflect site-specific differences in shimming procedures and operator expertise, rather than differences in the instrument performance. Future development of higher-quality shimming procedures on all systems would undoubtedly enhance image quality and improve standardization. The second issue was differences in peak transmit B1 power, which required a modification of the refocusing pulse at Site 3 that slightly modified the RF excitation profile and led to a reduced volumetric homogeneity, particularly in the temporal regions (Table S1 and Fig. 7). An additional vendor-specific difference was the lack of support for the DICOM standard for export of raw data from the scanner, which remains an impediment to standardization. This necessitated development of custom software to read data from Sites 1 and 3 scanners and to fix some of the sequence parameters in the processing software as these could not be inserted into the data header information.
Analysis of individual metabolite maps offers the potential for increased diagnostic sensitivity and specificity over ratio measurements, though imposes additional requirements for a signal normalization (44,45). The use of an internal water reference acquired at the same time as the metabolite acquisition is convenient; however, there remain limitations to this approach. The first is that the sequence used different RF excitation pulses for the metabolite (spin-echo) and water reference SI (gradient-echo). As a result, potential errors in RF power levels setting will impact the relative amplitude of these signals and thereby the signal normalization procedures. The second limitation is the assumption of normal water content in grey- and white-matter, which is used to derive the image scaling factor. This can be addressed by adding a measurement of absolute water content (46,47), although this remains a challenge to obtain the information in a sufficiently short time to not impact clinical efficacy.
This study has demonstrated that a normative database developed at one site can be used for comparisons of individual subject data made at other sites, regardless of the MR instrument being used. With further validation of these findings and increased availability of the analysis tools and normative database, it is proposed that acceptance of MRSI will be increased. A limitation, however, of the normalization method and the use of institutional units is the need for additional data collection if reference values are required for other experimental parameters. A further constraint imposed by standardization is that any changes to the sequence design or processing methods should not affect signal amplitudes. One desirable change for the current implementation is the use of parallel imaging approaches to shorten acquisition times (48), for which it is anticipated that the loss of SNR associated with a reduced k-space acquisition can be offset with newer detection systems, without any impact to the resultant signal quantitation.
A limitation of this study is that reproducibility in human subjects has not been evaluated at each site, which would ideally require the same group of subjects to be repeatedly scanned at all sites. A previous study using the same EPSI acquisition on a single instrument (Site 2) reported intra-subject coefficient of variation values for lobar-scale mean values of 5.2% and median values for individual voxel variation of 9.7% (23). Probably the most important factors that impact inter-site reproducibility are the SNR and repeatability of the shimming methods, and their impact on spectral fitting, and in this regard the mean CRLB values did not differ significantly between sites. An additional limitation is that the regional analysis was limited to lobar-scale regions, since this study was aimed at sampling a wide volume of the brain. Improved performance could undoubtedly be obtained for B0 shimming if spatial selection was limited to a smaller region that did not include inferior portions of the temporal lobes and cerebellum, and additional studies will be needed to examine inter-site reproducibility for analysis of specific brain structures. There also remain sources of variability that were not accounted for in this study, including that the normalization method did not account for potential differences of tissue water concentration or relaxation times between subjects, and that different system calibration and shimming procedures were used at each site.
The between-site comparisons demonstrated good agreements in a reference phantom, whereas the results for the normal subjects indicated significant differences in the temporal lobes and cerebellum, with an indication that results for GM are less reliable than for WM. The most significant difference between sites was seen in the cerebellum at Site 3. The regional mean metabolite CRLB values within the cerebrum were within an acceptable range (<10%) at all sites, and a measure of reliability, the intra-site correlation coefficients (ICC) for Cre indicated acceptable performance (mean 0.72, 0.88, and 0.67 for Sites 1 to 3 respectively), with the lowest values also being found in the temporal lobe for Site 3.
There remain several improvements that would potentially benefit the performance of the methods used in this study. Undoubtedly, improved shimming, including the availability of third-order shim terms (49,50), motion and dynamic B0 correction (51) would be of considerable benefit. Improvements in RF pulse design (and power) could be used to excite a larger volume and improve sampling in the cerebellum. Improved methods for tissue segmentation to increase the accuracy of CSF volume estimation would improve the tissue-regression analysis result, notably for cortical GM regions, and direct measurement of water density would improve reliability of metabolite signal normalization. A common difficulty at all sites was the requirement to export the raw data files (~12Gb for the 8-channel detection coil), which can be addressed in future developments by implementing the EPSI reconstruction on the scanner or exporting the data to a remote computer during the scan.
In conclusion, this study has demonstrated the feasibility of a platform-independent quantitative approach for whole-brain metabolite mapping using an established pulse sequence and fully automated, comprehensive processing software. Signal normalization was achieved by incorporating an interleaved water reference MRSI acquisition, which also provided for correction of instrument-dependent variations and instabilities, and integration with MRI-derived information was used to improve metabolite image reconstruction and account for CSF partial volume contribution on data analysis. It is suggested that increased standardization will encourage wider implementation of MRSI that will enable better evaluation of these methods for clinical studies.
Supplementary Material
Acknowledgments
This research is supported by the National Institutes of Health grant R01EB000822. The authors gratefully acknowledge contributions of James Norman, Brian J. Soher, PhD, and Colin Studholme, PhD to the development of components of the MIDAS software.
Grant Sponsor: National Institutes of Health (Grant number: R01EB000822)
Footnotes
Portions of this work were presented at the 21st Scientific Meeting of the International Society for Magnetic Resonance in Medicine in Salt Lake City, Utah, USA (20-26th April 2013).
REFERENCES
- 1.Barker PB, Bizzi A, De Stefano N, Gullapalli R, Lin DDM. Clinical MR Spectroscopy: Techniques and Applications. Cambridge University Press; Cambridge: 2009. [Google Scholar]
- 2.Chang L, Ernst T, Poland RE, Jenden DJ. In vivo proton magnetic resonance spectroscopy of the normal aging human brain. Life Sciences. 1996;58(22):2049–2056. doi: 10.1016/0024-3205(96)00197-x. [DOI] [PubMed] [Google Scholar]
- 3.Schuff N, Ezekiel F, Gamst A, Amend D, Capizzano AA, Maudsley AA, Weiner MW. Region and tissue differences of metabolites in normally aged brain using 1H magnetic resonance spectroscopic imaging. Magn Reson Med. 2001;45:899–907. doi: 10.1002/mrm.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagae-Poetscher LM, Bonekamp D, Barker PB, Brant LJ, Kaufmann WE, Horska A. Asymmetry and gender effect in functionally lateralized cortical regions: a proton MRS imaging study. J Magnetic Resonance Imaging. 2004;19(1):27–33. doi: 10.1002/jmri.10429. [DOI] [PubMed] [Google Scholar]
- 5.Maudsley AA, Domenig C, Govind V, Darkazanli A, Studholme C, Arheart K, Bloomer C. Mapping of brain metabolite distributions by volumetric proton MR spectroscopic imaging (MRSI) Magn Reson Med. 2009;61(3):548–559. doi: 10.1002/mrm.21875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maudsley AA, Darkazanli A, Alger JR, et al. Comprehensive processing, display and analysis for in vivo MR spectroscopic imaging. NMR Biomed. 2006;19(4):492–503. doi: 10.1002/nbm.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stagg CJ, Knight S, Talbot K, Jenkinson M, Maudsley AA, Turner MR. Whole-brain magnetic resonance spectroscopic imaging measures are related to disability in ALS. Neurology. 2013;80(7):610–615. doi: 10.1212/WNL.0b013e318281ccec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roy B, Gupta RK, Maudsley AA, et al. Utility of multiparametric 3T MRI for glioma characterization. Neuroradiology. 2013;55(5):603–613. doi: 10.1007/s00234-013-1145-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maudsley AA, Domenig C, Ramsay RE, Bowen BC. Application of volumetric MR spectroscopic imaging for localization of neocortical epilepsy. Epilepsy Res. 2010;88(2-3):127–138. doi: 10.1016/j.eplepsyres.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levin BE, Katzen HL, Maudsley AA, Post J, Myerson MS, Govind V, Nahab F, Scanlon B, Mittel BS. Whole-brain proton MR spectroscopic imaging in Parkinson’s Disease. Journal of Neuroimaging. 2012;24(1):39–44. doi: 10.1111/j.1552-6569.2012.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Govind V, Sharma KR, Maudsley AA, Arheart KL, Saigal G, Sheriff S. Comprehensive Evaluation of Corticospinal Tract Metabolites in Amyotrophic Lateral Sclerosis Using Whole-Brain (1)H MR Spectroscopy. PLoS One. 2012;7(4):e35607. doi: 10.1371/journal.pone.0035607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Govind V, Gold S, Kaliannan K, Saigal G, Falcone S, Arheart KL, Harris L, Jagid J, Maudsley AA. Whole-brain proton MR spectroscopic imaging of mild-to-moderate traumatic brain injury and correlation with neuropsychological deficits. J Neurotrauma. 2010;27(3):483–496. doi: 10.1089/neu.2009.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Posse S, DeCarli C, Le Bihan D. Three-dimensional echo-planar MR spectroscopic imaging at short echo times in the human brain. Radiology. 1994;192:733–738. doi: 10.1148/radiology.192.3.8058941. [DOI] [PubMed] [Google Scholar]
- 14.McLean MA, Woermann FG, Barker GJ, Duncan JS. Quantitative Analysis of Short Echo Time 1H-MRSI of Cerebral Gray and White Matter. Magn Reson Med. 2000;44(10):401–411. doi: 10.1002/1522-2594(200009)44:3<401::aid-mrm10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 15.Soher BJ, van Zijl PC, Duyn JH, Barker PB. Quantitative proton MR spectroscopic imaging of the human brain. Magn Reson Med. 1996;35:356–363. doi: 10.1002/mrm.1910350313. [DOI] [PubMed] [Google Scholar]
- 16.Kreis R, Ernst T, Ross BD. Absolute quantitation of water and metabolites in the human brain. II. Metabolite concentrations. J Magn Reson B. 1993;102:9–19. [Google Scholar]
- 17.Wijnen JP, van Asten JJ, Klomp DW, Sjobakk TE, Gribbestad IS, Scheenen TW, Heerschap A. Short echo time 1H MRSI of the human brain at 3T with adiabatic slice-selective refocusing pulses; reproducibility and variance in a dual center setting. J Magn Reson Imaging. 2010;31(1):61–70. doi: 10.1002/jmri.21999. [DOI] [PubMed] [Google Scholar]
- 18.Scheidegger O, Wingeier K, Stefan D, Graveron-Demilly D, van Ormondt D, Wiest R, Slotboom J. Optimized quantitative magnetic resonance spectroscopy for clinical routine. Magn Reson Med. 2013;70(1):25–32. doi: 10.1002/mrm.24455. [DOI] [PubMed] [Google Scholar]
- 19.Tate AR, Majos C, Moreno A, Howe FA, Griffiths JR, Arus C. Automated classification of short echo time in in vivo 1H brain tumor spectra: a multicenter study. Magn Reson Med. 2003;49(1):29–36. doi: 10.1002/mrm.10315. [DOI] [PubMed] [Google Scholar]
- 20.Tate AR, Underwood J, Acosta DM, et al. Development of a decision support system for diagnosis and grading of brain tumours using in vivo magnetic resonance single voxel spectra. NMR Biomed. 2006;19(4):411–434. doi: 10.1002/nbm.1016. [DOI] [PubMed] [Google Scholar]
- 21.Ebel A, Maudsley AA. Detection and correction of frequency instabilities for volumetric 1H echo-planar spectroscopic imaging. Magn Reson Med. 2005;53(2):465–469. doi: 10.1002/mrm.20367. [DOI] [PubMed] [Google Scholar]
- 22.Spielman DM, Pauly JM, Macovski A, Glover GH, Enzmann DR. Lipid-suppressed single- and multisection proton spectroscopic imaging of the human brain. J Magn Reson Imaging. 1992;2:253–262. doi: 10.1002/jmri.1880020302. [DOI] [PubMed] [Google Scholar]
- 23.Maudsley AA, Domenig C, Sheriff S. Reproducibility of serial whole-brain MR spectroscopic imaging. NMR Biomed. 2010;23:251–256. doi: 10.1002/nbm.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ebel A, Soher BJ, Maudsley AA. Assessment of 3D 1H NMR echo-planar spectroscopic imaging using automated spectral analysis. Magn Reson Med. 2001;46:1072–1078. doi: 10.1002/mrm.1301. [DOI] [PubMed] [Google Scholar]
- 25.Kim DH, Adalsteinsson E, Glover GH, Spielman DM. Regularized higher-order in vivo shimming. Magn Reson Med. 2002;48(4):715–722. doi: 10.1002/mrm.10267. [DOI] [PubMed] [Google Scholar]
- 26.Schar M, Vonken EJ, Stuber M. Simultaneous B(0)- and B(1)+-map acquisition for fast localized shim, frequency, and RF power determination in the heart at 3 T. Magn Reson Med. 2010;63(2):419–426. doi: 10.1002/mrm.22234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metzger G, Hu X. Application of interlaced Fourier transform to echo-planar spectroscopic imaging. J Magn Reson. 1997;125:166–170. doi: 10.1006/jmre.1997.1114. [DOI] [PubMed] [Google Scholar]
- 28.Brown MA. Time-domain combination of MR spectroscopy data acquired using phased-array coils. Magn Reson Med. 2004;52(5):1207–1213. doi: 10.1002/mrm.20244. [DOI] [PubMed] [Google Scholar]
- 29.Haupt CI, Schuff N, Weiner MW, Maudsley AA. Removal of lipid artifacts in 1H spectroscopic imaging by data extrapolation. Magn Reson Med. 1996;35(5):678–687. doi: 10.1002/mrm.1910350509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soher BJ, Young K, Govindaraju V, Maudsley AA. Automated spectral analysis III: application to in vivo proton MR spectroscopy and spectroscopic imaging. Magn Reson Med. 1998;40(6):822–831. doi: 10.1002/mrm.1910400607. [DOI] [PubMed] [Google Scholar]
- 31.Aygula Z, Soher BJ, Young K, Maudsley AA. GAVA - A graphical pulse sequence simulation, display and storage environment. Proceedings of the International Society for Magnetic Resonance in Medicine; Toronto. 2003.p. 852. [Google Scholar]
- 32.Alger JR, Symko SC, Bizzi A, Posse S, DesPres DJ, Armstrong MR. Absolute quantitation of short TE brain 1H-MR spectra and spectroscopic imaging data. J Comput Assist Tomogr. 1993;17(2):191–199. doi: 10.1097/00004728-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, Posse S, Jung RE, Morrison LA. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med. 2006;55(6):1219–1226. doi: 10.1002/mrm.20901. [DOI] [PubMed] [Google Scholar]
- 34.Wansapura JP, Holland SK, Dunn RS, Ball WSJ. NMR Relaxation times in the human brain at 3.0 Tesla. J Magn Reson Imaging. 1999;9(4):531–538. doi: 10.1002/(sici)1522-2586(199904)9:4<531::aid-jmri4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 35.Rooney WD, Johnson G, Li X, Cohen ER, Kim SG, Ugurbil K, Springer CS., Jr. Magnetic field and tissue dependencies of human brain longitudinal H2O relaxation in vivo. Magn Reson Med. 2007;57(2):308–318. doi: 10.1002/mrm.21122. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imag. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 37.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 38.Studholme C, Hill DL, Hawkes DJ. Automated three-dimensional registration of magnetic resonance and positron emission tomography brain images by multiresolution optimization of voxel similarity measures. Med Phys. 1997;24(1):25–35. doi: 10.1118/1.598130. [DOI] [PubMed] [Google Scholar]
- 39.Rousseau F, Maudsley AA, Ebel A, Darkazanli A, Weber P, Sivasankaran K, Yu Y, Studholme C. Evaluation of sub-voxel registration accuracy between MRI and 3D MR spectroscopy of the brain; Proc Soc Photo Opt Instrum Eng; San Diego. 2005.pp. 1213–1221. [Google Scholar]
- 40.Collins DL, Zijdenbos AP, Kollokian V, Sled JG, Kabani NJ, Holmes CJ, Evans AC. Design and construction of a realistic digital brain phantom. IEEE Trans Med Imag. 1998;17:463–468. doi: 10.1109/42.712135. [DOI] [PubMed] [Google Scholar]
- 41.Association NEM. Determination of image uniformity in diagnostic magnetic resonance images. NEMA Standards Publication. 2008;3(1):1–17. [Google Scholar]
- 42.Bedell BJ, Narayana PA. Volumetric analysis of white matter, gray matter, and CSF using fractional volume analysis. Magn Reson Med. 1998;39(6):961–969. doi: 10.1002/mrm.1910390614. [DOI] [PubMed] [Google Scholar]
- 43.Ebel A, Maudsley AA. Improved spectral quality for 3D MR spectroscopic imaging using a high spatial resolution acquisition strategy. Magn Reson Imaging. 2003;21(2):113–120. doi: 10.1016/s0730-725x(02)00645-8. [DOI] [PubMed] [Google Scholar]
- 44.Li BS, Wang H, Gonen O. Metabolite ratios to assumed stable creatine level may confound the quantification of proton brain MR spectroscopy. Magn Reson Imaging. 2003;21(8):923–928. doi: 10.1016/s0730-725x(03)00181-4. [DOI] [PubMed] [Google Scholar]
- 45.Danielsen ER, Michaelis T, Ross BD. Three methods of calibration in quantitative proton MR spectroscopy. J Magn Reson B. 1995;106:287–291. doi: 10.1006/jmrb.1995.1046. [DOI] [PubMed] [Google Scholar]
- 46.Gasparovic C, Neeb H, Feis DL, Damaraju E, Chen H, Doty MJ, South DM, Mullins PG, Bockholt HJ, Shah NJ. Quantitative spectroscopic imaging with in situ measurements of tissue water T1, T2, and density. Magn Reson Med. 2009;62(3):583–590. doi: 10.1002/mrm.22060. [DOI] [PubMed] [Google Scholar]
- 47.Sabati M, Maudsley AA. Fast and high-resolution quantitative mapping of tissue water content with full brain coverage for clinically-driven studies. Magn Reson Imaging. 2013;31(10):1752–1759. doi: 10.1016/j.mri.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sabati M, Zhan J, Govind V, Arheart KL, Maudsley AA. Impact of reduced k-space acquisition on pathologic detectability for volumetric MR spectroscopic imaging. J Magn Reson Imaging. 2014;39(1):224–234. doi: 10.1002/jmri.24130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spielman DM, Adalsteinsson E, Lim KO. Quantitative assessment of improved homogeneity using higher-order shims for spectroscopic imaging of the brain. Magn Reson Med. 1998;40(3):376–382. doi: 10.1002/mrm.1910400307. [DOI] [PubMed] [Google Scholar]
- 50.Pan JW, Lo KM, Hetherington HP. Role of very high order and degree B0 shimming for spectroscopic imaging of the human brain at 7 tesla. Magn Reson Med. 2012;68(4):1007–1017. doi: 10.1002/mrm.24122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bogner W, Hess AT, Gagoski B, Tisdall MD, van der Kouwe AJ, Trattnig S, Rosen B, Andronesi OC. Real-time motion- and B-correction for LASER-localized spiral-accelerated 3D-MRSI of the brain at 3T. Neuroimage. 2013;88C:22–31. doi: 10.1016/j.neuroimage.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.