Abstract
Bioactive lipids govern cellular homeostasis and pathogenic inflammatory processes. Current dogma holds that bioactive lipids, such as prostaglandins and lipoxins, are inactivated by 15-hydroxyprostaglandin dehydrogenase (15PGDH). In contrast, the present results reveal that catabolic “inactivation” of hydroxylated polyunsaturated fatty acids (PUFAs) yields electrophilic α,β-unsaturated ketone derivatives. These endogenously produced species are chemically reactive signaling mediators that induce tissue protective events. Electrophilic fatty acids diversify the proteome through post-translational alkylation of nucleophilic cysteines in key transcriptional regulatory proteins and enzymes that govern cellular metabolic and inflammatory homeostasis. 15PGDH regulates these processes as it is responsible for the formation of numerous electrophilic fatty acids including the arachidonic acid metabolite, 15-oxoeicosatetraenoic acid (15-oxoETE). Herein, the role of 15-oxoETE in regulating signaling responses is reported. In cell cultures, 15-oxoETE activates Nrf2-regulated antioxidant responses (AR) and inhibits NF-κB-mediated pro-inflammatory responses via IKKβ inhibition. Inhibition of glutathione S-transferases using ethacrynic acid incrementally increased the signaling capacity of 15-oxoETE by decreasing 15-oxoETE-GSH adduct formation. This work demonstrates that 15PGDH plays a role in the regulation of cell and tissue homeostasis via the production of electrophilic fatty acid signaling mediators.
Keywords: 15PGDH, 15-oxoETE, bioactive lipid, electrophile, 15-KETE
1. Introduction
15-hydroxyprostaglandin dehydrogenase (15-PGDH, EC 1.1.1.141) is a cytosolic, NAD+-dependent member of the short-chain dehydrogenase/reductase family (1-3). 15PGDH is found in most mammalian tissues with the highest activities in lung, kidney and placenta (4). 15PGDH has been recognized as a key enzyme in the metabolism of prostaglandins and other fatty acid signaling mediators, thus involving 15PGDH in processes of inflammation and proliferation. 15PGDH was identified as a high affinity enzyme responsible for the conversion of pro-proliferative prostaglandin E2 (PGE2) to its oxidized, “inactive” product, 15-ketoPGE2 (4-6). This inactivation is accomplished by the ∼103-fold lower affinity of 15-ketoPGE2 for the PGE2 G-protein coupled receptors (GPCRs), the EP receptors (7). While 15PGDH's role in the conversion of PGE2 to 15-ketoPGE2 has been studied in great detail in the context of cancer, the significance of other substrates has only recently emerged. For example, 15(S)-hydroxyeicosatetraenoic acid (15(S)-HETE) and 11(R)-HETE are substrates for 15PGDH, yielding 15-oxoETE and 11-oxoETE, respectively (8,9). Also, the tri-hydroxy containing fatty acid derivatives termed lipoxin A4 and resolvin E1 are putatively inactivated by 15PGDH catalyzed conversion to oxo metabolites (10-12). The inactivation of PGE2 by 15PGDH is a critical step in halting tumor cell proliferation; however, not only is PGE2 reduction important, but its 15PGDH oxidation product 15-ketoPGE2 associates with the peroxisome proliferator activator receptor γ (PPARγ), up-regulates p21WAF1/Cip1, the cyclin-dependent kinase inhibitor, and inhibits cell proliferation via p53-independent mechanisms in hepatocellular carcinoma (13,14). Similar to 15-ketoPGE2, both 15-oxoETE and 11-oxoETE display anti-proliferative properties in cell culture (8,9). Treatment of human umbilical vein endothelial cells (HUVECs) resulted in growth inhibition with an IC50 for 11-oxoETE of 2.1 µM (8). 11-oxoETE was also dose-dependently inhibitory to the proliferation of human colonic adenocarcinoma lines (LoVo, HCA-7) and lung brochoalveolar adenocarcinoma cells (A549) in the low µM range (15).
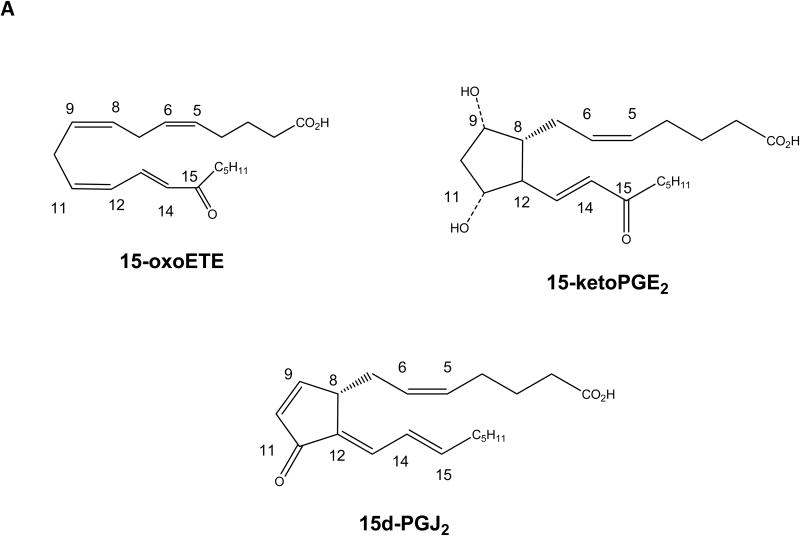
Significantly, these 15PGDH-derived products all contain an α,β-unsaturated carbonyl that confers electrophilicity, thus generating a chemically reactive product. 15PGDH-generated electrophiles such as 15-ketoPGE2 and 15-oxoETE are similar in structure to the electrophilic cyclopentenone prostaglandin, 15-deoxy-prostaglandin J2 (15d-PGJ2) (Fig. 1a). Electrophilic fatty acids (EFA) primarily include nitro-alkenes and α,β-unsaturated carbonyls, both of which have been studied in the context of their potent pleiotropic anti-inflammatory signaling actions (16). EFA signal through GPCR-independent mechanisms by forming a Michael addition adduct with functionally significant nucleophilic cysteines of transcriptional regulatory proteins and enzymes (17). EFA modulate the Keap1-Nrf2 (18), NF-κB (19), PPARγ (20), and heat shock factor pathways (21), as well inhibiting pro-inflammatory enzymes including xanthine oxidoreductase (22), matrix metalloproteinases (23) and soluble epoxide hydrolase (24).
Fig 1.
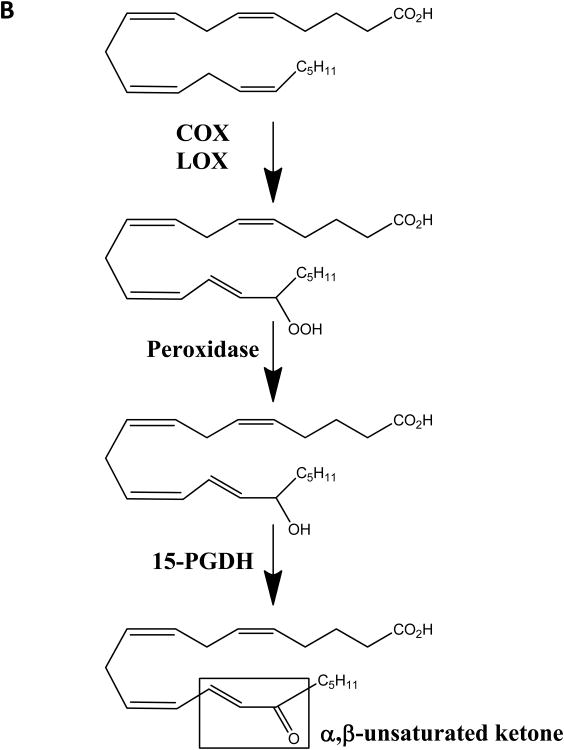
Structures of electrophilic fatty acids and 15-oxoETE formation. (A) Chemical structures of 15-oxoETE, 15-ketoPGE2 and 15d-PGJ2 provided for comparison. (B) The currently elucidated COX-2/15-LOX and 15PGDH dependent pathway for generation of 15-oxoETE. Arachidonic acid (AA) is converted by cyclooxygenase-2 (COX) or 15-lipoxygenase (LOX) to 15-hydroperoxyeicosatetraenoic acid (15-HpETE) which is reduced by a peroxidase to 15-HETE. 15PGDH oxidizes 15-HETE to 15-oxoETE, which contains an α,β-unsaturated ketone that confers 15-oxoETE with electrophilic properties.
The metabolic pathway for 15-oxoETE formation from arachidonic acid has been well described in cell lines expressing cyclooxygenase-2 (COX-2), 15-lipoxygenase (15-LO), and 15PGDH (9,25) (Fig. 1b). Thus, an understanding of the regulatory functions of 15PGDH in inflammation via the generation of bioactive metabolites are of interest in a number of inflammatory settings where COX or LO enzymes are expressed. We report evidence of 15-oxoETE as a mediator of inflammatory signaling by showing that 15-oxoETE up-regulates antioxidant response element (ARE) genes under Nrf2 transcriptional regulation and inhibits pro-inflammatory NF-κB-mediated signaling via IKKβ inhibition. The modulation of these mechanisms of inflammatory signaling is attributed to the electrophilic properties of 15-oxoETE.
2. Materials and Methods
2.1 Materials
15-oxoeicosatetraenoic acid (15-oxoETE) was purchased from Cayman Biochemical (Ann Arbor, MI). Ethacrynic acid and secondary antibodies were purchased from Santa Cruz Biotechnologies (Dallas, TX). LPS derived from E.coli 0127:B8, phorbol myristoyl acetate (PMA), actin primary antibody, wedelolactone and diethyl ether were purchased from Sigma-Aldrich (St. Louis, MO). Primary HO-1 and the IKKβ Kinase assay kit were purchased from Cell Signaling (Beverly, MA). The NQO1 primary antibody was purchased from Abcam (Cambridge, MA), GCLM from Proteintech (Chicago, IL), and GAPDH from Trevigen (Gaithersburg, MD). Solvents for liquid chromatography mass spectrometry (LC-MS) including pure water and acetonitrile were purchased from Burdick and Jackson (Morristown, NJ). Glacial acetic acid, Tris(2-carboxyethyl)phosphine hydrochloride (TCEP), Optima grade methanol and chloroform were purchased from Fisher Scientific (Pittsburgh, PA). The luciferase reporter assay was purchased from Promega (Madison, WI) and the human recombinant TNFα was purchased from Life technologies (Grand Island, NY).
2.2 Cell culture
THP-1 cells were purchased from American Type Culture Collection (ATCC, Manassas, VA) and grown per ATCC instructions. HEK293T cells stably expressing 5x-NF-κB driven firefly luciferase were a kind gift of the laboratory of Dr. John Hogenesch. THP-1 cells were grown in RPMI 1640 media and HEK293T cells were grown in DMEM, both with 10% FBS and 1% penicillin/streptomycin. Both cell lines were maintained at 37°C in 5% CO2.
2.3 15-oxoETE Treatment
THP-1 cells were cultured in 6 or 12 well dishes. At confluency, THP-1 cells were treated overnight with 100 nM of PMA. After PMA differentiation, THP-1 cells were treated with increasing concentrations of 15-oxoETE (1-50 µM) for 6 hr or with 25 µM 15-oxoETE at various time points between 1.5 and 24 hr. In some experiments THP-1 cells were pretreated for 1 hr with 100 µM ethacrynic acid (EA) to inhibit GST activity before treatment with 15-oxoETE (25 µM). For all experiments, media was replaced with RPMI containing 1% FBS and penicillin/streptomycin and THP-1 cells were differentiated.
2.4 Protein extraction and Western blotting
For western analyses, cells were scraped into RIPA buffer and protein was extracted. Protein concentrations were measured by the Pierce BCA assay (Thermo, Rockford, IL). The blots were probed for HO-1, NQO1, and GCLM expression. Actin was used for normalization of protein expression. Western blot densitometry was analyzed on a BioRad Imager (Hercules, CA).
2.5 RT-PCR
Relative quantitation of TNFα, IL-1β and IL-6 mRNA was determined on an Applied Biosystems StepOnePlus System (Life Technologies, Grand Island, NY) using their Taqman gene expression assay system. Briefly THP-1 cells were collected in 250 µL of Triazol and RNA was extracted. RNA was reversed transcribed to cDNA for RT-PCR analysis. Actin was used for normalization and mRNA is reported as relative expression.
2.6 NF-κB luciferase reporter assay
HEK293T cells stably expressing firefly luciferase driven by a 5x-κB reporter were incubated with 15-oxoETE (100 nM to 1 µM) for 5 minutes before treatment with 40 ng/mL TNFα for 6 hr. Treatment media was the same as maintenance media with 0.25% DMSO. For indicated samples, a pretreatment of 100 µM of EA was used 1 hr before treatment with 15-oxoETE. Cells were then assayed using the Promega luciferase assay system according to the manufacturer's instructions and luminescence was read in a Viktor 3 plate reader (Perkin Elmer. Akron, OH).
2.7 IKKβ Inhibition
HTScan IKKβ Kinase assay kit was used as directed with a minor modification to screen for IKKβ inhibition. Briefly, recombinant IKKβ was incubated with 15-oxoTE (2, 10, and 100 µM) or 100 µM wedelolactone (26) for 15 min in 1X kinase buffer prepared separately from the kit using the reducing agent TCEP instead of DTT. Biotin-tagged IκBα (1.5 mM) and ATP (200 mM) were added and the mix was incubated for 30 min at room temperature. The reaction was quenched with 50 mM EDTA, pH 8. The cocktail was loaded into streptavidin coated clear 96-well plates, allowed to bind for 2 hr at room temperature, and washed with PBS/T three times. The plate was incubated with 100 mL 1:1000 anti-phospho-IκBα antibody in TBS/T with 5% BSA for 2 hr, and washed three times with 200 mL PBS/T. Finally, the plate was incubated with 100 mL of secondary anti-rabbit antibody (1:1000) coupled to HRP for 1 hr at room temperature, developed, and read in a UV-Vis spectrophotometer.
2.8 Liquid chromatography mass spectrometry
15-oxoETE and 15-oxoETE-GSH were measured using reversed-phase liquid chromatography mass spectrometry in THP-1 cell lysate and media. Briefly, free fatty acid metabolites were extracted from the media and cell lysate using a chloroform methanol mixture (2:1). Before the extraction 20 ng of 5-oxoETE-d7 internal standard was added to each sample. The samples were allowed to equilibrate for 5 min before shaking for 10 min. Next, samples were centrifuged at 2800 × g for 10 min. Internal standard was added to the aqueous phase and it was desalted using Oasis HLB (1 cc) solid phase extraction (SPE) cartridges. The SPE was conditioned with one volume of methanol followed by one volume of water. The sample was washed with one volume of water and analytes were eluted with one volume of methanol. Organic and aqueous extracts were transferred to a clean vial and dried under a stream of nitrogen. Samples were reconstituted in 100 µL methanol before analysis. A Shimadzu HPLC (Columbia, MD) coupled to a CTC PAL autosampler (Leap Technologies, Carrboro, NC) and an AB Sciex (Framingham, MA) 5000 triple quadrupole mass spectrometer was used for the quantification of fatty acids. Sample (10 µL) was separated on a Waters X-bridge C18 (2.1 × 150 mm, 3.5 µ pore size). The solvent system employed aqueous 0.1% acetic acid (A) and 0.1% acetic acid in acetonitrile (B). For the organic phase analysis, the 60 min gradient with a flow rate of 0.25 mL/min started at 35% B and ramped to 90% B over 46 min. This was followed by a wash using 100% B for 6 min. The gradient then returned to starting conditions at 35% B for 8 min. The aqueous phase analysis was also run with a 60 min gradient starting at 10% B, which was held for 5 min before increasing to 98% B at 46 min. The column was washed with 100% B for 2 min and equilibrated at starting conditions. MS analyses by electrospray ionization were run in negative mode for 15-oxoETE and positive mode for GSH adducts. Selected reaction monitoring was used for sample analysis and quantification. The following transitions were used: 15-oxoETE 319.2 → 219.2, 5-oxoETE-d7 324.2 → 210.2, and 15-oxoETE-GSH 626.2 → 308.2 and 626.2 → 497.2. 15-oxoETE and 15-oxoETE-GSH were quantified using a 15-oxoETE standard curve. 15-oxoETE-GSH was characterized as previously described (8,25). Media and intracellular concentrations are reported in mol/L, and intracellular concentrations have been normalized to a THP-1 cell volume of 3 µL/106 cells (27).
A product ion spectrum of 15-oxoETE-GSH was obtained using an LTQ mass spectrometer (Thermo Scientific, Waltham, MA). The LC conditions were the same as described above for the quantification of 15-oxoETE-GSH, but the injection volume was 20 µL Full MS2 scans were performed in positive ion mode with an acquisition time of 30 ms, an ActQ of 0.25, and collision energy of 35. The ion spray voltage was maintained at 4 kV and the heater and capillary temperatures were set to 300°C and 270°C, respectively. The sheath, auxiliary and sweep gases were adjusted to 20, 18, and 12 arbitrary units.
2.9 Statistical analyses
Results are representative of an average of 3 individual experiments and statistical significance was determined by student t-test or one-way ANOVA with a Bonferroni post-test as indicated in the figure legend.
3. Results
3.1 15-oxoETE induces heme oxygenase-1 protein expression in THP-1 cells
To optimize protein expression analyses, THP-1 cells were treated with increasing concentrations of 15-oxoETE (1 to 50 µM) for 6 hr. HO-1 expression was used as a marker of Nrf2-mediated gene transcription and was significantly increased with 25 and 50 µM 15-oxoETE with no observed cytotoxicity (Fig 2a). Since 25 µM 15-oxoETE significantly increased HO-1 protein expression this concentration was chosen to perform a time course for HO-1 protein expression. While HO-1 expression is significantly increased over control treatment at 6 hr with sustained induction until 24 hr, the highest expression can be seen at 12 hr (Fig. 2b).
Fig. 2.
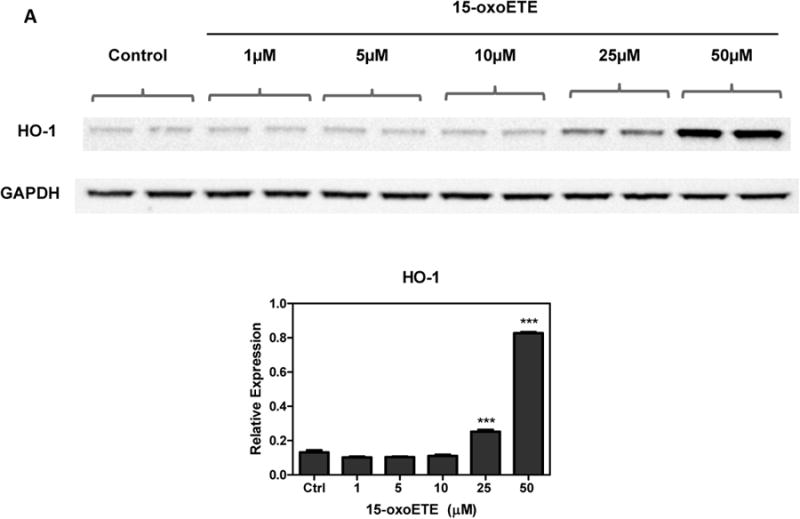
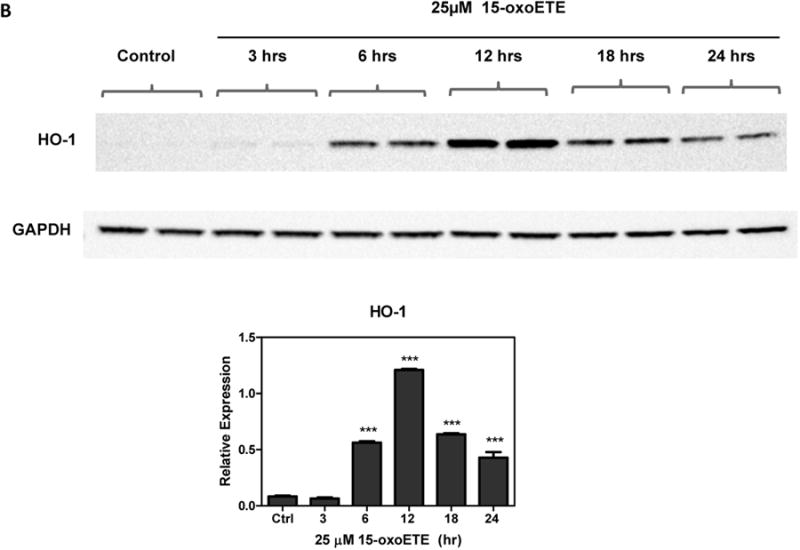
15-oxoETE up-regulates hemeoxygense-1. (A) THP-1 cells were incubated with increasing concentrations of 15-oxoETE for 6 hr (B) THP-1 cells were incubated with 25 µM 15-oxoETE from 3 to 24 hr. HO-1 expression was used as a marker of activation of the Nrf2-mediated antioxidant response. One way ANOVA with a Bonferroni post-test was used for statistical analysis.
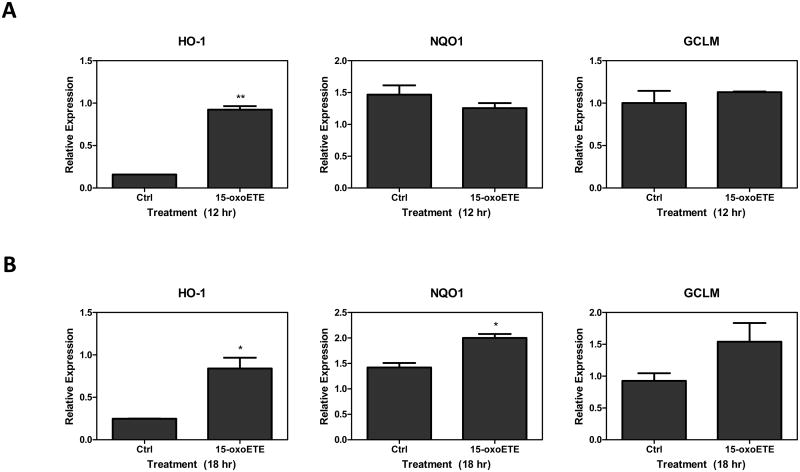
3.2 15-oxoETE activates Nrf2-dependent gene expression in THP-1 cells
THP-1 cells were treated with 25 µM 15-oxoETE for 12 and 18 hr to examine HO-1, NADPH-quinone oxidoreductase (NQO1) and glutamate-cysteine ligase modifier (GCLM) protein expression (Fig. 3a-b). At 12 hr, only HO-1 protein expression was significantly induced compared to control while at 18 hr, both HO-1 and NQO1 expression was significantly induced compared to control. GCLM expression increased at 18 hr compared to its expression at 12 hr; however, this increase is not statistically significant (Fig 3a-b).
Fig. 3.
15-oxoETE up-regulates the Nrf2-mediated anti-oxidant response. THP-1 cells were incubated with 25 µM 15-oxoETE for 12 hr (A) and 18 hr (B). Student's t-test was used for statistical analysis.
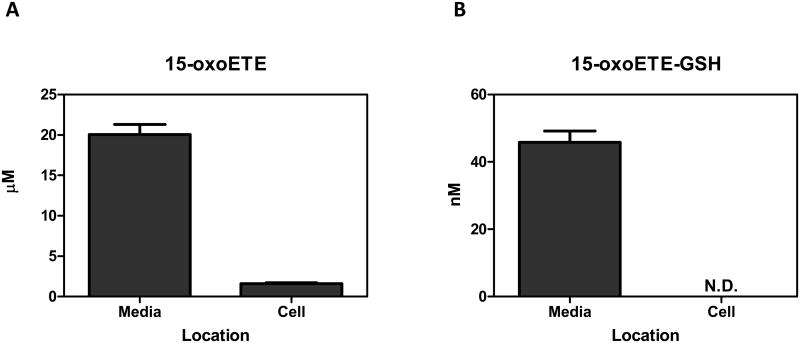
3.3 15-oxoETE bioavailability increases with GST inhibition
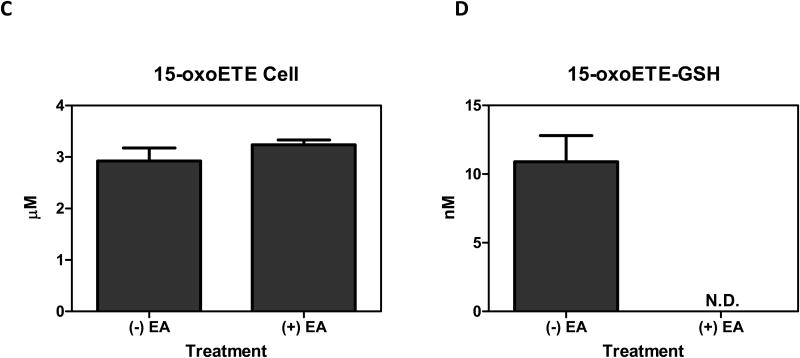
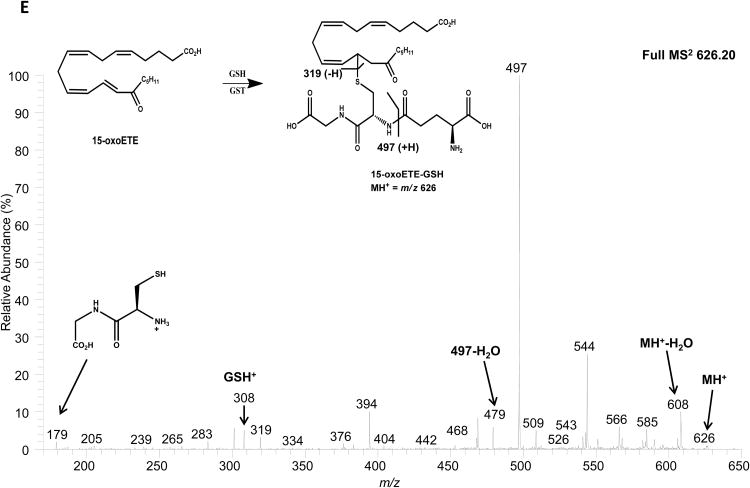
15-oxoETE concentrations were measured in media and cell lysate. After the addition of 25 µM 15-oxoETE, approximately 20 µM was recovered from the media after 12 hr of treatment. The intracellular concentration of free 15-oxoETE was 1.59 ± 0.18 µM (Fig. 4a). 15-oxoETE signaling actions are derived from its ability to form Michael adducts with reactive nucleophilic cysteines in transcription factors and pro-inflammatory enzymes. An abundant nucleophile that is readily available for 15-oxoETE to react with is GSH, with 15-oxoETE-GSH adduct formation catalyzed by glutathione S-transferases (GSTs) (25). 15-oxoETE-GSH adduct levels were determined to be 45.9 nM ± 3.35 nM in the media fraction while 15-oxoETE-GSH was not detectable in the cell lysate at 12 hr after the addition of 25 µM 15-oxoETE (Fig. 4b). THP-1 cells were pretreated with the GST inhibitor ethacrynic acid (EA, 100 µM) for 1 hr before treatment with 25 µM 15-oxoETE for 1.5 hr. Intracellular 15-oxoETE and 15-oxoETE-GSH adduct levels were measured. The inhibition of GSTs with EA incrementally increased the free intracellular 15-oxoETE pool that is readily available for adduction from 2.92 ± 0.36 µM to 3.24 ± 0.14 µM whereas the 15-oxoETE-GSH adduct was not detectable (Fig. 4c-d). The small change in free 15-oxoETE is consistent with results recently published by Codreanu et al. showing that differentiation of THP-1 cells resulted in a substantial decrease in GSH content from 1 mM to 0.02 mM and that electrophile addition did not further reduce GSH concentration (28). The 15-oxoETE-GSH adduct was identified using an LTQ mass spectrometer by its product ion spectra in positive ESI mode. Product ions representing MH+-H20 (m/z 608), loss of glutamate (m/z 497), loss of glutamate-H2O (m/z 479), 15oxoETE-H+ (m/z 319), GSH+ (m/z 308), and cysteinylglycine (m/z 179) are represented (Fig. 4e).
Fig. 4.
Intracellular 15-oxoETE levels are low. 15-oxoETE (A) and 15-oxoETE-GSH adduct (B) levels were measured in media and cell lysate after a 12 hr incubation with 25 µM 15-oxoETE. Pre-treatment of THP-1 cells with 100 µM ethacrynic acid before 25 µM 15-oxoETE treatment for 1.5 hr resulted in an incremental increase in free intracellular 15-oxoETE (C) and a decrease in 15-oxoETE-GSH adduct formation (D). (E) Product ion spectrum of 15-oxoETE-GSH.
3.4 15-oxoETE inhibits NF-κB via IKKβ inhibition
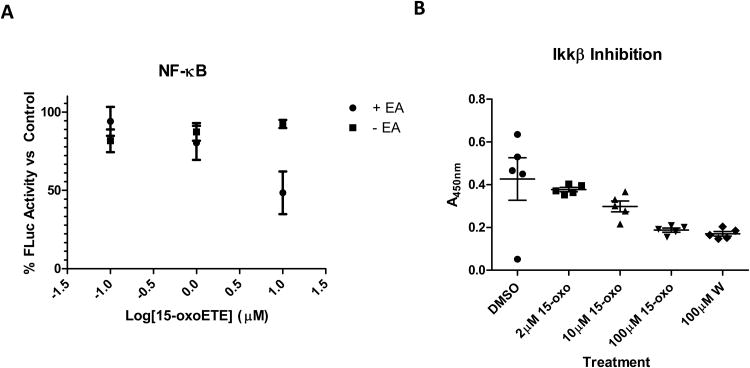
In addition to up-regulation of anti-inflammatory signaling pathways, the inhibition of NF-κB-mediated pro-inflammatory signaling was investigated. The mechanism accounting for 15-oxoETE inhibition of NF-κB was explored using an in vitro luciferase reporter and IKKβ binding assays. HEK293T cells stably expressing a 5x—κB driven firefly luciferase reporter were treated with increasing doses of 15-oxoETE from 100 nM to 1 µM. 15-oxoETE dose-dependently suppressed tumor necrosis factor α (TNF α)-induced luciferase expression in the presence of the glutathione S-transferase inhibitor ethacrynic acid (Fig. 5a). With EA pre-treatment, 1 µM 15-oxoETE reduced luciferase activity by 80% versus control.
Fig. 5.
15-oxoETE inhibits NF-κB activity. (A) HEK293T cells stably expressing a 5x-κB driven firefly luciferase reporter were pre-treated with or without 100 µM of the GST inhibitor ethacrynic acid for 1 hr then treated with 15-oxoETE for 5 min at increasing doses followed by TNFα (40 ng/mL) for 6 hr. Luciferase activity was determined in a luminometer then normalized to the vehicle (0.25% DMSO) treated control. (B) Recombinant human IKKβ was incubated with increasing concentrations of 15-oxoETE or wedelolactone (100 µM), followed by addition of biotin tagged substrate (IκBα) and ATP.
To probe potential mechanisms of NF-κB inhibition, the effects of 15-oxoETE on the signaling kinase IKKβ were examined with a recombinant kinase assay using antibody based detection of the phosphorylated substrate peptide. Increasing concentrations of 15-oxoETE from 2 to 100 µM resulted in decreased IKKβ kinase activity, with an inhibition greater than 50% at 100 µM. Wedelolactone was used as a positive control (26), yielding comparable inhibition to the equivalent concentration of 15-oxoETE (Fig. 5b).
3.5 15-oxoETE inhibits pro-inflammatory cytokine expression
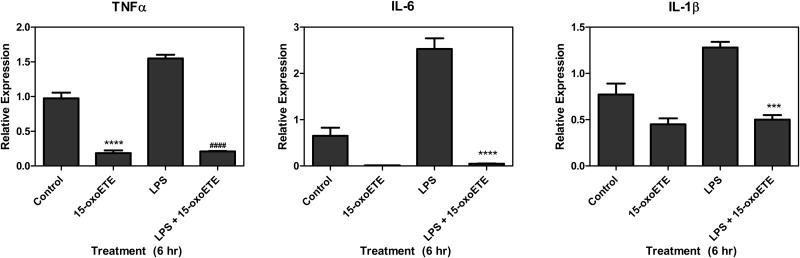
To further explore the actions of 15-oxoETE inhibition of NF-κB-mediated signaling, pro-inflammatory cytokine gene expression was examined. THP-1 cells were treated with LPS (100 ng/mL) with and without 25 µM 15-oxoETE for 6 hr. LPS stimulation and qRT-PCR analysis showed increased TNFα, interleukin (IL)-6 and IL-1β mRNA expression compared to vehicle control and cells treated with only 15-oxoETE (Fig. 6). Simultaneous treatment of THP-1 cells with LPS and 15-oxoETE resulted in significant abrogation of LPS-mediated cytokine induction with decreases of 86%, 98% and 61% in the relative expression of TNFα, IL-6 and IL-1β (Fig. 6). Interestingly, 15-oxoETE decreased TNFα, IL-6 and IL-1β message compared to control with a significant decrease in TNFα expression.
Fig. 6.
15-oxoETE inhibits NF-κB cytokines. THP-1 cells were treated with 15-oxoETE (25 µM), LPS (100 ng/mL) and LPS + 15-oxoETE for 6 hr. Relative expression of TNFα, IL-6 and IL-1β mRNA was determined. One way ANOVA with a Bonferroni post-test was used for statistical analysis.
4. Discussion
The present data reveal that a putatively “inactive” prostaglandin metabolite and oxidation products of other hydroxylated fatty acids (e.g., lipoxins, resolvins) will be conferred with additional pleiotropic anti-inflammatory signaling properties by 15PGDH. Central to this effect is the enzymatic oxidation of the hydroxyl-fatty acid to an electrophilic α,β-unsaturated carbonyl species. 15PGDH is a crucial enzyme in the regulation of prostaglandin metabolism, because of its inactivation of PGE2-dependent signaling. The implications of this reaction are especially critical in cancer, as 15PGDH expression is often lost with a concomitant increase in COX-2 and microsomal prostaglandin E synthase protein expression and activity (29-32). This loss of 15PGDH expression aberrantly increases PGE2 production and leads to unchecked tumor cell proliferation.
Notably, the unique biological activity of the 15PGDH-derived 15-ketoPGE2 has not been appreciated until the recent recognition of its potent PPARγ ligand activity (13,14). This new perspective indicates that 15-ketoPGE2 is conferred with its own biological activity that opposes the physiological effects of the parent PGE2, consistent with the loss of pro-inflammatory signaling after PGE2 dehydrogenation. Studies in hepatocellular carcinoma and cholangiocarcinoma indicate that 15-ketoPGE2 formation inhibits cell growth via PPARγ activation and downstream association with cyclin dependent kinases. In the case of the cholangiocarcinoma model, 15-ketoPGE2 generation also facilitates Smad 2/3 dissociation from PPARγ, promoting a Smad 2/3 complex with transforming growth factor β (13,14).
15-oxoeicosatetraenoic acid, similar in structure to 15-ketoPGE2, is also a product of 15PGDH that contains a bioactive α,β-unsaturated carbonyl. The precursor to 15-oxoETE, 15-HETE, mediates both salutary and pathogenic actions in atherosclerosis (33-35), asthma (36-39) and other inflammatory disorders (40-42). Again, the generation and independent signaling actions of the oxidized metabolite of 15-HETE has not been appreciated, as previous studies did not examine 15PGDH activity or the formation of 15-oxoETE. It is postulated that some of the beneficial effects attributed to 15-HETE could be due to 15-oxoETE formation and signaling. Recent studies have defined the pathway of 15-oxoETE formation (9,25) and at the very least, 15-oxoETE displays anti-proliferative actions towards endothelial cells in culture (9).
The concentrations of an electrophilic signaling mediator needed to recapitulate an in vivo response under in vitro conditions are typically 102 to 103-fold greater (43). This is particularly the case for lipophilic prostaglandins and chemically-reactive species such as oxo- and nitro-fatty acids, which can absorb to the walls of culture flasks and associate with medium constituents before interacting with the plasma membrane and gaining intracellular access via diffusion or active transport. Once intracellular, electrophilic fatty acids will adduct low and high molecular weight nucleophile-containing constituents, become esterified to complex lipids and undergo further metabolism via double bond reduction, β-oxidation and glucuronidation (17). In spite of this gauntlet of signal suppressing reactions, the activation of the nuclear lipid receptor PPARγ by oxo- and nitro-fatty acids is detectable within hours (20,44,45). This latter event raises another important point about electrophilic lipid signaling, as ligand-target protein interactions do not conform to saturation kinetics. Reports of the physiological levels in humans under normal conditions have found that free 15-oxoETE levels ranged between 47 and 738 pM in bronchoalveolar lavage fluid with a median of 101 pM (46) and 69 to 749 pM in blood (47,48). Similarly, low levels of 15d-PGJ2 (low pM in media and pg/106 cells) have been reported in 3T3-L1 adipocytes, which is below the concentration necessary for PPARγ activation (49). However, the covalent adduction of electrophiles to protein targets, such as the reaction of EFA with the Cys285 in the ligand binding domain of PPARγ, allows for the accumulation of electrophile-protein target adducts over time to have a profound impact on signaling pathways. The results in Fig. 1b support this concept, as the induction of HO-1 expression increases over time, likely due to the cumulative formation of the 15-oxoETE-Keap1 adduct. A separate study monitoring 15d-PGJ2 induction of endothelial cell HO-1 expression revealed that as much as 99% of exogenous 15d-PGJ2 did not enter the cell, yet HO-1 expression was induced (43). In the present study, intracellular levels of 15-oxoETE were 2.92 ± 0.36 µM at 1.5 hr and 1.59 ± 0.18 µM after 12 hr of incubation despite the addition of 25 µM 15-oxoETE to the THP-1 cells. A comparable study using Q-3-derived oxo-fatty acids to modulate Nrf2 and NF-κB signaling in RAW264.7 cells revealed that 18 hr after the addition of 5, 10 and 20 µM oxo-fatty acid, intracellular concentrations of 112 to 760 nM were detected (18). Incubation of rat intestinal epithelial cells with 1 µM calcium ionophore (A-23187) resulted in endogenous 15-oxoETE levels of 0.84 ± 0.06 pmol/106 cells (25). Measurement of a specific covalent electrophile-protein target can provide a more accurate assessment of concentrations required to initiate signaling. For example, when µM concentrations of biotin tagged 15d-PGJ2 are added to endothelial cells, only pM amounts of protein adducts are detected (43). In aggregate, these observations affirm that reactive lipid species present in vivo at pM to nM concentrations can impact the activity of electrophile-sensitive enzymes and signaling networks.
Unlike many autacoids, the α,β-unsaturated carbonyl-containing electrophiles are not known GPCR ligands. Instead, these electrophilic species are pleiotropic in their signaling actions by virtue of the broad spectrum of enzymatic and transcriptional regulatory mechanisms that have electrophile-reactive, functionally significant nucleophilic centers (50). Targets with susceptible redox-reactive cysteines that undergo Michael addition with electrophiles include the Cys273 and Cys288 in KEAP1 (Kelch-like ECH-associated protein 1) (21,50,51). This sensor protein is responsible for sequestering Nrf2 in the cytosol and regulating its proteasomal degradation (51). Electrophilic lipid adduction facilitates the release of Nrf2 and its translocation to the nucleus, where it promotes antioxidant response element (ARE) gene expression, including up-regulation of HO-1, NQO1, and GCLM amongst others (51,52). While Cys151 is a critical target for sulforaphane, tert-butyl hydroquinone, and N-iodoacetyl-N-biotinylhexylene-diamine, there are 26 other cysteines in Keap1 that are also potential sites for Michael addition (51). Additional studies of electrophilic fatty acid responses have shown that Nrf2 activation can also occur through Cys151-independent mechanisms (53,54). For example, nitro-oleic acid (NO2-OA) reaction with Cys273 and Cys288 is responsible for ∼50% of nitroalkene-dependent Nrf2-activation. These responses are consistent with those induced by the cyclopentenone prostaglandins PGA2 and 15d-PGJ2 (53,54). Moreover, Cys151-independent translocation of Nrf2 by NO2-OA also increased binding between Keap1 and the Cul3, promoting the Nrf2 escape from proteasomal degradation (51). It therefore seems likely that 15-oxoETE activates Nrf2 translocation through similar Cys151-independent pathways; however, the exact site(s) of 15-oxoETE alkylation remains to be determined through proteomic analysis. Recent work in developing novel tools for proteomic analysis of adducts, such as ω-alkynyl arachidonic acid, may prove useful in these future studies (55).
Electrophiles also induce heat shock factor-dependent heat shock protein expression and potently suppress pro-inflammatory cytokine and adhesion molecule expression through inhibition of NF-κB (17,21,45,51). Consistent with this, electrophilic fatty acids such as nitroalkene derivatives and 15d-PGJ2 inhibit multiple aspects of canonical NF-κB signaling (17,56). Both 15d-PGJ2 and nitroalkenes form Michael adducts with Cys38, located in the DNA binding domain, of the p65 subunit of NF-κB (19,57). Similarly, the p50 subunit DNA binding domain also contains a reactive cysteine at position 61 (58). Upstream of NF-κB, 15d-PGJ2, the A4 and J4 series neuroprostanes, and 4-hydroxynonenal have been shown to covalently adduct C179 in the activation loop of IKKβ, resulting in IKK inhibition, IκBα stabilization, and inhibition of NF-κB translocation (59-61). Results from this study suggest that 15-oxoETE may also target C179 of IKKβ, and thus potentially inhibit NF-κB at other redox sensitive levels of signaling including modification of the transcription factors; however, proteomic analysis is required for confirmation.
5. Conclusion
The data reported herein support that 15-oxoETE and other oxo-fatty acids are functionally-significant signaling mediators that have been conferred with an electrophilic character via the dehydrogenase activity of 15PGDH. 15-oxoETE activates anti-inflammatory Nrf2 signaling and down-regulates NF-κB-mediated pro-inflammatory signaling (Scheme 1). The pleiotropic nature of 15-oxoETE bioactivity warrants further investigation of anti-proliferative and anti-inflammatory signaling actions in specific disease models where 15-oxoETE may have therapeutic potential.
Scheme 1.
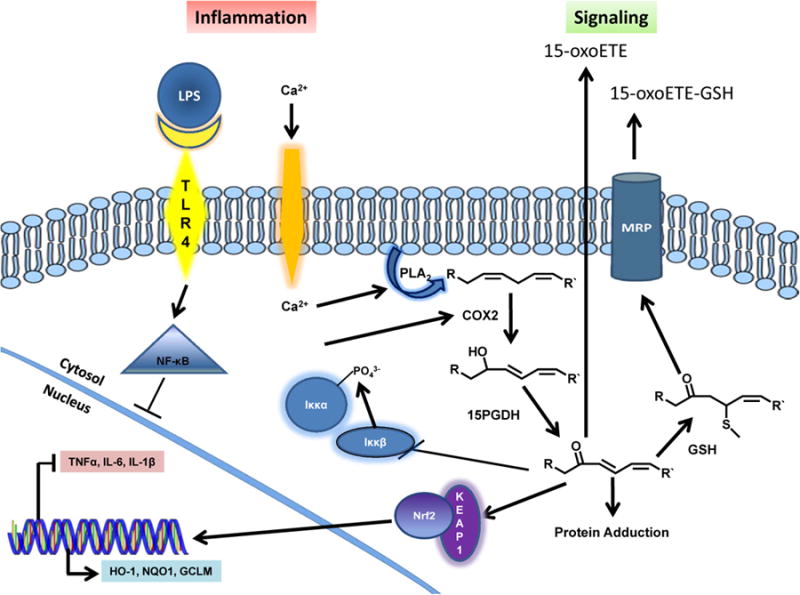
An illustration of 15-oxoETE-mediated modulation of inflammation via Nrf2 and NF-κB signaling pathways.
Supplementary Material
Highlights.
15PGDH metabolism of 15-HETE results in the formation of 15-oxoETE
15-oxoETE contains an α,β-unsaturated ketone and is electrophilic
Electrophilic fatty acids are bioactive, pleiotropic signaling mediators
15-oxoETE modulates inflammatory signaling via Nrf2 and NF-κB
Acknowledgments
This work was supported by NIH grants R01-HL058115 and R01-HL64937 (BF), P01HL103455 (M. Gladwin), T32-GM-008076 (NWS), P42ES023720 and P30ES013508 (IAB).
Abbreviations
- 15d-PGJ2
15-deoxy-prostaglandin J2
- 15PGDH
15-hydroxyprostaglandin dehydrogenase
- 15-HETE
15-hydroxyeicosatetraenoic acid
- 15-oxoETE
15-oxoeicosatetraenoic acid
- 15-ketoPGE2
15-keto-prostaglandin E2
- EFA
electrophilic fatty acids
- EA
ethacrynic acid
- GSH
glutathione
- GSTs
glutathione S-transferases
- IL
interleukin
- PGE2
prostaglandin E2
Footnotes
Conflict of Interest Statement: Bruce A. Freeman declares interest in Complexa Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bray JE, Marsden BD, Oppermann U. The human short-chain dehydrogenase/reductase (SDR) superfamily: a bioinformatics summary. Chemico-biological interactions. 2009;178:99–109. doi: 10.1016/j.cbi.2008.10.058. [DOI] [PubMed] [Google Scholar]
- 2.Persson B, Kallberg Y, Bray JE, Bruford E, Dellaporta SL, Favia AD, Duarte RG, Jornvall H, Kavanagh KL, Kedishvili N, Kisiela M, Maser E, Mindnich R, Orchard S, Penning TM, Thornton JM, Adamski J, Oppermann U. The SDR (short-chain dehydrogenase/reductase and related enzymes) nomenclature initiative. Chemico-biological interactions. 2009;178:94–98. doi: 10.1016/j.cbi.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niesen FH, Schultz L, Jadhav A, Bhatia C, Guo K, Maloney DJ, Pilka ES, Wang M, Oppermann U, Heightman TD, Simeonov A. High-affinity inhibitors of human NAD-dependent 15-hydroxyprostaglandin dehydrogenase: mechanisms of inhibition and structure-activity relationships. PLoS One. 2010;5:e13719. doi: 10.1371/journal.pone.0013719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tai HH, Ensor CM, Tong M, Zhou H, Yan F. Prostaglandin catabolizing enzymes. Prostaglandins & other lipid mediators. 2002;68-69:483–493. doi: 10.1016/s0090-6980(02)00050-3. [DOI] [PubMed] [Google Scholar]
- 5.Bergstroem S, Samuelsson B. Prostaglandins. Annual review of biochemistry. 1965;34:101–108. doi: 10.1146/annurev.bi.34.070165.000533. [DOI] [PubMed] [Google Scholar]
- 6.Coggins KG, Latour A, Nguyen MS, Audoly L, Coffman TM, Koller BH. Metabolism of PGE2 by prostaglandin dehydrogenase is essential for remodeling the ductus arteriosus. Nat Med. 2002;8:91–92. doi: 10.1038/nm0202-91. [DOI] [PubMed] [Google Scholar]
- 7.Smyth EM, Grosser T, Wang M, Yu Y, FitzGerald GA. Prostanoids in health and disease. Journal of lipid research. 2009;50 Suppl:S423–428. doi: 10.1194/jlr.R800094-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Zhang S, Arora JS, Snyder NW, Shah SJ, Blair IA. 11-Oxoeicosatetraenoic acid is a cyclooxygenase-2/15-hydroxyprostaglandin dehydrogenase-derived antiproliferative eicosanoid. Chem Res Toxicol. 2011;24:2227–2236. doi: 10.1021/tx200336f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei C, Zhu P, Shah SJ, Blair IA. 15-oxo-Eicosatetraenoic acid, a metabolite of macrophage 15-hydroxyprostaglandin dehydrogenase that inhibits endothelial cell proliferation. Mol Pharmacol. 2009;76:516–525. doi: 10.1124/mol.109.057489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serhan CN, Fiore S, Brezinski DA, Lynch S. Lipoxin A4 metabolism by differentiated HL-60 cells and human monocytes: conversion to novel 15-oxo and dihydro products. Biochemistry. 1993;32:6313–6319. doi: 10.1021/bi00076a002. [DOI] [PubMed] [Google Scholar]
- 11.Serhan CN, Petasis NA. Resolvins and Protectins in Inflammation Resolution. Chem Rev. 2011 doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, Blumberg RS, Serhan CN. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci U S A. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu D, Han C, Wu T. 15-hydroxyprostaglandin dehydrogenase (15-PGDH)-derived 15-keto-PGE2 inhibits cholangiocarcinoma cell growth through interaction with PPARgamma, SMAD2/3 and TAP63. J Biol Chem. 2013 doi: 10.1074/jbc.M113.453886. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Lu D, Han C, Wu T. 15-PGDH inhibits hepatocellular carcinoma growth through 15-keto-PGE/PPARgamma-mediated activation of p21. Oncogene. 2013 doi: 10.1038/onc.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snyder NW, Revello SD, Liu X, Zhang S, Blair IA. Cellular uptake and antiproliferative effects of 11-oxo-eicosatetraenoic acid. Journal of lipid research. 2013;54:3070–3077. doi: 10.1194/jlr.M040741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delmastro-Greenwood M, Freeman BA, Wendell SG. Redox-dependent anti-inflammatory signaling actions of unsaturated fatty acids. Annual review of physiology. 2014;76:79–105. doi: 10.1146/annurev-physiol-021113-170341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schopfer F, Cipollina C, Freeman BA. Formation and signaling actions of electrophilic lipids. Chem Rev. 2011;111:5997–6021. doi: 10.1021/cr200131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groeger AL, Cipollina C, Cole MP, Woodcock SR, Bonacci G, Rudolph TK, Rudolph V, Freeman BA, Schopfer FJ. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat Chem Biol. 2010;6:433–441. doi: 10.1038/nchembio.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui T, Schopfer FJ, Zhang J, Chen K, Ichikawa T, Baker PR, Batthyany C, Chacko BK, Feng X, Patel RP, Agarwal A, Freeman BA, Chen YE. Nitrated fatty acids: Endogenous anti-inflammatory signaling mediators. J Biol Chem. 2006;281:35686–35698. doi: 10.1074/jbc.M603357200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Itoh T, Fairall L, Amin K, Inaba Y, Szanto A, Balint BL, Nagy L, Yamamoto K, Schwabe JW. Structural basis for the activation of PPARgamma by oxidized fatty acids. Nature structural & molecular biology. 2008 doi: 10.1038/nsmb.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kansanen E, Jyrkkanen HK, Volger OL, Leinonen H, Kivela AM, Hakkinen SK, Woodcock SR, Schopfer FJ, Horrevoets AJ, Yla-Herttuala S, Freeman BA, Levonen AL. Nrf2-dependent and -independent responses to nitro-fatty acids in human endothelial cells: identification of heat shock response as the major pathway activated by nitro-oleic acid. J Biol Chem. 2009;284:33233–33241. doi: 10.1074/jbc.M109.064873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelley EE, Batthyany CI, Hundley NJ, Woodcock SR, Bonacci G, Del Rio JM, Schopfer FJ, Lancaster JR, Jr, Freeman BA, Tarpey MM. Nitro-oleic Acid, a Novel and Irreversible Inhibitor of Xanthine Oxidoreductase. J Biol Chem. 2008;283:36176–36184. doi: 10.1074/jbc.M802402200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonacci G, Schopfer FJ, Batthyany CI, Rudolph TK, Rudolph V, Khoo NK, Kelley EE, Freeman BA. Electrophilic fatty acids regulate matrix metalloproteinase activity and expression. J Biol Chem. 2011;286:16074–16081. doi: 10.1074/jbc.M111.225029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charles RL, Rudyk O, Prysyazhna O, Kamynina A, Yang J, Morisseau C, Hammock BD, Freeman BA, Eaton P. Protection from hypertension in mice by the Mediterranean diet is mediated by nitro fatty acid inhibition of soluble epoxide hydrolase. Proc Natl Acad Sci U S A. 2014;111:8167–8172. doi: 10.1073/pnas.1402965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SH, Rangiah K, Williams MV, Wehr AY, DuBois RN, Blair IA. Cyclooxygenase-2-mediated metabolism of arachidonic acid to 15-oxo-eicosatetraenoic acid by rat intestinal epithelial cells. Chem Res Toxicol. 2007;20:1665–1675. doi: 10.1021/tx700130p. [DOI] [PubMed] [Google Scholar]
- 26.Yuan F, Chen J, Sun PP, Guan S, Xu J. Wedelolactone inhibits LPS-induced pro-inflammation via NF-kappaB pathway in RAW 264.7 cells. Journal of biomedical science. 2013;20:84. doi: 10.1186/1423-0127-20-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tullius MV, Harth G, Horwitz MA. Glutamine synthetase GlnA1 is essential for growth of Mycobacterium tuberculosis in human THP-1 macrophages and guinea pigs. Infection and immunity. 2003;71:3927–3936. doi: 10.1128/IAI.71.7.3927-3936.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Codreanu SG, Ullery JC, Zhu J, Tallman KA, Beavers WN, Porter NA, Marnett LJ, Zhang B, Liebler DC. Alkylation damage by lipid electrophiles targets functional protein systems. Molecular & cellular proteomics : MCP. 2014;13:849–859. doi: 10.1074/mcp.M113.032953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myung SJ, Rerko RM, Yan M, Platzer P, Guda K, Dotson A, Lawrence E, Dannenberg AJ, Lovgren AK, Luo G, Pretlow TP, Newman RA, Willis J, Dawson D, Markowitz SD. 15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proc Natl Acad Sci U S A. 2006;103:12098–12102. doi: 10.1073/pnas.0603235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes D, Otani T, Yang P, Newman RA, Yantiss RK, Altorki NK, Port JL, Yan M, Markowitz SD, Mazumdar M, Tai HH, Subbaramaiah K, Dannenberg AJ. NAD+-dependent 15-hydroxyprostaglandin dehydrogenase regulates levels of bioactive lipids in non-small cell lung cancer. Cancer Prev Res (Phila) 2008;1:241–249. doi: 10.1158/1940-6207.CAPR-08-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding Y, Tong M, Liu S, Moscow JA, Tai HH. NAD+-linked 15-hydroxyprostaglandin dehydrogenase (15-PGDH) behaves as a tumor suppressor in lung cancer. Carcinogenesis. 2005;26:65–72. doi: 10.1093/carcin/bgh277. [DOI] [PubMed] [Google Scholar]
- 32.Wolf I, O'Kelly J, Rubinek T, Tong M, Nguyen A, Lin BT, Tai HH, Karlan BY, Koeffler HP. 15-hydroxyprostaglandin dehydrogenase is a tumor suppressor of human breast cancer. Cancer research. 2006;66:7818–7823. doi: 10.1158/0008-5472.CAN-05-4368. [DOI] [PubMed] [Google Scholar]
- 33.Potula HS, Wang D, Quyen DV, Singh NK, Kundumani-Sridharan V, Karpurapu M, Park EA, Glasgow WC, Rao GN. Src-dependent STAT-3-mediated expression of monocyte chemoattractant protein-1 is required for 15(S)-hydroxyeicosatetraenoic acid-induced vascular smooth muscle cell migration. J Biol Chem. 2009;284:31142–31155. doi: 10.1074/jbc.M109.012526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wittwer J, Hersberger M. The two faces of the 15-lipoxygenase in atherosclerosis. Prostaglandins Leukot Essent Fatty Acids. 2007;77:67–77. doi: 10.1016/j.plefa.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Gertow K, Nobili E, Folkersen L, Newman JW, Pedersen TL, Ekstrand J, Swedenborg J, Kuhn H, Wheelock CE, Hansson GK, Hedin U, Haeggstrom JZ, Gabrielsen A. 12- and 15-lipoxygenases in human carotid atherosclerotic lesions: associations with cerebrovascular symptoms. Atherosclerosis. 2011;215:411–416. doi: 10.1016/j.atherosclerosis.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 36.Zhao J, O'Donnell VB, Balzar S, St Croix CM, Trudeau JB, Wenzel SE. 15-Lipoxygenase 1 interacts with phosphatidylethanolamine-binding protein to regulate MAPK signaling in human airway epithelial cells. Proc Natl Acad Sci U S A. 2011;108:14246–14251. doi: 10.1073/pnas.1018075108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao J, Maskrey B, Balzar S, Chibana K, Mustovich A, Hu H, Trudeau JB, O'Donnell V, Wenzel SE. Interleukin-13-induced MUC5AC is regulated by 15-lipoxygenase 1 pathway in human bronchial epithelial cells. Am J Respir Crit Care Med. 2009;179:782–790. doi: 10.1164/rccm.200811-1744OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gulliksson M, Brunnstrom A, Johannesson M, Backman L, Nilsson G, Harvima I, Dahlen B, Kumlin M, Claesson HE. Expression of 15-lipoxygenase type-1 in human mast cells. Biochim Biophys Acta. 2007;1771:1156–1165. doi: 10.1016/j.bbalip.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Shankaranarayanan P, Nigam S. IL-4 induces apoptosis in A549 lung adenocarcinoma cells: evidence for the pivotal role of 15-hydroxyeicosatetraenoic acid binding to activated peroxisome proliferator-activated receptor gamma transcription factor. J Immunol. 2003;170:887–894. doi: 10.4049/jimmunol.170.2.887. [DOI] [PubMed] [Google Scholar]
- 40.Soumya SJ, Binu S, Helen A, Reddanna P, Sudhakaran PR. 15(S)-HETE-induced angiogenesis in adipose tissue is mediated through activation of PI3K/Akt/mTOR signaling pathway. Biochemistry and cell biology = Biochimie et biologie cellulaire. 2013;91:498–505. doi: 10.1139/bcb-2013-0037. [DOI] [PubMed] [Google Scholar]
- 41.Wu MY, Yang RS, Lin TH, Tang CH, Chiu YC, Liou HC, Fu WM. Enhancement of PLGF production by 15-(S)-HETE via PI3K-Akt, NF-kappaB and COX-2 pathways in rheumatoid arthritis synovial fibroblast. European journal of pharmacology. 2013;714:388–396. doi: 10.1016/j.ejphar.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 42.Masoodi M, Pearl DS, Eiden M, Shute JK, Brown JF, Calder PC, Trebble TM. Altered colonic mucosal Polyunsaturated Fatty Acid (PUFA) derived lipid mediators in ulcerative colitis: new insight into relationship with disease activity and pathophysiology. PLoS One. 2013;8:e76532. doi: 10.1371/journal.pone.0076532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oh JY, Giles N, Landar A, Darley-Usmar V. Accumulation of 15-deoxy-delta(12,14)-prostaglandin J2 adduct formation with Keap1 over time: effects on potency for intracellular antioxidant defence induction. The Biochemical journal. 2008;411:297–306. doi: 10.1042/bj20071189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nettles KW. Insights into PPARgamma from structures with endogenous and covalently bound ligands. Nature structural & molecular biology. 2008;15:893–895. doi: 10.1038/nsmb0908-893. [DOI] [PubMed] [Google Scholar]
- 45.Schopfer FJ, Cole MP, Groeger AL, Chen CS, Khoo NK, Woodcock SR, Golin-Bisello F, Motanya UN, Li Y, Zhang J, Garcia-Barrio MT, Rudolph TK, Rudolph V, Bonacci G, Baker PR, Xu HE, Batthyany CI, Chen YE, Hallis TM, Freeman BA. Covalent peroxisome proliferator-activated receptor gamma adduction by nitro-fatty acids: selective ligand activity and anti-diabetic signaling actions. J Biol Chem. 2010;285:12321–12333. doi: 10.1074/jbc.M109.091512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lundstrom SL, Levanen B, Nording M, Klepczynska-Nystrom A, Skold M, Haeggstrom JZ, Grunewald J, Svartengren M, Hammock BD, Larsson BM, Eklund A, Wheelock AM, Wheelock CE. Asthmatics exhibit altered oxylipin profiles compared to healthy individuals after subway air exposure. PLoS One. 2011;6:e23864. doi: 10.1371/journal.pone.0023864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, Bandyopadhyay S, Jones KN, Kelly S, Shaner RL, Sullards CM, Wang E, Murphy RC, Barkley RM, Leiker TJ, Raetz CR, Guan Z, Laird GM, Six DA, Russell DW, McDonald JG, Subramaniam S, Fahy E, Dennis EA. Lipidomics reveals a remarkable diversity of lipids in human plasma. Journal of lipid research. 2010;51:3299–3305. doi: 10.1194/jlr.M009449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, Sinelnikov I, Krishnamurthy R, Eisner R, Gautam B, Young N, Xia J, Knox C, Dong E, Huang P, Hollander Z, Pedersen TL, Smith SR, Bamforth F, Greiner R, McManus B, Newman JW, Goodfriend T, Wishart DS. The human serum metabolome. PLoS One. 2011;6:e16957. doi: 10.1371/journal.pone.0016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bell-Parikh LC, Ide T, Lawson JA, McNamara P, Reilly M, FitzGerald GA. Biosynthesis of 15-deoxy-delta12,14-PGJ2 and the ligation of PPARgamma. The Journal of clinical investigation. 2003;112:945–955. doi: 10.1172/JCI18012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dennehy MK, Richards KA, Wernke GR, Shyr Y, Liebler DC. Cytosolic and nuclear protein targets of thiol-reactive electrophiles. Chem Res Toxicol. 2006;19:20–29. doi: 10.1021/tx050312l. [DOI] [PubMed] [Google Scholar]
- 51.Kansanen E, Bonacci G, Schopfer FJ, Kuosmanen SM, Tong KI, Leinonen H, Woodcock SR, Yamamoto M, Carlberg C, Yla-Herttuala S, Freeman BA, Levonen AL. Electrophilic Nitro-fatty Acids Activate NRF2 by a KEAP1 Cysteine 151-independent Mechanism. J Biol Chem. 2011;286:14019–14027. doi: 10.1074/jbc.M110.190710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Villacorta L, Zhang J, Garcia-Barrio MT, Chen XL, Freeman BA, Chen YE, Cui T. Nitro-linoleic acid inhibits vascular smooth muscle cell proliferation via the Keap1/Nrf2 signaling pathway. Am J Physiol Heart Circ Physiol. 2007;293:H770–H776. doi: 10.1152/ajpheart.00261.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, Nakayama Y, Eguchi M, Wada Y, Kumagai Y, Yamamoto M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol. 2009;29:493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsujita T, Li L, Nakajima H, Iwamoto N, Nakajima-Takagi Y, Ohashi K, Kawakami K, Kumagai Y, Freeman BA, Yamamoto M, Kobayashi M. Nitro-fatty acids and cyclopentenone prostaglandins share strategies to activate the Keap1-Nrf2 system: a study using green fluorescent protein transgenic zebrafish. Genes Cells. 2011;16:46–57. doi: 10.1111/j.1365-2443.2010.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beavers WN, Serwa R, Shimozu Y, Tallman KA, Vaught M, Dalvie ED, Marnett LJ, Porter NA. omega-Alkynyl lipid surrogates for polyunsaturated fatty acids: free radical and enzymatic oxidations. J Am Chem Soc. 2014;136:11529–11539. doi: 10.1021/ja506038v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scher JU, Pillinger MH. 15d-PGJ2: the anti-inflammatory prostaglandin? Clin Immunol. 2005;114:100–109. doi: 10.1016/j.clim.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 57.Straus DS, Pascual G, Li M, Welch JS, Ricote M, Hsiang CH, Sengchanthalangsy LL, Ghosh G, Glass CK. 15-deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc Natl Acad Sci U S A. 2000;97:4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lambert C, Li J, Jonscher K, Yang TC, Reigan P, Quintana M, Harvey J, Freed BM. Acrolein inhibits cytokine gene expression by alkylating cysteine and arginine residues in the NF-kappaB1 DNA binding domain. J Biol Chem. 2007;282:19666–19675. doi: 10.1074/jbc.M611527200. [DOI] [PubMed] [Google Scholar]
- 59.Musiek ES, Brooks JD, Joo M, Brunoldi E, Porta A, Zanoni G, Vidari G, Blackwell TS, Montine TJ, Milne GL, McLaughlin B, Morrow JD. Electrophilic cyclopentenone neuroprostanes are anti-inflammatory mediators formed from the peroxidation of the omega-3 polyunsaturated fatty acid docosahexaenoic acid. J Biol Chem. 2008;283:19927–19935. doi: 10.1074/jbc.M803625200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rossi A, Kapahi P, Natoli G, Takahashi T, Chen Y, Karin M, Santoro MG. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature. 2000;403:103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 61.Ji C, Kozak KR, Marnett LJ. IkappaB kinase, a molecular target for inhibition by 4-hydroxy-2-nonenal. J Biol Chem. 2001;276:18223–18228. doi: 10.1074/jbc.M101266200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.