Abstract
Objective
Ex vivo models are routinely used to investigate the barrier function of the vocal fold epithelium. However, there are limited reports on assays that can be used to investigate the effect of clinically-relevant challenges on vocal fold epithelial tissue viability. Our objective was to determine the utility of two assays routinely used in cell culture – a cellular metabolic activity assay and a cell membrane integrity assay - to investigate the viability of ex vivo porcine vocal fold epithelium.
Study Design
Prospective, ex vivo animal study
Methods
Porcine vocal folds were exposed to acrolein, hydrochloric acid, or hydrogen peroxide challenge. An untreated, sham challenge was included as a control. Assays including metabolic activity, cell membrane integrity, and histology were used to determine whether challenges reduced epithelial viability as compared to sham.
Results
Cell membrane integrity and metabolic activity assays detected reductions in viability following hydrochloric acid and hydrogen peroxide challenges but not acrolein challenge as compared to sham. No challenge produced significant changes in epithelial appearance as evidenced by light microscopy.
Conclusion
Metabolic activity and cell membrane integrity assays are valuable tools that can be used to evaluate the viability of ex vivo vocal fold epithelial tissue following clinically-relevant challenges. As viability is reduced, the ability of epithelial tissue to maintain its barrier function is compromised. Accurate assessment of viability may provide us clues into understanding mechanisms underlying vocal fold epithelial injury and disease.
Keywords: Ex vivo model, vocal fold epithelium, viability assay
INTRODUCTION
In ex vivo models, vocal folds are excised from larynges post mortem and then utilized for experimental testing.1,2 This model has numerous advantages including cost-effectiveness as well as ready tissue availability from human autopsy or animals intended for slaughter.3 Ex vivo models have emerged as one of the primary means to study the vocal fold epithelium. As the outermost layer of the vocal folds, the epithelium forms a barrier that is critical for the protection of the underlying lamina propria from a wide range of mechanical, chemical, and biologic challenges.4 The ability of the epithelium to form an effective barrier is influenced by various factors including tissue viability or the tissue’s ability to maintain or recover its potentialities.5 Specifically, as viability is reduced, epithelial barrier function is compromised. However, our knowledge regarding whether clinically-relevant challenges to the epithelium impact viability is limited. Consequently, there is a need to identify and test assays that can be used to assess the viability of ex vivo vocal fold epithelial tissue.
Viable tissues are defined as those that are capable of living.6 Viability assays, therefore, measure attributes of a tissue when it is alive.7 Tissues have multiple attributes in which viability can be assessed.8,9 As a result, a multiparametric approach is necessary for accurate assessment of ex vivo tissue viability. Assays available to assess tissue viability are typically classified into groups based upon specific attributes being assessed,6,7 with two principle groups being structural and metabolic assays. Routine histology to investigate whether challenges induce gross morphological damage is a structural viability assay that has been used previously with ex vivo vocal fold epithelium.10,11 However, other structural viability assays such as those examining cell membrane integrity or metabolic viability assays have yet to be tested for use in ex vivo vocal fold tissue. While electron microscopy may be useful for examining cell membrane integrity,12,13 reductions in viability are difficult to quantify using this method. Viability assays that investigate cell membrane integrity using a specialized stain to detect membrane damage and cellular metabolic activity are easily quantifiable, well-established in cell cultures, and have been used previously with a wide variety of cell types including vocal fold fibroblasts.14,15 Investigations are needed that seek to evaluate whether cell membrane integrity and metabolic activity assays can be used to evaluate the effect of clinically relevant challenges on the viability of ex vivo vocal fold epithelium.
The objective of this study was to determine the utility of two assays routinely used in cell culture – a cellular metabolic activity assay and a cell membrane integrity assay - to investigate the viability of ex vivo porcine vocal fold epithelium. Similar assays have been successfully used to assess the viability of ex vivo skin, cornea, and buccal tissues.16–18 The utility of a viability assay can be determined by investigating whether the assay is able to successfully detect reductions in tissue viability following a challenge as compared to an untreated tissue.8 Consequently, we tested whether three clinically relevant challenges would reduce viability as measured by epithelial metabolic activity and cell membrane integrity as compared to an untreated, sham challenge. Challenges included: acrolein, an environmentally pervasive pollutant,19 hydrochloric acid, the acidic component of laryngopharyngeal reflux,11 and hydrogen peroxide, a common reactive oxygen species.10 We hypothesized that these assays would detect reductions in epithelial viability following challenges as compared to sham. As numerous investigations have stressed the importance of using a multiparametric approach to assess tissue viability,8 parallel histological analysis of ex vivo vocal fold tissue was also performed to investigate whether challenges induce gross morphological damage. Findings from this investigation will provide researchers with methods to investigate two unique attributes, metabolic activity and cell membrane integrity, of ex vivo vocal fold viability. As viability is reduced, the ability of a tissue to maintain itself and function is significantly compromised.7 Consequently, accurate assessment of vocal fold epithelial viability can strongly influence interpretation of experimental findings and may also provide us with clues into understanding pathophysiology of vocal fold epithelial injury and disease.
MATERIALS & METHODS
Vocal Fold Preparation
A porcine model (Sus scrofa domesticus) was used in the current investigation. Morphologically, porcine vocal folds are similar to human vocals20,21 and have been used previously to investigate the effects of numerous clinically relevant challenges on the barrier function of ex vivo vocal fold epithelium.2,10,22 Porcine larynges (N=33) from male and female animals approximately 6-months of age were obtained from commercial slaughterhouses. Larynges originated from various commercial breeds including: Berkshire, Duroc, Spotted Pig, and Yorkshire. Larynges were collected within 30 minutes of animal sacrifice and transported to the laboratory on ice in freshly prepared phosphate buffered saline (PBS, pH 7.4). Vocal folds were harvested following validated procedures.1,10,11,23 Larynges were first separated into two hemilarynges by dissection along the mid-sagittal plane. Shallow incisions, using a scalpel, were then made 1 cm above and 1 cm below the midmembranous vocal fold. Fine forceps and surgical scissors were then utilized to carefully separate the epithelium and superficial lamina propria as a sheet from the underlying connective tissue and muscle. A 6 mm diameter disc of midmembranous vocal fold tissue from each hemilarynx was then obtained using a punch biopsy device.
Challenges
Immediately following dissection, the epithelial surface of the vocal fold discs were incubated for 60 minutes at 37°C in one of the following challenges: acrolein (400µm), hydrochloric acid (HCl, pH3), or hydrogen peroxide (H2O2, 1M). Challenges were prepared in fresh PBS immediately prior to each experiment. An untreated, sham challenge (PBS-alone) was included as a control. Similar challenges have been utilized previously to investigate the adverse effects of clinically-relevant insults on the vocal fold epithelial barrier10,11,19 and were specifically chosen for the purpose of the current investigation because these were hypothesized to reduce epithelial viability. While the concentration of acrolein and pH of HCl is representative of what occurs in vivo,24–26 a lower concentration of H2O2 has been used in previous studies in the vocal folds to mimic physiologic conditions.10 H2O2 was selected as a positive control for significantly reduced viability. H2O2 has been consistently shown in the literature to decrease epithelial cell viability as measured by a metabolic activity and cell membrane integrity assays.27–29 While lower concentrations have been used with cell cultures, the concentration was increased to account for the use of tissues. Chemicals were purchased from Sigma Aldrich (St. Louis, MI).
Metabolic Activity Assay
MTT assay uses cellular metabolism as an indicator of viability.5,18 MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) is a yellow, water soluble tetrazolium salt that is converted in viable cells to dark purple insoluble formazan by enzymes in active mitochondria. The amount of measured formazan is directly proportional to the number of living cells. Vocal fold dissected from 14 larynges were utilized for the metabolic activity assay. Vocal folds were exposed for 60 minutes to sham (N=6), acrolein (N=6), HCl (N=6), or H2O2 (N=6) challenge. To ensure that the MTT assays accurately discriminates between viable and non-viable tissue, additional vocal folds (N=3) were treated for 60 minutes with 10% DMSO.5 Vocal fold exposure to DMSO prior to the addition of the MTT solution significantly inhibits the conversion of tetrazolium salt to formazan. Consequently, DMSO-challenged tissues are measured as non-viable. Challenged tissues were then subjected to the MTT assay. Vocal folds were rinsed twice with PBS and placed in individual wells of a six-well tissue culture plate. Freshly prepared MTT solution (2 ml at 2 mg/ml concentration) was added to each well, and the plate was rotated at 100 rpm for 2 hours at 37°C. After incubation, remaining MTT solution was removed and vocal folds were rinsed twice for 1 minute with 1 ml of PBS. Vocal folds were then finely minced with surgical scissors and formazan precipitate was extracted from the tissue with 4 ml of dimethyl sulfoxide (DMSO). Minced vocal folds with DMSO were rotated at 100 rpm for 80 minutes. Aliquots (200µl) of formazan precipitate were taken for assessment of viability. Formazan absorbance was measured using a SmartSpec Plus spectrophotometer (Bio-Rad Laboratories, Hercules, CA) at 540 nm with DMSO as a blank. Viability index for each vocal fold was calculated by dividing the measured formazan absorbance by tissue weight (abs/mg).
Membrane Integrity Assay
A Hoechst/Ethidium stain combination was utilized to investigate the integrity of vocal fold epithelial cell membranes. Hoechst 33342 (Hoechst), a nucleic acid dye, is a cell permeant nuclear stain that emits a fluorescent blue light in all cells, viable or non-viable, when excited by ultraviolet light at 350 nm.30 Ethidium homodimer −1 (Ethidium) produces a bright red fluorescence exclusively in the nuclei of non-viable cells with damaged cell membranes when excited at 528 nm.31 Vocal folds dissected from 13 larynges were utilized for the membrane integrity assay. Vocal folds were incubated for 60 minutes in sham (N=10), acrolein (N=5), HCl (N=5), or H2O2 (N=5) challenge. Immediately following challenges, vocal folds were rinsed with fresh PBS and placed epithelial side down in an 8-well coverslip plate. Vocal folds were incubated for 40 minutes at room temperature in a Hoechst (10µg/ml) and Ethidium (4µM) stain combination. Stains were removed and vocal folds gently rinsed with PBS to remove excess dye. The vocal fold epithelial surface was immediately viewed and imaged using a Nikon Eclipse TE 2000-S (Tokyo, Japan) or EVOS® digital inverted (Advanced Microscopy Group, Bothell, WA) fluorescent microscope. Three image sets (Hoechst image, Ethidium image) from randomly selected fields were acquired for each vocal fold at 20× magnification. Positively stained Hoechst nuclei (blue) and Ethidium nuclei (red) were counted by a blinded observer using ImageJ cell counting software (Version 1.45s, National Institutes of Health, USA). For each vocal fold, the number of Hoechst and Ethidium stained cells were summed across the three image sets. Cell damage rate was calculated by dividing the total number of damaged cells (Ethidium stained) by the total number of cells (Hoechst stained) and multiplying by 100 to yield a percentage of damage cells. Cell counts for 10% of randomly selected vocal folds were repeated. Intra- and interrater reliability was assessed using two-way, mixed intraclass correlation coefficients (ICC). Intra- (ICC = 0.95) and interrater ICCs (ICC = 0.97) were in the excellent range.
Histology
Vocal folds dissected from 6 larynges were utilized for histology. Vocal folds were incubated in sham (N=3), acrolein (N=3), HCl (N=3), or H2O2 (N=3) challenge. Vocal folds were then fixed in 10% neutral buffered formalin, dehydrated via an ethyl alcohol series, and embedded in paraffin. Tissue was sectioned (5µm) and stained with hematoxylin and eosin (H&E). Epithelial and subepithelial appearance of the vocal fold was graded for damage by a pathologist blinded to the identity of the challenge. Vocal folds were graded on the following damage criteria: epithelial shedding, epithelial erosion, epithelial edema, epithelial shrinkage, epithelial detachment, vacuolization, and interstitial edema. Criteria were given a damage score on the following 0 to 5 scale where: 0= none, 1=minimal, 2=mild, 3=moderate, 4=marked, and 5=severe. A similar scale has been used previously in porcine vocal folds to investigate the effects of simulated reflux and reactive oxygen species on vocal fold structure.10,11 10% of slides were selected at random for assessment of intrarater reliability. All scores yielded nonsignificant differences (p=0.33) for repeat grading using a paired t-test.
Statistical Analysis
Results of the metabolic activity assay (viability index), cell membrane integrity assay (cell damage rate), and histology (damage score) were expressed as means ± standard deviations (SD). For the metabolic activity assay and cell membrane integrity assay, separate, one-way ANOVAs were used to determine whether viability differed between sham, acrolein, HCl, and H2O2 challenges. If the p value was significant, then pair wise comparisons were examined (Tukey’s HSD tests) to identify which challenges reduced viability as compared to sham. For histological scored criteria, Kruskal-Wallis tests were to compare the effects of challenges on vocal fold structure. For a criteria to be consider scored, at least one vocal fold had to receive a score > 0. Separate tests were used for each scored criterion. A p < 0.05 was considered statistically significant difference. All statistical analyses were performed using SPSS Version 22 software (IBM, Chicago, IL).
RESULTS
Metabolic Activity Assay
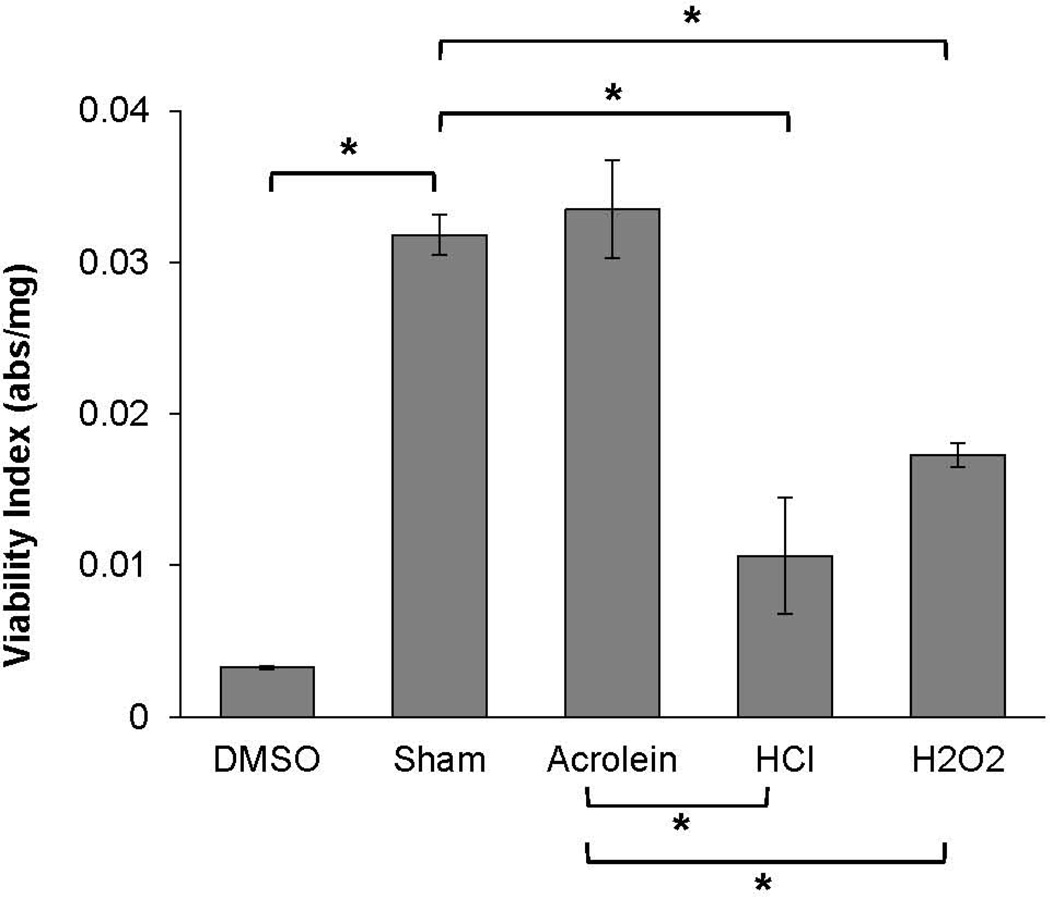
In order to confirm that the MTT assay accurately discriminates between viable and non-viable cells, viability indices for vocal folds challenged with DMSO were compared to sham-challenged tissues. As expected, viability indices were significantly reduced following DMSO as compared to sham challenge (p < 0.001, Figure 1). Consequently, we went on to investigate whether MTT assay would detect reductions in viability following acrolein, HCl, and H2O2 challenges as compared to sham. Viability indices significantly differed across sham (0.032 abs/mg ± 0.003), acrolein (0.034 abs/mg ± 0.008), HCl (0.011 abs/mg ± 0.009), and H2O2 (0.017 abs/mg ± 0.002) challenges (F(3, 20) =, p < 0.001, Figure 1). Tukey post-hoc comparisons revealed viability indices significantly decreased following HCl and H2O2 challenges as compared to sham (HCl vs sham: p < 0.001, H2O2 vs sham: p = 0.01) and acrolein (HCl vs acrolein: p < 0.001, H2O2 vs acrolein: p = 0.005) challenge. Lower viability indices as compared to sham are indicative of reduced cellular metabolism and viability following HCl and H2O2 challenge. Viability indices were not significantly different between acrolein and sham challenges vocal folds (p = 0.99).
Figure 1.
Viability indices (abs/mg) of porcine vocal folds exposed to sham, acrolein, HCl, or H2O2 challenge or DMSO. Data are presented as mean and standard error of the mean. Viability indices significantly decreased following HCl and H2O2 challenges as compared to sham and acrolein. * p < 0.05
Membrane Integrity Assay
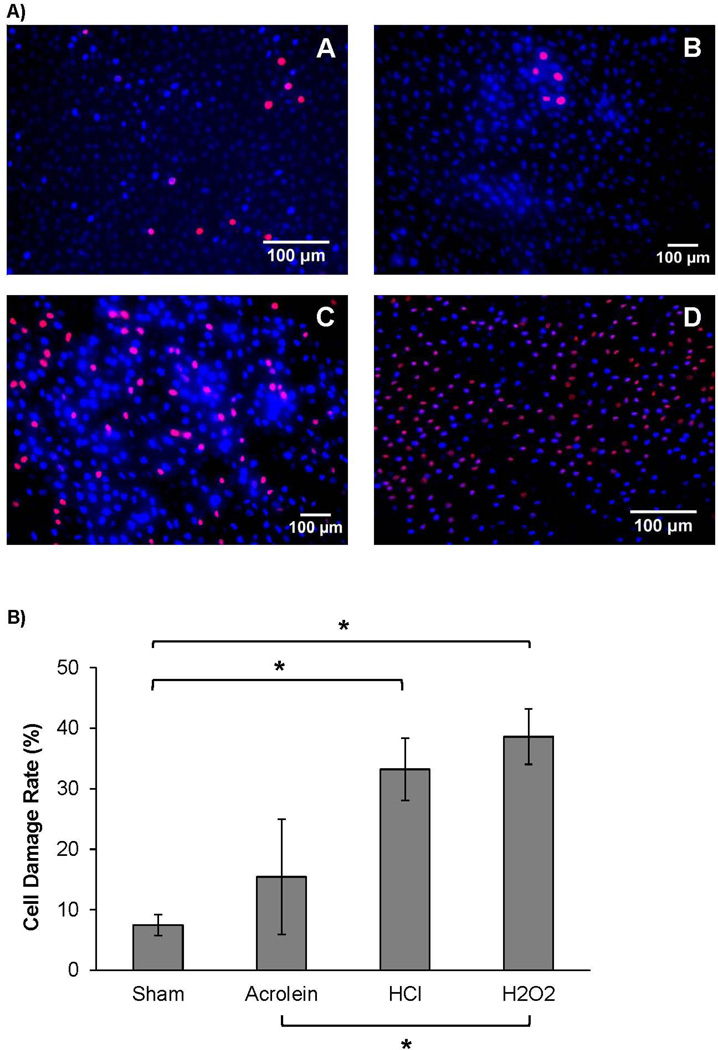
Cell damage rates differed significantly across sham (7.46% ± 5.57), acrolein (15.43 ± 21.27), HCl (33.20 ± 11.49), and H2O2 (38.60 ± 10.21) challenges (F(3,21) = 9.86, p < 0.001, Figure 2A). Tukey’s HSD post-hoc comparisons revealed that HCl and H2O2 challenges resulted in significantly greater cell damage rates as compared to sham challenge (HCl vs sham: p = 0.004, H2O2 vs sham: p = 0.001, Figure 2B). H2O2 challenge also caused significantly greater cell membrane damage as compared to acrolein challenge (p = 0.03). Greater cell damage rates as compared to sham are indicative of epithelial cell membrane damage and reduced viability following HCl and H2O2 challenge. Cell damage rates between acrolein and sham challenged vocal folds were not significantly different (p = 0.63).
Figure 2.
A) Representative fluorescent micrographs of the effect of sham (A), acrolein (B), HCl (C), and H2O2 (D) challenges on porcine vocal fold epithelial cell membrane integrity (Hoechst (blue), ethidium (red), 20×). Diffuse labeling of damaged cells (red) is observed in the HCl and H2O2, but not sham or acrolein challenged tissue. B) Epithelial cell damage rate (%) of porcine vocal folds exposed to sham, acrolein, HCl, or H2O2 challenge, Data are presented as mean and standard error of the mean. Damage rate significantly increased following HCl and H2O2 challenges as compared to sham and H2O2 challenge as compared to acrolein. * p < 0.05
Histology
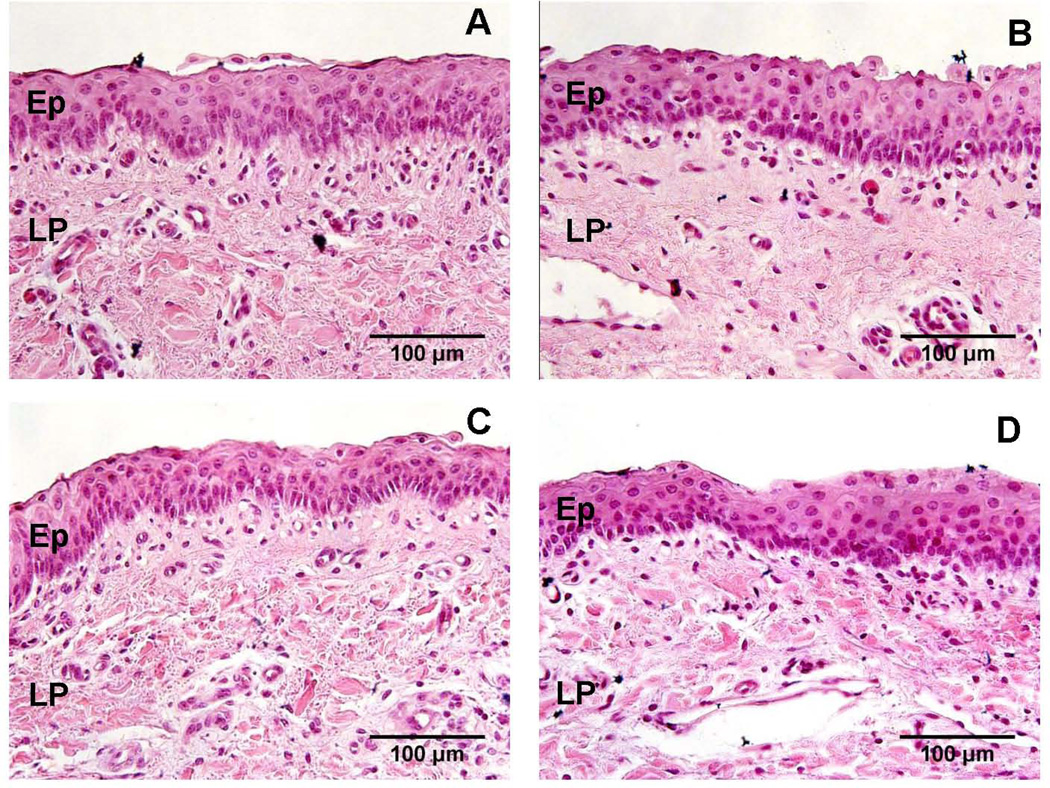
All vocal folds had stratified squamous epithelium, as confirmed by the pathologist. Two damage criteria not scored included epithelial shedding and epithelial detachment. All other criteria presented with mean damage scores less than or equal to one suggesting overall minimal tissue damage was detected using histology (Figure 3). Damage scores between sham, acrolein, HCl, and H2O2-challenged vocal folds were not significantly different for any scored criteria: epithelial erosion (H(3) = 1.22, p = 0.75), epithelial edema (H(3) = 2.21, p = 0.53), epithelial thinning (H(3) = 2.20, p = 0.53), vacuolization (H(3) = 0.41, p = 0.94), and interstitial edema (H(3) = 2.20, p = 0.53).
Figure 3.
Representative light micrographs of the effect of sham (A), acrolein (B), HCl (C), and H2O2 (D) challenges on vocal fold structure (H&E stain, 40×). No significant damage was observed following any challenge. Ep = stratified squamous epithelium, LP = lamina propria.
DISCUSSION
Use of ex vivo tissue has permitted significant advancements in our understanding of vocal fold epithelial biology. Epithelium is necessary for vocal fold health by providing a critical barrier function.4 Yet, there are few reports in the literature on methods to measure the viability of vocal fold epithelial tissue following challenges. As viability is reduced, the ability of an epithelium to maintain its barrier function is compromised. Being able to accurately investigate viability may provide us clues into understanding mechanisms underlying vocal fold epithelial injury and disease. Various classifications of viability assays including structural and metabolic-based have been developed for use with a large array of cell lines. Fewer have been tested for use in the study of tissue.18 In the current investigation, we utilized a membrane integrity assay and cellular metabolic activity assay to evaluate the viability of ex vivo porcine vocal fold epithelium. Specifically, we were interested in examining if these assays could be used to investigate whether three clinically relevant challenges would reduce epithelial viability as compared to an untreated, sham challenge. Reductions in viability as measured by both cellular metabolic activity and cell membrane integrity were observed following HCl and H2O2, but not acrolein challenge as compared to the sham challenge. No significant changes in histologic appearance were observed following any challenge.
Metabolic activity and cell membrane integrity assays successfully discriminated between experimental challenges with reproducible results across vocal fold samples. Findings between assays were also notably consistent with both demonstrating that only H2O2 and HCl challenges reduced viability within the timeframe investigated in the current investigation. Larger-scale, gross morphological changes can be associated with tissue viability.9 As a result, routine histology was also performed to assess ex vivo vocal fold epithelial viability. While histology may provide a general impression of viability by scoring degrees of tissue damage, it depends on subjective rater assessment and cannot be quantified in a manner consistent with robust viability assays.6 No significant tissue damage following any challenge was detected using histology. It may be that measures of metabolic activity and cell membrane integrity are attributes more sensitive to reductions in epithelial viability. It is also possible that acute challenges, such as those used here, are not of significant duration to induce gross morphological changes.
Tetrazolium salt assays such as the MTT have been used extensively as assessments of viability.9 MTT is a functional assay that works by measuring the activity of enzymes involved in cellular metabolism. An advantage of the MTT assay is that relatively small amounts of tissue can be processed and analyzed semi-automatically within a short time period. Furthermore, results are easily quantifiable and interpreted. It is of note that when preparing vocal folds for the MTT assay, dissection limitations preclude the complete separation of the superficial lamina propria from the vocal fold epithelium. This entire tissue is then utilized for assessment. In the current study, only the epithelial side of the vocal fold tissue was exposed to challenges. Consequently, it is likely reductions in viability following HCl and H2O2 exposure is secondary to changes occurring in the epithelium.
A Hoechst/Ethidium stain combination was used to investigate the integrity of vocal fold epithelial cell membranes. This structural assay detects cell membrane damage, a well-recognized attribute of cell viability, which is unable to be visualized using routine histology alone. Based upon our findings, a cell membrane integrity assay, similar to the metabolic activity assay, is a suitable method to investigate vocal fold epithelial viability. However, this assay does present with some limitations. Ethidium stain produces a bright red fluorescence in the nuclei of cells with damaged cell membranes and has been used previously as an indicator of epithelial cell death.32,33 However, ethidium does not discriminate between cell death that results from apoptosis or necrosis. Future studies will attempt to adapt dual staining protocols such as fluorescent Annexin V and propidium iodide to discriminate between apoptotic and necrotic cell death in ex vivo vocal fold tissue.34 In addition, vocal fold epithelium is composed of multiple cell layers.1 When using this assay, as described, only the outermost layer of vocal fold epithelial cells can be visualized. Future investigations may employ use of 3- dimensional microscopy techniques in order to examine the multiple cell layers of the vocal fold epithelium. This technique combined with cell membrane staining has been used previously with the stratified squamous epithelium of the cornea30 and may also be useful in vocal folds.
In the current study, we examined two classifications of viability assays: structural and metabolic. However, other assay classifications are also relevant for testing the viability of ex vivo vocal fold epithelium. Transepithelial resistance (TER) is functional measure of epithelial viability.35 TER measures the “tightness” of the epithelium to the passage of electrolytes.4 Viable vocal fold epithelia demonstrate a high TER suggestive of “tight” epithelial barrier. If a challenge to the vocal fold epithelium reduces TER, epithelia are “leaky” and considered no longer viable. HCl challenge has been shown previously to significantly decrease TER suggesting reduced viability.11 Acrolein challenge, on the other hand, does not influence TER and functional viability.19 This is consistent with our findings from the metabolic activity and cell membrane integrity assays. However, as viability assays are derived from various classifications, these measurements do not always correlate with one other.9 This stresses the importance of using multiple assays of different classifications to investigate various cellular attributes during viability assessment of ex vivo vocal fold epithelial tissue.
CONCLUSION
Metabolic activity assay and membrane integrity activity assay are valuable tools that can be used to evaluate the viability of ex vivo vocal fold epithelial tissue following clinically-relevant challenges. To the best of our knowledge, this is the first time these assays have been adapted for use with vocal fold tissue. Assessment of cellular metabolic activity and membrane integrity should be included in future ex vivo studies as reproducible and sensitive measures of epithelial viability following a wide-array of chemical, mechanical, bacterial, or viral challenges.
ACKNOWLEDGMENTS
This work was supported by NIH Grants R01 DC012773, R01 DC011759, R03 DC008690, and T32 DC009401 from the National Institute on Deafness and other Communicative Disorders (NIDCD). We thank Drew Roenneburg for technical support during histology preparation and Dr. David Yang for his assistance during histological scoring.
Footnotes
Conflict of Interest and Financial Disclosures: None
This research was presented at the American Broncho-Esophagological Society Meeting at the Annual Combined Otolaryngological Society Meeting, May 15–16, 2014, Las Vegas, NV
We also appreciate the contributions of April Barr and Sarah Wang towards data analysis. Porcine larynges were received from Monon Meat Packing, Monon, IN, Black Earth Meats, Black Earth, WI and Hoesley’s Meats, New Glarus, WI.
REFERENCES
- 1.Fisher K, Telser A, Phillips J, Yeates D. Regulation of vocal fold transepithelial water fluxes. J Appl Physiol. 2001;91:1401–1411. doi: 10.1152/jappl.2001.91.3.1401. [DOI] [PubMed] [Google Scholar]
- 2.Bulmer D, Ali M, Brownlee I, Dettmar P, Pearson J. Laryngeal mucosa: Its susceptability to damage by acid and pepsin. Laryngoscope. 2010;120:777–782. doi: 10.1002/lary.20665. [DOI] [PubMed] [Google Scholar]
- 3.Steimer A, Laue M, Franke H, Haltner-Ukomado E, Lehr CM. Porcine alveolar epithelial cells in primary culture: Morphological, bioelectrical and immunocytochemical characterization. Pharm Res. 2006;23:2078–2093. doi: 10.1007/s11095-006-9057-7. [DOI] [PubMed] [Google Scholar]
- 4.Levendoski EE, Leydon C, Thibeault SL. Vocal Fold Epithelial Barrier in Health and Injury A Research Review. J Speech Lang Hear Res. doi: 10.1044/2014_JSLHR-S-13-0283. Published Online: July 25, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicolazzo JA, Reed BL, Finnin BC. The effect of various in vitro conditions on the permeability characteristics of the buccal mucosa. J Pharm Sci. 2003;92:2399–2410. doi: 10.1002/jps.10505. [DOI] [PubMed] [Google Scholar]
- 6.Pegg DE. Viability assays for preserved cells, tissues, and organs. Cryobiology. 1989;26:212–231. doi: 10.1016/0011-2240(89)90016-3. [DOI] [PubMed] [Google Scholar]
- 7.Bank HL, Schmehl MK. Parameters for evaluation of viability assays: accuracy, precision, specificity, sensitivity, and standardization. Cryobiology. 1989;26:203–211. doi: 10.1016/0011-2240(89)90015-1. [DOI] [PubMed] [Google Scholar]
- 8.Castagnoli C, Alotto D, Cambieri I, et al. Evaluation of donor skin viability: fresh and cryopreserved skin using tetrazolioum salt assay. Burns. 2003;29:759–767. doi: 10.1016/j.burns.2003.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Park JC, Hwang YS, Suh H. Viability evaluation of engineered tissues. Yonsei Med J. 2000;41:836–844. doi: 10.3349/ymj.2000.41.6.836. [DOI] [PubMed] [Google Scholar]
- 10.Alper R, Fu X, Erickson-Levendoski E, Zheng W, Sivasankar M. Acute stress to vocal fold epithelia from reactive oxygen species. Laryngoscope. 2011;121:2180–2184. doi: 10.1002/lary.22157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erickson E, Sivasankar M. Simulated reflux decreases vocal fold epithelial barrier resistance. Laryngoscope. 2010;120:1569–1575. doi: 10.1002/lary.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnston N, Knight J, Dettmar P, Lively M, Koufman J. Pepsin and carbonic anhydrase isoenzyme III as diagnostic markers for laryngopharyngeal reflux disease. Laryngoscope. 2004;114:2129–2134. doi: 10.1097/01.mlg.0000149445.07146.03. [DOI] [PubMed] [Google Scholar]
- 13.Leydon C, Selekman JA, Palecek S, Thibeault SL. Human embryonic stem cell-derived epithelial cells in a novel in vitro model of vocal mucosa. Tissue Eng Part A. 2013;19:2233–2241. doi: 10.1089/ten.tea.2012.0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jia X, Yeo Y, Clifton RJ, et al. Hyaluronic acid-based microgels and microgel networks for vocal fold regeneration. Biomacromolecules. 2006;7:3336–3344. doi: 10.1021/bm0604956. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Thibeault SL. Biocompatibility of a synthetic extracellular matrix on immortalized vocal fold fibroblasts in 3-D culture. Acta Biomater. 2010;6:2940–2948. doi: 10.1016/j.actbio.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ge L, Sun L, Chen J, et al. The viability change of pigskin in vitro. Burns. 2010;36:533–538. doi: 10.1016/j.burns.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Imbert D, Cullander C. Assessment of cornea viability by confocal laser scanning microscopy and MTT assay. Cornea. 1997;16:666–674. [PubMed] [Google Scholar]
- 18.Imbert D, Cullander C. Buccal mucosa in vitro experiments. I. Confocal imaging of vital staining and MTT assays for the determination of tissue viability. J Control Release. 1999;58:39–50. doi: 10.1016/s0168-3659(98)00143-6. [DOI] [PubMed] [Google Scholar]
- 19.Levendoski EE, Sivasankar MP. Vocal fold ion transport and mucin expression following acrolein exposure. J Membr Biol. 2014;247:441–450. doi: 10.1007/s00232-014-9651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barker E, Haverson K, Stokes C, Birchall M, Bailey M. The larynx as an immunological organ: Immunological architecture in the pig as a large animal model. Clin Exp Immunol. 2005;143:6–14. doi: 10.1111/j.1365-2249.2005.02950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gill G, Buda A, Moorghen M, Dettmar P, Pignatelli M. Characterisation of adherens and tight junctional molecules in normal animal larynx; determining a suitable model for studying molecular abnormalities in human laryngopharyngeal reflux. J Clin Pathol. 2005;58:1265–1270. doi: 10.1136/jcp.2004.016972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Branski R, Zhou H, Kraus D, Sivasankar M. The effects of cigarette smoke condensate on vocal fold transepithelial resistance and inflammatory signaling in vocal fold fibroblasts. Laryngoscope. 2011;121:601–605. doi: 10.1002/lary.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durkes A, Sivasankar MP. Bicarbonate availability for vocal fold epithelial defense to acidic challenge. Ann Otol Rhinol Laryngol. 2014;123:71–76. doi: 10.1177/0003489414521143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faroon O, Roney N, Taylor J, Ashizawa A, Lumpkin MH, Plewak DJ. Acrolein health effects. Toxicol Ind Health. 2008;24:447–490. doi: 10.1177/0748233708094188. [DOI] [PubMed] [Google Scholar]
- 25.Ayer H, Yeager D. Irritants in cigarette smoke plumes. Am J Public Health. 1982;72:1283–1285. doi: 10.2105/ajph.72.11.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston N, Bulmer D, Gill G, et al. Cell biology of laryngeal epithelial defenses in health and disease: Further studies. Ann Otol Rhinol Laryngol. 2003;112:481–491. doi: 10.1177/000348940311200601. [DOI] [PubMed] [Google Scholar]
- 27.Lukinova N, Iacovelli J, Dentchev T, et al. Iron chelation protects the retinal pigment epithelial cell line ARPE-19 against cell death triggered by diverse stimuli. Invest Ophthalmol Vis Sci. 2009;50:1440–1447. doi: 10.1167/iovs.08-2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhuang S, Yan Y, Daubert RA, Han J, Schnellmann RG. ERK promotes hydrogen peroxide-induced apoptosis through caspase-3 activation and inhibition of Akt in renal epithelial cells. Am J Physiol Renal Physiol. 2007;292:F440–F447. doi: 10.1152/ajprenal.00170.2006. [DOI] [PubMed] [Google Scholar]
- 29.Ohguro N, Fukuda M, Sasabe T, Tano Y. Concentration dependent effects of hydrogen peroxide on lens epithelial cells. Br J Ophthalmol. 1999;83:1064–1068. doi: 10.1136/bjo.83.9.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pipparelli A, Thuret G, Toubeau D, et al. Pan-corneal endothelial viability assessment: application to endothelial grafts predissected by eye banks. Invest Ophthalmol Vis Sci. 2011;52:6018–6025. doi: 10.1167/iovs.10-6641. [DOI] [PubMed] [Google Scholar]
- 31.Emgard M, Blomgren K, Brundin P. Characterisation of cell damage and death in embryonic mesencephalic tissue: A study on ultrastructure, vital stains and protease activity. Neuroscience. 2002;115:1177–1187. doi: 10.1016/s0306-4522(02)00480-3. [DOI] [PubMed] [Google Scholar]
- 32.Saffarzadeh M, Juenemann C, Queisser MA, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. Plos ONE. 2012;7:e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stalmans P, Van Aken EH, Veckeneer M, Feron EJ, Stalmans I. Toxic effect of indocyanine green on retinal pigment epithelium related to osmotic effects of the solvent. Am J Ophthalmol. 2002;134:282–285. doi: 10.1016/s0002-9394(02)01468-x. [DOI] [PubMed] [Google Scholar]
- 34.Sawai H, Domae N. Discrimination between primary necrosis and apoptosis by necrostatin-1 in Annexin V-positive/propidium iodide-negative cells. Biochem Biophys Res Commun. 2011;411:569–573. doi: 10.1016/j.bbrc.2011.06.186. [DOI] [PubMed] [Google Scholar]
- 35.Barthe L, Woodley J, Houin G. Gastrointestinal absorption of drugs: Methods and studies. Fundam Clin Pharmacol. 1999;13:154–168. doi: 10.1111/j.1472-8206.1999.tb00334.x. [DOI] [PubMed] [Google Scholar]