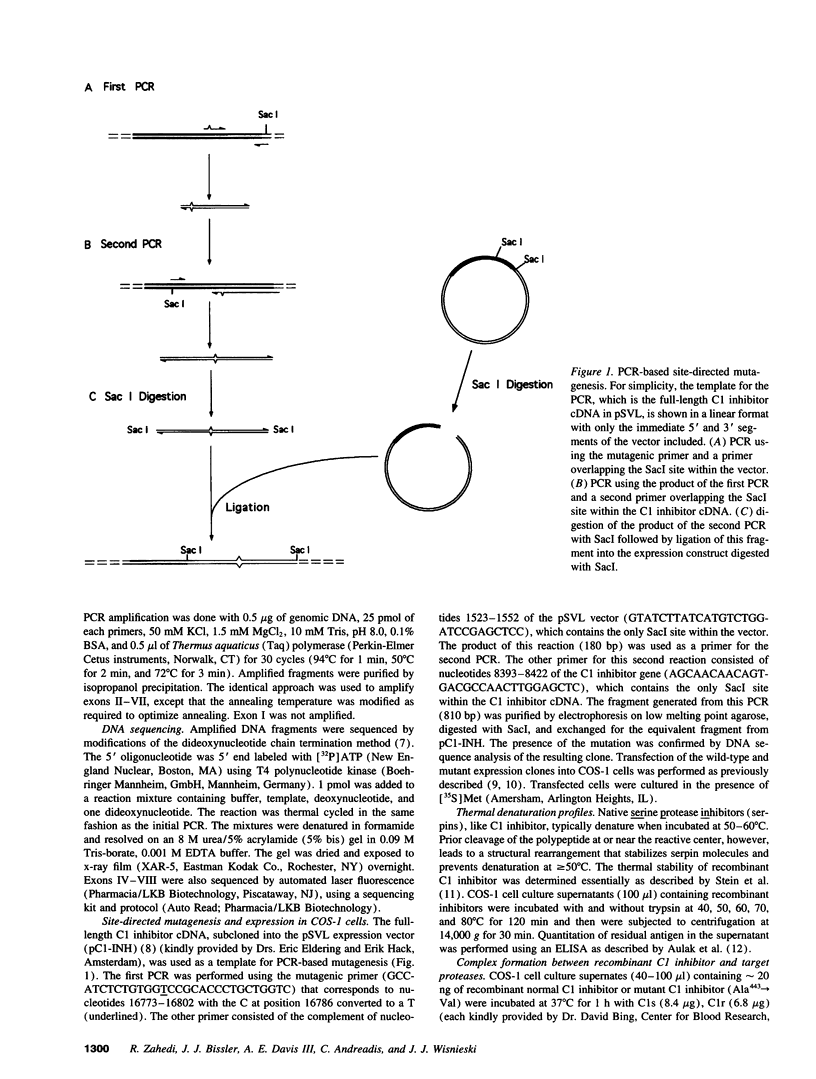
Abstract
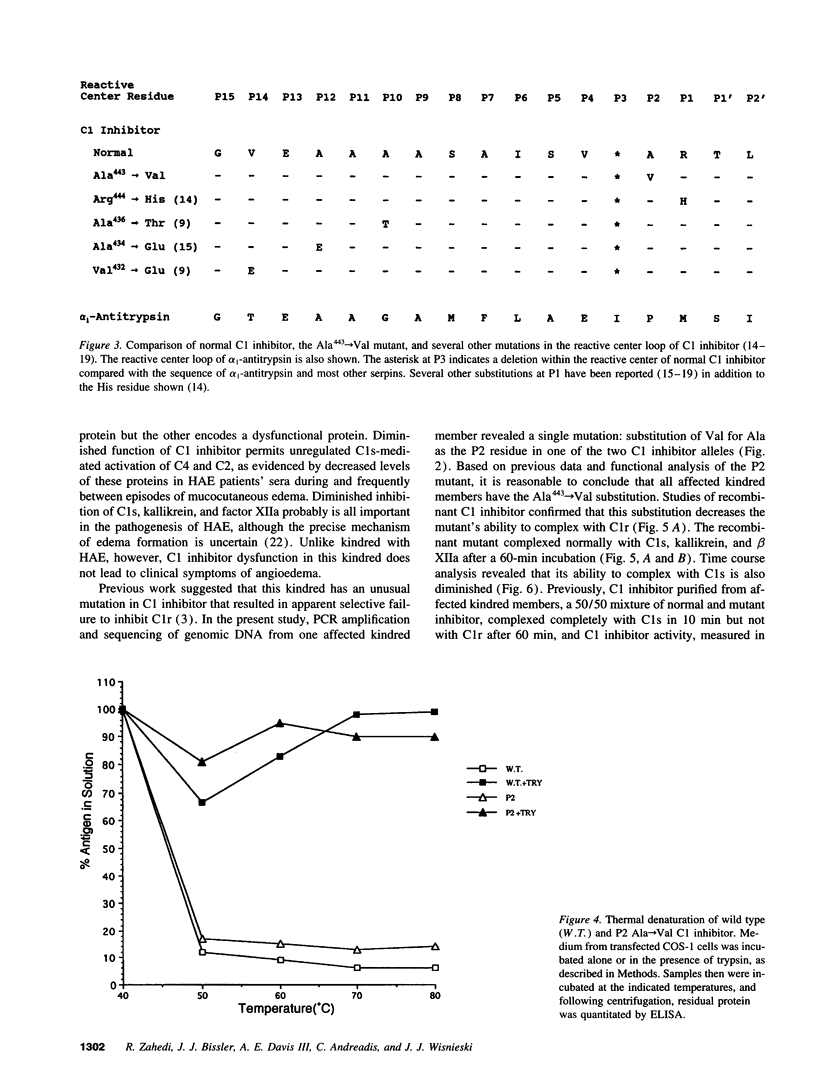
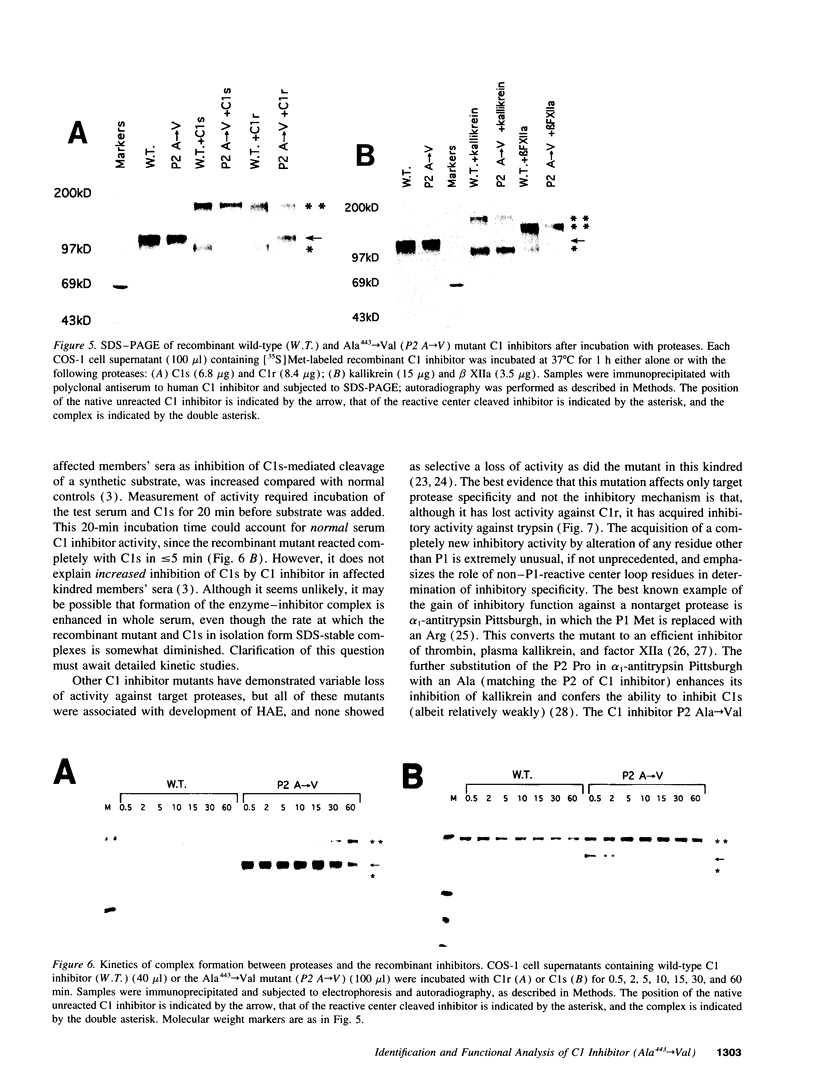
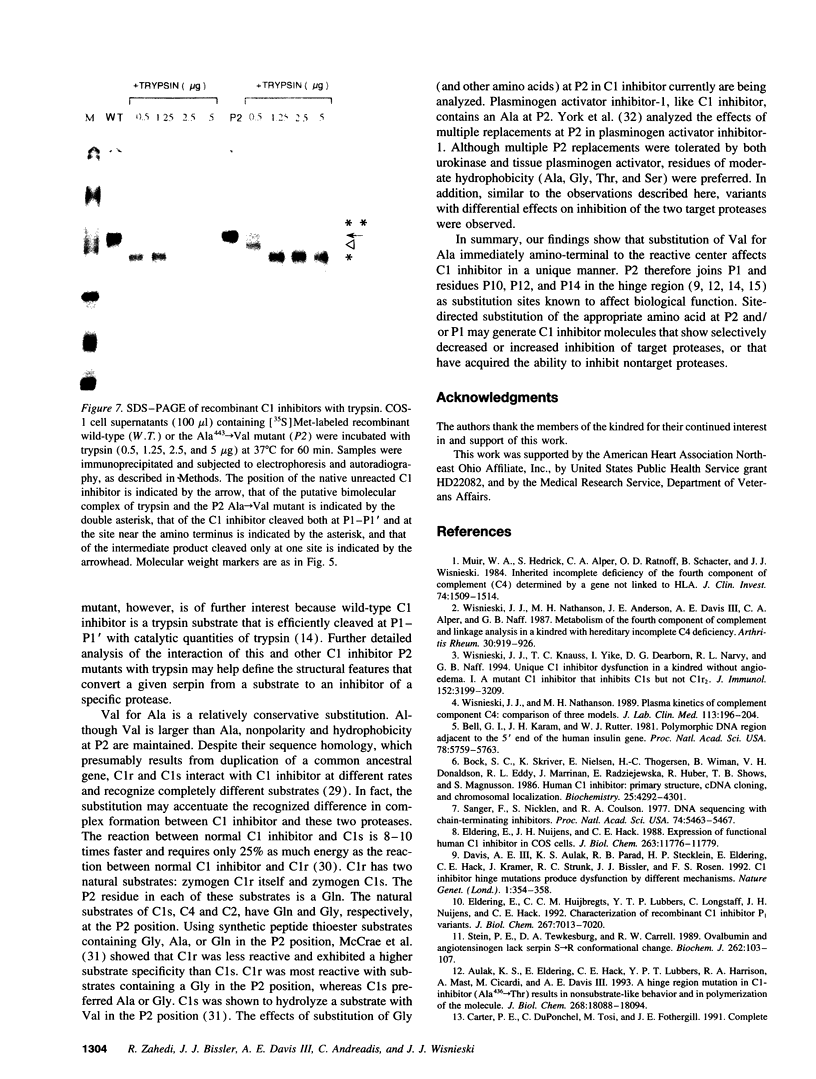
We have determined the cause of an unusual C1 inhibitor abnormality in a large kindred. We previously found that half of serum C1 inhibitor molecules in affected kindred members are normal. The other half complexed with C1s but showed little complex formation with C1r. These molecules also appeared to be relatively resistant to digestion by trypsin. Taken together, the findings suggested that members of this kindred are heterozygous for an unusual C1 inhibitor mutation. Sequencing of genomic DNA from the kindred revealed that thymine has replaced cytosine in the codon for Ala443 (P2 residue) in one C1 inhibitor allele, resulting in substitution with a Val residue. To test the effect of this substitution, a mutant C1 inhibitor containing Ala443-->Val was constructed by site-directed mutagenesis and expressed in COS-1 cells. Both the Ala443-->Val mutant and the wild-type C1 inhibitor complexed completely with C1s, kallikrein, and coagulation Factor XIIa after incubation at 37 degrees C for 60 min. In contrast, the mutant inhibitor failed to complex completely with C1r under the same conditions. Time course analysis showed that the ability of the mutant to complex with C1s is also impaired: although it complexed completely in 60 min, the rate of complex formation during a 0-60-min incubation was decreased compared with wild-type C1 inhibitor. The mutant inhibitor also formed a complex with trypsin, a serine protease that cleaves, and is not inhibited by, wild-type C1 inhibitor. The Ala443-->Val mutation therefore converts C1 inhibitor from a substrate to an inhibitor of trypsin. These studies emphasize the role of the P2 residue in the determination of target protease specificity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arlaud G. J., Thielens N. M., Aude C. A. Structure and function of C1r and C1s: current concepts. Behring Inst Mitt. 1989 Jul;(84):56–64. [PubMed] [Google Scholar]
- Aulak K. S., Cicardi M., Harrison R. A. Identification of a new P1 residue mutation (444Arg----Ser) in a dysfunctional C1 inhibitor protein contained in a type II hereditary angioedema plasma. FEBS Lett. 1990 Jun 18;266(1-2):13–16. doi: 10.1016/0014-5793(90)81494-9. [DOI] [PubMed] [Google Scholar]
- Aulak K. S., Eldering E., Hack C. E., Lubbers Y. P., Harrison R. A., Mast A., Cicardi M., Davis A. E., 3rd A hinge region mutation in C1-inhibitor (Ala436-->Thr) results in nonsubstrate-like behavior and in polymerization of the molecule. J Biol Chem. 1993 Aug 25;268(24):18088–18094. [PubMed] [Google Scholar]
- Aulak K. S., Harrison R. S. Rapid and sensitive techniques for identification and analysis of 'reactive-centre' mutants of C1-inhibitor proteins contained in type II hereditary angio-oedema plasmas. Biochem J. 1990 Nov 1;271(3):565–569. doi: 10.1042/bj2710565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulak K. S., Pemberton P. A., Rosen F. S., Carrell R. W., Lachmann P. J., Harrison R. A. Dysfunctional C1-inhibitor(At), isolated from a type II hereditary-angio-oedema plasma, contains a P1 'reactive centre' (Arg444----His) mutation. Biochem J. 1988 Jul 15;253(2):615–618. doi: 10.1042/bj2530615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I., Karam J. H., Rutter W. J. Polymorphic DNA region adjacent to the 5' end of the human insulin gene. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5759–5763. doi: 10.1073/pnas.78.9.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock S. C., Skriver K., Nielsen E., Thøgersen H. C., Wiman B., Donaldson V. H., Eddy R. L., Marrinan J., Radziejewska E., Huber R. Human C1 inhibitor: primary structure, cDNA cloning, and chromosomal localization. Biochemistry. 1986 Jul 29;25(15):4292–4301. doi: 10.1021/bi00363a018. [DOI] [PubMed] [Google Scholar]
- Carter P. E., Duponchel C., Tosi M., Fothergill J. E. Complete nucleotide sequence of the gene for human C1 inhibitor with an unusually high density of Alu elements. Eur J Biochem. 1991 Apr 23;197(2):301–308. doi: 10.1111/j.1432-1033.1991.tb15911.x. [DOI] [PubMed] [Google Scholar]
- Davis A. E., 3rd, Aulak K., Parad R. B., Stecklein H. P., Eldering E., Hack C. E., Kramer J., Strunk R. C., Bissler J., Rosen F. S. C1 inhibitor hinge region mutations produce dysfunction by different mechanisms. Nat Genet. 1992 Aug;1(5):354–358. doi: 10.1038/ng0892-354. [DOI] [PubMed] [Google Scholar]
- Davis A. E., 3rd C1 inhibitor and hereditary angioneurotic edema. Annu Rev Immunol. 1988;6:595–628. doi: 10.1146/annurev.iy.06.040188.003115. [DOI] [PubMed] [Google Scholar]
- Donaldson V. H., Harrison R. A., Rosen F. S., Bing D. H., Kindness G., Canar J., Wagner C. J., Awad S. Variability in purified dysfunctional C1(-)-inhibitor proteins from patients with hereditary angioneurotic edema. Functional and analytical gel studies. J Clin Invest. 1985 Jan;75(1):124–132. doi: 10.1172/JCI111664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson V. H., Wagner C. J., Tsuei B., Kindness G., Bing D. H., Harrison R. A., Rosen F. S. Interactions of plasma kallikrein and C1-s with normal and dysfunctional C1(-)-inhibitor proteins from patients with hereditary angioneurotic edema: analytic gel studies. Blood. 1987 Apr;69(4):1096–1101. [PubMed] [Google Scholar]
- Eldering E., Huijbregts C. C., Lubbers Y. T., Longstaff C., Hack C. E. Characterization of recombinant C1 inhibitor P1 variants. J Biol Chem. 1992 Apr 5;267(10):7013–7020. [PubMed] [Google Scholar]
- Eldering E., Nuijens J. H., Hack C. E. Expression of functional human C1 inhibitor in COS cells. J Biol Chem. 1988 Aug 25;263(24):11776–11779. [PubMed] [Google Scholar]
- Frangi D., Aulak K. S., Cicardi M., Harrison R. A., Davis A. E., 3rd A dysfunctional C1 inhibitor protein with a new reactive center mutation (Arg-444-->Leu). FEBS Lett. 1992 Apr 13;301(1):34–36. doi: 10.1016/0014-5793(92)80204-t. [DOI] [PubMed] [Google Scholar]
- McRae B. J., Lin T. Y., Powers J. C. Mapping the substrate binding site of human C1r and C1s with peptide thioesters. Development of new sensitive substrates. J Biol Chem. 1981 Dec 10;256(23):12362–12366. [PubMed] [Google Scholar]
- Muir W. A., Hedrick S., Alper C. A., Ratnoff O. D., Schacter B., Wisnieski J. J. Inherited incomplete deficiency of the fourth component of complement (C4) determined by a gene not linked to human histocompatibility leukocyte antigens. J Clin Invest. 1984 Oct;74(4):1509–1514. doi: 10.1172/JCI111564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen M. C., Brennan S. O., Lewis J. H., Carrell R. W. Mutation of antitrypsin to antithrombin. alpha 1-antitrypsin Pittsburgh (358 Met leads to Arg), a fatal bleeding disorder. N Engl J Med. 1983 Sep 22;309(12):694–698. doi: 10.1056/NEJM198309223091203. [DOI] [PubMed] [Google Scholar]
- Patston P. A., Roodi N., Schifferli J. A., Bischoff R., Courtney M., Schapira M. Reactivity of alpha 1-antitrypsin mutants against proteolytic enzymes of the kallikrein-kinin, complement, and fibrinolytic systems. J Biol Chem. 1990 Jun 25;265(18):10786–10791. [PubMed] [Google Scholar]
- Pemberton P. A., Harrison R. A., Lachmann P. J., Carrell R. W. The structural basis for neutrophil inactivation of C1 inhibitor. Biochem J. 1989 Feb 15;258(1):193–198. doi: 10.1042/bj2580193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen G. S., Catanese J. J., Kress L. F., Travis J. Primary structure of the reactive site of human C1-inhibitor. J Biol Chem. 1985 Feb 25;260(4):2432–2436. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira M., Ramus M. A., Jallat S., Carvallo D., Courtney M. Recombinant alpha 1-antitrypsin Pittsburgh (Met 358----Arg) is a potent inhibitor of plasma kallikrein and activated factor XII fragment. J Clin Invest. 1986 Feb;77(2):635–637. doi: 10.1172/JCI112347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim R. B., Arlaud G. J., Colomb M. G. Kinetics of reaction of human C1-inhibitor with the human complement system proteases C1r and C1s. Biochim Biophys Acta. 1980 Apr 11;612(2):433–449. doi: 10.1016/0005-2744(80)90126-6. [DOI] [PubMed] [Google Scholar]
- Skriver K., Radziejewska E., Silbermann J. A., Donaldson V. H., Bock S. C. CpG mutations in the reactive site of human C1 inhibitor. J Biol Chem. 1989 Feb 25;264(6):3066–3071. [PubMed] [Google Scholar]
- Skriver K., Wikoff W. R., Patston P. A., Tausk F., Schapira M., Kaplan A. P., Bock S. C. Substrate properties of C1 inhibitor Ma (alanine 434----glutamic acid). Genetic and structural evidence suggesting that the P12-region contains critical determinants of serine protease inhibitor/substrate status. J Biol Chem. 1991 May 15;266(14):9216–9221. [PubMed] [Google Scholar]
- Stein P. E., Tewkesbury D. A., Carrell R. W. Ovalbumin and angiotensinogen lack serpin S-R conformational change. Biochem J. 1989 Aug 15;262(1):103–107. doi: 10.1042/bj2620103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnieski J. J., Knauss T. C., Yike I., Dearborn D. G., Narvy R. L., Naff G. B. Unique C1 inhibitor dysfunction in a kindred without angioedema. I. A mutant C1 INH that inhibits C1-s but not C1-r. J Immunol. 1994 Mar 15;152(6):3199–3209. [PubMed] [Google Scholar]
- Wisnieski J. J., Nathanson M. H., Anderson J. E., Davis A. E., 3rd, Alper C. A., Naff G. B. Metabolism of C4 and linkage analysis in a kindred with hereditary incomplete C4 deficiency. Arthritis Rheum. 1987 Aug;30(8):919–926. doi: 10.1002/art.1780300812. [DOI] [PubMed] [Google Scholar]
- Wisnieski J. J., Nathanson M. H. Plasma kinetics of complement component C4: comparison of three models. J Lab Clin Med. 1989 Feb;113(2):196–203. [PubMed] [Google Scholar]
- York J. D., Li P., Gardell S. J. Combinatorial mutagenesis of the reactive site region in plasminogen activator inhibitor I. J Biol Chem. 1991 May 5;266(13):8495–8500. [PubMed] [Google Scholar]