Abstract
Aldo-keto reductases (AKRs) are an expanding family of NAD(P)(H)-dependent oxidoreductases that catalyze the reduction of either carbonyl groups or α,β-unsaturated ketones on a variety of endogenous and exogenous substrates. The enzymes catalyze a sequential ordered bi-bi kinetic mechanism, in which cofactor is bound first and released last. Using human steroid 5β-reductase (AKR1D1) as a representative enzyme, the influence of substrate structure on the rate-limiting steps of AKR catalysis has been previously determined. The rate of the chemistry step was found to differ by two orders of magnitude when different steroid substrates were used in single turnover experiments with AKR1D1. This difference was reflected in multiple turnover experiments. C17-C21 steroid substrates exhibited a fast chemistry step followed by slow product release as suggested by “burst” phase kinetics. By contrast, C27 steroids have a slower chemical step that determines the rate of the reaction and “burst-phase” kinetics are no longer observed. Here we present single turnover kinetic experiments and find that they support the existence of two different binding poses for fast substrates due to their biphasic nature. We also re-interpret the loss of “burst-phase” kinetics in the multiple turnover experiments as due to long range effects of the steroid side-chain interacting with distal parts of the steroid pocket to perturb the reaction trajectory for hydride transfer and thus reduce kcat. The ability of steroid structure and hence binding pose to influence rate determination in steroid transforming AKRs is discussed as a general phenomenon.
Keywords: Pre-steady state kinetics, hydride transfer, cofactor release. bile-acids, steroid hormones
1. Introduction
Aldo-keto reductases (AKRs) are a superfamily of NAD(P)(H)-dependent oxidoreductases that catalyze the reduction of either carbonyl groups to their corresponding alcohols or α,β-unsaturated ketones to their corresponding alkanones. AKRs are present in all domains of life and fall into 16 families based on sequence homology (http://www.med.upenn.edu/akr/). They are involved in the metabolism of a wide variety of endogenous substrates including sugars, lipids, retinals, steroids, and prostaglandins as well as xenobiotics such as dicarbonyls and chemical carcinogen metabolites, e.g., polycyclic aromatic hydrocarbons trans-dihydrodiols [1]. Fifteen AKR members have been identified in human, many of which belong to the AKR1 family. Human AKRs function as aldehyde reductases, aldose reductases, steroid transforming enzymes, β-subunits of the voltage-gated dependent potassium channel, and aflatoxin aldehyde reductases [2].
AKR1D, is a unique subfamily and represents the steroid 5β-reductases. They catalyze a stereospecific irreversible double bond reduction instead of carbonyl oxidoreduction. The human enzyme AKR1D1 metabolizes all bile acid precursors and steroid hormones containing the Δ4-3-ketone unsaturated A-ring functionality. This reaction is essential for bile acid maturation, which results in the formation of primary bile acids, chenodeoxycholic acid and cholic acid. These bile acids emulsify dietary fat and lipophilic vitamins due to their detergent-like properties and cause positive feedback inhibition of bile acid biosynthesis at the level of 7α-hydroxylase [3]. 5β-Reduction is also important for steroid hormone metabolism. It generates physiologically active 5β-steroids that are involved in neurotransmission, erythropoiesis, and parturition and also initiates steroid clearance in by forming tetrahydrosteroids for conjugation [4].
Like all the other AKR family members, AKR1D1 is a cytosolic monomeric protein of 37 kDa and possesses the signature (α/β)8 triosephosphate isomerase barrel core structure [5]. The active site resides on the C-terminus of the β-strands with long flexible loops forming the ligand binding site. AKR1D1 is believed to follow a sequential ordered bi-bi kinetic mechanism, in which NADPH is bound first and NADP+ leaves last. This assumption is based on sequence homology to other AKRs, and kinetic and crystallographic evidence that cofactor binds to AKR1D1 in the absence of steroid but not vice-versa. In AKR catalysis, cofactor binding and release are usually accompanied by conformational changes that are characteristically slow steps, whereas the rate of the chemistry step can vary significantly depending on the substrate. The turnover number of the reaction kcat represents the slowest rate-limiting step during catalysis, which can change depending on the relative rates of the chemical step, product and cofactor release steps [6, 7]. This sometimes causes inconsistent results between different studies due to variations in the reaction conditions.
Recombinant AKR1D1 was first purified to homogeneity and systemically characterized by us. The enzyme is capable of reducing a variety of steroid substrates including C18, C19, C21, and C27 series as long as the α,β-unsaturated Δ4-3-ketone moiety is present in the steroid A-ring [8]. Steady-state kinetic measurements reveal significant differences in turnover numbers (kcat) for different steroid substrates, depending on the structure, especially the length of the C17 side chain (Table 1). For example, the C21 corticosteroids with shorter side-chains are among the fastest substrates for AKR1D1, whereas the C27 bile acid precursors with longer side-chains are the slowest substrates. The difference in kcat values between cortisone and cholestenone is 20-fold [8]. This observation suggests that a significant change has occurred in the rate-determining step. We previously reported a transient kinetic study to examine the basis of these differences [4].
Table 1.
Rate constants determined from transient single turnover experiments.
| Parametersa | A1/Atotal (s−1) | kobs1 (s−1) | kobs2 (s−1) | kcat (s−1)d |
|---|---|---|---|---|
| Fast substrates – biphasic progress curves | ||||
|
| ||||
| aldosterone | 0.47 | 1.27 ± 0.02 | 0.128 ± 0.002 | 0.15 ± 0.01 |
| cortisoneb | 0.32 | 0.78 ± 0.01 | 0.0943 ± 0.0004 | 0.195 ± 0.002 |
| testosterone | 0.27 | 0.32 ± 0.01 | 0.0704 ± 0.0009 | 0.14 ± 0.03 |
| corticosterone | 0.30 | 0.119 ± 0.003 | 0.0176 ± 0.0002 | 0.032 ± 0.002 |
| Slow substrates – monophasic progress curves | ||||
|
| ||||
| 7α-hydroxycholestenonec | NA | 0.00523 ± 0.00001 | NA | 0.033 ± 0.002 |
| cholestenone | NA | 0.00133 ± 0.00001 | NA | 0.0100 ± 0.0007 |
Single turnover experiments were performed at 37 °C in 100 mM potassium phosphate buffer (pH 7.0), containing 4% acetonitrile as cosolvent.
For cortisone reduction at pH 6, kobs1 = 1.69 s−1, kobs2 = 0.21 s−1[9].
For 7α-hydroxycholestenone reduction at pH 6, kobs1 = 0.021 s−1 [9].
kcat was determined at 100 mM potassium phosphate buffer (pH 6.0) [8].
Here we present single turnover experiments to cover a series of steroid substrates and reexamine our multiple turnover experiments to dissect the kinetic mechanism of AKR1D1. Our findings suggest that the rate of the chemistry step of 5β-reduction is sensitive to steroid substrate structure, which in turn determines the rate limiting step. We find evidence in our single turnover experiments for two binding poses for the fast substrates based on their biphasic nature. We also find that in our multiple turnover experiments there is loss of “burst-phase” kinetics for the slow substrates that have an extended C17 side-chain. We conclude that different binding poses for steroid substrates likely dictate rate determination and that these effects can occur distal to the active site. We discuss these findings within the broader context of other steroid transforming AKRs.
2. Materials and Methods
Materials
All steroids were obtained from Steraloids (Wilton, NH, U.S.A.). Pyridine nucleotides were purchased from Roche Applied Science (Indianapolis, IN, USA). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO) and were of ACS (American Chemical Society) grade or better.
Single turnover experiments
All stopped flow experiments were performed on an Applied Photophysics SX.18 and SX.19 MV stopped-flow spectrophotometer (Leatherhead, UK). AKR1D1 was pre-incubated with stoichiometric amount of NADPH to allow the formation of the E•NADPH complex. The reaction was initiated upon mixing with the steroid solution. A typical reaction contained 1.0 μM AKR1D1, 0.8 μM NADPH and 35 μM steroid in 100 mM potassium phosphate buffer (pH 7.0), containing 4% acetonitrile as cosolvent. The progress curves were monitored fluorometrically (λexcitation = 340 nm, λemission = 450 nm) for 20–1000s depending on the rate of the reactions. The curves were fitted to either a single-exponential (Equation 1) or double-exponential equation (Equation 2), where y is the fluorescence signal, A, A1, and A2 are the amplitudes, a is the intercept, and kobs is rate constant of the chemistry step.
| (Equation 1) |
| (Equation 2) |
Multiple turnover experiments
AKR1D1 was pre-incubated with a saturating amount of NADPH and then mixed with steroid substrate to initiate the reaction [9]. A typical reaction contained 2.5 μM AKR1D1, 18 μM NADPH and 25 μM steroid in 100 mM potassium phosphate buffer (pH 6.0), containing 4% acetonitrile as cosolvent. The progress curves were monitored fluorometrically (λexcitation = 340 nm, λemission = 450 nm) for 20–100s and fitted to either the “burst” (Equation 3) or the linear equations (Equation 4), where kburst is the rate constant for the exponential “burst-phase”, and kss is the rate constant for the linear steady-state phase.
| (Equation 3) |
| (Equation 4) |
For a representative fast substrate cortisone and a slow substrate 7α-hydroxycholestenone, experiments were performed over a range of steroid concentrations. Secondary plots of kburst and kss versus [S] displayed saturation kinetics and were fitted to the hyperbolic equation (Equation 5), where kburst/ss can be either kburst or kss, klim is the limiting rate constant, and K1/2 is the apparent half-saturation constant.
| (Equation 5) |
NADP+ release rate
The dissociation rate constant of NADP+ from AKR1D1 was determined using a ligand chase experiment [9]. NADP+ from the AKR1D1•NADP+ complex (1 μM AKR1D1 and 5 μM NADP+) was displaced in a large excess of NADPH (50 μM) in 100 mM potassium phosphate buffer (pH 6.0), containing 4% acetonitrile as cosolvent. The formation of the E•NADPH complex was monitored by the increase in the energy transfer band (λexcitation = 295 nm, λemission = 450 nm) on the stopped-flow instrument. The kinetic traces were fitted to a single-exponential equation (Equation 1).
3. Results
3. 1. The rate limiting step of AKR1D1 is affected by the steroid substrate
Transient single turnover experiments show variation in kchem
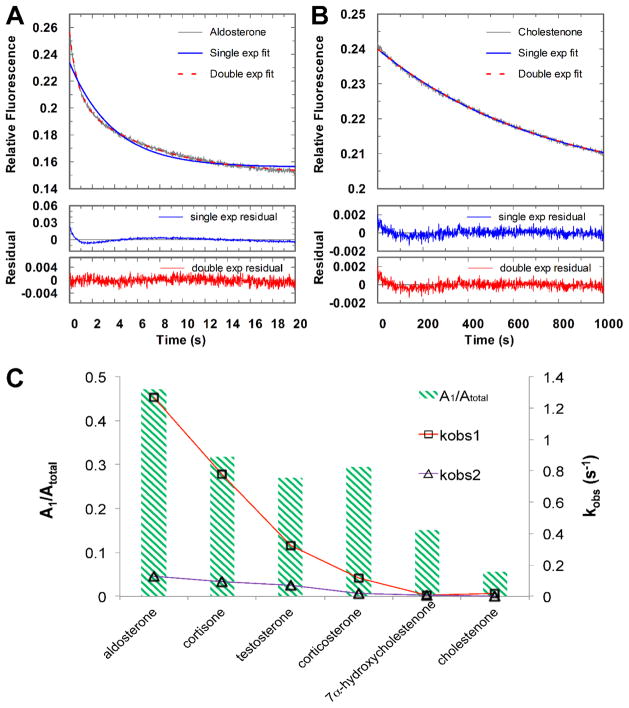
Steady-state experiments revealed a 20-fold variation in turnover number for different steroid substrates. To reconcile these data, the kinetic mechanism of 5β-reduction by AKR1D1 was dissected using pre-steady state kinetics. Single turnover experiments were employed to measure the rate of the chemistry step (kchem), which is defined by hydride transfer and proton donation. Six different steroid substrates were examined. In our setup, the enzyme concentration was kept equal or slightly higher than the cofactor concentration to limit the reaction to the first turnover. The progress curves for steroid reduction were obtained by following the disappearance of NADPH fluorescence. At saturating concentration of the steroid substrate, the kinetic traces could be categorized into two groups.
One group were best fitted to a double exponential equation, indicating two populations of enzyme activity were involved in the chemical step (Figure 1A). The biphasic behavior could not be readily explained by enzyme stability or cofactor status. The rate constants of the fast phase kobs1 of 1.27 to 0.119 s−1 are 5- to 10-fold higher than the slow phase kobs2 of 0.128 to 0.0176 s−1 (Table 1). Steroids in this group coincided with the ones with a higher turnover number in the steady-state, e.g., aldosterone, cortisone, testosterone, and corticosterone.
Figure 1.
Representative progressive curves for the single turnover reactions of aldosterone reduction (A) and cholestenone reduction (B) catalyzed by WT AKR1D1 and a replot of the observed rate constants kobs1 and kobs2 (on the right axis) and amplitudes (on the left axis) from the single turnover reactions by fitting the kinetic traces to double exponential equation for all the substrates (C). A1 and kobs1 are from the fast phase, A2 and kobs2 are from the slow phase. Atotal is the sum of A1 and A2. The reactions were performed at 100 potassium buffer at pH 7 at 37 °C.
The second group were best fitted to a single exponential equation (Figure 1B). Steroids in this group were the slower substrates, e.g. 7α-hydroxycholestenone and cholestenone. The rate constants of 7α-hydroxycholestenone and cholestenone are 0.00523 and 0.00133 s−1, respectively. If the kinetic traces from both groups were force-fitted to the double exponential equation, it is obvious that the rates of both the fast and slow phases correlate with the turnover number of the steroid. The proportion of the two phases as noted by the amplitudes of these transients was dependent on the particular steroid substrate. With the fastest substrate aldosterone, the amplitudes for the fast phase (A1) accounted for about 50% of the total signal (Atotal) (Table 1). The fraction of the amplitude represented by the fast phase decreased when slower substrates were employed. The rate constant kobs1 and the amplitude for the fast phase continued to decline until the two phases could not be well distinguished for the slowest substrates 7α-hydroxycholestenone and cholestenone (Figure 1C).
Despite the biphasic behavior observed for the fast substrates, the significant difference in the chemical rates between the fast and slow substrates is evident. Even if the slower phase of the fast substrate is chosen for comparison, there is a two orders of magnitude difference in the kobs values between the fastest substrate aldosterone (0.128 s−1) and the slowest substrate cholestenone (0.00133 s−1). This supports our hypothesis based on the steady-state data that the rate of the chemistry step relies heavily on the structure of steroid. The striking variation in the rate of the chemistry step is responsible for the sizable difference in kcat.
Transient multiple turnover experiments indicate change in rate determination
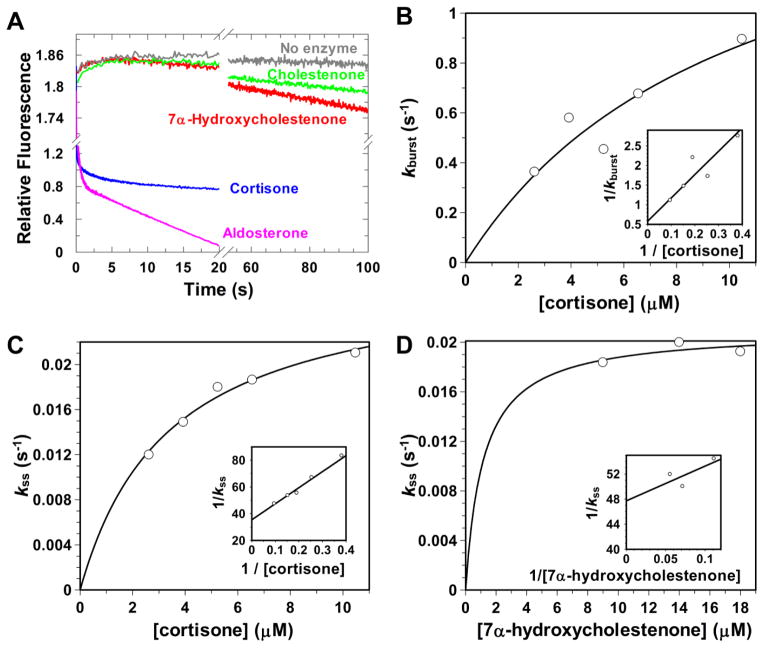
The macroscopic rate constant kcat is determined by multiple factors including the chemical step, release of steroid products and cofactor. To elucidate the impact of these steps on the overall reaction, 5β-reduction was also examined using multiple turnover experiments. Multiple turnover experiments using a pre-formed AKR1D1•NADPH complex monitor all the steps in the reaction sequence including steroid binding, chemistry, and product release. Using 25 μM steroid in the presence of saturating NADPH, the kinetic progress traces could again be divided into two groups.
One group showed “burst-phase” kinetics and were only observed with the fast steroid substrates, aldosterone, cortisone, and testosterone (Figure 2A). The presence of “burst-phase” kinetics indicated that the reaction proceeds through a fast chemistry step with a quick buildup of an intermediate followed by a slow release step. The rate constant of the “burst-phase” (kburst) represents the rate of the chemistry step (kchem). The rate constant of the linear phase kss represents kcat. The fast steroid substrates of AKR1D1 allow an optimal chemical rate as indicated by the single turnover experiments. The initial burst occurs at a rate (kburst) between 1.98 and 0.71 s−1 with an accumulation of the E•NADP+•5β-dihydrosteroid ternary complex. These rates are in agreement with the rate constants of the fast phase kobs1 in single turnover experiments. The reaction then slowed down to the linear phase with a rate (kss) between 0.10 and 0.050 s−1 (Table 2). The rate of the chemistry step is much higher than product release and therefore only contributes < 20% to the rate determination of 5β-reduction. For the fast substrates, the rate of 5β-reduction is largely determined by the product release step.
Figure 2.
Representative progressive curves for the multiple turnover reactions catalyzed by WT AKR1D1 (A) and secondary plots of the observed rate constants kburst and kss from multiple turnover reactions versus steroid concentrations fitted to a hyperbolic function (B–D). The reactions were performed at 100 potassium buffer at pH 6 at 37 °C.
Table 2.
Rate constants determined from transient multiple turnover and steady-state experiments (5, 6).
| Parameters | kburst (s−1) | kss (s−1) |
|---|---|---|
|
Burst kinetics
|
||
| aldosterone | 1.98 ± 0.05 | 0.094 ± 0.002 |
| cortisone | 1.39 ± 0.04 | 0.104 ± 0.002 |
| testosterone | 0.71 ± 0.03 | 0.050 ± 0.002 |
|
Linear kinetics
|
||
| 7α-hydroxycholestenone | NA | 0.0160 ± 0.0002 |
| cholestenone | NA | 0.0060 ± 0.0002 |
Experiments were performed at 37 °C in 100 mM potassium phosphate buffer (pH 6.0), containing 4% acetonitrile as cosolvent.
In contrast to the fast substrates, the other group of the kinetic traces generated by the slow C27 substrates 7α-hydroxycholestenone and cholestenone eliminated the “burst-phase” and were depicted by a straight line (Figure 2A). Linear kinetics indicates that the rate of the reaction is controlled by a single slow step, the chemical step unless the product release step is occurring at a similar rate. For 5β-reduction, the rate constant of the linear phase kss represents kchem. The kss values are 0.016 s−1 and 0.006 s−1 for 7α-hydroxycholestenone and cholestenone respectively, and are comparable with their kobs1 in the single turnover experiments. For the slow substrates, the rate of the chemical step is almost the sole contributor to turnover number.
The multiple turnover reactions were further examined over a range of steroid concentrations for the representative substrates cortisone and 7α-hydroxycholestenone (Figure 2B–D). For the fast substrate cortisone, secondary fit of kburst and kss versus steroid concentrations to a hyperbolic equation yielded limiting rate constants of 1.73 s−1 and 0.16 s−1. 7α-Hydroxycholestenone was only assessed with limited range of steroid concentrations due to the detection limit of the stopped-flow instrument. A limiting rate constant of kss was determined to be 0.020 s−1. The maximum values of kss for cortisone and 7α-hydroxycholestenone both agree with their kcat values respectively.
3. 2. NADP+ and steroid product release
Rates of Product Release Confirm a Change in Rate-Determining Step
Since the turnover of fast substrates may be rate limited by NADP+ release, this step was also monitored directly using ligand chase experiments. In this setup, NADP+ was replaced from a pre-formed E•NADP+ complex by NADPH under transient kinetic conditions. The kinetic trace observed was best fit to a single exponential equation with a koff of 0.36 ± 0.01 s−1. The rate of steroid product release can also be estimated using Equation 6, where kchem is the rate of the chemistry step, kr,Sp is the release of the steroid product, and kr, NADP+ is the release of NADP+ [10].
| (Equation 6) |
For cortisone, the rate of release of 5β-dihydrocortisone was determined to be 0.55 s −1, comparable to the rate of release of cofactor.
For the fast steroid substrates the rate of the chemistry step is usually faster than the product release rates. But for the slow substrates, the rate of the chemistry step can approach or be even slower than the rate of product release. When the chemical rate and the product release rates converge to similar values, a transition point is reached so that the change of rate-limiting step between the fast and slow substrates occurs. This is seen in the multiple turnover experiments. The change from “burst-phase” kinetic traces to linear kinetic traces is due to decrease in the chemical rate that becomes rate limiting.
4. Discussion
Steroid structure affects the rate determination of AKR
Our studies show that steroid substrate structures determine the rate-limiting step in AKR1D1 by affecting the rate of the chemistry step. The biphasic behavior observed in the single turnover reactions with fast substrates in AKR1D1 suggests that two different rates of activity exist, which is likely due to different binding positions of the steroid. We hypothesize that AKR1D1 accommodates steroid substrates in two slightly different binding poses, both of which give similar binding energy minimia. However, since hydride transfer is very sensitive to the transfer distance and dihedral angle, this fine-tuning of steroid position lead to two kinetically distinguishable chemical steps. The faster substrates may be able to occupy the preferred position more often than the slower substrates, yielding a higher kobs and amplitude for the fast phase. Multiple turnover experiments also support the assertion that steroid structure and hence binding pose influences rate-determination. All the fast substrates lack an extended side-chain and exhibit “burst-phase” kinetics showing the chemistry step is fast. By contrast the slow substrates with extended side-chains now have low kcat values and the chemistry step is rate determining.
These data are all consistent with the chemical rate being controlled by differences in the binding position of the substrate. The steroid binding channels in AKRs are open to the solvent and would accept substrates of various sizes. The smaller size of the fast steroid substrates may allow them to fully fit in the active site while the C17 tail of the slow C27 substrates has to cling to the outer surface of the enzyme. Steady state kinetic data showed that the longer steroids tend to have lower Km values, suggesting that the additional interaction between the longer steroid and the enzyme plays a role in substrate binding. This interaction might have an impact on the precise location of the steroid, which could influence the trajectory for hydride transfer. Hydride transfer is very sensitive to the donor-acceptor distance and alignment [11].
We believe that rate determination governed by steroid structure is a general phenomenon for steroid transforming AKRs. For rat liver 3α-hydroxysteroid dehydrogenase, AKR1C9, the rate-limiting step is dependent on steroid structure as well. In the reduction of 5α-androstane-3,17-dione by AKR1C9, the rate of the chemical step is over 15-fold higher than kcat and the release of cofactor controls the overall rate [6], whereas in the reduction of 5α-dihydrotestosterone, chemical transformation is rate limiting [12].
There are also examples in AKRs showing that mutations of non-catalytic residues in the steroid binding site can perturb steroid binding and affect the rate of the chemistry step. This was revealed in alanine scanning mutagenesis studies of the steroid binding site in AKR1C9. The NADP+ dependent oxidation of 5α-androstane-3α,17β-diol catalyzed by the AKR1C9 W227A mutant caused a 5- to 30-fold increase in steroid Kd. This impairment of steroid binding caused by a mutation in the steroid binding pocket disrupted the optimal hydride transfer trajectory and lead to decrease in the rate of the chemistry step by three orders of magnitude [12]. Similarly, the NADP+ dependent oxidation of androsterone catalyzed by the AKR1C9 F118A resulted in impaired steroid positioning and depressed chemical rate, which led to a change in the rate-limiting step from product release to the chemical step [13].
Other factors affect the rate determination of AKR
In addition to steroid structure, there are other factors that influence the rate-limiting step of AKRs. While steroid structure mainly affects the rate of the chemistry step, these other factors often influence the product release steps. Using cofactor as an example, the NADP+ dependent oxidation of 5α-androstane-3α,17β-diol catalyzed by AKR1C9 is limited by cofactor release. However, when NAD+ is substituted, the rate of NAD+ release was significantly higher than the rate of release of NADP+ and allowed the rate of the chemical step to become rate deterrmining. The kcat is in fact 2-fold higher in the presence of NAD+ than NADP+ [12].
Buffer conditions, including the buffer species, ionic strength, and pH, also affect the rate of catalysis and sometimes change the rate-limiting step. The requirement for a general acid/base tyrosine for AKR oxidoreduction makes enzymes in the family sensitive to pH perturbation. Other than pH, one special feature about the AKRs is that the enzymes are very sensitive to ionic strength of the reaction system. The cofactor binding in AKRs relies on the salt linkage between the cofactor binding loops and electrostatic interactions between the 2′AMP in the tail of the NADP(H) with a conserved arginine (Arg279 in AKR1D1). Low salt concentration strengthens these interactions and causes an increase in cofactor binding affinity. For AKR1D1, the Kd decreased 140-fold from 0.324 ± 0.008 μM to 0.0023 ± 0.0006 μM when the buffer is changed from 100 mM to 10 mM potassium phosphate. This change is accompanied by a two-fold decrease in the cofactor release rate from 0.276 ± 0.007 s−1 to 0.164 ± 0.007 s−1 at pH 7. A corresponding two-fold decrease in the velocity of the cortisone reduction was also observed since this reaction is limited by the cofactor release rate. At 10 μM cortisone at fixed NADPH concentration the rate was reduced from 0.060 ± 0.001 s−1 to 0.0352 ± 0.0004 s−1 when the salt concentration was reduced (unpublished data). We have previously reported that the reduction of 7α-hydroxycholestenone catalyzed by AKR1D1 in 100 mM potassium phosphate at pH 6 is limited by the chemical step. When the reaction system was changed to 10 mM potassium phosphate buffer at pH 7, multiple turnover reactions showed “burst-phase” kinetic traces. At the suboptimal pH of 7, the lower ionic strength reduced the rate of cofactor release so that it was now rate determining.
Directionality of the AKR reaction may also affect rate determination. 5β-Reduction catalyzed by AKR1D1 is irreversible. In contrast most AKRs catalyze a reversible oxidoreduction. In the reversible reactions the rate-limiting steps for the forward and reverse reactions can be the same or different. For example, in the NAD(P)H dependent interconversion of 5α-androstane-3,17-dione and androsterone catalyzed by AKR1C9 both directions are controlled by cofactor release [6]. However, a more commonly seen scenario is that the reduction and oxidation directions can have different rate-limiting steps. In the NADP(H) dependent interconversion of 5β-dihydrotestosterone and 5α-androstane-3α,17β-diol catalyzed by AKR1C9, the chemical step is rate-limiting in the reduction direction but product release is rate-limiting in the oxidation direction [12]. When the same reaction is catalyzed by AKR1C2, human type 3 3α-hydroxysteroid dehydrogenase, the chemical step, the release of the steroid product, and the release of the cofactor product all contribute to the rate-determination in the reduction direction, whereas the chemical step dominates kcat in the oxidation direction [7]. For the interconversion between xylose and xylitol, AKR1B1 and yeast Candida tenuis xylose reductase AKR2B5, both have the cofactor release as the rate-limiting step in the reduction direction whereas hydride transfer is the rate-limiting step in the oxidation, direction. This is quite remarkable since AKR1B1 uses NADP(H) and AKR2B5 uses NAD(H) as cofactor [14, 15].
5. Conclusion
We conclude that steroid structure and binding pose determines the rate-limiting step in AKR1D1 catalysis. For fast substrates the binding pose is optimal and product release is rate determining. For slow substrates long range effects of the steroid side-chain influence the reaction trajectory so that chemistry becomes rate determining. The rate limiting step in AKR1D1 is also sensitive to cofactor species and buffer pH. These concepts can be generalized to all the AKRs. The rate-determining step in AKR is not fixed, but dependent on the particular reaction and condition. Caution should be exercised when interpreting pH-kcat profile to elucidate catalytic mechanism. In this instance other tools should be employed including pH rate profiles under transient conditions, site-directed mutagenesis and the use of primary and solvent kinetic isotope effect measurements. These approaches have been used success with AKR1B1, AKR1C9 and AKR2B5 [14–16].
Highlight.
Transient state kinetics were performed on human 5β-reductase (AKR1D1)
The rate-limiting step of AKR1D1 is dependent on the structure of steroid substrate
Single turnover experiments exhibit bi-phasic behavior indicating the existence of two binding poses
Multiple turnover experiments show “burst-phase” or linear phase traces that are steroid dependent
Steroid interactions distal from the active site decrease optimal reaction rates
Acknowledgments
This work was supported by NIH grants 1R01-DK47015, 1R01CA090744, and P30-ES13508 to TMP, F32-DK089827 to MC, and a pilot project from P30-ES13508 to YJ.
Abbreviations
- AKR
aldo-keto reductase
- AKR1B1
human aldose reductase
- AKR1C2
human type 3 3α-hydroxysteroid dehydrogenase
- AKR1C9
rat 3α-hydroxysteroid dehydrogenase
- AKR1D1
human steroid 5α-reductase
- AKR2B5
yeast Candida tenuis xylose reductase
Footnotes
6. Conflict of interest statement
The authors declare no conflicts of interest associated with this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jin Y, Penning TM. Aldo-keto reductases and bioactivation/detoxication. Annu Rev Pharmacol Toxicol. 2007;47:263–292. doi: 10.1146/annurev.pharmtox.47.120505.105337. [DOI] [PubMed] [Google Scholar]
- 2.Penning TM, Drury JE. Human aldo-keto reductases: Function, gene regulation, and single nucleotide polymorphisms. Arch Biochem Biophys. 2007;464:241–250. doi: 10.1016/j.abb.2007.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 4.Jin Y, Chen M, Penning TM. Rate of steroid double-bond reduction catalysed by the human steroid 5β-reductase (AKR1D1) is sensitive to steroid structure: implications for steroid metabolism and bile acid synthesis. Biochem J. 2014;462:163–171. doi: 10.1042/BJ20140220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Costanzo L, Drury JE, Penning TM, Christianson DW. Crystal structure of human liver Δ4-3-ketosteroid 5β-reductase (AKR1D1) and implications for substrate binding and catalysis. J Biol Chem. 2008;283:16830–16839. doi: 10.1074/jbc.M801778200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper WC, Jin Y, Penning TM. Elucidation of a complete kinetic mechanism for a mammalian hydroxysteroid dehydrogenase (HSD) and identification of all enzyme forms on the reaction coordinate. J Biol Chem. 2007;282:33484–33493. doi: 10.1074/jbc.M703414200. [DOI] [PubMed] [Google Scholar]
- 7.Jin Y, Penning TM. Multiple steps determine the overall rate of the reduction of 5α-dihydrotestosterone catalyzed by human type 3 3α-hydroxysteroid dehydrogenase: Implications for the elimination of androgens. Biochemistry. 2006;45:13054–13063. doi: 10.1021/bi060591r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen M, Drury JE, Penning TM. Substrate specificity and inhibitor analyses of human steroid 5b-reductase (AKR1D1) Steroids. 2011;76:484–490. doi: 10.1016/j.steroids.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin Y, Chen M, Penning TM. Rate of steroid double-bond reduction catalysed by the human steroid 5β-reductase (AKR1D1) is sensitive to steroid structure: implications for steroid metabolism and bile acid synthesis. Biochem J. 2014;462:163–171. doi: 10.1042/BJ20140220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plapp BV. On calculation of rate and dissociation constants from kinetic constants for the Ordered Bi Bi mechanism of liver alcohol dehydrogenase. Arch Biochem Biophys. 1973;156:112–114. doi: 10.1016/0003-9861(73)90347-0. [DOI] [PubMed] [Google Scholar]
- 11.Mesecar AD, Stoddard BL, Koshland DE. Orbital steering in the catalytic power of enzymes: small structural changes with large catalytic consequences. Science. 1997;277:202–206. doi: 10.1126/science.277.5323.202. [DOI] [PubMed] [Google Scholar]
- 12.Heredia VV, Penning TM. Dissection of the physiological interconversion of 5a-DHT and 3α-diol by rat 3α-HSD via transient kinetics shows that the chemical step is rate-determining: Effect of mutating cofactor and substrate-binding pocket residues on catalysis. Biochemistry. 2004;43:12028–12037. doi: 10.1021/bi0489762. [DOI] [PubMed] [Google Scholar]
- 13.Heredia VV, Cooper WC, Kruger RG, Jin Y, Penning TM. Alanine scanning mutagenesis of the testosterone binding site of rat 3α-hydroxysteroid dehydrogenase demonstrates contact residues influence the rate-determining step. Biochemistry. 2004;43:5832–5841. doi: 10.1021/bi0499563. [DOI] [PubMed] [Google Scholar]
- 14.Nidetzky B, Klimacek M, Mayr P. Transient-state and steady-state kinetic studies of the mechanism of NADH-dependent aldehyde reduction catalyzed by xylose reductase from the yeast Candida tenuis. Biochemistry. 2001;40:10371–10381. doi: 10.1021/bi010148a. [DOI] [PubMed] [Google Scholar]
- 15.Grimshaw CE, Bohren KM, Lai CJ, Gabbay KH. Human aldose reductase: rate constants for a mechanism including interconversion of ternary complexes by recombinant wild-type enzyme. Biochemistry. 1995;34:14356–14365. doi: 10.1021/bi00044a012. [DOI] [PubMed] [Google Scholar]
- 16.Schlegel B, Jez J, Penning T. Mutagenesis of 3α-hydroxysteroid dehydrogenase reveals a “push-pull” mechanism for proton transfer in aldo-keto reductases. Biochemistry. 1998;37:3538–3548. doi: 10.1021/bi9723055. [DOI] [PubMed] [Google Scholar]