Abstract
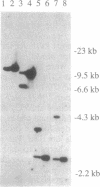
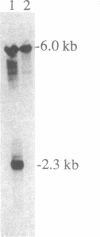
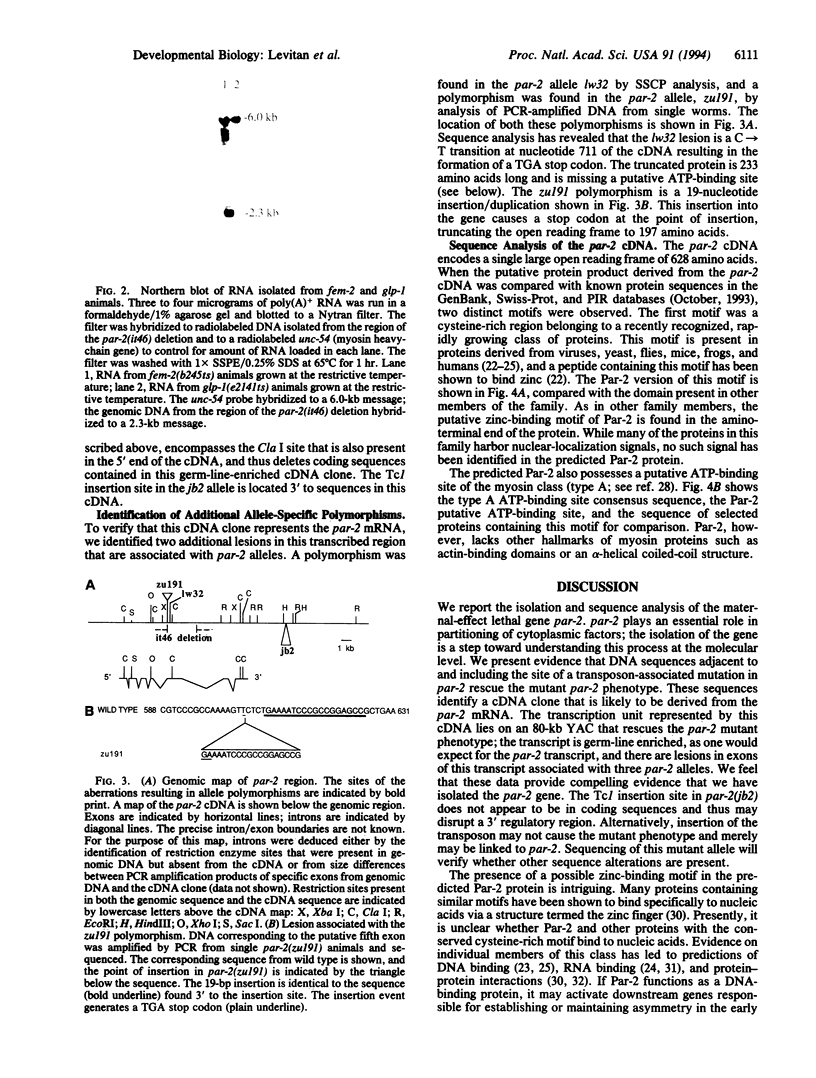
The par-2 gene of Caenorhabditis elegans functions in early embryogenesis to ensure an asymmetric first cleavage and the segregation of cytoplasmic factors. Both processes appear to be required to generate daughter blastomeres with distinct developmental potential. We isolated an allele of par-2 by using a screen for maternal-effect lethal mutations in a strain known for its high frequency of transposition events. A transposable element was found to be linked to this allele. Sequences flanking the site of transposon insertion were cloned and found to rescue the par-2 mutant phenotype. DNA in the par-2 region hybridized to a 2.3-kb germ-line-enriched mRNA. The cDNA corresponding to this germ-line-enriched message was cloned, sequenced, and used to identify the molecular lesions associated with three par-2 alleles. Sequence analysis of the par-2 cDNA revealed that the predicted protein contained two distinct motifs found in other known proteins: an ATP-binding site and a cysteine-rich motif which identifies the par-2 gene product as a member of a growing class of putative zinc-binding proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin J., Kimble J. glp-1 is required in the germ line for regulation of the decision between mitosis and meiosis in C. elegans. Cell. 1987 Nov 20;51(4):589–599. doi: 10.1016/0092-8674(87)90128-0. [DOI] [PubMed] [Google Scholar]
- Bellini M., Lacroix J. C., Gall J. G. A putative zinc-binding protein on lampbrush chromosome loops. EMBO J. 1993 Jan;12(1):107–114. doi: 10.1002/j.1460-2075.1993.tb05636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg J. M. Zinc fingers and other metal-binding domains. Elements for interactions between macromolecules. J Biol Chem. 1990 Apr 25;265(12):6513–6516. [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974 May;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J., Saari B., Anderson P. Activation of a transposable element in the germ line but not the soma of Caenorhabditis elegans. Nature. 1987 Aug 20;328(6132):726–728. doi: 10.1038/328726a0. [DOI] [PubMed] [Google Scholar]
- Coulson A., Waterston R., Kiff J., Sulston J., Kohara Y. Genome linking with yeast artificial chromosomes. Nature. 1988 Sep 8;335(6186):184–186. doi: 10.1038/335184a0. [DOI] [PubMed] [Google Scholar]
- Emmons S. W., Yesner L., Ruan K. S., Katzenberg D. Evidence for a transposon in Caenorhabditis elegans. Cell. 1983 Jan;32(1):55–65. doi: 10.1016/0092-8674(83)90496-8. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Freemont P. S., Hanson I. M., Trowsdale J. A novel cysteine-rich sequence motif. Cell. 1991 Feb 8;64(3):483–484. doi: 10.1016/0092-8674(91)90229-r. [DOI] [PubMed] [Google Scholar]
- Haupt Y., Alexander W. S., Barri G., Klinken S. P., Adams J. M. Novel zinc finger gene implicated as myc collaborator by retrovirally accelerated lymphomagenesis in E mu-myc transgenic mice. Cell. 1991 May 31;65(5):753–763. doi: 10.1016/0092-8674(91)90383-a. [DOI] [PubMed] [Google Scholar]
- Hill D. P., Strome S. Brief cytochalasin-induced disruption of microfilaments during a critical interval in 1-cell C. elegans embryos alters the partitioning of developmental instructions to the 2-cell embryo. Development. 1990 Jan;108(1):159–172. doi: 10.1242/dev.108.1.159. [DOI] [PubMed] [Google Scholar]
- Jones J. S., Weber S., Prakash L. The Saccharomyces cerevisiae RAD18 gene encodes a protein that contains potential zinc finger domains for nucleic acid binding and a putative nucleotide binding sequence. Nucleic Acids Res. 1988 Jul 25;16(14B):7119–7131. doi: 10.1093/nar/16.14.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemphues K. J., Priess J. R., Morton D. G., Cheng N. S. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell. 1988 Feb 12;52(3):311–320. doi: 10.1016/s0092-8674(88)80024-2. [DOI] [PubMed] [Google Scholar]
- Kimble J., Edgar L., Hirsh D. Specification of male development in Caenorhabditis elegans: the fem genes. Dev Biol. 1984 Sep;105(1):234–239. doi: 10.1016/0012-1606(84)90279-3. [DOI] [PubMed] [Google Scholar]
- Kirby C., Kusch M., Kemphues K. Mutations in the par genes of Caenorhabditis elegans affect cytoplasmic reorganization during the first cell cycle. Dev Biol. 1990 Nov;142(1):203–215. doi: 10.1016/0012-1606(90)90164-e. [DOI] [PubMed] [Google Scholar]
- Krause M., Hirsh D. A trans-spliced leader sequence on actin mRNA in C. elegans. Cell. 1987 Jun 19;49(6):753–761. doi: 10.1016/0092-8674(87)90613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R. C., Feinbaum R. L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993 Dec 3;75(5):843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- Lovering R., Hanson I. M., Borden K. L., Martin S., O'Reilly N. J., Evan G. I., Rahman D., Pappin D. J., Trowsdale J., Freemont P. S. Identification and preliminary characterization of a protein motif related to the zinc finger. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2112–2116. doi: 10.1073/pnas.90.6.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C. C., Kramer J. M., Stinchcomb D., Ambros V. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991 Dec;10(12):3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Price B. D., Chang Z., Smith R., Bockheim S., Laughon A. The Drosophila neuralized gene encodes a C3HC4 zinc finger. EMBO J. 1993 Jun;12(6):2411–2418. doi: 10.1002/j.1460-2075.1993.tb05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schierenberg E. Cell determination during early embryogenesis of the nematode Caenorhabditis elegans. Cold Spring Harb Symp Quant Biol. 1985;50:59–68. doi: 10.1101/sqb.1985.050.01.010. [DOI] [PubMed] [Google Scholar]
- Slobbe R. L., Pluk W., van Venrooij W. J., Pruijn G. J. Ro ribonucleoprotein assembly in vitro. Identification of RNA-protein and protein-protein interactions. J Mol Biol. 1992 Sep 20;227(2):361–366. doi: 10.1016/0022-2836(92)90890-v. [DOI] [PubMed] [Google Scholar]
- Strome S. Generation of cell diversity during early embryogenesis in the nematode Caenorhabditis elegans. Int Rev Cytol. 1989;114:81–123. doi: 10.1016/s0074-7696(08)60859-1. [DOI] [PubMed] [Google Scholar]
- Strome S., Wood W. B. Generation of asymmetry and segregation of germ-line granules in early C. elegans embryos. Cell. 1983 Nov;35(1):15–25. doi: 10.1016/0092-8674(83)90203-9. [DOI] [PubMed] [Google Scholar]
- Su L., Hershberger R. J., Weissman I. L. LYAR, a novel nucleolar protein with zinc finger DNA-binding motifs, is involved in cell growth regulation. Genes Dev. 1993 May;7(5):735–748. doi: 10.1101/gad.7.5.735. [DOI] [PubMed] [Google Scholar]
- Sweeney F. P., Pocklington M. J., Orr E. The yeast type II myosin heavy chain: analysis of its predicted polypeptide sequence. J Muscle Res Cell Motil. 1991 Feb;12(1):61–68. doi: 10.1007/BF01781175. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Lehmann R. Nanos is the localized posterior determinant in Drosophila. Cell. 1991 Aug 23;66(4):637–647. doi: 10.1016/0092-8674(91)90110-k. [DOI] [PubMed] [Google Scholar]
- Williams B. D., Schrank B., Huynh C., Shownkeen R., Waterston R. H. A genetic mapping system in Caenorhabditis elegans based on polymorphic sequence-tagged sites. Genetics. 1992 Jul;131(3):609–624. doi: 10.1093/genetics/131.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]