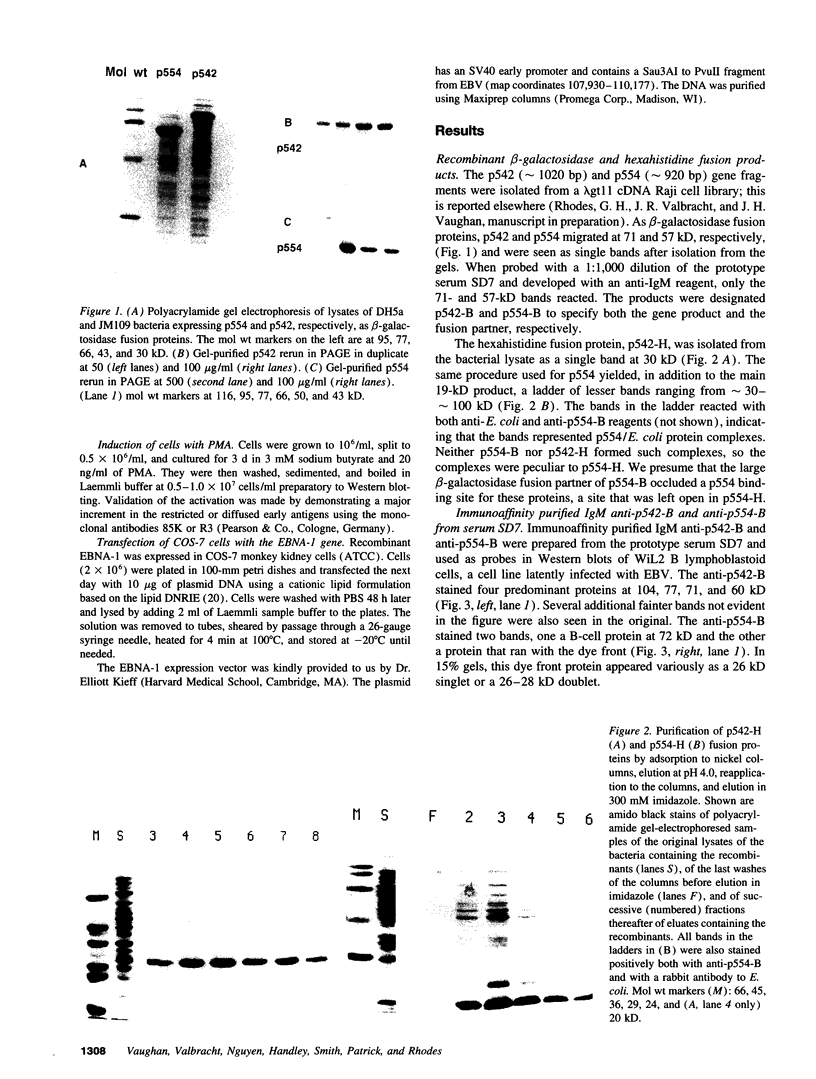
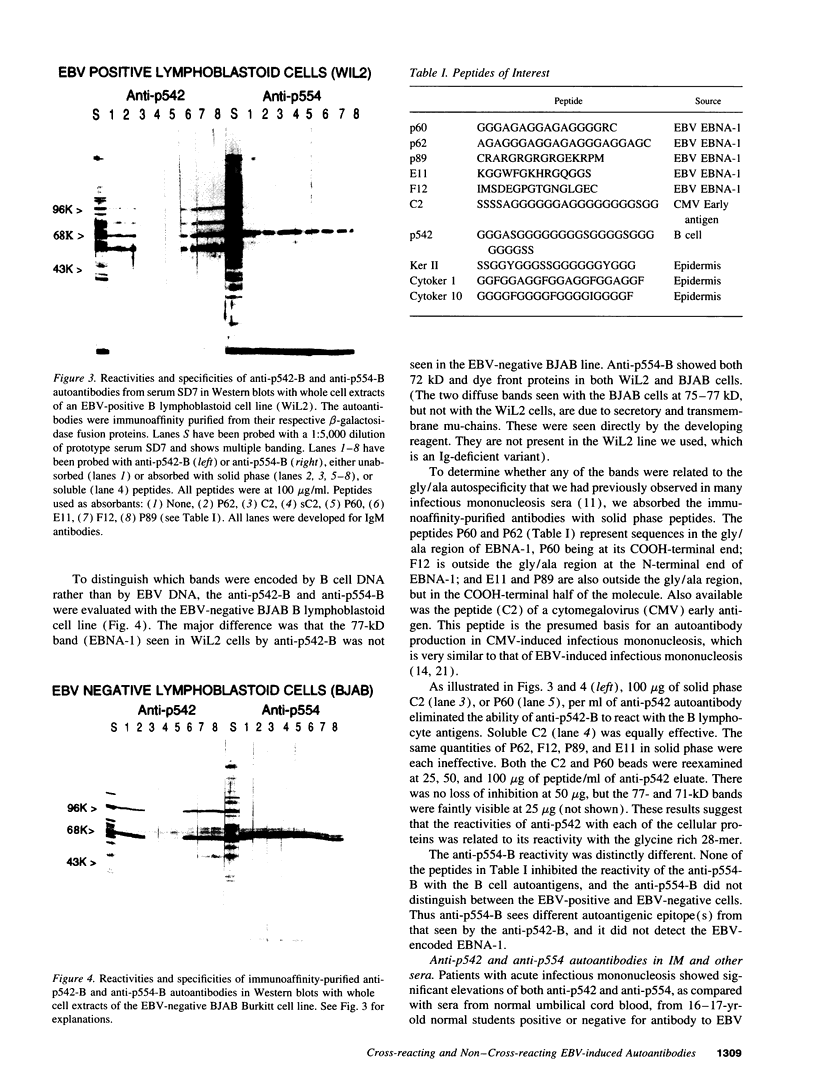
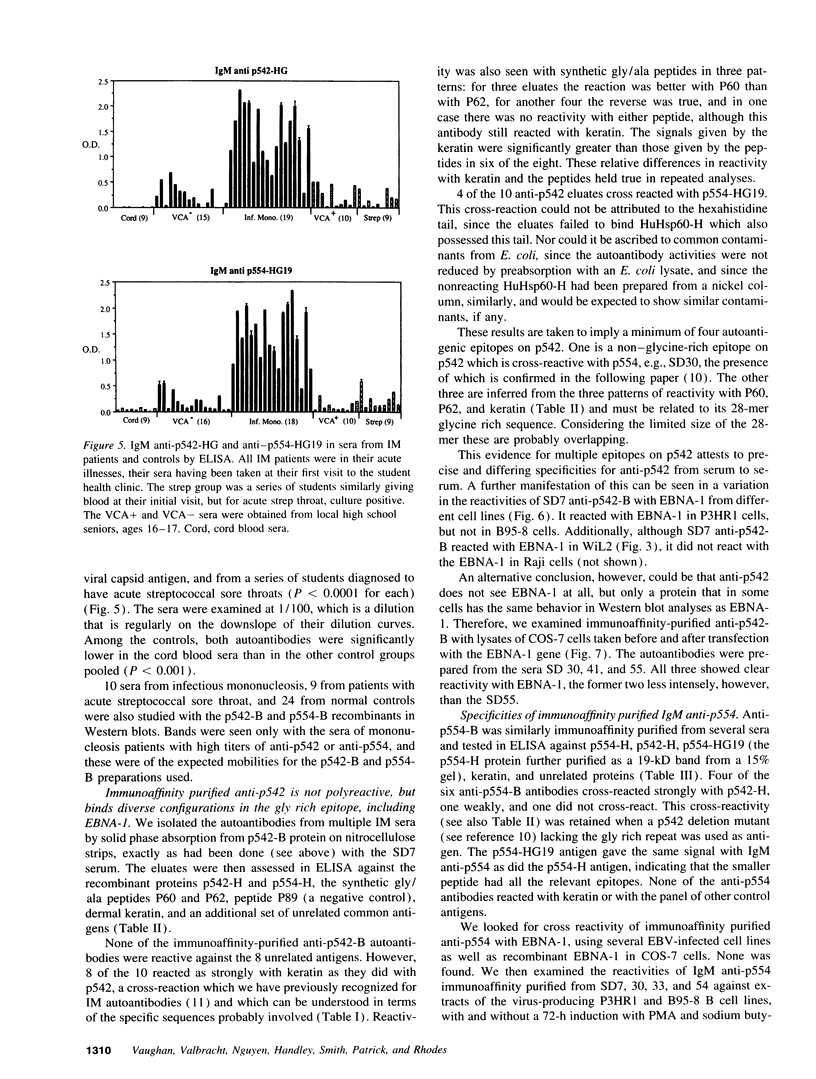
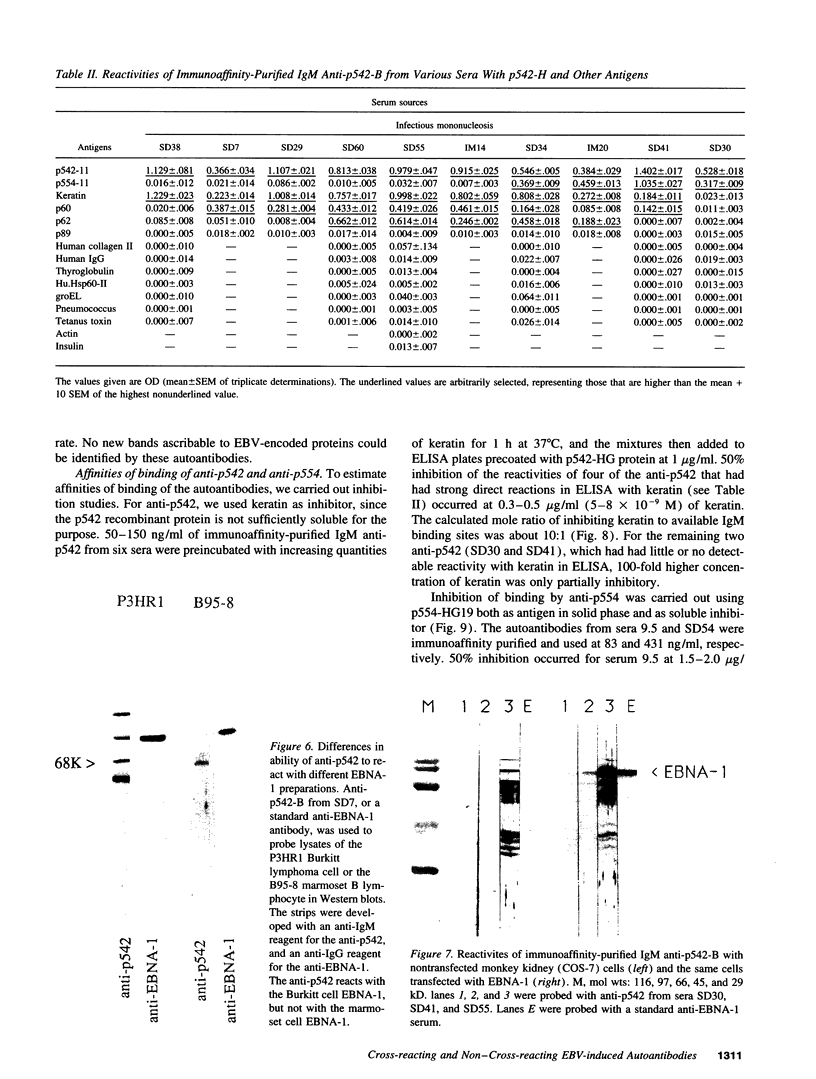
Abstract
In previous studies of infectious mononucleosis, we found IgM autoantibodies which react with hematopoietic cell antigens. Many of these were inhibited by synthetic glycine/alanine peptides representing the glycine/alanine repeat of Epstein-Barr virus nuclear antigen-1. We have cloned and expressed fragments of genes encoding two of these autoantigens. One gene (p542) encodes a protein containing a glycine-rich 28-mer, which is its chief autoantigenic epitope and which represents a newly identified class of evolutionarily conserved autoepitopes. The other gene (p554) encodes a protein that is not demonstrably cross-reactive with Epstein-Barr virus nuclear antigen-1 or with any other EBV protein, but forms complexes with other proteins. Immunoaffinity-purified anti-p542 and anti-p554 have relatively high binding affinities, as evidenced by inhibition at 10(6)-10(8) M-1, and neither autoantibody showed polyreactivity with other common antigens. The data thus suggest that neither autoantibody is simply an expression of polyclonal B cell activation. We conclude that the two autoantigens stimulate autoantibody synthesis by different mechanisms. One autoantigen shares homology to a viral protein which generates cross-reacting antibodies to the autoantigenic epitope. The other has no recognizable cross-reaction with the infecting pathogen and may become immunogenic through complexing with other proteins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acampora D., D'Esposito M., Faiella A., Pannese M., Migliaccio E., Morelli F., Stornaiuolo A., Nigro V., Simeone A., Boncinelli E. The human HOX gene family. Nucleic Acids Res. 1989 Dec 25;17(24):10385–10402. doi: 10.1093/nar/17.24.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Faber V. Antibodies to smooth muscle and other tissue components in infectious mononucleosis. Scand J Infect Dis. 1978;10(1):1–5. doi: 10.3109/inf.1978.10.issue-1.01. [DOI] [PubMed] [Google Scholar]
- Baboonian C., Venables P. J., Williams D. G., Williams R. O., Maini R. N. Cross reaction of antibodies to a glycine/alanine repeat sequence of Epstein-Barr virus nuclear antigen-1 with collagen, cytokeratin, and actin. Ann Rheum Dis. 1991 Nov;50(11):772–775. doi: 10.1136/ard.50.11.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenfeld P., Haratz N., Klein G., Sulitzeanu D. Cross-reactivity between the EBNA-1 p107 peptide, collagen, and keratin: implications for the pathogenesis of rheumatoid arthritis. Clin Immunol Immunopathol. 1990 Jan;54(1):14–25. doi: 10.1016/0090-1229(90)90002-8. [DOI] [PubMed] [Google Scholar]
- Felgner J. H., Kumar R., Sridhar C. N., Wheeler C. J., Tsai Y. J., Border R., Ramsey P., Martin M., Felgner P. L. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J Biol Chem. 1994 Jan 28;269(4):2550–2561. [PubMed] [Google Scholar]
- Garzelli C., Pacciardi A., Carmignani M., Conaldi G., Basolo F., Falcone G. A human monoclonal autoantibody isolated from a patient with infectious mononucleosis reactive with both self antigens and Epstein-Barr virus nuclear antigen (EBNA). Immunol Lett. 1989 Sep;22(3):211–216. doi: 10.1016/0165-2478(89)90193-4. [DOI] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Houghten R. A., Chang W. C., Li C. H. Human beta-endorphin: synthesis and characterization of analogs iodinated and tritiated at tyrosine residues 1 and 27. Int J Pept Protein Res. 1980 Oct;16(4):311–320. doi: 10.1111/j.1399-3011.1980.tb02592.x. [DOI] [PubMed] [Google Scholar]
- Huidbüchel E., Blaschek M., Seigneurin J. M., Lamour A., Berthelot J. M., Youinou P. Anti-organelle and anti-cytoskeletal autoantibodies in the serum of Epstein-Barr virus-infected patients. Ann Med Interne (Paris) 1991;142(5):343–346. [PubMed] [Google Scholar]
- Imajoh S., Kawasaki H., Suzuki K. The amino-terminal hydrophobic region of the small subunit of calcium-activated neutral protease (CANP) is essential for its activation by phosphatidylinositol. J Biochem. 1986 Apr;99(4):1281–1284. doi: 10.1093/oxfordjournals.jbchem.a135593. [DOI] [PubMed] [Google Scholar]
- Kataaha P. K., Holborow E. J., Edwards J. M. Incidence of anti-intermediate filament antibody in serum samples of students with suspected glandular fever. J Clin Pathol. 1985 Mar;38(3):351–354. doi: 10.1136/jcp.38.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataaha P. K., Mortazavi-Milani S. M., Russell G., Holborow E. J. Anti-intermediate filament antibodies, antikeratin antibody, and antiperinuclear factor in rheumatoid arthritis and infectious mononucleosis. Ann Rheum Dis. 1985 Jul;44(7):446–449. doi: 10.1136/ard.44.7.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp S., Bröker M., Amann E. pSEM vectors: high level expression of antigenic determinants and protein domains. Biotechniques. 1990 Mar;8(3):280–281. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linder E., Kurki P., Andersson L. C. Autoantibody to "intermediate filament" in infectious mononucleosis. Clin Immunol Immunopathol. 1979 Dec;14(4):411–417. doi: 10.1016/0090-1229(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Luka J., Kreofsky T., Pearson G. R., Hennessy K., Kieff E. Identification and characterization of a cellular protein that cross-reacts with the Epstein-Barr virus nuclear antigen. J Virol. 1984 Dec;52(3):833–838. doi: 10.1128/jvi.52.3.833-838.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE S., SPACKMAN D. H., STEIN W. H. Automatic recording apparatus for use in the chromatography of amino acids. Fed Proc. 1958 Dec;17(4):1107–1115. [PubMed] [Google Scholar]
- Martin T., Duffy S. F., Carson D. A., Kipps T. J. Evidence for somatic selection of natural autoantibodies. J Exp Med. 1992 Apr 1;175(4):983–991. doi: 10.1084/jem.175.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead G. M., Cowin P., Whitehouse J. M. Antitubulin antibody in healthy adults and patients with infectious mononucleosis and its relationship to smooth muscle antibody (SMA). Clin Exp Immunol. 1980 Feb;39(2):328–336. [PMC free article] [PubMed] [Google Scholar]
- Menezes J., Leibold W., Klein G., Clements G. Establishment and characterization of an Epstein-Barr virus (EBC)-negative lymphoblastoid B cell line (BJA-B) from an exceptional, EBV-genome-negative African Burkitt's lymphoma. Biomedicine. 1975 Jul;22(4):276–284. [PubMed] [Google Scholar]
- Misra R., Venables P. J., Plater-Zyberk C., Watkins P. F., Maini R. N. Anti-cardiolipin antibodies in infectious mononucleosis react with the membrane of activated lymphocytes. Clin Exp Immunol. 1989 Jan;75(1):35–40. [PMC free article] [PubMed] [Google Scholar]
- Parry D. A., Steinert P. M. Intermediate filament structure. Curr Opin Cell Biol. 1992 Feb;4(1):94–98. doi: 10.1016/0955-0674(92)90064-j. [DOI] [PubMed] [Google Scholar]
- Reith W., Herrero-Sanchez C., Kobr M., Silacci P., Berte C., Barras E., Fey S., Mach B. MHC class II regulatory factor RFX has a novel DNA-binding domain and a functionally independent dimerization domain. Genes Dev. 1990 Sep;4(9):1528–1540. doi: 10.1101/gad.4.9.1528. [DOI] [PubMed] [Google Scholar]
- Rhodes G., Carson D. A., Valbracht J., Houghten R., Vaughan J. H. Human immune responses to synthetic peptides from the Epstein-Barr nuclear antigen. J Immunol. 1985 Jan;134(1):211–216. [PubMed] [Google Scholar]
- Rhodes G., Rumpold H., Kurki P., Patrick K. M., Carson D. A., Vaughan J. H. Autoantibodies in infectious mononucleosis have specificity for the glycine-alanine repeating region of the Epstein-Barr virus nuclear antigen. J Exp Med. 1987 Apr 1;165(4):1026–1040. doi: 10.1084/jem.165.4.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes G., Smith R. S., Rubin R. E., Vaughan J., Horwitz C. A. Identical IgM antibodies recognizing a glycine-alanine epitope are induced during acute infection with Epstein-Barr virus and cytomegalovirus. J Clin Lab Anal. 1990;4(6):456–464. doi: 10.1002/jcla.1860040613. [DOI] [PubMed] [Google Scholar]
- Sutton R. N., Emond R. T., Thomas D. B., Doniach D. The occurrence of autoantibodies in infectious mononucleosis. Clin Exp Immunol. 1974 Jul;17(3):427–436. [PMC free article] [PubMed] [Google Scholar]
- Vaughan J. H., Nguyen M. D., Valbracht J. R., Patrick K., Rhodes G. H. Epstein-Barr virus-induced autoimmune responses. II. Immunoglobulin G autoantibodies to mimicking and nonmimicking epitopes. Presence in autoimmune disease. J Clin Invest. 1995 Mar;95(3):1316–1327. doi: 10.1172/JCI117782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winiarski J. Antibodies to platelet membrane glycoprotein antigens in three cases of infectious mononucleosis-induced thrombocytopenic purpura. Eur J Haematol. 1989 Jul;43(1):29–34. doi: 10.1111/j.1600-0609.1989.tb01247.x. [DOI] [PubMed] [Google Scholar]