Abstract
Introduction
The majority of patients enrolled in treatment for substance use disorders (SUDs) also use tobacco. Many will continue to use tobacco even during abstinence from other drugs and alcohol, often leading to smoking-related illnesses. Despite this, little research has been conducted to assess the influence of being a smoker on SUD treatment outcomes and changes in smoking during a treatment episode.
Methods
In this secondary analysis, cigarette smoking was evaluated in participants completing outpatient SUD treatment as part of a multi-site study conducted by the National Drug Abuse Treatment Clinical Trials Network. Analyses included the assessment of changes in smoking and nicotine dependence via the Fagerström Test for Nicotine Dependence during the 12-week study among all smokers (Aim #1), specifically among those in the experimental treatment group (Aim #2), and the moderating effect of being a smoker on treatment outcomes (Aim #3).
Results
Participants generally did not reduce or quit smoking throughout the course of the study. Among a sub-set of participants with higher baseline nicotine dependence scores randomized to the control arm, scores at the end of treatment were lower compared to the experimental arm, though measures of smoking quantity did not appear to decrease. Further, being a smoker was associated with poorer treatment outcomes compared to non-smokers enrolled in the trial.
Conclusions
This study provides evidence that patients enrolled in community-based SUD treatment continue to smoke, even when abstaining from drugs and alcohol. These results add to the growing literature encouraging the implementation of targeted, evidence-based interventions to promote abstinence from tobacco among SUD treatment patients.
Keywords: substance use disorders, smoking, tobacco, cessation, internet-delivered treatment, Therapeutic Education System
1. Introduction
The majority of patients enrolled in treatment for substance use disorders (SUDs) also use tobacco with reported rates as high as 97% (Bobo, 1989; Guydish et al., 2011; Kalman, 1998; McClure, Acquavita, Dunn, Stoller, & Stitzer, 2014; Nahvi, Richter, Li, Modali, & Arnsten, 2006; Pajusco et al., 2012). This is significantly higher than the smoking rate in the general population, which is currently 19.3% in the United States (Centers for Disease Control and Prevention, 2012). Those enrolled in treatment for SUDs are more likely to die due to smoking-related illnesses than from complications from their primary drug of choice (Baca & Yahne, 2009; Hser, McCarthy, & Anglin, 1994; Hurt et al., 1996). Attempts to explain the relationship between nicotine and the use of other substances have involved conceptual models including, biological vulnerabilities due to nicotine during adolescents (Kelley & Rowan, 2004; Lydon, Wilson, Child, & Geier, 2014; Santos, Marin, Cruz, Delucia, & Planeta, 2009), common neural pathways, substrates, and dysregulation contributing to addiction (Kalivas, Lalumiere, Knackstedt, & Shen, 2009), and pharmacological interactions between drugs potentially increasing their reinforcing properties (Mello, Lukas, & Mendelson, 1985; Mello, Mendelson, Sellers, & Kuehnle, 1980; Mutschler, Stephen, Teoh, Mendelson, & Mello, 2002). Additional conceptual models, not specific to nicotine, have focused on reinforcer pathology (Bickel, Johnson, Koffarnus, MacKillop, & Murphy, 2014) and reward seeking (Arias-Carrion & Salama, 2012), to name only a few.
Despite high rates of smoking among SUD treatment patients and the well-known negative health effects of smoking, the majority who enter SUD treatment as cigarette smokers will not contact resources to assist them in quitting and tobacco cessation services are not always available onsite for those who might be interested. Several studies administered across treatment settings have reported low levels of both availability and use of smoking cessation services in participating SUD programs (Eby & Laschober, 2013; Friedmann, Jiang, & Richter, 2008; Fuller et al., 2007; Knudsen & Studts, 2011; Laschober & Eby, 2013; Richter, Choi, McCool, Harris, & Ahluwalia, 2004), though services are becoming more common with the introduction of state mandates and smoking cessation guidelines for SUD treatment clinics (Guydish et al., 2012; Williams et al., 2005).
Recent evidence appears to suggest that non-smokers or former smokers may have better drug abstinence outcomes or proxies of outcomes compared to smokers. This is concerning given that the majority of SUD patients are smokers, and they may be starting treatment episodes already at a disadvantage. Among users of both tobacco and cannabis, a recent review showed poorer cannabis cessation outcomes compared to cannabis only users (Peters, Budney, & Carroll, 2012), and a human laboratory-based study showed that co-users of tobacco and cannabis were more likely to relapse (to cannabis) compared to non-smoking cannabis users (Haney et al., 2013). Smoking during opioid detoxification was shown to increase opioid craving (Mannelli, Wu, Peindl, & Gorelick, 2013). It has also been found that cocaine-dependent patients who stopped smoking in response to smoking cessation treatment provided concurrently with SUD treatment had improved cocaine-use outcomes relative to those who continued to smoke (Winhusen, Kropp, Theobald, & Lewis, 2014). The aforementioned results suggest that there is a potentially important relationship between tobacco use and SUD treatment outcomes. Studies to this point have been substance-specific, and may be limited in generalizability. The current report, however, includes data from a large, geographically diverse outpatient SUD treatment population, and may provide additional insight, generalizability, and support for the previous findings that smokers appear to have poorer SUD treatment outcomes.
Additionally, little is known about changes in smoking during a SUD treatment episode and published evaluations have been limited to adolescent populations. One study found that smoking persisted throughout SUD treatment and increased at the 12-month follow-up visit (Coleman-Cowger & Catlin, 2013). Another study showed that among adolescents in treatment for cannabis use disorders, moderate and heavy cigarette smokers decreased their cigarettes per day only slightly during treatment, but showed similar smoking rates at follow-up, while mild smokers decreased their cigarettes per day during treatment and follow-up (Shelef, Diamond, Diamond, & Myers, 2009). Among adolescents enrolled in a cannabis cessation pharmacotherapeutic clinical trial, there were no changes in cigarette smoking during treatment (McClure, Baker, & Gray, 2014), while another report showed that adolescent cannabis users with attention-deficit/hyperactivity disorder who reduced their cannabis use by at least 50% following treatment also significantly decreased their cigarette smoking (Gray et al., 2011). The scarcity of data on this topic suggests that the majority of treatment trials among SUD populations do not assess or do not report the impact of SUD treatment on cigarette smoking and other tobacco use, presenting a missed opportunity for future trials and intervention improvement.
In order to contribute to the literature on the complex issue of cigarette smoking among SUD treatment patients, the current report explored cigarette smoking within the context of a randomized controlled effectiveness trial of a web-delivered psychosocial treatment (WEB-TX) conducted within the National Drug Abuse Treatment Clinical Trials Network (NIDA CTN) (Campbell et al., 2014; Campbell et al., 2012). This secondary analysis had three main aims; 1) assess if smoking and nicotine dependence changed over the course of the 12-week study, 2) explore if the treatment group (Therapeutic Education System [TES]) showed reductions in smoking and changes in nicotine dependence over the treatment period compared to the control group (treatment as usual [TAU]), and 3) determine if being a smoker moderated the effect of treatment with TES (versus TAU) on abstinence from drugs and alcohol.
Though TES as a treatment intervention for drugs and alcohol does not target cigarette smoking specifically, the content material of TES focuses on skills-based learning for achieving and maintaining abstinence from drugs (e.g., drug refusal, coping with craving and withdrawal, avoiding triggers, etc.). It follows that interventions of this sort targeting SUDs may extend to cigarette smoking through knowledge and acquired skills that may prove useful in cessation efforts. In a broader sense, it is also possible that the improvement of SUD symptomology is associated with reductions in tobacco use or cessation. Evaluation of the relations between smoking and substance use is valuable for the continued improvement of treatment strategies to address both concurrently.
2. Methods
2.1 Participants and Procedures
The parent trial (WEB-TX; CTN-0044) was conducted within 10 outpatient, geographically-diverse, community-based SUD treatment programs (Campbell et al., 2014; Campbell et al., 2012). Enrolled participants were adult men and women (N=507) who were within the first 30 days of their current treatment episode. After screening and baseline assessment, participants were randomized to receive 12 weeks of either standard TAU (n=252) or TAU + TES (n=255), whereby TES replaced two hours of standard care per week. TES (Bickel, Marsch, Buchhalter, & Badger, 2008; Marsch et al., 2014) consisted of a web-delivered version of the Community Reinforcement Approach (Onken, 1997) that incorporated voucher-based contingency management (Higgins et al., 1994; Peirce et al., 2006; Petry et al., 2005; Stitzer, Petry, & Peirce, 2010) to promote abstinence from drugs and alcohol. TES has 62 computer-delivered, interactive, multimedia modules, which covered skills for achieving and maintaining abstinence. Participants made two weekly research assessment visits for 12 weeks and completed 3- and 6-month follow-up visits. Primary outcomes for this study (Campbell et al., 2014) and further study details (Campbell et al., 2013; Campbell et al., 2012) are described elsewhere, but briefly, participants randomized to TES had greater abstinence rates and lower treatment dropout rates compared to a standard outpatient treatment control condition (Campbell et al., 2014). The trial was registered with Clinicaltrials.gov (NCT01104805).
2.2 Measures
Smoking
Cigarette smoking was self-reported during the trial. Smoking status and nicotine dependence were assessed at the baseline visit, weeks 4, 8, and 12 during treatment, and at the 3- and 6-month follow-up visits. Participants were asked if they currently smoked cigarettes and if they were using any smoking cessation medication (i.e., nicotine replacement, buproprion, varenicline, or other). If participants endorsed smoking, they completed the six-item Fagerström Test for Nicotine Dependence (FTND, range 0–10) (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991). Responses on the FTND were summed resulting in a score of nicotine dependence, with 10 indicating the most severe level of dependence. Two specific items, often referred to as the Heaviness of Smoking Index (HSI) (Heatherton, Kozlowski, Frecker, Rickert, & Robinson, 1989; Kozlowski, Porter, Orleans, Pope, & Heatherton, 1994), from the FTND were examined in a separate analysis. The first item was number of cigarettes per day, which was categorized into four levels based on the standard response categories used in the FTND questionnaire: 10 or less, 11–20, 20–30, and 31+. The second item was time to first cigarette after waking, also categorized into four levels: within 5 min, 6–30 min, 31–60, and after 60 min.
Substance Use
Abstinence from drugs and alcohol was assessed twice per week during the treatment phase. Abstinence was defined as: 1) a negative urine drug test (for 10 substances), and 2) self-reported abstinence from drugs and alcohol based on the Timeline Follow Back method (Sobell, Sobel, Bogardis, Leo, & Skinner, 1992). Abstinence data were considered missing if: 1) the urine screen was missing or; 2) the urine screen was negative and the self-report was missing. The outcome for the purposes of this analysis was a binary measure of abstinence (yes or no). Treatment responders were defined as participants who were abstinent from all drugs and drinking days during the final four weeks of treatment (weeks 9–12) with no missing data points. The last four weeks of active treatment was the pre-specified indicator of treatment success and consistent with the primary outcome publication (Campbell et al., 2014).
2.3 Statistical Analyses
Demographic and clinical characteristics were described for the total sample (N=507) and by smoking status at baseline using means, standard deviations, and frequencies; chi-square and t-tests were used to test differences between baseline smokers and non-smokers on these variables. Additionally, the categorical variables of cigarettes per day and time to first cigarette after waking were analyzed for changes from baseline to week 12 (Aim #1). The proportion of participants endorsing each category and change in category was assessed using chi-square tests.
Four outcome variables were analyzed: smoking status at the end of treatment (EOT; week 12) (binary; among N=507 enrolled participants, n=448 cases with smoking status data at EOT) (Aim #2); number of cigarettes per day at EOT (categorical; among n=391 baseline smokers, n=344 with smoking data available at EOT) (Aim #2); FTND scores at week 4, 8, and 12 (continuous longitudinal; among n=391 baseline smokers, n=366 with ≥ 1 FTND assessment) (Aim #2), and weekly abstinence from drugs and alcohol during the last four weeks of treatment (binary longitudinal; among N=507 enrolled participants, n=469 with ≥ 1 observation during the last 4 weeks of treatment) (Aim #3). Treatment arm, baseline drug use status (positive vs. negative assessed by urine drug and breath alcohol screen) and baseline smoking status or baseline FTND score, as appropriate, were included as predictors in statistical models. Generalized linear models were applied for the outcomes with appropriate link functions (logit for binary outcome, cumulative logit for categorical outcome, and identity for continuous outcome). Interactions were tested (between treatment arm, baseline drug use status, and baseline smoking status or baseline FTND scores) and included in the final models only if significant (p<.05). In addition, for the model testing smoking status at week 12, treatment response (i.e., participants who were completely abstinent during this time with no missing data) was also tested in an attempt to replicate prior research (i.e., Gray et al., 2011). This was not significant (p<.05) and therefore not included in the final model. Time was included in the models testing the longitudinal outcomes: FTND and abstinence. Site and subject were treated as random effects. The correlation between the repeated measurements over time within subject was modeled using the first-order auto regressive structure. Missing data were assumed missing at random. SAS version 9.3 was utilized for all analyses.
3. Results
3.1 Demographic and Smoking Characteristics
Demographic and smoking characteristics are shown in Table 1 for the entire study sample (N=507) and for self-reported cigarette smokers (n=391; 77%) and non-smokers (n=116; 23%) at baseline. Participants who were cigarette smokers at baseline tended to be younger, more likely to be female, less educated, and unemployed. No significant difference was found in baseline drug use status by smoking status. Smokers and non-smokers were equally likely to be randomized to each treatment arm. When separated by primary substance of abuse, the proportion of smokers to non-smokers was generally consistent with overall averages, with the exception of the opioid group, which had a higher rate of smokers compared to other substances of abuse, while the stimulant group had a lower rate of smokers. At baseline, only 16 smokers (4.1%) reported current use of a smoking cessation medication, and even fewer reported currently using a medication at the end of 12 weeks (12; 3.5%). During the 12 weeks of active treatment and participation in SUD treatment, approximately 19 (5% out of 344 reporting) baseline smokers reported being non-smokers at week 12. However, 11 (11% out of 104 reporting) baseline non-smokers reported being smokers at week 12.
Table 1.
Baseline characteristics by cigarette smoking status (N=507).
| Total Sample (N=507) |
Smokers (n=391) |
Non-smokers (n=116) |
t/X2 | p-value | |
|---|---|---|---|---|---|
| Variables | Mean(SD)/n(%) | ||||
| Age (years) | 34.89 (10.89) | 33.89 (10.14) | 38.28 (12.58) | 3.45 | <.001 |
| Female (%) | 192 (37.9) | 168 (43.1) | 24 (20.7) | 19.03 | <.001 |
| Race/Ethnicity (%) White Black/African American Hispanic/Latino Multi-racial/Other |
267 (52.7) 112 (22.1) 55 (10.9) 73 (14.4) |
213 (54.5) 87 (22.3) 41 (10.5) 50 (12.8) |
54 (46.6) 25 (21.6) 14 (12.1) 23 (19.8) |
4.37 | .22 |
| Education (%) < High School High School/GED > High School |
118 (23.3) 310 (61.1) 79 (15.6) |
108 (27.6) 233 (59.6) 50 (12.8) |
10 (8.6) 77 (66.4) 29 (25.0) |
23.11 | <.001 |
| Single/Never Married (%) | 308 (60.8) | 244 (62.4) | 64 (55.2) | 1.96 | .16 |
| Unemployed (%) | 298 (58.8) | 240 (61.4) | 58 (50.0) | 4.78 | .03 |
| Primary Substance (%) Alcohol Cocaine Stimulants Marijuana Opioids Other |
104 (20.5) 102 (20.1) 69 (13.6) 114 (22.5) 108 (21.3) 10 (2.0) |
80 (20.5) 83 (21.2) 46 (11.8) 82 (21.0) 91 (23.3) 9 (2.3) |
24 (20.7) 19 (16.4) 23 (19.8) 32 (27.6) 17 (14.7) 1 (0.9) |
11.12 | .049 |
| Negative Baseline Drug Use Status (%) | 275 (54.2) | 217 (55.5) | 58 (50.0) | 1.09 | .30 |
| Smoking medication (%) Baseline Week 12 (N=448) |
16 (3.2) 13 (2.9) |
16 (4.1) 12 (3.5) (n=344) |
0 (0.0) 1 (1.0) (n=104) |
4.90 1.81 |
.03 .18 |
| Smoking at week 12 (%) (N=448) |
336 (75.0) | 325 (94.5) (n=344) |
11 (10.6) (n=104) |
299.8 | <.001 |
| Treatment Assignment (%) TAU TES |
252 (49.7) 255 (50.3) |
199 (50.9) 192 (49.1) |
53 (45.7) 63 (54.3) |
0.97 | .32 |
| Baseline Abstinence Status and Treatment Assignment (%) Drug Negative + TES Drug Negative + TAU Drug Positive + TES Drug Positive + TAU |
136 (26.8) 139 (27.4) 119 (23.5) 113 (22.3) |
108 (27.6) 109 (27.9) 84 (21.5) 90 (23.0) |
28 (24.1) 30 (25.9) 35 (30.2) 23 (19.8) |
3.82 | .28 |
3.2 Changes in Smoking during Treatment (Aim #1)
The categorical variables of cigarettes per day and time to first cigarette were assessed via individual items on the FTND and were examined among baseline smokers. At week 12, responses to these items were categorized as an improvement or decrement in severity, or no change from baseline. Improvements or decrements were defined as participants changing categories during treatment to reflect changes in nicotine dependence (e.g., fewer cigarettes per day and/or more time to first cigarette). Table 2 shows the proportions of participants who showed either categorical decrease, increase, or no changes on cigarettes per day, and also in time to first cigarette at EOT. Decreases in cigarettes per day and longer time to first cigarette occurred for approximately 22% and 26% of participants, respectively. Increases in cigarettes per day and less time to first cigarette (considered detrimental) occurred in 12% and 17% of participants, respectively. The majority of the sample did not change their number of cigarettes smoked per day (66%) or time to first cigarette (57%).The only significant predictor of cigarettes smoked per day at EOT was baseline cigarettes smoked per day (X2(1)=107.27, p<.001).
Table 2.
Changes in cigarettes per day and time to first cigarette from baseline to week 12 (end of treatment [EOT]) among baseline smokers (n=391) with an EOT observation (n=344). Categories were based on FTND responses during baseline and changes in categories were based on responses at week 12.
| Cigarettes per Day | ||||
|---|---|---|---|---|
| Baseline Categories | N | % – Decreased | % – Same level | % – Increased |
| 1–10 | 194 | 8.3 | 73.7 | 18.0 |
| 11–20 | 131 | 35.1 | 59.5 | 5.4 |
| 21–30 | 14 | 85.7 | 14.3 | 0.0 |
| 31+ | 5 | 40.0 | 60.0 | 0.0 |
| Total | 344 | 22.1 | 65.7 | 12.1 |
| Time to First Cigarette | ||||
| Baseline Categories | N | % – More time | % – Same time | % – Less time |
| < 5 min | 70 | 34.2 | 65.8 | 0.0 |
| 6–30 min | 63 | 24.1 | 55.2 | 20.7 |
| 31–60 min | 87 | 22.2 | 39.7 | 38.1 |
| 60+ min | 123 | 18.6 | 60.0 | 21.4 |
| Total | 343 | 26.2 | 57.2 | 16.6 |
3.3 Smoking Changes between Treatment Groups (Aim #2)
3.3.1 Smoking Status
Smoking at EOT was modeled by treatment arm, baseline drug use status, and baseline smoking status. A significant two-way interaction of treatment assignment and baseline drug use status (X2(1)=4.34, p=.04) was observed on self-reported smoking status at EOT (Figure 1). EOT smoking appeared to be lower among participants who were drug positive at baseline and assigned to TES compared to participants receiving TAU, but this observed treatment effect did not reach statistical significance (OR=0.40, 95% CI: 0.13, 1.28, p=.12). There was also no significant difference between TES and TAU among baseline drug negative participants (OR=2.18, 95% CI: 0.77, 6.14, p=.14). As expected, baseline smoking status (i.e., being a smoker) was significantly associated with continued smoking at EOT (X2(1)=147.55, p<.001).
Figure 1.
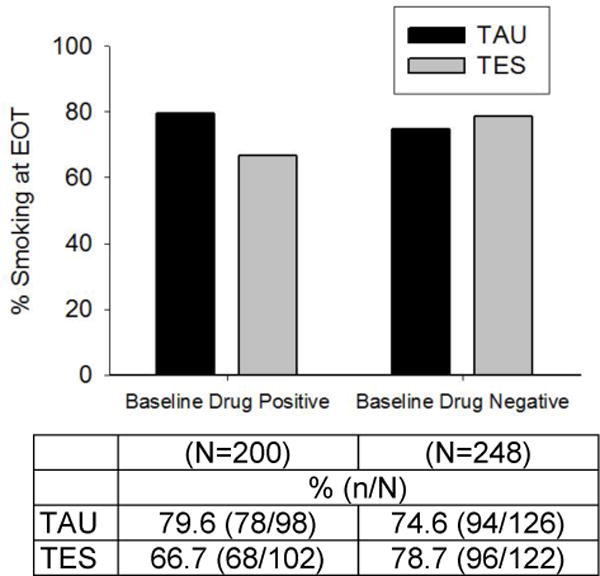
Observed smoking proportions at week 12 (end of treatment [EOT]) by baseline drug use status (positive vs. negative assessed via urine drug and breath alcohol screen) and treatment arm (Therapeutic Education System [TES] vs. treatment as usual [TAU] (n=448). A significant two-way interaction of treatment arm and baseline drug use status (X2(1)=4.34, p=.04) was observed on the proportion of participants who self-reported smoking at EOT.
3.3.2 Nicotine Dependence
Average FTND nicotine dependence scores for smokers at baseline showed that participants were moderately nicotine dependent (3.49 ± 2.3). Dependence severity did not differ significantly by treatment arm at baseline or throughout treatment. There was no significant change over time in nicotine dependence scores among all cigarette smokers, and average FTND scores at EOT were 3.30 (SD=2.4).
The model testing the outcome of FTND scores during treatment included a three-way interaction between treatment arm, baseline drug use status, and baseline FTND (F(1, 661)=4.73, p=.03).To illustrate this significant interaction, FTND baseline scores were split into four categories (quartiles) to demonstrate the observed interaction between treatment arm and baseline drug use status for the lowest quartile (≤1, bottom 24% of scores; Figure 2A) and the highest quartile (≥6, top 23% of scores; Figure 2B). There were no significant differences by treatment arm or baseline drug use status for those with lower baseline FTND scores. Participants with higher baseline FTND demonstrated lower EOT FTND scores in TAU compared to TES, but only among those who were drug negative at baseline. There were no significant differences by treatment arm among baseline drug positive participants in the top quartile for baseline FTND.
Figure 2.
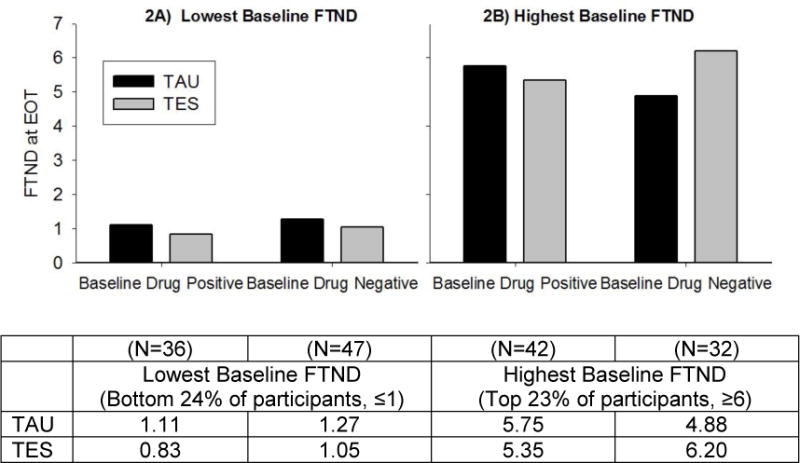
Nicotine dependence scores at week 12 (end of treatment [EOT]) by bottom (≤1 FTND) and top (≥6 FTND) quartiles of baseline nicotine dependence severity, baseline drug use status (positive vs. negative), and treatment arm (Therapeutic Education System [TES] vs. treatment as usual [TAU]) (n=366). Statistical analyses found a significant three-way interaction between treatment arm, baseline drug use status, and baseline FTND (F(1, 661)=4.73, p=.03).
3.4 Abstinence from Drugs and Alcohol (Aim #3)
Smoking was assessed as a possible moderator of treatment on the outcome of abstinence in the last four weeks of treatment. The final model yielded one significant two-way interaction: treatment and baseline smoking status (F(1, 2450)=3.76, p=.053).To illustrate this interaction, Figure 3 shows the proportion of participants who were abstinent from drugs and alcohol in the last four weeks of treatment, separated by treatment arm and baseline smoking status. There was a significant treatment difference favoring TES over TAU among non-smokers (OR=6.61, 95% CI: 1.85, 23.59, p=.004), but no differences by treatment arm among baseline smokers (OR=1.56, 95% CI: 0.77, 3.16, p=.21). Baseline drug use status was also associated with the abstinence outcome (F(1, 2450)=62.20, p<.001); those who were drug negative at baseline were more likely to be abstinent in the last four weeks of treatment.
Figure 3.
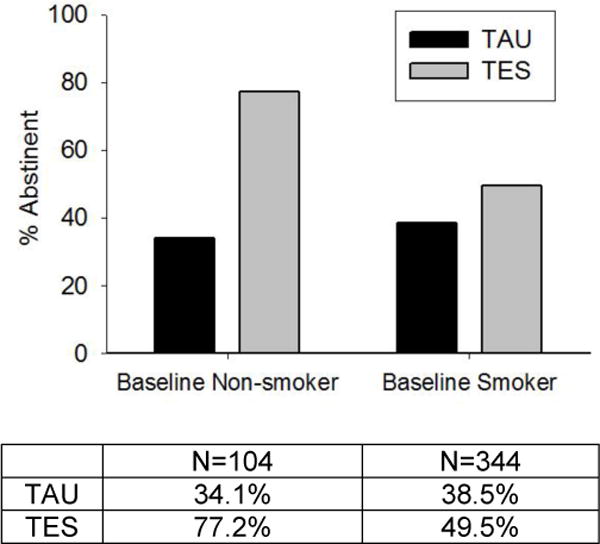
The observed proportion of participants abstinent from drugs and alcohol use days in the last 4 weeks of treatment (weeks 9–12) by baseline smoking status and treatment arm (Therapeutic Education System [TES] vs. treatment as usual [TAU]) (n=469). The final model yielded one significant two-way interaction: treatment and baseline smoking status (F(1, 2450)=3.76, p=.053).
4. Discussion
This secondary analysis examined cigarette smoking during outpatient SUD treatment among a large and geographically diverse sample of patients enrolled in community treatment clinics across the United States. Some study results were confirmatory of previous literature, including the high rates of smoking prevalence among the study sample (77%) (Bobo, 1989; Guydish et al., 2011; Kalman, 1998; McClure, Acquavita, et al., 2014; Nahvi et al., 2006; Pajusco et al., 2012). Unfortunately, the vast majority of participants (96%) were not using smoking cessation medications at treatment entry and this did not change during the 12-week study. Among baseline smokers, only 19 participants reported being non-smokers at week 12 (5%), and perhaps more troubling, 11 baseline non-smokers reported being smokers at week 12 (11%). These results serve to further justify the need to target smoking cessation during SUD treatment as the majority of patients smoke and few are using smoking cessation medications.
Study results revealed that nicotine dependence severity and cigarettes per day generally did not change over the course of the 12-week study. Upon further inspection, however, results showed that participants randomized to TES with greater nicotine dependence and who were also drug negative at baseline had higher FTND scores compared to their TAU counterparts at EOT. Given a treatment effect of TES on drug and alcohol abstinence, one possible conclusion that could be drawn is that participants in TES were substituting more tobacco in place of drugs and alcohol as a potential coping strategy for their abstinence. We did not find a change in cigarettes per day among this sub-set of participants, however, so this potential behavioral link and drug substitution hypothesis requires further study and more sensitive assessments to measure increases in smoking. It should also be noted that the higher FTND scores may reflect changes in other smoking-related behavior captured by this assessment, and not specific to quantity of tobacco being used. This difference in FTND at EOT among baseline drug negative participants is of interest, especially since increases in tobacco use represent very serious health-related concerns, and requires further study and increased attention, if reliably present.
While the TES intervention was efficacious for treating drug and alcohol use (Campbell et al., 2014), it did not specifically address cigarette smoking and, not surprisingly, intervention effects did not appear to extend to cigarette smoking. This supports the need for specialized interventions that target cessation from cigarettes and tobacco for use in SUD treatment patients. These interventions are desperately needed in SUD treatment and there is ample evidence to suggest that smoking cessation interventions delivered during SUD treatment episodes do not jeopardize abstinence and may even enhance long-term abstinence from drugs and alcohol (Bobo, McIlvain, Lando, Walker, & Leed-Kelly, 1998; Frosch, Shoptaw, Nahom, & Jarvik, 2000; Hughes, 1993; Joseph, Nichol, & Anderson, 1993; Prochaska, Delucchi, & Hall, 2004; Richter & Arnsten, 2006; Tsoh, Chi, Mertens, & Weisner, 2011; Winhusen et al., 2013).
The results from the current study also suggest that being a cigarette smoker may be adversely related to SUD treatment outcomes. Among participants who were non-smokers at baseline, there was a significant difference in abstinence outcomes at EOT favoring TES over TAU. This difference was not present among baseline smokers. This result is consistent with the literature suggesting better drug abstinence outcomes (or proxies of outcomes) in non-smokers or former smokers (Haney et al., 2013; Mannelli et al., 2013; Peters et al., 2012; Winhusen et al., 2014), but demonstrates this relationship in a large, geographically diverse outpatient SUD (non-opioid) adult population. This relationship appears to be robust, and it may be possible that reducing smoking early in the SUD treatment may serve to improve treatment response. This represents an interesting avenue for future work to explore.
This secondary analysis had several limitations. First, biochemical verification of smoking status was not confirmed as part of study procedures. All smoking data were based on self-report and we cannot determine if non-smokers were former smokers and when they had quit. Second, tobacco policies and smoking cessation services and resources at the participating treatment facilities were not systematically assessed. While TES did not directly address tobacco, it is possible that study sites had different smoking cessation services and education available to participants, which may have influenced results. Also, sites most likely differed in terms of tobacco restrictions, policies, and cultures surrounding tobacco use while in SUD treatment, that were not accounted for in the current analysis. Third, demographic findings from our sample are descriptive and may not be generalizable to other SUD patients and treatment clinics. Finally, FTND was the primary measure to capture and quantify smoking, which is not ideal as an outcome measure of smoking. Cigarettes per day via Timeline Follow-Back methods were not collected as part of study procedures, which may have provided a more sensitive measure of reductions or increases in smoking during the trial.
4.1 Conclusions
TES appeared to exert a greater effect on the outcome of drug and alcohol use for non-smokers compared to smokers in the current report. Also, for a sub-set of participants randomized to TES, FTND scores were higher compared to participants in TAU at EOT. This suggests that although participants were abstinent from drugs and alcohol, they were still using tobacco and may even be increasing their tobacco use as way to cope with or manage reductions in substance use, though this statement is speculative and requires further investigation. It is also possible that other smoking-related behavior is changing, contributing to higher FTND scores without an increase in tobacco use. Cigarette smoking was not targeted by the treatment intervention and may not have been addressed in the usual care received by participants in the treatment programs. Therefore, it is not surprising that participants generally did not quit smoking during the trial. Prior research suggests that individuals enrolled in treatment for SUDs are receptive to smoking cessation interventions (McClure, Acquavita, et al., 2014; Nahvi et al., 2006; Richter, Gibson, Ahluwalia, & Schmelzle, 2001), and the results from the current study provide further evidence that patients enrolled in outpatient SUD treatment are in need of targeted smoking cessation interventions.
A logical next step for intervention development and refinement may be the incorporation of modules targeting smoking cessation within web-delivered programs, such as TES. There are several promising internet-delivered interventions for smoking cessation (Civljak, Stead, Hartmann-Boyce, Sheikh, & Car, 2013), which may hold the potential for use in outpatient SUD treatment settings as part of a comprehensive treatment plan. Future research should continue to work to incorporate evidence-based smoking cessation into comprehensive SUD treatment, and web-based interventions should be explored given their potential to deliver effective treatments in a cost-effective manner. SUD treatment patients are continuing to smoke during a treatment episode and resources to promote abstinence from tobacco should be encouraged among community treatment programs to avoid the almost inevitable smoking-related illnesses that these participants will likely encounter.
Highlights.
The majority of those with substance use disorders are also tobacco users.
Little is known about the influence of smoking on treatment outcomes.
Smokers had poorer treatment outcomes compared to non-smokers.
Participants did not reduce their smoking throughout the course of the study.
Evidence-based tobacco interventions are needed among SUD patients.
Acknowledgments
The authors wish to acknowledge the funding sources for this study. The execution of this study was supported by grants from the National Drug Abuse Treatment Clinical Trials Network: U10DA013035 (Edward V. Nunes), U10DA015831 (Kathleen M. Carroll and Roger D. Weiss), U10DA013034 (Maxine L. Stitzer and Robert P. Schwartz), U10 DA013720 (José Szapocznik and Lisa R. Metsch), U10DA013732 (Theresa Winhusen), U10DA020024 (Madhukar H. Trivedi), U10DA013714 (Dennis M. Donovan and John Roll), and U10DA015815 (James L. Sorensen and Dennis McCarty). Effort for this secondary analysis was supported by NIDA grants K01DA036739 (Erin A. McClure), K12DA031794 (Kathleen T. Brady), U01DA031779 (Kevin M. Gray), U10DA013727 (Kathleen T. Brady), and K24DA022412 (Edward V. Nunes). The funding source had no role other than financial support. The authors also wish to thank all participating study sites and staff for their hard work and dedication to the successful execution of this study protocol.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest. Dr. Nunes has received medication from research studies from Alkermes/Cephalon, Inc. and Reckitt-Benckiser and has received from Brainsway devices under investigation and reimbursement for travel expenses for investigators’ meeting; he has previously received medication for a research study from Duramed Pharmaceuticals. He was paid an honorarium and received reimbursement for travel expenses for attendance at a Lilly Advisory Board Meeting in January 2012 and received educational materials from Otsuka America Pharmaceutical, Inc. in 2013. The authors have no additional conflicts of interest to report.
References
- Arias-Carrion O, Salama M. Reward-seeking behavior and addiction: cause or cog? Current drug abuse reviews. 2012;5(3):178–189. doi: 10.2174/1874473711205030178. [DOI] [PubMed] [Google Scholar]
- Baca CT, Yahne CE. Smoking cessation during substance abuse treatment: what you need to know. Journal of Substance Abuse Treatment. 2009;36(2):205–219. doi: 10.1016/j.jsat.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Johnson MW, Koffarnus MN, MacKillop J, Murphy JG. The behavioral economics of substance use disorders: reinforcement pathologies and their repair. Annual Review of Clinical Psychology. 2014;10:641–677. doi: 10.1146/annurev-clinpsy-032813-153724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA, Buchhalter AR, Badger GJ. Computerized behavior therapy for opioid-dependent outpatients: a randomized controlled trial. Experimental and clinical psychopharmacology. 2008;16(2):132–143. doi: 10.1037/1064-1297.16.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobo JK. Nicotine Dependence and Alcoholism Epidemiology and Treatment. Journal of Psychoactive Drugs. 1989;21(3):323–329. doi: 10.1080/02791072.1989.10472174. [DOI] [PubMed] [Google Scholar]
- Bobo JK, McIlvain HE, Lando HA, Walker RD, Leed-Kelly A. Effect of smoking cessation counseling on recovery from alcoholism: findings from a randomized community intervention trial. Addiction. 1998;93(6):877–887. doi: 10.1046/j.1360-0443.1998.9368779.x. [DOI] [PubMed] [Google Scholar]
- Campbell AN, Nunes EV, Matthews AG, Stitzer M, Miele GM, Polsky D, Ghitza UE. Internet-Delivered Treatment for Substance Abuse: A Multisite Randomized Controlled Trial. American Journal of Psychiatry. 2014 doi: 10.1176/appi.ajp.2014.13081055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell AN, Nunes EV, McClure EA, Hu MC, Turrigiano E, Goldman B, Stabile PQ. Characteristics of an outpatient treatment sample by primary substance of abuse. Journal of addiction medicine. 2013;7(5):363–371. doi: 10.1097/ADM.0b013e31829e3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell AN, Nunes EV, Miele GM, Matthews A, Polsky D, Ghitza UE, Crowell AR. Design and methodological considerations of an effectiveness trial of a computer-assisted intervention: an example from the NIDA Clinical Trials Network. Contemporary clinical trials. 2012;33(2):386–395. doi: 10.1016/j.cct.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Current cigarette smoking among adults -United States, 2011. MMWR. Morbidity and mortality weekly report. 2012;61(44):889–894. [PubMed] [Google Scholar]
- Civljak M, Stead LF, Hartmann-Boyce J, Sheikh A, Car J. Internet-based interventions for smoking cessation. Cochrane Database Syst Rev. 2013;7:CD007078. doi: 10.1002/14651858.CD007078.pub4. [DOI] [PubMed] [Google Scholar]
- Coleman-Cowger VH, Catlin ML. Changes in tobacco use patterns among adolescents in substance abuse treatment. Journal of Substance Abuse Treatment. 2013;45(2):227–234. doi: 10.1016/j.jsat.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Eby LT, Laschober TC. Perceived implementation of the Office of Alcoholism and Substance Abuse Services (OASAS) tobacco-free regulation in NY State and clinical practice behaviors to support tobacco cessation: a repeated cross-sectional study. Journal of Substance Abuse Treatment. 2013;45(1):83–90. doi: 10.1016/j.jsat.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann PD, Jiang L, Richter KP. Cigarette smoking cessation services in outpatient substance abuse treatment programs in the United States. Journal of Substance Abuse Treatment. 2008;34(2):165–172. doi: 10.1016/j.jsat.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frosch DL, Shoptaw S, Nahom D, Jarvik ME. Associations between tobacco smoking and illicit drug use among methadone-maintained opiate-dependent individuals. Experimental and clinical psychopharmacology. 2000;8(1):97–103. doi: 10.1037//1064-1297.8.1.97. [DOI] [PubMed] [Google Scholar]
- Fuller BE, Guydish J, Tsoh J, Reid MS, Resnick M, Zammarelli L, McCarty D. Attitudes toward the integration of smoking cessation treatment into drug abuse clinics. Journal of Substance Abuse Treatment. 2007;32(1):53–60. doi: 10.1016/j.jsat.2006.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, Riggs PD, Min SJ, Mikulich-Gilbertson SK, Bandyopadhyay D, Winhusen T. Cigarette and cannabis use trajectories among adolescents in treatment for attention-deficit/hyperactivity disorder and substance use disorders. Drug and Alcohol Dependence. 2011;117(2–3):242–247. doi: 10.1016/j.drugalcdep.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guydish J, Passalacqua E, Tajima B, Chan M, Chun J, Bostrom A. Smoking prevalence in addiction treatment: a review. Nicotine Tob Res. 2011;13(6):401–411. doi: 10.1093/ntr/ntr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guydish J, Tajima B, Kulaga A, Zavala R, Brown LS, Bostrom A, Chan M. The New York policy on smoking in addiction treatment: findings after 1 year. American Journal of Public Health. 2012;102(5):e17–25. doi: 10.2105/ajph.2011.300590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Bedi G, Cooper ZD, Glass A, Vosburg SK, Comer SD, Foltin RW. Predictors of marijuana relapse in the human laboratory: robust impact of tobacco cigarette smoking status. Biological Psychiatry. 2013;73(3):242–248. doi: 10.1016/j.biopsych.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. British Journal of Addiction. 1989;84(7):791–799. doi: 10.1111/j.1360-0443.1989.tb03059.x. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badger GJ. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Archives of General Psychiatry. 1994;51(7):568–576. doi: 10.1001/archpsyc.1994.03950070060011. [DOI] [PubMed] [Google Scholar]
- Hser YI, McCarthy WJ, Anglin MD. Tobacco use as a distal predictor of mortality among long-term narcotics addicts. Preventive Medicine. 1994;23(1):61–69. doi: 10.1006/pmed.1994.1009. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Treatment of smoking cessation in smokers with past alcohol/drug problems. Journal of Substance Abuse Treatment. 1993;10(2):181–187. doi: 10.1016/0740-5472(93)90043-2. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, Melton LJ., 3rd Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. JAMA : the journal of the American Medical Association. 1996;275(14):1097–1103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- Joseph AM, Nichol KL, Anderson H. Effect of Treatment for Nicotine Dependence on Alcohol and Drug-Treatment Outcomes. Addictive Behaviors. 1993;18(6):635–644. doi: 10.1016/0306-4603(93)90017-4. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl 1):169–173. doi: 10.1016/j.neuropharm.2008.07.011. (Journal Article) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman D. Smoking cessation treatment for substance misusers in early recovery: A review of the literature and recommendations for practice. Substance Use and Misuse. 1998;33(10):2021–2047. doi: 10.3109/10826089809069815. [DOI] [PubMed] [Google Scholar]
- Kelley BM, Rowan JD. Long-term, low-level adolescent nicotine exposure produces dose-dependent changes in cocaine sensitivity and reward in adult mice. International Journal of Developmental Neuroscience. 2004;22(5–6):339–348. doi: 10.1016/j.ijdevneu.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Knudsen HK, Studts JL. Availability of nicotine replacement therapy in substance use disorder treatment: longitudinal patterns of adoption, sustainability, and discontinuation. Drug and Alcohol Dependence. 2011;118(2–3):244–250. doi: 10.1016/j.drugalcdep.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski LT, Porter CQ, Orleans CT, Pope MA, Heatherton T. Predicting smoking cessation with self-reported measures of nicotine dependence: FTQ, FTND, and HSI. Drug and Alcohol Dependence. 1994;34(3):211–216. doi: 10.1016/0376-8716(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Laschober TC, Eby LT. Counselor and clinical supervisor perceptions of OASAS tobacco-free regulation implementation extensiveness, perceived accountability, and use of resources. Journal of Psychoactive Drugs. 2013;45(5):416–424. doi: 10.1080/02791072.2013.845329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon DM, Wilson SJ, Child A, Geier CF. Adolescent brain maturation and smoking: what we know and where we’re headed. Neuroscience and Biobehavioral Reviews. 2014;45:323–342. doi: 10.1016/j.neubiorev.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannelli P, Wu LT, Peindl KS, Gorelick DA. Smoking and opioid detoxification: behavioral changes and response to treatment. Nicotine Tob Res. 2013;15(10):1705–1713. doi: 10.1093/ntr/ntt046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsch LA, Guarino H, Acosta M, Aponte-Melendez Y, Cleland C, Grabinski M, Edwards J. Web-based behavioral treatment for substance use disorders as a partial replacement of standard methadone maintenance treatment. Journal of Substance Abuse Treatment. 2014;46(1):43–51. doi: 10.1016/j.jsat.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EA, Acquavita SP, Dunn KE, Stoller KB, Stitzer ML. Characterizing smoking, cessation services, and quit interest across outpatient substance abuse treatment modalities. Journal of Substance Abuse Treatment. 2014;46(2):194–201. doi: 10.1016/j.jsat.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EA, Baker NL, Gray KM. Cigarette smoking during an N-acetylcysteine-assisted cannabis cessation trial in adolescents. American Journal of Drug and Alcohol Abuse. 2014 doi: 10.3109/00952990.2013.878718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Lukas SE, Mendelson JH. Buprenorphine effects on cigarette smoking. Psychopharmacology. 1985;86(4):417–425. doi: 10.1007/BF00427902. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Sellers ML, Kuehnle JC. Effects of heroin self-administration on cigarette smoking. Psychopharmacology. 1980;67(1):45–52. doi: 10.1007/BF00427594. [DOI] [PubMed] [Google Scholar]
- Mutschler NH, Stephen BJ, Teoh SK, Mendelson JH, Mello NK. An inpatient study of the effects of buprenorphine on cigarette smoking in men concurrently dependent on cocaine and opioids. Nicotine Tob Res. 2002;4(2):223–228. doi: 10.1080/14622200210124012. [DOI] [PubMed] [Google Scholar]
- Nahvi S, Richter K, Li X, Modali L, Arnsten J. Cigarette smoking and interest in quitting in methadone maintenance patients. Addictive Behaviors. 2006;31(11):2127–2134. doi: 10.1016/j.addbeh.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Onken LS, B JD, Boren JJ. NIDA Research Monograph. Vol. 165. Rockville, MD: National Institute on Drug Abuse; 1997. Beyond Therapeutic Alliance: Keeping the Drug-dependent Individual in Treatment. [Google Scholar]
- Pajusco B, Chiamulera C, Quaglio G, Moro L, Casari R, Amen G, Lugoboni F. Tobacco addiction and smoking status in heroin addicts under methadone vs. buprenorphine therapy. International journal of environmental research and public health. 2012;9(3):932–942. doi: 10.3390/ijerph9030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JM, Petry NM, Stitzer ML, Blaine J, Kellogg S, Satterfield F, Li R. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: a National Drug Abuse Treatment Clinical Trials Network study. Arch Gen Psychiatry. 2006;63(2):201–208. doi: 10.1001/archpsyc.63.2.201. [DOI] [PubMed] [Google Scholar]
- Peters EN, Budney AJ, Carroll KM. Clinical correlates of co-occurring cannabis and tobacco use: a systematic review. Addiction (Abingdon, England) 2012;107(8):1404–1417. doi: 10.1111/j.1360-0443.2012.03843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Peirce JM, Stitzer ML, Blaine J, Roll JM, Cohen A, Li R. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: a national drug abuse treatment clinical trials network study. Archives of General Psychiatry. 2005;62(10):1148–1156. doi: 10.1001/archpsyc.62.10.1148. [DOI] [PubMed] [Google Scholar]
- Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. Journal of Consulting and Clinical Psychology. 2004;72(6):1144–1156. doi: 10.1037/0022-006x.72.6.1144. [DOI] [PubMed] [Google Scholar]
- Richter KP, Arnsten JH. A rationale and model for addressing tobacco dependence in substance abuse treatment. Substance abuse treatment, prevention, and policy. 2006;1:23. doi: 10.1186/1747-597x-1-23. (Journal Article) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter KP, Choi WS, McCool RM, Harris KJ, Ahluwalia JS. Smoking cessation services in U.S. methadone maintenance facilities. Psychiatric services (Washington, D.C.) 2004;55(11):1258–1264. doi: 10.1176/appi.ps.55.11.1258. [DOI] [PubMed] [Google Scholar]
- Richter KP, Gibson CA, Ahluwalia JS, Schmelzle KH. Tobacco use and quit attempts among methadone maintenance clients. American Journal of Public Health. 2001;91(2):296–299. doi: 10.2105/ajph.91.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos GC, Marin MT, Cruz FC, Delucia R, Planeta CS. Amphetamine-and nicotine-induced cross-sensitization in adolescent rats persists until adulthood. Addict Biol. 2009;14(3):270–275. doi: 10.1111/j.1369-1600.2009.00153.x. [DOI] [PubMed] [Google Scholar]
- Shelef K, Diamond GS, Diamond GM, Myers MG. Changes in tobacco use among adolescent smokers in substance abuse treatment. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors. 2009;23(2):355–361. doi: 10.1037/a0014517. [DOI] [PubMed] [Google Scholar]
- Sobell M, Sobel lL, Bogardis J, Leo G, Skinner W. Problem drinkers’ perceptions of whether treatment goals should be self-selected or therapist-selected. Behav Ther. 1992;23:43–52. [Google Scholar]
- Stitzer ML, Petry NM, Peirce J. Motivational incentives research in the National Drug Abuse Treatment Clinical Trials Network. Journal of Substance Abuse Treatment. 2010;38(Suppl 1):S61–69. doi: 10.1016/j.jsat.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoh JY, Chi FW, Mertens JR, Weisner CM. Stopping smoking during first year of substance use treatment predicted 9-year alcohol and drug treatment outcomes. Drug and Alcohol Dependence. 2011;114(2–3):110–118. doi: 10.1016/j.drugalcdep.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Foulds J, Dwyer M, Order-Connors B, Springer M, Gadde P, Ziedonis DM. The integration of tobacco dependence treatment and tobacco-free standards into residential addictions treatment in New Jersey. Journal of Substance Abuse Treatment. 2005;28(4):331–340. doi: 10.1016/j.jsat.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Winhusen TM, Brigham GS, Kropp F, Lindblad R, Gardin JG, 2nd, Penn P, Ghitza U. A randomized trial of concurrent smoking-cessation and substance use disorder treatment in stimulant-dependent smokers. Journal of Clinical Psychiatry. 2013 doi: 10.4088/JCP.13m08449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen TM, Kropp F, Theobald J, Lewis DF. Achieving smoking abstinence is associated with decreased cocaine use in cocaine-dependent patients receiving smoking-cessation treatment. Drug and Alcohol Dependence. 2014;134:391–395. doi: 10.1016/j.drugalcdep.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]


