Abstract
Prior to the genome-wide association era, candidate gene studies were a major approach in schizophrenia genetics. In this invited review, we consider the current status of 25 historical candidate genes for schizophrenia (e.g., COMT, DISC1, DTNBP1, and NRG1). The initial study for 24 of these genes explicitly evaluated common variant hypotheses about schizophrenia. Our evaluation included a meta-analysis of the candidate gene literature, incorporation of the results of the largest genomic study yet published for schizophrenia, ratings from informed researchers who have published on these genes, and ratings from 24 schizophrenia geneticists. On the basis of current empirical evidence and mostly consensual assessments of informed opinion, it appears that the historical candidate gene literature did not yield clear insights into the genetic basis of schizophrenia. A likely reason why historical candidate gene studies did not achieve their primary aims is inadequate statistical power. However, the considerable efforts embodied in these early studies unquestionably set the stage for current successes in genomic approaches to schizophrenia.
Keywords: schizophrenia, genetics, candidate gene, review, meta-analysis
Introduction
In this review, we consider the current status of candidate genes for schizophrenia that were prominent in the literature prior to the genome-wide association study (GWAS) era. This review was invited by Prof Julio Licinio, the editor of Molecular Psychiatry.
Due to the high heritability of schizophrenia1, there have been many efforts to discover the causative genetic factors, and candidate gene studies have been a major approach. For example, the SZGene database 2 (obtained 11/2009) listed 1406 candidate gene papers investigating over 700 genes. In these studies, one or more genetic markers in genes hypothesized to be involved in the etiology of schizophrenia were genotyped in cases with schizophrenia and controls. Prior to the advances brought about by the Human Genome Project3 and the International HapMap Project 4, it was difficult and expensive to genotype a comprehensive list of genetic variants in a genomic region. Investigators thus tended to genotype a few genetic markers in a candidate gene selected based on prevailing theories of the etiology of schizophrenia (e.g., antipsychotic pharmacology) or positional candidate genes from linkage or cytogenetic studies.
The candidate gene strategy had a few notable successes in identifying genetic variation for other complex diseases. Influential examples included replicated associations of Alzheimer’s disease with common variation in APOE 5 and alcohol dependence with common variants in alcohol metabolic genes. 6 It was thus not unreasonable to hope that similar studies might work for schizophrenia. Realization of this expectation proved difficult. A pattern emerged whereby an initial claim of association for a seemingly plausible, even exciting, candidate gene for schizophrenia was followed by a mixed pattern of non-replications and replication. Thus, candidate gene association studies for schizophrenia became controversial. 7–10
The goal of this review is to evaluate the current status of historical candidate genes for schizophrenia. The motivation is straight-forward: there are hundreds of papers on these genes, several of these genes have motivated considerable biological experimentation, and as recent large-scale studies have expanded our knowledge base, it is reasonable to review this topic. Early candidate gene studies evaluated tens of genetic markers in hundreds of subjects and more recent studies conducted genome-wide comparisons of millions of genetic markers in tens of thousands of subjects. The largest published study is of 34,000 cases from the Psychiatric Genomics Consortium, PGC) which identified 108 genome-wide significant loci. 11
Almost all of these historical candidate gene studies evaluated the role of common variation (one part of a spectrum of variants involved in schizophrenia). Our evaluation includes: (a) meta-analysis of candidate gene studies, (b) PGC schizophrenia mega-analysis results,11 (c) expert evaluations from researchers on specific candidate genes, and (d) survey ratings from schizophrenia genomics investigators. The latter two approaches are not “scientific” in a strict sense, but rather provide a general guide to the “significance” and “impact” that the earlier findings currently have in the field.
Methods
We selected 25 genes prominent in the pre-GWAS era. The first schizophrenia GWAS appeared in 2007 but, given that many candidate gene association studies were published in 2008, we evaluated candidate gene studies published in calendar year 2008 or earlier. The 25 genes we selected were either featured in reviews of the genetics of schizophrenia 10, 12–14 or were highly studied (≥20 papers recorded in SZGene). The genes and the rationale for being a candidate gene for schizophrenia are given in Table 1. We continued several important assumptions made by virtually all candidate gene studies (see Limitations). First, these studies evaluated “schizophrenia” as a dichotomous entity. Second, as with the primary studies, we assumed that genetic variants act on the gene it was in or near. This assumption is crucial and will be inaccurate for a currently unknown proportion of genetic variants. Third, as discussed below, almost all of these studies evaluated common genetic variation.
Table 1.
Candidate genes of historical importance in schizophrenia research
| Gene | Product | Reviews | Pre-GWAS | Rationale |
|---|---|---|---|---|
| AKT1 | v-akt murine thymoma viral oncogene homolog 1 | 2 | 13 | Mood disorder pharmacology 59 |
| APOE | Apolipoprotein E | 1 | 32 | Implicated in Alzheimer’s disease 75 |
| BDNF | Brain-derived neurotrophic factor | 0 | 40 | Neurodevelopment hypothesis 76 |
| CHRNA7 | Cholinergic receptor, nicotinic, α7 | 1 | 12 | Linkage analysis 77 |
| COMT | Catechol-O-methyltransferase | 4 | 81 | 22q11 CNV 78 |
| DAO | D-amino-acid oxidase | 2 | 10 | Linkage analysis, glutamate hypothesis 79 |
| DAOA | D-amino acid oxidase activator | 3 | 27 | Linkage analysis, glutamate hypothesis 79 |
| DISC1 | Disrupted in schizophrenia 1 | 3 | 22 | Translocation in a pedigree 80 |
| DRD2 | Dopamine receptor D2 | 1 | 67 | Antipsychotic pharmacology 81 |
| DRD3 | Dopamine receptor D3 | 2 | 71 | Dopamine hypothesis 20 |
| DRD4 | Dopamine receptor D4 | 0 | 45 | Antipsychotic pharmacology 82 |
| DTNBP1 | Dystrobrevin binding protein 1 | 3 | 32 | Linkage analysis 83 |
| GRM3 | Glutamate receptor, metabotropic 3 | 1 | 15 | Glutamate hypothesis 21 |
| HTR2A | Serotonin receptor 2A | 2 | 57 | Antipsychotic pharmacology 84 |
| KCNN3 | Potassium intermediate/small conductance calcium-activated channel, subfamily N, member 3 | 0 | 23 | Discovery of a CAG repeat 85 |
| MTHFR | Methylenetetrahydrofolate reductase | 0 | 20 | Psychiatric symptoms with MTHFR dysfunction 86 |
| NOTCH4 | Notch 4 | 0 | 24 | Linkage analysis 87 |
| NRG1 | Neuregulin 1 | 3 | 41 | Linkage analysis 88 |
| PPP3CC | Protein phosphatase 3, catalytic subunit, γ isozyme | 1 | 9 | Linkage analysis/mouse phenotype 89 |
| PRODH | Proline dehydrogenase (oxidase) 1 | 3 | 10 | 22q11 CNV (incorrectly called “PRODH2” 90 |
| RGS4 | Regulator of G-protein signaling 4 | 3 | 22 | Differential expression in cases 91 |
| SLC6A3 | Dopamine transporter | 0 | 22 | Dopamine hypothesis 92 |
| SLC6A4 | Serotonin transporter | 1 | 32 | Implicated in mood disorders 93 |
| TNF | Tumor necrosis factor | 0 | 21 | Immune hypothesis 94 |
| ZDHHC8 | Zinc finger, DHHC-type 8 | 2 | 9 | 22q11 CNV 95 |
Reviews: the number of times a gene was in any of four selected reviews of schizophrenia genetics circa 2005. 10, 12–14 Pre-GWAS: the number of schizophrenia candidate gene papers studying this gene in calendar year 2008 or earlier. 2, 16 Rationale: the stated explanation for considering this gene as a candidate gene for schizophrenia according to the original publication. With the exception of DISC1, all studies evaluated common variant hypotheses.
We evaluated these 25 historical candidate genes for schizophrenia in four ways. First, we conducted fixed-effects meta-analyses for all genetic markers in these 25 genes using summary data on subjects of European ancestry in the SZGene database. 2 Second, we included PGC results for schizophrenia (9.5 million markers in 34,241 cases, 45,604 controls, and 1,235 trios followed by replication analyses of 263 SNPs in 1,513 cases and 66,236 controls). 11 We report the results for the same SNP appearing in SZGene and for the SNP with the smallest P-value in a gene (± 25 kb).
Third, we elicited perspectives from “informed investigators” who had published most extensively on or were the original or firmest proponents of a particular candidate gene in schizophrenia. These individuals were identified using PubMed searches: (gene [All Fields] OR “protein name” [All Fields]) AND (“schizophrenia” [MeSH Terms] OR “schizophrenia” [All Fields]). Informed investigators were contacted to request their summary judgment of the current status of one particular gene as a genetic risk factor for schizophrenia (1=very unlikely and 5=very likely). Genetic risk could refer to common, uncommon, rare, private, or de novo genetic variation. A draft of Table 2 was supplied upon request. Informed investigators were given the opportunity to include text in the Supplement to explain their rating.
Table 2.
Empirical findings for 25 candidate genes.
| Gene | Marker | SZGene OR (95% CI) | SZGene P | PGC OR (95% CI) | PGC P | PGC Pmin (± 25kb) | Informed Investigator Rating ‡ | Schizophrenia geneticists Rating ‡ |
|---|---|---|---|---|---|---|---|---|
| AKT1 | rs3730358 | 1.01 (0.91–1.13) | 0.82 | 1.02 (0.99–1.06) | 0.17 | 0.0003 | 5 | 2.5 |
| APOE | ε.2/3/4 | 0.99 (0.82–1.20) | 0.95 | 0.99 (0.96–1.02) † | 0.48 | 0.0095 | 1.7 | |
| BDNF | 270C/T | 0.68 (0.52–0.87) | 0.0028 | 1.01 (0.97–1.06) † | 0.55 | 8.0x10−5 | 3.0 | |
| rs6265 | 0.95 (0.87–1.04) | 0.29 | 0.95 (0.92–0.97) | 8.0x10−5 | ||||
| CHRNA7 | rs28531779 | 0.97 (0.72–1.30) | 0.82 | 1.01 (0.96–1.05) | 0.79 | 0.0096 | 5 | 2.9 |
| COMT | rs4680 | 1.00 (0.96–1.05) | 0.92 | 0.99 (0.97–1.01) | 0.56 | 0.0065 | 1 § | 2.4 |
| DAO | rs3918346 | 1.00 (0.89–1.12) | 0.94 | 1.03 (1.00–1.05) | 0.035 | 0.0004 | 3 | 2.2 |
| DAOA | rs3916965 | 0.95 (0.90–1.01) | 0.11 | 1.00 (0.98–1.02) | 0.96 | 0.015 | 3 | 2.0 |
| DISC1 | rs999710 | 1.07 (1.00–1.14) | 0.045 | 1.01 (0.99–1.03) | 0.29 | 0.00095 | 4.5 | 2.7 |
| DRD2 | rs1801028 | 0.85 (0.71–1.03) | 0.10 | 0.95 (0.89–1.03) | 0.22 | 8.3x10−9 | 4 | 4.1 |
| DRD3 | rs6280 | 1.03 (0.97–1.08) | 0.33 | 0.99 (0.97–1.01) | 0.31 | 0.015 | 2 | 2.3 |
| DRD4 | rs4646983 | 1.13 (0.76–1.67) | 0.56 | No data | No data | 0.0026 | 2.2 | |
| DTNBP1 | rs3213207 | 1.10 (1.02–1.19) | 0.015 | 1.04 (1.01–1.08) | 0.012 | 0.0073 | 2 | 2.4 |
| GRM3 | rs2228595 | 1.21 (0.96–1.52) | 0.099 | 1.01 (0.97–1.06) | 0.58 | 1.0x10−10 | 4.0 | |
| HTR2A | rs6311 | 1.14 (1.06–1.23) | 0.0005 | 1.01 (0.99–1.04) | 0.18 | 0.011 | 4 | 2.3 |
| KCNN3 | 1333T/C | 1.12 (0.33–3.76) | 0.86 | 0.95 (0.93–0.98) † | 3.3x10−5 | 6.8x10−6 | 3.0 | |
| MTHFR | rs1801133 | 1.09 (1.01–1.17) | 0.026 | 1.01 (0.98–1.03) | 0.55 | 0.016 | 2.1 | |
| NOTCH4 | rs367398 | 1.00 (0.87–1.15) | 0.99 | No data | No data | 1.1x10−18 | 3.2 | |
| NRG1 | rs62510682 | 0.94 (0.88–1.01) | 0.074 | 0.97 (0.95–1.00) | 0.024 | 0.0012 | 3 | 2.9 |
| PPP3CC | rs7837713 | 0.99 (0.81–1.21) | 0.91 | 1.01 (0.97–1.06) | 0.62 | 0.00017 | 2.0 | |
| PRODH | rs383964 | 1.09 (0.88–1.35) | 0.42 | 1.02 (0.97–1.07) | 0.41 | 0.0092 | 2.0 | |
| RGS4 | rs2661319 | 0.93 (0.88–0.99) | 0.013 | 1.01 (0.99–1.03) | 0.47 | 0.0061 | 2 ¶ | 2.1 |
| SLC6A3 | VNTR (rs28363170) | 0.97 (0.82–1.16) | 0.77 | 0.98 (0.94–1.01) † | 0.24 | 0.0103 | 2.0 | |
| SLC6A4 | 5-HTTVNTR | 1.11 (1.01–1.21) | 0.024 | 0.91 (0.86–0.96) † | 4.2x10−4 | 0.00042 | 2.5 | |
| 5-HTTLPR | 1.01 (0.94–1.09) | 0.75 | 1.03 (1.00–1.07) † | 0.058 | ||||
| TNF | rs1800629 | 1.00 (0.86–1.17) | 0.98 | 0.91 (0.89–0.94) | 5.6x10−10 | 1.7x10−18 | 3.0 | |
| ZDHHC8 | rs175174 | 1.00 (0.90–1.11) | 0.96 | 0.98 (0.96–1.01) | 0.17 | 4.1x10−6 | 2.4 |
SZGene OR (odds ratio) and 95% CI (confidence interval) from our meta-analysis of SZGene 2. Shown are the best marker per gene (full list in Table S1) plus two widely-studied markers (rs6265 and 5-HTTLPR). PGC OR and 95% CI from the PGC mega-analysis 11. PGC P=P-value. PGC Pmin=minimum P-value ±25 kb of a gene.
For non-SNP markers, the smallest PGC P-value within 25 kb of a variant is shown. Shaded SZGene cells are nominally significant but far from genome-wide significance. Shaded PGC cells are genome-wide significant. Ratings that are ≥4 are shaded.
Raters were asked for a 1–5 ranking (1=very unlikely and 5=very likely): “What is your current summary judgment that genomic studies implicate GENE as a genetic risk factor for schizophrenia?” Supplemental Note provides detail. Schizophrenia geneticists ratings are means for N=24.
Rating as a main effect, but “4” as an epistatic effect (Supplemental Note).
Rating as involved in the pathophysiology of schizophrenia would be “4” (Supplemental Note).
Fourth, we obtained perspectives from “schizophrenia geneticists”. We used principal investigators from the PGC schizophrenia working group11 as a convenience sample. We obtained responses from 24 investigators for summary judgments using the same rating scheme as for the informed investigators. Many of these investigators study common, uncommon, rare, private, or de novo genetic variation.
Results
Table 1 summarizes 25 historically important candidate genes for schizophrenia. For 24 of 25 genes, the initial study conducted genotyping to evaluate the impact of common genetic variation on risk for schizophrenia. Some candidate genes were selected because of rare genetic events (e.g., COMT, PRODH, and ZDDHC8 are located in the 22q11 deletion CNV) but the study evaluate common genetic variation rather than rare variation. The DISC1 study genotyped rare variation in a Scottish pedigree. The key findings for three genes were unimpressive for schizophrenia per se but presented somewhat more significant findings for putative endophenotypes (CHRNA7 and COMT) or a broadly inclusive set of psychiatric disorders (DISC1). Eleven genes were positional candidates based on genome-wide linkage or structural variation (CHRNA7, COMT, DAO, DAOA, DISC1, DTNBP1, NOTCH4, NRG1, PPP3CC, PRODH, and ZDHHC8). Eight genes derived from a hypothesis about the etiology of schizophrenia based on pharmacology (AKT1, DRD2, DRD3, DRD4, GRM3, HTR2A, SLC6A3, and SLC6A4). Six genes were from miscellaneous hypotheses (APOE, BDNF, KCNN3, MTHFR, RGS4, and TNF).
Many of the reported common variant SNP or haplotype relative risks were exceptionally large: often >1.5 and >2 for DRD3, HTR2A, MTHFR, NRG1, and PRODH. Rigorous control for multiple testing of genetic markers, haplotypes, and/or phenotypes was evident in one study (ZDHHC8). None of the P-values in the primary studies were genome-wide significant 15 (P<5x10−8), and most were not notable after correction for the number of SNPs genotyped.
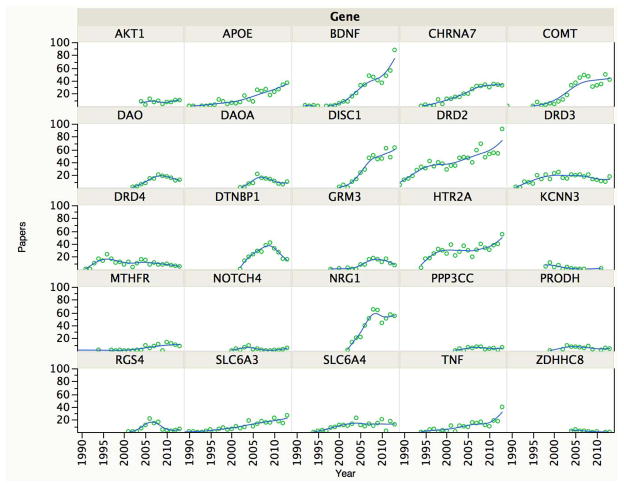
Figure 1 shows the number of times that each gene or its protein product co-occurred with schizophrenia in a paper indexed by PubMed per year. This serves as a rough metric for the importance/impact of a gene for the schizophrenia research community. For eight genes, the numbers of studies increased with time (APOE, BDNF, CHRNA7, COMT, DISC1, DRD2, HTR2A, and NRG1). Four genes peaked and then tapered off (DAO, DAOA, DTNBP1, and RGS4). For 13 genes, there have been relatively few reports (AKT1, DRD3, DRD4, GRM3, KCNN3, MTHFR, NOTCH4, PPP3CC, PRODH, SLC6A3, SLC6A4, TNF, and ZDHHC8).
Figure 1.
Candidate gene publications per gene and per year. For each gene, the number of publications is indicated on the Y-axis, and the year is the X-axis. The data shown are from a PubMed query: (gene [All Fields] OR “protein name”[All Fields]) AND (“schizophrenia”[MeSH Terms] OR “schizophrenia”[All Fields]). The goal of this PubMed query was to provide a rough gauge of the impact of a candidate gene on the field (which differs from the “pre-GWAS” column in Table 1).
Table 2 provides four evaluations for each historical candidate gene for schizophrenia. The first evaluation is a meta-analysis of candidate gene association studies in SZGene published in 2008 or earlier showing the most-studied genetic marker per gene (Table S1 shows results for all markers). We have previously shown that the candidate gene studies in SZGene had small samples and poor coverage of common genetic variation.16 No finding is near genome-wide significance 15 (P<5x10−8), and all P-values for this evaluation fall short by a factor of 10,000 or more. None is notable even on a gene-wise basis (which many would consider inappropriately liberal).
The second evaluation reports the results from the large PGC mega-analysis for schizophrenia. 11 We report results for the same SZGene polymorphisms (if available) plus the smallest P-value in the gene (±25 kb). The PGC and SZGene results agree on the absence of evidence of association for most of these genes (21/25). Four genes (DRD2, GRM3, NOTCH4, and TNF) are genome-wide significant in the PGC analysis, but were not implicated by the SZGene meta-analysis. The lack of association for NOTCH4 and TNF in the candidate gene literature is notable given that these genes are in the major histocompatibility complex (MHC). The MHC contains the most significant association common variant association (P=3.5x10−31) and the second largest OR (1.2) for schizophrenia. 11 Due to extensive linkage disequilibrium, the MHC contains thousands of genome-wide significant associations extending over 7 Mb. Indeed, there are over 60 genome-wide significant associations ±25 kb of NOTCH4 and four ±25 kb of the small TNF gene (2.8 kb). 11
The third evaluation was a rating by informed investigators for 12 genes. These individuals introduced the gene into the literature or had published extensively on it. The informed investigators provide fuller explanations for their rankings in the Supplement. Five genes (AKT1, CHRNA7, DISC1, DRD2, and HTR2A) were rated as highly likely to be a genetic risk factor for schizophrenia (rating ≥4 on a 1–5 scale). With the exception of DRD2, none of the informed ratings ≥4 is supported by empirical results from the older SZGene or the newer PGC mega-analysis.
The fourth evaluation consisted of ratings from 24 schizophrenia geneticists. The distribution of ratings is provided in Figure S2. The mean ratings were ≥4 for only DRD2 and GRM3. The mean ratings were discordant with those from informed investigators for AKT1, CHRNA7, DISC1, and HTR2A.
Discussion
Our knowledge of the genetic architecture of schizophrenia – the number of loci, allele frequencies, genotypic relative risks, and modes of action – has grown significantly in the past year. The largest GWAS to date suggests that schizophrenia is associated with many common genetic variants of small effect sizes. 11 Several rare CNVs have genotypic relative risks in the 5–20 range. 17 Rare exonic variants of stronger effect do play a role, but it now appears unlikely that schizophrenia has a genetic architecture dominated by such variants. 18, 19 A direct comparison found that common genetic variation accounted for far more of the variance in liability to schizophrenia than rare copy number variation or rare deleterious exonic variation.18
Given the importance of common variation in the etiology of schizophrenia and that 24 of 25 historical candidate genes for schizophrenia explicitly posited and evaluated the role of common variation, it is timely to assess the contributions of this literature to our knowledge of schizophrenia. With the advantages of hindsight (and noting that the authors of this review conducted many candidate gene studies including two in Table 1 20, 21), we offer a number of explanatory hypotheses regarding this literature.
Historical candidate genes for schizophrenia in light of current empirical results
First, it is now clear that historical candidate gene association studies for common genetic variation had grossly inadequate statistical power. For example, a candidate gene study of 1,000 cases and 1,000 controls has 0.03% power 22 to detect a genotypic relative risk (GRR) of 1.15 (assuming a log-additive model, lifetime prevalence=0.007, minor allele frequency=0.3, and α=5x10−8). A GRR of 1.15 is large for schizophrenia, and only 10 of 128 SNPs 11 reaching genome-wide significance had GRR > 1.15. When power is so low, the probability that a “significant” finding is a false positive is overwhelming. 23, 24
Second, the largest GWAS to date had essentially 100% power to identify common genetic variants with GRR > 1.15 (minor allele frequency > 0.10) or GRR > 1.19 (minor allele frequency > 0.05). We can thus exclude common genetic effects akin to those for APOE and Alzheimer’s disease (i.e., GRR of 3.7 for APOE*ε4 vs. ε3). 25 We can also conclude that the GRRs reported in many of the 24 common variant studies in Table 1 (often >1.5 and >2 for five genes) are inconsistent with what we now know about GRRs for common variation (Table 2, often for the same genetic marker reported in the initial study). Some common variants in Table 2 (e.g., the KCNN3 CAG repeat or complex haplotypes in NRG1) may not have been well-captured in SNP arrays; however, this criticism is mitigated by the lack of evidence from the SZGene meta-analyses for the same variants.
Third, even before the current generation of large genomic studies for schizophrenia, it was reassuring to note that candidate gene meta-analyses by other authors (Table S2) 26–41 and our SZGene meta-analyses (Table 2) were converging on the null. This is important because the candidate gene literature for common variation in schizophrenia is often believed to be replete with false positive claims: this generality is not supported by meta-analysis.
Fourth, the largest and most carefully conducted schizophrenia common variant association study does not provide empirical support for 21 of the 25 historical candidate genes as genetic risk factors for schizophrenia. 11 Two historical candidate genes (DRD2 and GRM3) have genome-wide significant evidence for common variant association with schizophrenia 11 although the candidate gene literature did not support these associations. Two additional candidate genes (TNF and NOTCH4) have genome-wide significant associations with schizophrenia. 11 These genes are in the extended MHC, a complex region with high gene density and extensive linkage disequilibrium, and the MHC-schizophrenia association may not implicate these genes. The candidate gene literature missed these associations although these should have been the most accessible common variant findings: the MHC was the first genome-wide significant GWAS signal for schizophrenia 42–44 and 11% of high-quality SNPs (6,570 of 57,891) in the extended MHC region exceeded genome-wide significance. 11 These false negatives from the pre-GWAS era likely resulted from extremely low statistical power and limited genotyping.
Fifth, one historical candidate gene (DISC1) studied a rare genetic event, the t(1;11) (q42.1;q14.3) translocation in a Scottish pedigree where the propositus did not have schizophrenia. The genetic linkage results in this pedigree point to a broad phenotype (LOD 7.1 for recurrent major depression, bipolar disorder, or schizophrenia). The status of DISC1 is controversial despite its entry into the literature nearly 15 years ago 45 (see also a rebuttal 46). The most critical issue is that no other genetic study has independently implicated DISC1 (i.e., met contemporary significance thresholds for rare exonic variation, rare CNVs, or common variation). 11, 18, 19, 47, 48 In contrast, many other rare variant associations have genetic replication evidence. For example, early-onset Alzheimer’s disease is caused by rare mutations in APP, PSEN1, and PSEN2.49 Unlike the singular DISC1 event, these associations are highly compelling as they replicate in many different pedigrees (90 families for APP, 405 for PSEN1, and 22 for PSEN2, see URLs). Similarly, the CNV associations for autism and schizophrenia replicate in large samples worldwide. 17
In conclusion, the current evidence from large and carefully conducted studies of genetic variation does not support the idea that the historical candidate gene literature led to robust and replicable genetic findings with the capacity to provide insights into the etiology of schizophrenia. Most genes (24 of 25) evaluated common variant hypotheses: the large effect sizes posited by initial studies were not confirmed, and four common variant associations that now meet genome-wide significance were missed. These conclusions have an important qualifier. Knowledge of the genetic basis of schizophrenia is incomplete but rapidly growing. Historically large studies were published in 2014, and considerable expansions of sample sizes for common and rare variant analyses are in progress. Some genes in Table 2 could become notable in the future.
Alternative perspectives on historical candidate genes for schizophrenia
The opinions of experts play a role in science particularly when there are few hard data, and have been important in psychiatry. 50 The prominence of some historical candidate genes for schizophrenia has increased despite a lack of strong support from genetic studies (Figure 1). Thus, we also surveyed opinions on these genes. First, for 12 genes, we obtained ratings from informed investigators (i.e., those who introduced a candidate gene into the literature or who published extensively on it, Table 2). We point readers to the Supplement for further explanations from the informed investigators. The informed investigator ratings agreed with the PGC results for seven genes. For five genes (AKT1, CHRNA7, DISC1, DRD2, and HTR2A), the informed investigator rating was high (a rating ≥4 on a 1–5 scale). For one of these five (DRD2), the PGC results concur. For the remaining four genes, the informed investigator ratings ≥4 were different from empirical results.
Second, we obtained ratings from 24 schizophrenia geneticists. The mean ratings were ≥4 for only DRD2 and GRM3. The mean ratings were inconsistent with those from informed investigators for AKT1, CHRNA7, DISC1, and HTR2A. Note that all ratings could incorporate any type of genetic variation. In general, we found that empirical data and opinion agreed for most of the 25 candidate genes, and the discrepancies for AKT1, CHRNA7, DISC1, and HTR2A stand out. Several informed investigators address this issue and believe that genetic results that do not meet widely accepted standards for significance in genetics or which lack replication can be augmented by biological data (Supplement). To this view, biological plausibility can provide salience to chance-level genetic results.
We contend that this “biological validation” argument is weak, subjective, prone to incorrect decisions, and liable to divert downstream research efforts by emphasizing the wrong targets. First, as documented in this paper, biology-driven candidate gene studies have not been particularly useful. Second, because we understand so little of the pathogenesis of schizophrenia, we have no biological gold standards or first principles. Put simply, there is neither a biology that we can demand of a ‘”true” associated gene nor a biology that is inconsistent with a “false” gene. Third, how then can we assess the validity of the biological connection being made? For genetics to achieve its goal of providing secure entry points into the biology of schizophrenia, findings must stand on their own merits without reference to other biological hypotheses or data. To do otherwise inevitably leads to circular reasoning (i.e., speculative biological supported by weak genetics supported by biological speculation).
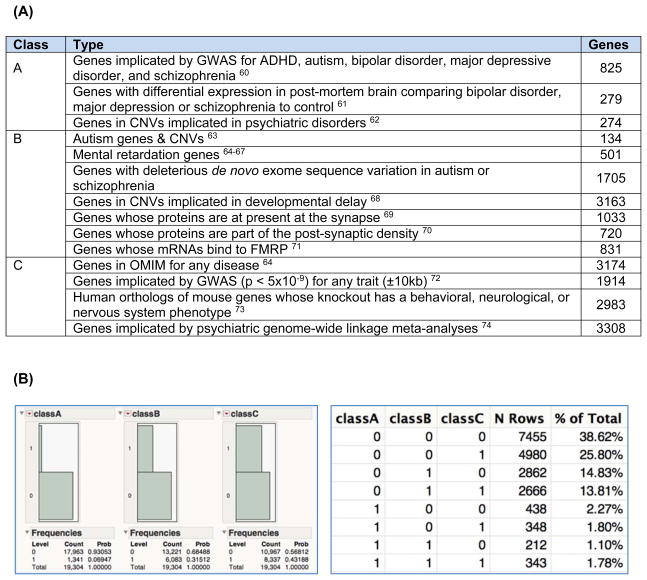
Fourth, the criterion of biological salience is surprisingly inclusive. A large fraction of human genes are of legitimate interest to an integrative neuroscientist: depending on inclusion criteria, ⅓ to ⅔ of human genes are of biological interest (Figure 2). Genomic studies can test millions of hypotheses –hundreds of genetic markers will have P-values in the 10−5 to 10−7 range from the play of chance. These “intriguing” genetic markers will, merely by chance, often be located near a biologically “cool” gene. This is a meaningless coincidence until proven otherwise by genetic evidence.
Figure 2. How many biologically interesting human genes are there?
This bioinformatic analysis addressed the question: how many human genes are of legitimate interest to an integrative neuroscientist or psychiatric geneticist? (A) We intersected 19,304 gene models from GENCODE (v17, “KNOWN” or “protein_coding”) with multiple data sources. Some genes can be in multiple categories. (B) Summary statistics (1=in set, 0=not in set): 35.6% of all genes are in classes A or B (=6869/19304), and 61.4% of all genes are in classes A, B, or C (=11849/19304). These numbers are conservative as adding “expression in brain at any developmental stage” would increase the numbers further. Thus, sizable proportions of all genes are of potential interest to a biologist. Biological interest is an imprecise criterion for the salience of a finding.
Fifth, this argument is counter to best practices in human genetics that demand rigorous significance thresholds and replication. 51–55 For GWAS, the 5x10−8 threshold is nearly universally accepted. For rare variants of strong effect, a recent Nature paper summarized the recommendations of an NHGRI working group: 56 (a) “we emphasize the critical primacy of robust statistical genetic support for the implication of new genes, which may then be supplemented with ancillary experimental or informatic evidence supporting a mechanistic role”; (b) “Just as for genome-wide association studies of common variants, replication of newly implicated disease genes in independent families or population cohorts is critical supporting evidence, and in most cases essential for a novel gene to be regarded as convincingly implicated in disease”; and (c) “Without rigorous standards we risk an acceleration of false-positive reports of causality, which would impede the translation of genomic research findings into the clinical diagnostic setting and hinder biological understanding of disease”.
Sixth, this argument is not accepted by many journals. Nature Genetics requires: “the genetic and statistical evidence for association should be sound. Molecular biological evidence for a functional variant is desirable in addition to, but will not substitute for, sound genetic evidence.” 57 PLoS Genetics states: “genetic arguments should stand on their own”. 58
Finally, we note that invoking the biological argument is unnecessary. If statistical significance is marginal or if replication is required, then a more definitive study should be designed and conducted in order to falsify the hypothesis. In many instances, this is achievable via collaboration and may be difficult for rarer genetic variants. We believe that schizophrenia genetics needs secure associations (significant beyond chance with precise replication) in order for genomic knowledge to be used as the essential anchor for understanding the biological basis of schizophrenia. Best practices in 2014 are thus very different from 2004. For example, the AKT1 paper appeared in Nature Genetics in 2004 59 with a considerable amount of biological and mouse model data but the genetic data are a SNP P-value of 0.05 (uncorrected for five genotyped SNPs) with no replication data.
Limitations - Can any historical candidate gene be formally excluded?
We have been careful to state that the current genomic evidence is inconsistent with an association for a particular gene. This is an evolving area, and it is possible that genes not now associated with schizophrenia will transition to significance with on-going expansions of sample sizes for common and rare variation. None of the historical candidate genes can be unequivocally excluded as a genetic risk factor for schizophrenia. However, we can state with high confidence that the large common variant genetic effects originally reported in many initial candidate gene studies are highly unlikely to be true.
Moreover, the location of some associations could provide a false clue, as genetic associations can act over long genomic distances – however, this assumption was made in the primary studies too. All current genomic technologies may miss some important types of genetic variation that play an etiological role.
It can also be argued that the genetic models used in the current generation of genomic studies for schizophrenia are inappropriate, that models should incorporate gene-environment, gene-gene, or even gene-gene-gene interactions. In a similar vein, it is possible that analyses that attempt to identify heterogeneity within the “schizophrenia” construct will prove informative. These hypotheses and alternative conceptualizations have merit and are now being investigated. Conducting these studies to a high standard is very difficult, and require unswerving adherence to accepted standards: thorough evaluation of bias, rigorous statistical significance thresholds, and replication are essential.
Conclusion
In summary, the current empirical evidence strongly supports the idea that the historical candidate gene literature yielded no robust and replicable insights into the etiology of schizophrenia. Even so, it is fair to note that these early studies unquestionably set the stage for the current era of genomic discovery for schizophrenia. These foundational efforts were a necessary step toward a better understanding of schizophrenia as a biological trait.
Supplementary Material
Acknowledgments
This work was supported by NIMH R01 MH077139 and U01 MH085520.
Footnotes
URLs
SzGene database (http://www.szgene.org) obtained in 11/2009 (active updating of SZGene ended in 12/2011). Genomic results available via the PGC (http://pgc.unc.edu), genomic visualization using Ricopili (http://www.broadinstitute.org/mpg/ricopili), and gene-centric data at GeneBook (http://atgu.mgh.harvard.edu/genebook). Alzheimer Disease & Frontotemporal Dementia Mutation Database (http://www.molgen.ua.ac.be).
Author Contributions
All authors reviewed and approved the final version of the manuscript.
Conflicts of Interest
The authors report no conflicts.
References
- 1.Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60(12):1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- 2.Allen NC, Bagade S, McQueen MB, Ioannidis JPA, Kavvoura FK, Khoury MJ, et al. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet. 2008;40(18583979):827–834. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- 3.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409(6822):860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 4.International HapMap Consortium. A haplotype map of the human genome. Nature. 2005;437(7063):1299–1320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou Z, Liu C, Che C, Huang H. Clinical Genetics of Alzheimer’s Disease. Biomed Res Int. 2014;2014:291862. doi: 10.1155/2014/291862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reich T, Hinrichs A, Culverhouse R, Bierut L. Genetic studies of alcoholism and substance dependence. Am J Hum Genet. 1999;65(3):599–605. doi: 10.1086/302561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan PF. Spurious genetic associations. Biol Psychiatry. 2007;61(10):1121–1126. doi: 10.1016/j.biopsych.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Ioannidis JP. Commentary: grading the credibility of molecular evidence for complex diseases. Int J Epidemiol. 2006;35(3):572–578. doi: 10.1093/ije/dyl003. discussion 593–576. [DOI] [PubMed] [Google Scholar]
- 9.Neale BM, Sham PC. The future of association studies: gene-based analysis and replication. Am J Hum Genet. 2004;75(3):353–362. doi: 10.1086/423901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lohmueller KE, Pearce CL, Pike M, Lander ES, Hirschhorn JN. Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet. 2003;33(2):177–182. doi: 10.1038/ng1071. [DOI] [PubMed] [Google Scholar]
- 11.Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological Insights From 108 Schizophrenia-Associated Genetic Loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Owen MJ, Craddock N, O’Donovan MC. Schizophrenia: genes at last? Trends Genet. 2005;21(9):518–525. doi: 10.1016/j.tig.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan PF. The genetics of schizophrenia. PLoS Medicine. 2005;2:614–618. doi: 10.1371/journal.pmed.0020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2004 doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- 15.Pe’er I, Yelensky R, Altshuler D, Daly MJ. Estimation of the multiple testing burden for genomewide association studies of nearly all common variants. Genet Epidemiol. 2008;32(4):381–385. doi: 10.1002/gepi.20303. [DOI] [PubMed] [Google Scholar]
- 16.Collins AL, Kim Y, Sklar P, O’Donovan MC, Sullivan PF. Hypothesis-driven candidate genes for schizophrenia compared to genome-wide association results. Psychol Med. 2012;42(3):607–616. doi: 10.1017/S0033291711001607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan PF, Daly MJ, O’Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet. 2012;13(8):537–551. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Purcell SM, Moran JL, Fromer M, Ruderfer D, Solovieff N, Roussos P, et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506(7487):185–190. doi: 10.1038/nature12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506(7487):179–184. doi: 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crocq MA, Mant R, Asherson P, Williams J, Hode Y, Mayerova A, et al. Association between schizophrenia and homozygosity at the dopamine D3 receptor gene. J Med Genet. 1992;29(12):858–860. doi: 10.1136/jmg.29.12.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marti SB, Cichon S, Propping P, Nothen M. Metabotropic glutamate receptor 3 (GRM3) gene variation is not associated with schizophrenia or bipolar affective disorder in the German population. Am J Med Genet. 2002;114(1):46–50. doi: 10.1002/ajmg.1624. [DOI] [PubMed] [Google Scholar]
- 22.Gauderman WJ. Sample size requirements for matched case-control studies of gene-environment interaction. Stat Med. 2002;21(1):35–50. doi: 10.1002/sim.973. [DOI] [PubMed] [Google Scholar]
- 23.Wacholder S, Chanock S, Garcia-Closas M, El Ghormli L, Rothman N. Assessing the probability that a positive report is false: an approach for molecular epidemiology studies. J Natl Cancer Inst. 2004;96(6):434–442. doi: 10.1093/jnci/djh075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14(5):365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 25.Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39(1):17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe Y, Nunokawa A, Someya T. Association of the BDNF C270T polymorphism with schizophrenia: updated meta-analysis. Psychiatry Clin Neurosci. 2013;67(2):123–125. doi: 10.1111/pcn.12018. [DOI] [PubMed] [Google Scholar]
- 27.Lee KY, Joo EJ, Jeong SH, Kang UG, Roh MS, Kim SH, et al. No association between AKT1 polymorphism and schizophrenia: a case-control study in a Korean population and a meta-analysis. Neurosci Res. 2010;66(3):238–245. doi: 10.1016/j.neures.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Loh HC, Chow TJ, Tang PY, Yong HS. No association between AKT1 gene variants and schizophrenia: a Malaysian case-control study and meta-analysis. Psychiatry Res. 2013;209(3):732–733. doi: 10.1016/j.psychres.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Shi J, Gershon ES, Liu C. Genetic associations with schizophrenia: meta-analyses of 12 candidate genes. Schizophr Res. 2008;104(1–3):96–107. doi: 10.1016/j.schres.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okochi T, Ikeda M, Kishi T, Kawashima K, Kinoshita Y, Kitajima T, et al. Meta-analysis of association between genetic variants in COMT and schizophrenia: an update. Schizophr Res. 2009;110(1–3):140–148. doi: 10.1016/j.schres.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 31.Costas J, Sanjuan J, Ramos-Rios R, Paz E, Agra S, Ivorra JL, et al. Heterozygosity at catechol-O-methyltransferase Val158Met and schizophrenia: new data and meta-analysis. J Psychiatr Res. 2011;45(1):7–14. doi: 10.1016/j.jpsychires.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 32.Muller DJ, Zai CC, Shinkai T, Strauss J, Kennedy JL. Association between the DAOA/G72 gene and bipolar disorder and meta-analyses in bipolar disorder and schizophrenia. Bipolar Disord. 2011;13(2):198–207. doi: 10.1111/j.1399-5618.2011.00905.x. [DOI] [PubMed] [Google Scholar]
- 33.Tan J, Lin Y, Su L, Yan Y, Chen Q, Jiang H, et al. Association between DAOA gene polymorphisms and the risk of schizophrenia, bipolar disorder and depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:89–98. doi: 10.1016/j.pnpbp.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Mathieson I, Munafo MR, Flint J. Meta-analysis indicates that common variants at the DISC1 locus are not associated with schizophrenia. Mol Psychiatry. 2012;17(6):634–641. doi: 10.1038/mp.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ni J, Lu W, Wu Z, Chen J, Yi Z, Zhang C. T102C polymorphism of serotonin 2A type receptor gene confers susceptibility to (early onset) schizophrenia in Han Chinese: an association study and meta-analysis. Asia Pac Psychiatry. 2013;5(1):24–30. doi: 10.1111/appy.12027. [DOI] [PubMed] [Google Scholar]
- 36.Gu L, Long J, Yan Y, Chen Q, Pan R, Xie X, et al. HTR2A-1438A/G polymorphism influences the risk of schizophrenia but not bipolar disorder or major depressive disorder: a meta-analysis. J Neurosci Res. 2013;91(5):623–633. doi: 10.1002/jnr.23180. [DOI] [PubMed] [Google Scholar]
- 37.Peerbooms OL, van Os J, Drukker M, Kenis G, Hoogveld L, de Hert M, et al. Meta-analysis of MTHFR gene variants in schizophrenia, bipolar disorder and unipolar depressive disorder: evidence for a common genetic vulnerability? Brain Behav Immun. 2011;25(8):1530–1543. doi: 10.1016/j.bbi.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 38.Nishi A, Numata S, Tajima A, Kinoshita M, Kikuchi K, Shimodera S, et al. Meta-analyses of Blood Homocysteine Levels for Gender and Genetic Association Studies of the MTHFR C677T Polymorphism in Schizophrenia. Schizophr Bull. 2014 doi: 10.1093/schbul/sbt154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu CY, Qian ZZ, Gong FF, Lu SS, Feng F, Wu YL, et al. Methylenetetrahydrofolate reductase (MTHFR) polymorphism susceptibility to schizophrenia and bipolar disorder: an updated meta-analysis. J Neural Transm. 2014 doi: 10.1007/s00702-014-1261-8. [DOI] [PubMed] [Google Scholar]
- 40.Gong YG, Wu CN, Xing QH, Zhao XZ, Zhu J, He L. A two-method meta-analysis of Neuregulin 1(NRG1) association and heterogeneity in schizophrenia. Schizophr Res. 2009;111(19362450):109–114. doi: 10.1016/j.schres.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 41.Xu M, St Clair D, He L. Testing for genetic association between the ZDHHC8 gene locus and susceptibility to schizophrenia: An integrated analysis of multiple datasets. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(7):1266–1275. doi: 10.1002/ajmg.b.31096. [DOI] [PubMed] [Google Scholar]
- 42.International Schizophrenia Consortium. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe’er I, et al. Common variants on chromosome 6p22. 1 are associated with schizophrenia. Nature. 2009;460:753–757. doi: 10.1038/nature08192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sullivan PF. Questions about DISC1 as a genetic risk factor for schizophrenia. Molecular Psychiatry. 2013;18:1050–1052. doi: 10.1038/mp.2012.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Porteous DJ, Thomson PA, Millar JK, Evans KL, Hennah W, Soares DC, et al. DISC1 as a genetic risk factor for schizophrenia and related major mental illness: response to Sullivan. Mol Psychiatry. 2014;19(2):141–143. doi: 10.1038/mp.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levinson DF, Duan J, Oh S, Wang K, Sanders AR, Shi J, et al. Copy number variants in schizophrenia: Confirmation of five previous findings and new evidence for 3q29 microdeletions and VIPR2 duplications. Am J Psychiatry. 2011;168:302–316. doi: 10.1176/appi.ajp.2010.10060876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Szatkiewicz J, O’Dushlaine C, Chen G, Chambert K, Moran J, Neale B, et al. Copy number variation in schizophrenia in Sweden. Molecular Psychiatry. 2014;19:762–773. doi: 10.1038/mp.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bertram L, Tanzi RE. Thirty years of Alzheimer’s disease genetics: the implications of systematic meta-analyses. Nat Rev Neurosci. 2008;9(10):768–778. doi: 10.1038/nrn2494. [DOI] [PubMed] [Google Scholar]
- 50.Kendler KS. Toward a scientific psychiatric nosology: strengths and limitations. Archives of General Psychiatry. 1990;47:969–973. doi: 10.1001/archpsyc.1990.01810220085011. [DOI] [PubMed] [Google Scholar]
- 51.Attia J, Ioannidis JP, Thakkinstian A, McEvoy M, Scott RJ, Minelli C, et al. How to use an article about genetic association: C: What are the results and will they help me in caring for my patients? Jama. 2009;301(3):304–308. doi: 10.1001/jama.2008.993. [DOI] [PubMed] [Google Scholar]
- 52.Attia J, Ioannidis JP, Thakkinstian A, McEvoy M, Scott RJ, Minelli C, et al. How to use an article about genetic association: B: Are the results of the study valid? Jama. 2009;301(2):191–197. doi: 10.1001/jama.2008.946. [DOI] [PubMed] [Google Scholar]
- 53.Ioannidis JP, Thomas G, Daly MJ. Validating, augmenting and refining genome-wide association signals. Nat Rev Genet. 2009;10(5):318–329. doi: 10.1038/nrg2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kraft P, Zeggini E, Ioannidis JP. Replication in genome-wide association studies. Stat Sci. 2009;24(4):561–573. doi: 10.1214/09-STS290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCarthy MI, Abecasis GR, Cardon LR, Goldstein DB, Little J, Ioannidis JP, et al. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nature Reviews Genetics. 2008;9(5):356–369. doi: 10.1038/nrg2344. [DOI] [PubMed] [Google Scholar]
- 56.MacArthur DG, Manolio TA, Dimmock DP, Rehm HL, Shendure J, Abecasis GR, et al. Guidelines for investigating causality of sequence variants in human disease. Nature. 2014;508(7497):469–476. doi: 10.1038/nature13127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Framework for a fully powered risk engine. Nat Genet. 2005;37(11):1153. doi: 10.1038/ng1105-1153. Editorial. [DOI] [PubMed] [Google Scholar]
- 58.Barsh GS, Copenhaver GP, Gibson G, Williams SM. Guidelines for genome-wide association studies. PLoS genetics. 2012;8(7):e1002812. doi: 10.1371/journal.pgen.1002812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet. 2004;36(2):131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- 60.Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnston-Wilson NL, Sims CD, Hofmann JP, Anderson L, Shore AD, Torrey EF, et al. Disease-specific alterations in frontal cortex brain proteins in schizophrenia, bipolar disorder, and major depressive disorder. The Stanley Neuropathology Consortium. Mol Psychiatry. 2000;5(2):142–149. doi: 10.1038/sj.mp.4000696. [DOI] [PubMed] [Google Scholar]
- 62.Sullivan PF, Daly MJ, O’Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nature Reviews Genetics. 2012;13:537–551. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Betancur C. Etiological heterogeneity in autism spectrum disorders: more than 100 genetic and genomic disorders and still counting. Brain Res. 2011;1380:42–77. doi: 10.1016/j.brainres.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 64.McKusick VA. Mendelian Inheritance in Man and its online version, OMIM. Am J Hum Genet. 2007;80(4):588–604. doi: 10.1086/514346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chiurazzi P, Schwartz CE, Gecz J, Neri G. XLMR genes: update 2007. Eur J Hum Genet. 2008;16(4):422–434. doi: 10.1038/sj.ejhg.5201994. [DOI] [PubMed] [Google Scholar]
- 66.Najmabadi H, Hu H, Garshasbi M, Zemojtel T, Abedini SS, Chen W, et al. Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature. 2011;478(7367):57–63. doi: 10.1038/nature10423. [DOI] [PubMed] [Google Scholar]
- 67.Inlow JK, Restifo LL. Molecular and comparative genetics of mental retardation. Genetics. 2004;166(2):835–881. doi: 10.1093/genetics/166.2.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cooper GM, Coe BP, Girirajan S, Rosenfeld JA, Vu TH, Baker C, et al. A copy number variation morbidity map of developmental delay. Nature genetics. 2011;43(9):838–846. doi: 10.1038/ng.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lips ES, Cornelisse LN, Toonen RF, Min JL, Hultman CM, Holmans PA, et al. Functional gene group analysis identifies synaptic gene groups as risk factor for schizophrenia. Molecular psychiatry. 2012;17(10):996–1006. doi: 10.1038/mp.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Croning MD, Marshall MC, McLaren P, Armstrong JD, Grant SG. G2Cdb: the Genes to Cognition database. Nucleic Acids Res. 2009;37(Database issue):D846–851. doi: 10.1093/nar/gkn700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146(2):247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hindorff LA, Sethupathy P, Junkins HA, Ramos EM, Mehta JP, Collins FS, et al. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci U S A. 2009;106(23):9362–9367. doi: 10.1073/pnas.0903103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blake JA, Bult CJ, Kadin JA, Richardson JE, Eppig JT. The Mouse Genome Database (MGD): premier model organism resource for mammalian genomics and genetics. Nucleic Acids Res. 2011;39(Database issue):D842–848. doi: 10.1093/nar/gkq1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Konneker T, Barnes T, Furberg H, Losh M, Bulik CM, Sullivan PF. A searchable database of genetic evidence for psychiatric disorders. American Journal of Medical Genetics (Neuropsychiatric Genetics) 2008;147:671–675. doi: 10.1002/ajmg.b.30802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harrington CR, Roth M, Xuereb JH, McKenna PJ, Wischik CM. Apolipoprotein E type epsilon 4 allele frequency is increased in patients with schizophrenia. Neurosci Lett. 1995;202(1–2):101–104. doi: 10.1016/0304-3940(95)12218-4. [DOI] [PubMed] [Google Scholar]
- 76.Sasaki T, Dai XY, Kuwata S, Fukuda R, Kunugi H, Hattori M, et al. Brain-derived neurotrophic factor gene and schizophrenia in Japanese subjects. Am J Med Genet. 1997;74(4):443–444. [PubMed] [Google Scholar]
- 77.Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, et al. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci U S A. 1997;94(2):587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98(12):6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chumakov I, Blumenfeld M, Guerassimenko O, Cavarec L, Palicio M, Abderrahim H, et al. Genetic and physiological data implicating the new human gene G72 and the gene for D-amino acid oxidase in schizophrenia. Proc Natl Acad Sci U S A. 2002;99(21):13675–13680. doi: 10.1073/pnas.182412499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 2000;9(9):1415–1423. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- 81.Comings DE, Comings BG, Muhleman D, Dietz G, Shahbahrami B, Tast D, et al. The dopamine D2 receptor locus as a modifying gene in neuropsychiatric disorders. JAMA. 1991;266(13):1793–1800. [PubMed] [Google Scholar]
- 82.Sommer SS, Lind TJ, Heston LL, Sobell JL. Dopamine D4 receptor variants in unrelated schizophrenic cases and controls. Am J Med Genet. 1993;48(2):90–93. doi: 10.1002/ajmg.1320480207. [DOI] [PubMed] [Google Scholar]
- 83.Straub RE, Jiang Y, MacLean CJ, Ma Y, Webb BT, Myakishev MV, et al. Genetic variation in the 6p22. 3 gene DTNBP1, the human ortholog of the mouse dysbindin gene, is associated with schizophrenia. Am J Hum Genet. 2002;71(2):337–348. doi: 10.1086/341750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Inayama Y, Yoneda H, Sakai T, Ishida T, Nonomura Y, Kono Y, et al. Positive association between a DNA sequence variant in the serotonin 2A receptor gene and schizophrenia. Am J Med Genet. 1996;67(1):103–105. doi: 10.1002/(SICI)1096-8628(19960216)67:1<103::AID-AJMG18>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 85.Chandy KG, Fantino E, Wittekindt O, Kalman K, Tong LL, Ho TH, et al. Isolation of a novel potassium channel gene hSKCa3 containing a polymorphic CAG repeat: a candidate for schizophrenia and bipolar disorder? Mol Psychiatry. 1998;3(1):32–37. doi: 10.1038/sj.mp.4000353. [DOI] [PubMed] [Google Scholar]
- 86.Arinami T, Yamada N, Yamakawa-Kobayashi K, Hamaguchi H, Toru M. Methylenetetrahydrofolate reductase variant and schizophrenia/depression. Am J Med Genet. 1997;74(5):526–528. doi: 10.1002/(sici)1096-8628(19970919)74:5<526::aid-ajmg14>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 87.Wei J, Hemmings GP. The NOTCH4 locus is associated with susceptibility to schizophrenia. Nat Genet. 2000;25(4):376–377. doi: 10.1038/78044. [DOI] [PubMed] [Google Scholar]
- 88.Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71(4):877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gerber DJ, Hall D, Miyakawa T, Demars S, Gogos JA, Karayiorgou M, et al. Evidence for association of schizophrenia with genetic variation in the 8p21. 3 gene, PPP3CC, encoding the calcineurin gamma subunit. Proc Natl Acad Sci U S A. 2003;100(15):8993–8998. doi: 10.1073/pnas.1432927100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu H, Heath SC, Sobin C, Roos JL, Galke BL, Blundell ML, et al. Genetic variation at the 22q11 PRODH2/DGCR6 locus presents an unusual pattern and increases susceptibility to schizophrenia. Proc Natl Acad Sci U S A. 2002;99(6):3717–3722. doi: 10.1073/pnas.042700699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chowdari KV, Mirnics K, Semwal P, Wood J, Lawrence E, Bhatia T, et al. Association and linkage analyses of RGS4 polymorphisms in schizophrenia. Hum Mol Genet. 2002;11(12):1373–1380. doi: 10.1093/hmg/11.12.1373. [DOI] [PubMed] [Google Scholar]
- 92.Li T, Yang L, Wiese C, Xu CT, Zeng Z, Giros B, et al. No association between alleles or genotypes at the dopamine transporter gene and schizophrenia. Psychiatry Res. 1994;52(1):17–23. doi: 10.1016/0165-1781(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 93.Collier DA, Arranz MJ, Sham P, Battersby S, Vallada H, Gill P, et al. The serotonin transporter is a potential susceptibility factor for bipolar affective disorder. Neuroreport. 1996;7(10):1675–1679. doi: 10.1097/00001756-199607080-00030. [DOI] [PubMed] [Google Scholar]
- 94.Boin F, Zanardini R, Pioli R, Altamura CA, Maes M, Gennarelli M. Association between -G308A tumor necrosis factor alpha gene polymorphism and schizophrenia. Mol Psychiatry. 2001;6(1):79–82. doi: 10.1038/sj.mp.4000815. [DOI] [PubMed] [Google Scholar]
- 95.Liu H, Abecasis GR, Heath SC, Knowles A, Demars S, Chen YJ, et al. Genetic variation in the 22q11 locus and susceptibility to schizophrenia. Proc Natl Acad Sci U S A. 2002;99(26):16859–16864. doi: 10.1073/pnas.232186099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.