Abstract
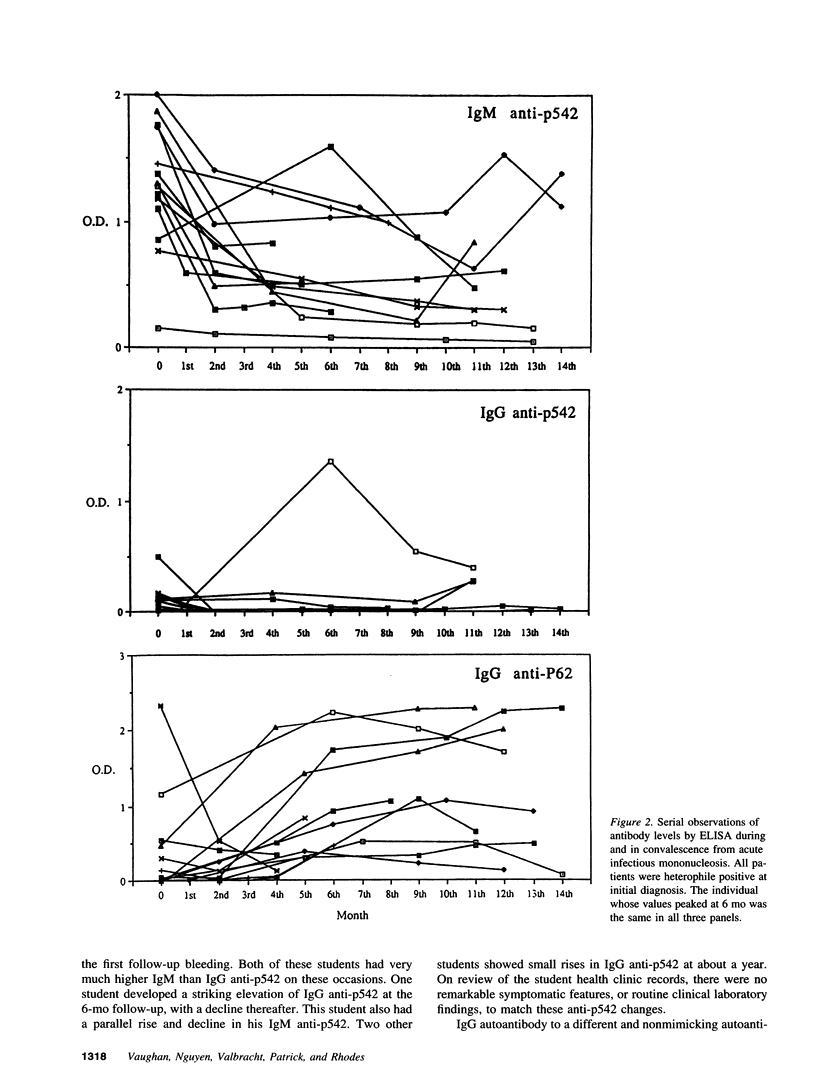
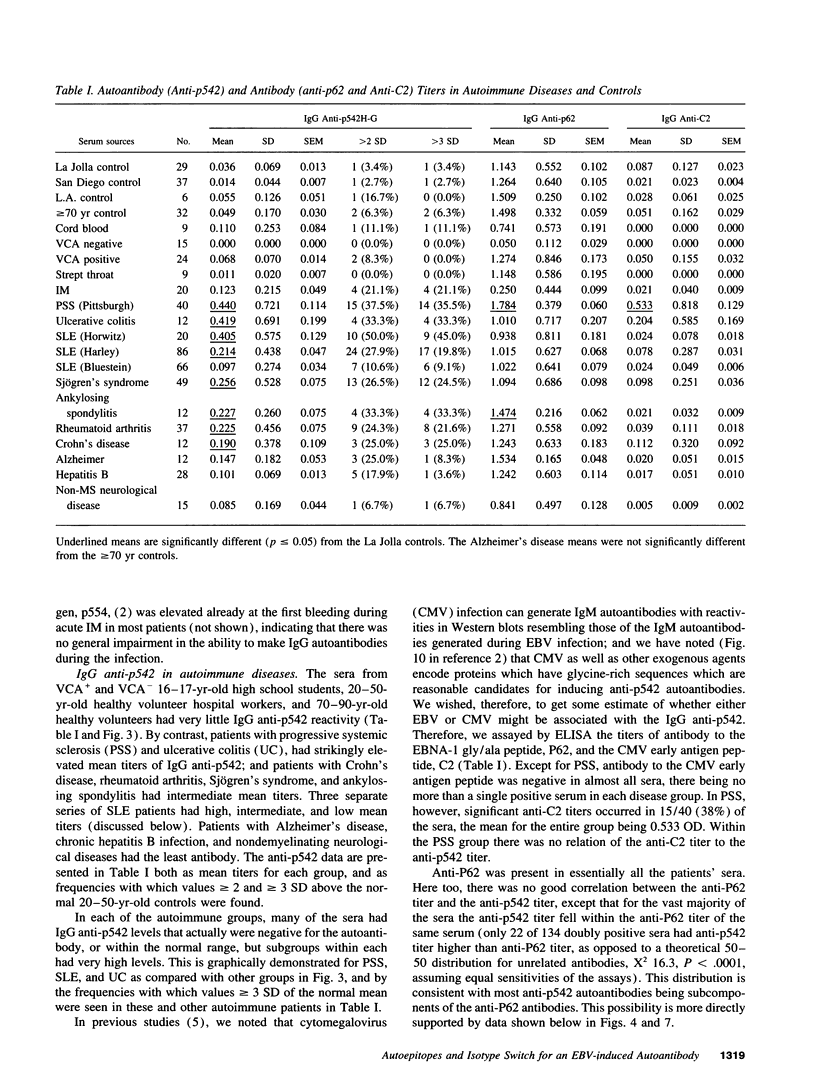
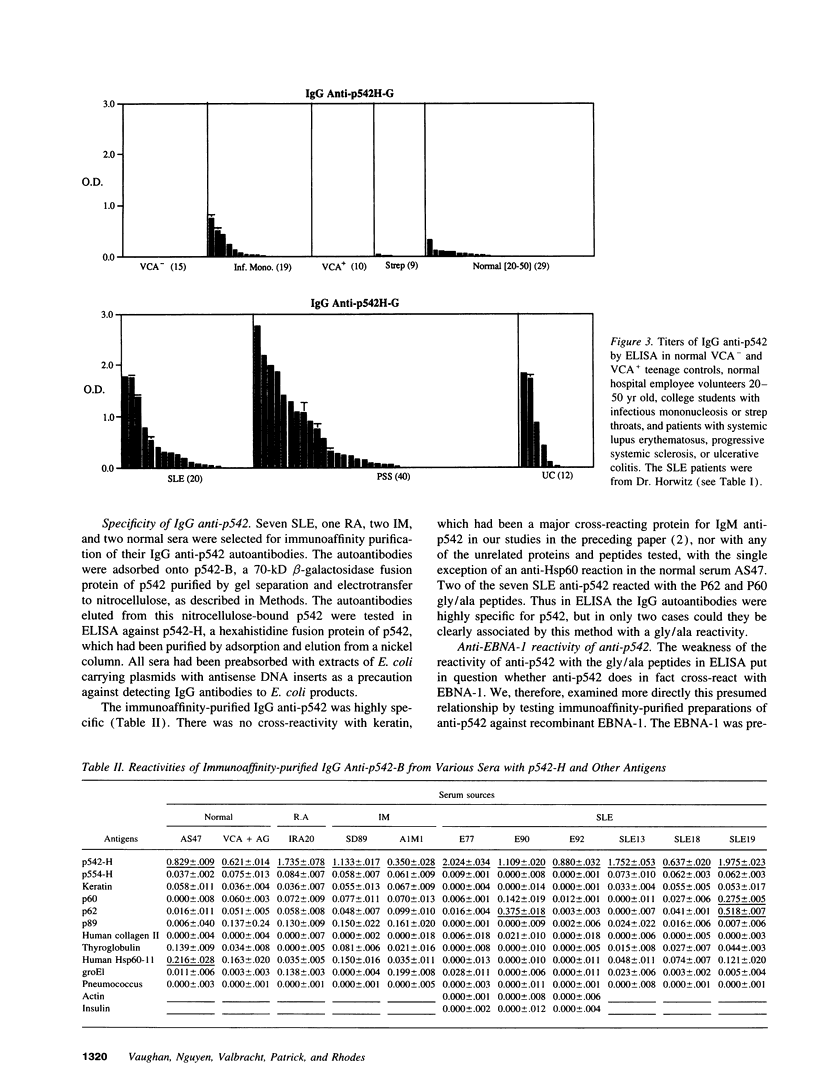
During infectious mononucleosis, IgM autoantibodies are generated to a protein, p542, which contains a glycine-rich 28-mer epitope cross-reactive with the Epstein-Barr nuclear antigen-1 through Epstein-Barr nuclear antigen-1's glycine/alanine repeat. In normal individuals it is uncommon to find IgG anti-p542, but among patients with progressive systemic sclerosis, systemic lupus erythematosus, and ulcerative colitis high IgG anti-p542 (> 3 SD above the mean of normal 20-50 yr controls) occurred frequently. Lesser elevations occurred in Sjögren's syndrome, rheumatoid arthritis, ankylosing spondylitis, and Crohn's disease, but none with chronic hepatitis B infection. The reactive epitopes on p542 were mapped with deletion mutants, which indicated that the glycine-rich 28-mer was the major antigenic determinant, with lesser antibody responses to other epitopes. We conclude that normally there is an inability to generate IgG autoantibodies to the cross-reactive (mimicking) epitope of the p542 host protein, but that this inability is overcome in a proportion of patients with autoimmune disease. We conclude also that non-cross-reactive autoepitopes exist on p542 protein, to which IgG autoantibodies can commonly be formed in autoimmune disorders. The mechanisms responsible for the latter must involve different mechanisms than those responsible for autoantibodies to the mimicking epitope.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein S., Pritchard-Briscoe H., Anderson T. A., Crosbie J., Gammon G., Loblay R. H., Basten A., Goodnow C. C. Induction of self-tolerance in T cells but not B cells of transgenic mice expressing little self antigen. Science. 1991 Mar 8;251(4998):1223–1225. doi: 10.1126/science.1900950. [DOI] [PubMed] [Google Scholar]
- Aris J. P., Blobel G. cDNA cloning and sequencing of human fibrillarin, a conserved nucleolar protein recognized by autoimmune antisera. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):931–935. doi: 10.1073/pnas.88.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings P. B., Hoch S. O., Vaughan J. H. Polymorphism of the EBNA/RANA antigen in Epstein-Barr virus-positive cell lines. Arthritis Rheum. 1984 Dec;27(12):1423–1427. doi: 10.1002/art.1780271214. [DOI] [PubMed] [Google Scholar]
- Bini P., Chu J. L., Okolo C., Elkon K. Analysis of autoantibodies to recombinant La (SS-B) peptides in systemic lupus erythematosus and primary Sjogren's syndrome. J Clin Invest. 1990 Feb;85(2):325–333. doi: 10.1172/JCI114441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray P. F., Luka J., Bray P. F., Culp K. W., Schlight J. P. Antibodies against Epstein-Barr nuclear antigen (EBNA) in multiple sclerosis CSF, and two pentapeptide sequence identities between EBNA and myelin basic protein. Neurology. 1992 Sep;42(9):1798–1804. doi: 10.1212/wnl.42.9.1798. [DOI] [PubMed] [Google Scholar]
- Chou C. H., Wang J., Knuth M. W., Reeves W. H. Role of a major autoepitope in forming the DNA binding site of the p70 (Ku) antigen. J Exp Med. 1992 Jun 1;175(6):1677–1684. doi: 10.1084/jem.175.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang H., Fischbach M., Talal N. Anti-idiotypic antiserum to monoclonal anti-Sm inhibits the autoantigen-induced proliferative response. J Immunol. 1985 Jun;134(6):3825–3830. [PubMed] [Google Scholar]
- Farmer G. W., Vincent M. M., Fuccillo D. A., Horta-Barbosa L., Ritman S., Sever J. L., Gitnick G. L. Viral investigations in ulcerative colitis and regional enteritis. Gastroenterology. 1973 Jul;65(1):8–18. [PubMed] [Google Scholar]
- Francoeur A. M., Peebles C. L., Gompper P. T., Tan E. M. Identification of Ki (Ku, p70/p80) autoantigens and analysis of anti-Ki autoantibody reactivity. J Immunol. 1986 Mar 1;136(5):1648–1653. [PubMed] [Google Scholar]
- Fritzler M. J. Autoantibodies in scleroderma. J Dermatol. 1993 May;20(5):257–268. doi: 10.1111/j.1346-8138.1993.tb01389.x. [DOI] [PubMed] [Google Scholar]
- Gohill J., Cary P. D., Couppez M., Fritzler M. J. Antibodies from patients with drug-induced and idiopathic lupus erythematosus react with epitopes restricted to the amino and carboxyl termini of histone. J Immunol. 1985 Nov;135(5):3116–3121. [PubMed] [Google Scholar]
- Hannestad K., Andreassen K., Kristoffersen G. Anti-idiotypic immune responses against adjuvant-free isologous IgM monoclonal antibodies and their augmentation by complex formation between IgM and albumin in bovine serum. Eur J Immunol. 1992 Feb;22(2):321–327. doi: 10.1002/eji.1830220206. [DOI] [PubMed] [Google Scholar]
- Hennessy K., Heller M., van Santen V., Kieff E. Simple repeat array in Epstein-Barr virus DNA encodes part of the Epstein-Barr nuclear antigen. Science. 1983 Jun 24;220(4604):1396–1398. doi: 10.1126/science.6304878. [DOI] [PubMed] [Google Scholar]
- Hennessy K., Kieff E. One of two Epstein-Barr virus nuclear antigens contains a glycine-alanine copolymer domain. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5665–5669. doi: 10.1073/pnas.80.18.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa H., Iho S., Yokochi T., Hoshino T. Detection of antibodies to the Epstein-Barr virus nuclear antigens in the sera from patients with systemic lupus erythematosus. Immunol Lett. 1988 Mar;17(3):249–252. doi: 10.1016/0165-2478(88)90037-5. [DOI] [PubMed] [Google Scholar]
- Lehmann P. V., Forsthuber T., Miller A., Sercarz E. E. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992 Jul 9;358(6382):155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- Lin R. H., Mamula M. J., Hardin J. A., Janeway C. A., Jr Induction of autoreactive B cells allows priming of autoreactive T cells. J Exp Med. 1991 Jun 1;173(6):1433–1439. doi: 10.1084/jem.173.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamula M. J., Lin R. H., Janeway C. A., Jr, Hardin J. A. Breaking T cell tolerance with foreign and self co-immunogens. A study of autoimmune B and T cell epitopes of cytochrome c. J Immunol. 1992 Aug 1;149(3):789–795. [PubMed] [Google Scholar]
- Martin T., Duffy S. F., Carson D. A., Kipps T. J. Evidence for somatic selection of natural autoantibodies. J Exp Med. 1992 Apr 1;175(4):983–991. doi: 10.1084/jem.175.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naparstek Y., André-Schwartz J., Manser T., Wysocki L. J., Breitman L., Stollar B. D., Gefter M., Schwartz R. S. A single germline VH gene segment of normal A/J mice encodes autoantibodies characteristic of systemic lupus erythematosus. J Exp Med. 1986 Aug 1;164(2):614–626. doi: 10.1084/jem.164.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngou J., Graafland H., Segondy M. Antibodies against polypeptides of purified Epstein-Barr virus in sera from patients with connective tissue diseases. J Autoimmun. 1992 Apr;5(2):243–249. doi: 10.1016/0896-8411(92)90203-3. [DOI] [PubMed] [Google Scholar]
- Ngou J., Segondy M., Seigneurin J. M., Graafland H. Antibody responses against polypeptide components of Epstein-Barr virus-induced early diffuse antigen in patients with connective tissue diseases. J Med Virol. 1990 Sep;32(1):39–46. doi: 10.1002/jmv.1890320107. [DOI] [PubMed] [Google Scholar]
- Ogata K., Ogata Y., Takasaki Y., Tan E. M. Epitopes on proliferating cell nuclear antigen recognized by human lupus autoantibody and murine monoclonal antibody. J Immunol. 1987 Nov 1;139(9):2942–2946. [PubMed] [Google Scholar]
- Olee T., Lu E. W., Huang D. F., Soto-Gil R. W., Deftos M., Kozin F., Carson D. A., Chen P. P. Genetic analysis of self-associating immunoglobulin G rheumatoid factors from two rheumatoid synovia implicates an antigen-driven response. J Exp Med. 1992 Mar 1;175(3):831–842. doi: 10.1084/jem.175.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Origgi L., Perego R., Hu C., Bertetti E., D'Agostino P., Asero R., Riboldi P. Anti-Epstein-Barr virus antibodies in systemic lupus erythematosus. Boll Ist Sieroter Milan. 1988;67(2):116–122. [PubMed] [Google Scholar]
- Paterson P. Y., Day E. D., Whitacre C. C., Berenberg R. A., Harter D. H. Endogenous myelin basic protein-serum factors (MBP-SFs) and anti-MBP antibodies in humans. Occurrence in sera of clinically well subjects and patients with multiple sclerosis. J Neurol Sci. 1981 Oct;52(1):37–51. doi: 10.1016/0022-510x(81)90132-5. [DOI] [PubMed] [Google Scholar]
- Petersen J., Rhodes G., Patrick K., Roudier J., Vaughan J. H. Human T cell responses to the Epstein-Barr nuclear antigen-1 (EBNA-1) as evaluated by synthetic peptides. Cell Immunol. 1989 Oct 15;123(2):325–333. doi: 10.1016/0008-8749(89)90293-1. [DOI] [PubMed] [Google Scholar]
- Petersen J., Rhodes G., Roudier J., Vaughan J. H. Altered immune response to glycine-rich sequences of Epstein-Barr nuclear antigen-1 in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheum. 1990 Jul;33(7):993–1000. doi: 10.1002/art.1780330711. [DOI] [PubMed] [Google Scholar]
- Pisetsky D. S. Systemic lupus erythematosus. Curr Opin Immunol. 1991 Dec;3(6):917–923. doi: 10.1016/s0952-7915(05)80014-7. [DOI] [PubMed] [Google Scholar]
- Rhodes G., Rumpold H., Kurki P., Patrick K. M., Carson D. A., Vaughan J. H. Autoantibodies in infectious mononucleosis have specificity for the glycine-alanine repeating region of the Epstein-Barr virus nuclear antigen. J Exp Med. 1987 Apr 1;165(4):1026–1040. doi: 10.1084/jem.165.4.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes G., Smith R. S., Rubin R. E., Vaughan J., Horwitz C. A. Identical IgM antibodies recognizing a glycine-alanine epitope are induced during acute infection with Epstein-Barr virus and cytomegalovirus. J Clin Lab Anal. 1990;4(6):456–464. doi: 10.1002/jcla.1860040613. [DOI] [PubMed] [Google Scholar]
- Rocha B., von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991 Mar 8;251(4998):1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- Roitt I. M., Hutchings P. R., Dawe K. I., Sumar N., Bodman K. B., Cooke A. The forces driving autoimmune disease. J Autoimmun. 1992 Apr;5 (Suppl A):11–26. doi: 10.1016/0896-8411(92)90015-i. [DOI] [PubMed] [Google Scholar]
- Roitt I. M., Torrigiani G. Identification and estimation of undegraded thyroglobulin in human serum. Endocrinology. 1967 Sep;81(3):421–429. doi: 10.1210/endo-81-3-421. [DOI] [PubMed] [Google Scholar]
- Shlomchik M., Mascelli M., Shan H., Radic M. Z., Pisetsky D., Marshak-Rothstein A., Weigert M. Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. J Exp Med. 1990 Jan 1;171(1):265–292. doi: 10.1084/jem.171.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore A., Klock R., Lee P., Snow K. M., Keystone E. C. Impaired late suppression of Epstein-Barr virus (EBV)-induced immunoglobulin synthesis: a common feature of autoimmune disease. J Clin Immunol. 1989 Mar;9(2):103–110. doi: 10.1007/BF00916937. [DOI] [PubMed] [Google Scholar]
- Steinberg A. D., Krieg A. M., Gourley M. F., Klinman D. M. Theoretical and experimental approaches to generalized autoimmunity. Immunol Rev. 1990 Dec;118:129–163. doi: 10.1111/j.1600-065x.1990.tb00815.x. [DOI] [PubMed] [Google Scholar]
- Szigeti R., Timar L., Weiland O., Henle W., Henle G., Hennessy K., Kieff E., Sulitzeanu D., Dillner J., Klein G. Epstein-Barr virus (EBV) antigen-specific leukocyte migration inhibition (LMI) in infectious mononucleosis (IM). I. Kinetics and response to a membrane protein on EBV-transformed cells. Clin Immunol Immunopathol. 1986 Dec;41(3):342–350. doi: 10.1016/0090-1229(86)90005-x. [DOI] [PubMed] [Google Scholar]
- Tilkin A. F., Vinci G., Michon J., Levy J. P. Autoreactive HLA-DR-specific autoreactive T-cell clones: possible regulatory function for B lymphocytes and hematopoietic precursors. Immunol Rev. 1990 Aug;116:171–181. doi: 10.1111/j.1600-065x.1990.tb00810.x. [DOI] [PubMed] [Google Scholar]
- Tosato G., Steinberg A. D., Blaese R. M. Defective EBV-specific suppressor T-cell function in rheumatoid arthritis. N Engl J Med. 1981 Nov 19;305(21):1238–1243. doi: 10.1056/NEJM198111193052102. [DOI] [PubMed] [Google Scholar]
- Tsokos G. C., Magrath I. T., Balow J. E. Epstein-Barr virus induces normal B cell responses but defective suppressor T cell responses in patients with systemic lupus erythematosus. J Immunol. 1983 Oct;131(4):1797–1801. [PubMed] [Google Scholar]
- Vaughan J. H., Valbracht J. R., Nguyen M. D., Handley H. H., Smith R. S., Patrick K., Rhodes G. H. Epstein-Barr virus-induced autoimmune responses. I. Immunoglobulin M autoantibodies to proteins mimicking and not mimicking Epstein-Barr virus nuclear antigen-1. J Clin Invest. 1995 Mar;95(3):1306–1315. doi: 10.1172/JCI117781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield A. J., Fox J. D., Sawyerr A. M., Taylor J. E., Sweenie C. H., Smith M., Emery V. C., Hudson M., Tedder R. S., Pounder R. E. Detection of herpesvirus DNA in the large intestine of patients with ulcerative colitis and Crohn's disease using the nested polymerase chain reaction. J Med Virol. 1992 Nov;38(3):183–190. doi: 10.1002/jmv.1890380306. [DOI] [PubMed] [Google Scholar]
- Yokochi T., Yanagawa A., Kimura Y., Mizushima Y. High titer of antibody to the Epstein-Barr virus membrane antigen in sera from patients with rheumatoid arthritis and systemic lupus erythematosus. J Rheumatol. 1989 Aug;16(8):1029–1032. [PubMed] [Google Scholar]