Abstract
The incidence of chronic wounds is increased among older adults, and the impact of chronic wounds on quality of life is particularly profound in this population. It is well established that wound healing slows with age. However, the basic biology underlying chronic wounds and the influence of age-associated changes on wound healing are poorly understood. Most studies have used in vitro approaches and various animal models, but observed changes translate poorly to human healing conditions. The impact of age and accompanying multi-morbidity on the effectiveness of existing and emerging treatment approaches for chronic wounds is also unknown, and older adults tend to be excluded from randomized clinical trials. Poorly defined outcomes and variables, lack of standardization in data collection, and variations in the definition, measurement, and treatment of wounds also hamper clinical studies. The Association of Specialty Professors, in conjunction with the National Institute on Aging and the Wound Healing Society, held a workshop, summarized in this paper, to explore the current state of knowledge and research challenges, engage investigators across disciplines, and identify key research questions to guide future study of age-associated changes in chronic wound healing.
Keywords: chronic wound, pressure ulcer, diabetic foot ulcer, venous leg ulcer, wound repair, wound healing
Introduction
Chronic wounds, which include venous leg ulcers (VLU), diabetic foot ulcers (DFU), arterial insufficiency, and pressure ulcers (PU), disproportionately afflict older adults and impose substantial morbidity and mortality on millions of older Americans. The great majority of chronic wounds are associated with conditions more common in older individuals, including vascular disease, venous insufficiency, unrelieved pressure, or diabetes mellitus. In addition, a disproportionate and increasing number of older adults undergo surgery and are at risk for wound complications. Fundamental questions remain about the impact of aging on wound healing and the mechanisms of wound repair and tissue regeneration in older adults. Furthermore, few well-designed clinical trials have explored the treatment of wounds in older adults, leaving clinicians with scant evidence to guide optimal wound management. However, with better scientific and clinical tools, along with an increasing number of highly motivated and talented investigators, we are reaching a critical juncture to address these issues.
This workshop convened a trans-disciplinary group of experts in the fields of wound repair and regeneration, skin aging, geriatric conditions, and gerontology from across the United States and Canada, as well as program staff and scientists from the National Institute on Aging, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Heart, Lung, and Blood Institute, and the National Institute of Nursing Research. The workshop aimed primarily to review current knowledge in key epidemiologic, basic science, and clinical topics; identify gaps in that knowledge; and develop a research agenda. Participants summarized research priorities and generated questions for future research (Table 1).
Table 1.
Research questions for wound healing in older adults.
| Category | Research Questions |
|---|---|
| Epidemiology and Quality of Life |
|
| Basic Biology of Wound Healing, Chronic Wounds, and Aging |
|
| Molecular and Cellular Processes, Inflammation |
|
| Molecular and Cellular Processes, Oxidative Stress |
|
| Molecular and Cellular Processes, Microbial Burden |
|
| Clinical Care, General |
|
| Clinical Care, Novel Therapeutic Approaches |
|
| Clinical Care, Nutrition |
|
Epidemiology of Chronic Wounds in Older Adults
The burden, particularly prevalence and incidence, of chronic wounds is unclear because of underreporting, poor definition of “chronic wound,” and inaccurate diagnostic coding for wound care. Many epidemiologic studies do not distinguish between prevalence and incidence, and they often focus on endpoints, such as lower-extremity amputations (LEA), which are easier to define and measure. Thus, estimates of prevalence and incidence vary across studies. Despite these limitations, studies indicate that the incidence of chronic wounds increases with age even into late life (1, 2). Studies using the General Practice Research Database in the United Kingdom have found that VLU incidence is three to four times higher, and PU incidence five to seven times higher, in persons older than 80 years compared with persons aged 65 to 70 years (1, 2). Care for chronic wounds costs about $10 billion annually (3), and it is likely that wound care in adults aged 65 and older accounts for the majority of these costs.
Chronic wounds have a profound effect on quality of life (QOL), as assessed by generic and wound-specific instruments or by health utility (4–6). The impact is similar to that seen with kidney or heart failure, and QOL decline is particularly precipitous among older adults. However, overall QOL among older populations with chronic wounds is poorly understood. Existing measures do not differentiate age-related differences in the impact of chronic wounds between community-dwelling older adults and those in long-term care. Data from the U.S. Wound Registry indicate that patients in outpatient wound centers have an average of eight comorbid conditions (7). However, there is no clear distinction between the QOL impact associated with chronic wounds and that associated with comorbidities (8, 9). Furthermore, QOL as a function of wound severity, etiology, and complications is poorly understood.
Although chronic wounds are cross-cultural, racial and ethnic disparities appear in wound severity at presentation and in subsequent treatment of wounds. However, these disparities are more likely to reflect socioeconomic differences and clinician bias. PU incidence among African American nursing home residents is more than 1.5 times that of white residents (10–13). That disparity likely arises from differences in diagnosis and care; PU incidence increases among white residents who live in nursing homes where the majority of residents are African American (10–13). LEA risk is also higher among African American and Native American patients, compared with non-Hispanic white patients, and it varies by culture among Hispanic patients (14–16). However, the incidence of diabetes is also higher among non-white individuals, and race and ethnicity is less of a predictor for LEA than other factors, such as differences in rates of peripheral vascular disease or smoking. Time to amputation is shorter for African American patients than for whites, but this disparity also might arise from differences in prevention and care: African American patients tend to receive less preventive care, while white patients are more likely to receive revascularizations (17–19).
Basic Science of Wound Repair and Healing
Biology of Wound Healing, Chronic Wounds, and Aging
The complex process of wound healing occurs in overlapping phases, including inflammation, proliferation, angiogenesis, epidermal restoration, and wound contraction and remodeling (20). Important cell types in this process include platelets, which recruit inflammatory cells and form a provisional matrix, and macrophages, which include several phenotypes and regulate the cytokine environment in the wound, which influences proliferative responses and wound closure (21). Matrix metalloproteinases (MMPs) are active throughout wound healing, aiding in phagocytosis, angiogenesis, cell migration during epidermal restoration, and tissue remodeling.
In chronic wounds, resident cells proliferate less and show morphology similar to that seen in senescent cells. Fibroblasts from chronic VLU, particularly ulcers of long duration, show poorer responses to platelet-derived growth factor (PDGF) (22), alterations in transforming growth factor beta (TFG-β) and TGF-β type II receptor expression (23), and abnormal phosphorylation of key signal transduction proteins (24). Decreased receptor expression in cells in these wounds is similar to that in cells exposed to low oxygen tension, suggesting that chronic wounds are hypoxic (24).
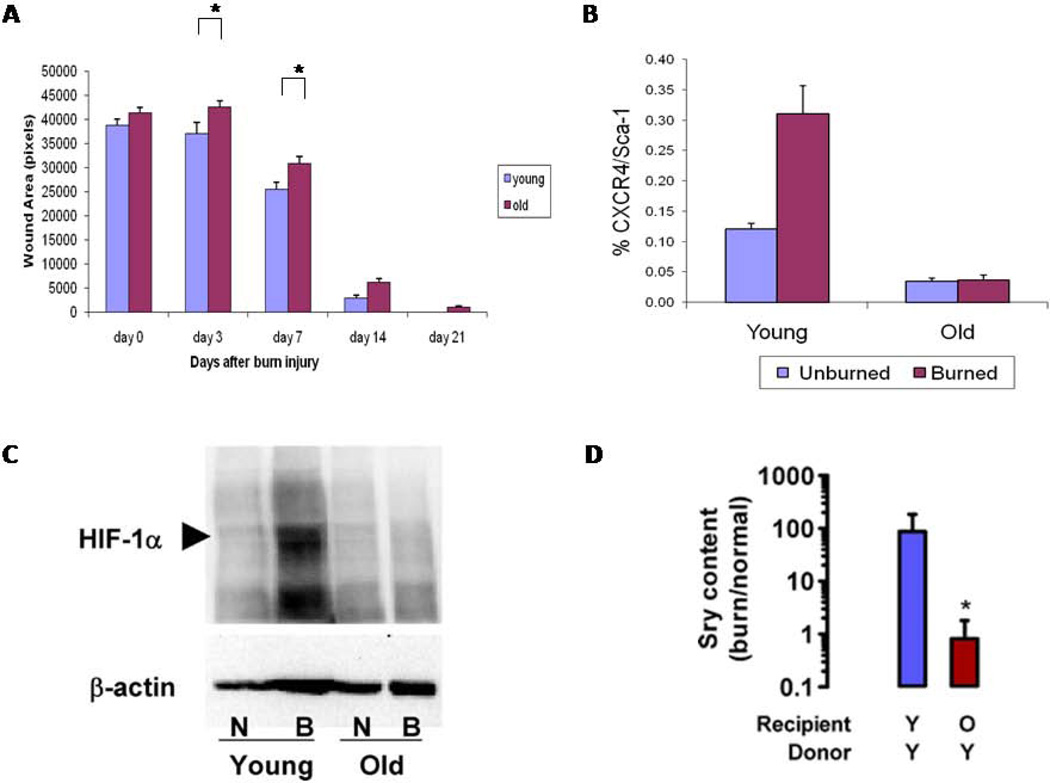
Aging also is associated with alterations in wound healing. In a diabetic mouse model, the healing of burns is delayed in older mice as a result of diminished hypoxia-inducible factor 1 (HIF1) expression, fewer bone marrow-derived angiogenic cells (BMDAC), and dampened response and homing among BMDAC that are present (Figure 1) (25, 26). Aging also is associated with delays in macrophage and T-cell infiltration, angiogenesis, and epithelialization.
Figure 1.
Burn wound repair is delayed in aged mice. A. Wound area was evaluated 0, 3, 7, 14 and 21 days following burn injury in 2 month old (young) vs 2 year old C57BL/6J mice. B. Bone marrow-derived angiogenic cells were identified by FACS as CXCR4+/Sca-1+. C. HIF1 concentrations in response to burn injury are reduced in aged mice, compared with younger ones. D. Bone marrow-derived angiogenic cells transferred from young male mice show impaired homing in older recipient mice, compared with younger ones. Donor cells were identified using the Sry gene as a marker. N, normal; B, burned.
Adapted from: Zhang X et al. Aging impairs the mobilization and homing of bone marrow-derived angiogenic cells to burn wounds. J Mol Med. 2011;89(10):985-95.
The properties of the extracellular matrix (ECM) and its contribution to wound-healing changes throughout the lifespan (Table 2) (27). Whereas younger skin can mount a robust response by producing ECM that can adapt to the mechanical demands of an injury, older skin shows considerable atrophy and a prolonged and blunted healing response (28) with heightened inflammation and differences in signal transduction, that result in inferior in ECM production. Healing in older animals also involves a protective and non-inflammatory response characterized by reduced matrix molecule production and reduced scarring. Work in an in vitro model of aged rat skin suggests that age-associated disadvantages in healing may arise from overexpression of MMPs, particularly MMP2 (29), consistent with findings that protease expression and activity are increased in older human adults (30). Age-related changes in hormonal status affect repair. MMPs, particularly MMP2, are elevated principally in older postmenopausal females, and estrogen replacement therapy can stimulate the migration and proliferation of keratinocytes and elaboration of matrix (30).
Table 2.
Properties in cutaneous extracellular matrix and wound healing across the lifespan (27).
| Age of skin | Properties |
|---|---|
| Fetal |
|
| Juvenile |
|
| Early adult |
|
| Aged adult |
|
ECM, extracellular matrix; MMP, matrix metalloproteinase; TGF-β, transforming growth factor beta
The microcirculation, defined as arterioles, capillaries and venules, plays a critical role in wound-healing. The microcirculation of aged skin demonstrates impaired vasoregulation, which reflects changes in inflammatory responses, lower numbers of progenitor cells, and declines in circulatory mediators (31). Age-associated delays in microvascular responses to stressors lead to impaired temperature regulation and increased likelihood of tissue hypoperfusion (31) that inhibits wounds from reaching the angiogenic stage of repair. Optimal healing strategies following surgery and other stressors must therefore use multifactorial approaches to address changes in the microcirculation in the older host. Potential strategies include better use of existing vessels to optimize vasodilation (e.g., physical activity, pneumatic compression, or pharmacologic mediators) (32–34), optimization of inflammatory and other cellular responses (e.g., stem cells) (35, 36), and strategies to address deficiencies in growth factors, sex steroids, and the ECM (37, 38).
Molecular and Cellular Processes in Wound Healing
Inflammation
Under normal wound healing conditions, early macrophages promote inflammation, and later macrophages clear neutrophils and switch to a reparative phenotype. In the wounds of diabetic mice, however, macrophages fail to clear dying neutrophils and therefore remain in a proinflammatory phenotype (39). Similarly, in both humans and mice, VLU contain high levels of iron; thus, macrophages take up more iron and remain in a proinflammatory state (40). Although impairment in the switch from the proinflammatory to reparative phenotype is clearly involved in chronic wounds, the intermediate steps between the two phenotypes are not clear. Whether an alteration in the macrophage switch affects wound healing in aging is unknown.
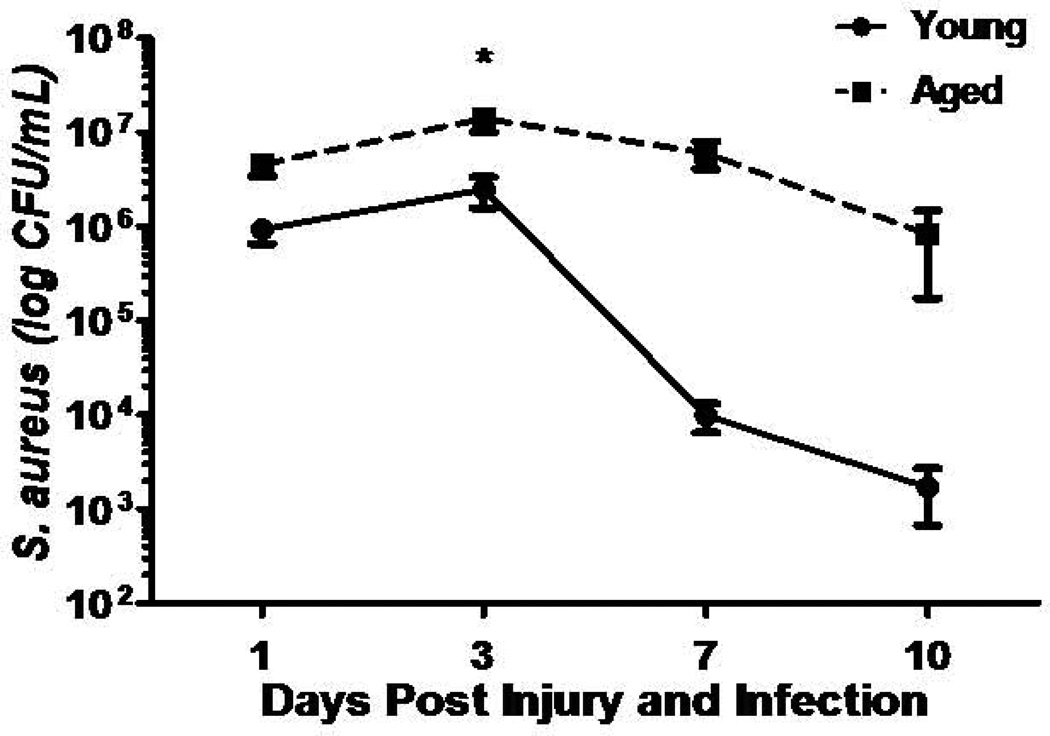
Excisional wounds heal more slowly in older mice than in young adult mice (41) as a result of increased macrophage infiltration, especially at earlier phases of wound repair. Age-associated aberrations in macrophage functions decrease or delay vascularization, collagen deposition, and collagen remodeling (42). In contrast, scald wounds heal more slowly in older mice as a result of lower chemokine levels (43). Neutrophil depletion, which enhances wound healing in younger mammals (44), delays wound closure in aged mice (45). All these changes may arise from age-associated increases in basal or constitutive inflammation, which occurs even in healthy individuals. Age-associated inflammation and delays in wound healing may have particular consequences for infection. In a mouse model inoculated with Staphylococcus aureus, older mice fail to clear the infection (Figure 2) and show less neutrophil chemotaxis, increased bacterial colonization, and slowed macrophage infiltration (46).
Figure 2.
Implications of age-associated inflammation for infection. In a mouse model, older mice inoculated with Staphylococcus aureus fail to clear infection, compared with younger mice.
Source: Brubaker AL, Rendon JL, Ramirez L, Choudhry MA, Kovacs EJ. Reduced neutrophil chemotaxis and infiltration contributes to delayed resolution of cutaneous wound infection with advanced age. Journal of immunology 2013;190(4):1746-57. Copyright 2013. The American Association of Immunologists, Inc.
Age-associated inflammation is characterized by sustained elevations in proinflammatory cytokines, such as IL-6 and tumor necrosis factor alpha, and by declines in growth factors that are important for wound healing. TGF-β, which remains elevated during chronic inflammation, may promote the transformation of acute wounds into chronic ones by contributing to fibrotic replacement and scarring and by inhibiting re-epithelialization (47). TGF-β expression is influenced by angiotensin receptor signaling. With diabetes or age, skin shows increased expression of angiotensin II and the proinflammatory, vasoconstrictive AT1 receptor signaling pathway (48–50). Treatment with the angiotensin receptor blocker (ARB) losartan improves muscle remodeling after injury (51), and diabetic individuals taking ARBs are less likely to undergo amputation, compared with those taking angiotensin-converting enzyme inhibitors (52). Thus, angiotensin receptor signaling likely increases fibrosis and satellite cell deactivation and may serve as a target for wound healing.
Mitochondrial dysfunction and oxidative stress
Mitochondria provide energy and produce reactive oxygen species to drive the increased mitotic and synthetic activity necessary for wound healing. Oxidative stress is also necessary for cellular signaling, the clearing of bacteria, the transition into the proliferative phase of wound healing, and enhancement of angiogenesis through the production of mediators such as nitric oxide and the HIF1α pathway (53). Because of the high energy needs during wound repair, and to avoid the overuse of mitochondria as the sole energy source, the process for ATP generation shifts from oxidative phosphorylation to glycolysis. Although glycolysis is less efficient, it likely protects the mitochondrial pool from increasing damage related to oxidative stress damage (54). Furthermore, efficient mitochondrial turnover mechanisms in the form of mitophagy and mitobiogenesis are required to maintain a healthy pool of mitochondria. Yet the skin is exposed to higher levels of extrinsic insults, which likely lead to dysfunctional mitochondria, decreased ATP production, and increased oxidative damage that triggers mitochondrial turnover. Skin mitochondria, particularly in exposed skin, show increased incidence of mitochondrial DNA mutations with older age (55), indicating not only an increase in the number of dysfunctional mitochondria but also defects in eliminating them. The chronic inflammation seen with age and chronic conditions increases the number of dysfunctional mitochondria, and older age has been associated with lower levels of antioxidants (56). However, the link between age-associated mitochondrial dysfunction and impaired wound healing is poorly studied.
Microbial Burden
The impact of microbial burden on wound healing is unknown and likely underestimated. Traditionally, studies of microbial burden have relied on culture-based techniques and therefore have excluded the vast majority of microbes. Because culture-based studies also exclude bacteria that rely on microbial community interactions, they provide little information about the biofilm, a factor thought to be critical in wound healing. Recent studies using 16S ribosomal RNA-based gene sequencing and quantitative PCR have revealed that the skin and wounds have rich microbiomes with marked variability across body regions, wound type, and sampling method (57–59). In particular, Staphylococcus, Anaerococcus, Corynebacterium, and anaerobic species appear to contribute to microbial burden and wound behavior; for example a genomic study in DFU suggests a negative correlation between Staphylococcus burden and the depth and duration of the ulcer (57). Bacterial community structure also has been correlated with clinical data. Among 30 patients with open fractures related to traumatic injury, bacterial community structure differs between patients who later develop complications and those who do not, as well as between upper- and lower-extremity wounds (60). Further study with genomic sequencing techniques and clinical correlations might therefore identify microbial burden associated with the development of chronic wounds. The best collection methods are still unknown, however, and more standardization is needed to facilitate comparisons across studies. Moreover, rodent studies suggest that aging affects bacterial clearance, but no microbiome research has focused on older adults.
Basic Science Research Considerations
In vitro models have yielded much information on the basic biology of wound healing, and more complex, reproducible, and relevant systems, such as the use of time-lapse photography to measure wound parameters, are available. However, the mechanical environment in which these models are studied differs from the human environment. Attempting to study too many variables in these models can hinder new understanding, yet models that mimic the combination of comorbid conditions that occur in aged humans are needed. Stem cell research is promising, but use of stem cells is hampered by concerns about immunogenicity, teratomas and other malignancies, the ability to maintain pluripotency, and limited supply. The use of embryonic stem cells also is hampered by ethical concerns.
Many studies in wound repair have relied on animal models, particularly mouse models, to increase understanding of the phases of wound healing and the changes that occur with age. However, skin morphology and the mechanisms of wound repair differ markedly between mice and humans. Pig skin is closest to human skin (61), but the utility of pig models is limited by a long lifespan and higher maintenance costs. Moreover, studies in animal models have focused primarily on excisional wounds; incisional wounds, abrasions, and burns are poorly studied. Furthermore, there are no models that mimic chronic wounds or the comorbidities commonly seen with human aging.
The identification of predictive, diagnostic, and/or indicative biomarkers for wound healing has been difficult because of the multifactorial pathogenesis and the heterogeneity of sampling spanning across and within wound type. Very little is known regarding the contribution of aging in the context of specific biomarkers among older adults. Gene expression profiles have been identified in biopsies from VLU, DFU, or other chronic wounds, yielding a large number of potential biomarkers of non-healing wounds (62–65). However, the correlations of these tissue-based markers with wound healing outcomes, and how to harness this information into predictive and diagnostic tools, are not clear. Rapid tests that detect increased MMP levels in wounds can identify a subset of patients with poor wound healing (66–70), but these tests are hampered by variability in obtaining and measuring specimens. It is still not clear which of these patients might heal with standard of care, whether observed differences represent cause or effect, or how age influences such tests. Furthermore, PCR-based identification of bacterial species is under development as an approach to point-of-care diagnostics related to poly-microbial and biofilm-infected wounds (71, 72). Substantial research is needed to identify, evaluate, and validate biomarkers related to wound healing both in general and specifically in older adults.
Clinical Research on Chronic Wounds
Novel Therapeutic Approaches
Cellular and Tissue Engineered Products
Cellular and tissue-engineered products are often combined with standard-of-care approaches such as moist wound healing, compression, and offloading. A new product that distinguishes itself from current cellular and tissue-engineered products is HP802-247, which delivers a specific, optimized ratio of primed allogeneic fibroblasts and keratinocytes directly to the ulcer in a fibrin spray (73, 74). Phase 2b study data indicate that HP802-247 promotes significant healing of VLU, with the odds of wound healing being 2.75 times greater for patients with HP802-247, compared with those receiving the vehicle control (73, 75). Confirmatory Phase 3 clinical trials are under way in both the United States and Europe. In general, development of evidence-based clinical protocols for the therapeutic use of cellular and tissue-engineered approaches has been challenged by a lack of well-controlled and comparative data, clear mechanism(s) of action, and clear definitions that distinguish between cellular therapies, advanced therapies, and dressings. Such development also has been hampered by the need for better defined regulatory and reimbursement pathways. Additionally, the potential influences of patient age on cellular and tissue-engineered products are poorly characterized.
Negative Pressure Wound Therapy (NPWT
Although data from randomized controlled trials and meta-analyses suggest that NPWT is effective in older adults (76–82), few studies have focused specifically on older adults, and there are not enough data for a clear recommendation. Many variables that may influence wound healing are defined inadequately in these studies (83), and the mechanism of action for NPWT is poorly understood. Primary effects may include macro-deformation, or wound contraction, and micro-deformation, or the microscopic interaction between the wound and dressing. Potential secondary effects include increased cell proliferation and granulation tissue, perhaps as a result of changes in bacterial levels or cell stress (84–90).
Hyperbaric Oxygen Therapy
The benefit of hyperbaric oxygen therapy is even less clear. In a recent meta-analysis of six randomized controlled trials and six observational studies, the observational studies showed a benefit, but the randomized trials did not (91), and none of the studies focused on older adults. A retrospective study also failed to show efficacy or effectiveness (92). Some animal data suggest that hyperbaric oxygen therapy is effective at all ages (93, 94). New mechanistic studies of hyperbaric oxygen therapy are focusing on stem cells. Vasculogenic and mesenchymal stem cells incur damage with both chronological and replicative aging, resulting in poorer differentiation (95–97) and reduced mobilization (98–100). However, few clinical data have correlated circulating cells with wound healing (101).
Electrical Stimulation
Physical therapy approaches also show promise for wound healing. Although electrotherapy has not been assessed in large clinical trials, a meta-analysis of several small trials in patients of all ages has found that it effectively promotes wound closure (102). Electrotherapy improves blood flow and prevents PU in a population affected by spinal cord injury (103), and it may improve take of grafts and flaps, improve vascularization, reduce necrosis, and increase angiogenesis in patients with VLU or critical limb ischemia (104–106).
Ultrasound
Low-frequency (22.5 to 35kHz) ultrasound applied in contact rapidly debrides the wound surface and is a fairly comfortable procedure (107); many patients decline pre-treatment with lidocaine after one or two treatments. Several studies have shown that low-frequency contact ultrasound works synergistically with antibiotics to provide a better kill rate of antibiotic -resistant strains of bacteria and biofilms, compared with antibiotics alone (108–111). Low-frequency ultrasound also reduces antimicrobial resistance in vitro (111). Data from a small clinical study among 17 patients aged 32 to 83 years with ulcers of mixed etiologies suggest that low-frequency ultrasound promotes healing for all wounds without antibiotics (109). The effectiveness of low-frequency ultrasound in healing chronic wounds of older adults is unclear. Larger randomized clinical trials are under way in Canada (NCT01973361) and Australia (112), but additional large, multicenter trials are needed.
Nutrition
Older adults categorized as undernourished are at increased risk for developing PU (Pressure Ulcers) and other complex wounds (113, 114). However, this association may be confounded by factors other than inadequate nutrient intake (115). Commonly used putative markers of nutritional deficiency have low sensitivity and specificity as nutritional indicators in these older high-risk populations (115). Most of these individuals have multiple additional comorbidities, such as ongoing inflammation, disuse atrophy, or other metabolic disturbances, and these comorbidities can have a greater impact than nutritional intake in altering the putative nutritional markers (116). In addition, despite a wealth of nutritional studies, no consensus has been reached on optimal nutritional care for older adults with chronic wounds. The recommended daily protein allowance assumes that adults are healthy, consume high-quality protein, and have an adequate energy intake (117). Recognizing that protein requirements are influenced by inflammation, the adequacy of energy intake, and other stressors common in older patients with complex wounds, the Agency for Healthcare Research and Quality developed recommendations for protein intake for patients with uncomplicated PU, but these estimates are based on anecdotal evidence (117, 118). There is conflicting evidence that dietary interventions or commercial supplementation are effective in preventing PUs or in accelerating healing (118–121). Of the few studies that report evidence of benefit, most are methodologically weak and their findings have yet to be verified (119, 121–124). Further research is needed in this area. However, disentangling nutritional needs from all other factors affecting host metabolic response to injury, especially when multiple comorbid conditions are present, remains a challenge (114, 120, 125).
Clinical Research Considerations
The majority of wound care is performed in the outpatient setting, and clinical trials therefore focus on outpatient care. However, the presence of a wound significantly affects in-patient costs, length of hospital stay, and discharge planning. Thus future clinical studies will require a clear definition of hospital-based wound healing in older adults. Such a definition has been hindered by variations in data collection and in the definition, measurement, and treatment of wounds in older adults. Measurable outcomes also must be defined and several have been suggested. A well-structured electronic medical record that follows the patient through the continuum of care will facilitate the measurement of these variables for clinical outcomes and research.
Approval of products or devices by the U.S. Food and Drug Administration (FDA) is a major driver in the design and conduct of clinical trials (126). However, the approval process in general is long and expensive, and only 1 in 25 products are eventually approved. Approval is even more constrained for wound care. Only three products for wound care have been approved by the FDA in the past 20 years (127), and the FDA has defined only one endpoint—complete healing—for wounds. Thus traditional, FDA-driven, randomized trials, while effective for assessing efficacy, may not inform clinical decision-making for wound care (126). Other study designs, such as pragmatic or comparative effectiveness approaches, might be more appropriate (128).
A critical issue in clinical trial design for older adults centers on inclusion criteria. Clinical trial populations tend to be homogenous, and many comorbidities associated with older age are excluded. Age itself can be an exclusion criterion, but it is not clear that it should be. A meta-analysis of ten trials has found that it is wound chronicity, rather than age, that plays a strong role in healing among patients receiving standard care for DFU (129). Another study has found that the area and duration of the wound, but not age, influence healing of VLU following spray therapy (130). Thus age does not appear to be a significant factor in the response to wound healing treatment. It can be an important predictor, however, as illustrated by the formula derived from a clinical database for an Ulcerated Leg Severity Assessment (131).
Unanswered Questions, Future Directions, and Research Challenges
Future research will require common definitions and standardized procedures for data collection, and it will need to address the analytical challenges associated with studying older adults, such as population heterogeneity, missing data from death or dropout, limited sample sizes, and variable follow-up times. Valid clinical and patient-centered measures, particularly those of most value to the patient, also are needed. With better measures and more data, additional endpoints might be accepted by the FDA for clinical trials in wound care, particularly in older adults. Common comorbidities are a major concern in the field of geriatrics and therefore should be explored both in clinical trials and in basic and preclinical studies. Issues related to polypharmacy also should be explored. Specific research questions regarding wound healing in older adults are listed in Table 1.
Because the concept of chronic wounds crosses many disciplines, more collaboration is needed to answer common questions. Transdisciplinary collaboration between clinicians and basic scientists can facilitate development of animal models that more closely mimic human wound closure. Interaction between wound care clinicians and basic scientists can identify optimal strategies to obtain and use clinical samples, and multicenter collaborations among wound care clinicians, geriatricians, and gerontologists will improve clinical trial design for older adults and incorporate measures of quality of life. In addition, investigators studying wound healing can learn from other fields, such as cancer, and engagement with government, industry, data-mining companies, and consulting groups might provide access to public and proprietary databases and information focused on public health. Potential resources are listed in Table 4.
Table 4.
Available or Forthcoming Resources
| Resource | Purpose | |
|---|---|---|
| Infrastructure, Databases, and Registries | ||
| U.S. Wound Registry (www.uswoundregistry.com) |
|
|
| Stony Brook University Clinical Decision Support system | Institutional review board-approved, electronic medical record-based system to facilitate enrollment in clinical studies | |
| Measures | ||
|
Generic quality of life instruments | |
|
Wound-specific quality of life instruments | |
| Funding Sources | ||
| R21/R33 mechanism, National Institute on Aging |
|
|
EQ-5D, EuroQol; SF-36, 36-Item Short Form Health Survey
Future research on wound healing in older adults also will benefit from efforts to address structural challenges in the research enterprise. Well-conducted education and implementation science studies can improve the ability of front-line providers to provide critical wound care, aid in convincing hospital and nursing home administrators of the value of educational programs, and increase implementation of preventive approaches. Perverse incentives related to the fee-for-service model, which has traditionally ignored prevention, also must be addressed. Moreover, development of a formal wound care specialty would promote consensus on standard wound care, provide a more unified approach to wound research, and perhaps improve and expand cross-discipline educational approaches.
Table 3.
Potential Outcomes for Clinical Studies of Wound-Healing in Older Adults.
|
Acknowledgement
This workshop was supported by generous grants to ASP from the National Institute on Aging (1 U13 AG040938 01) and the John A. Hartford Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIA or the National Institutes of Health. In addition, the views expressed in written conference materials or publications and by speakers or moderators do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention by trade names, commercial practices, or organizations imply endorsement by the U.S. Government.
We are grateful to Nancy Woolard for her assistance with organizing the workshop. To see the agenda, a list of workshop moderators and attendees, and workshop presentations, please visit: http://www.im.org/p/cm/ld/fid=599.
List of Abbreviations
- ARB
angiotensin receptor blocker
- BMDAC
bone marrow-derived angiogenic cells
- DFU
diabetic foot ulcer
- ECM
extracellular matrix
- FDA
U.S. Food and Drug Administration
- HIF1
hypoxia inducible factor 1
- LEA
lower-extremity amputation
- NPWT
negative pressure wound therapy
- PU
pressure ulcer
- QOL
quality of life
- TGF-β
transforming growth factor beta
- VLU
venous leg ulcer
Footnotes
Conflicts of interest were acknowledged as follows:
Dr. Gould reports non-financial support from MiMedx, Cytomedix, Celleration, and Cardinal Health.
Dr. Abadir has a patent Novel, protective, anti-inflammatory receptor and its use in preservation of mitochondrial function, wound healing and repair pending.
Dr. Davidson reports grants from National Institutes of Health and the Department of Veterans Affairs.
Dr. Fife is the Executive Director of The Chronic Disease Registry (d/b/a the U.S. Wound Registry, USWR), a 501C(3) organization which provided some of the data for this presentation, specifically the data on the use of biological dressings among older patients and their associated healing rates.
Dr. Grice reports grants from Janssen Research and Development; personal fees from GOJO, Amway International, GlaxoSmithKline, and L'Oreal.
Dr. High reports grants from Chimerix, Sanofi-Pasteur, Optimer, Astellas, is a consultant for University of Virginia and the University of Minnesota, other financial or material support from McGraw-Hill Publishers and Uptodate, Inc.
Dr. Jacobson is a Smith and Nephew employee which produces the cell therapy product described in the presentation.
Dr. McFarland Horne reports grants from John A. Hartford Foundation during the conduct of the study.
Dr. Tomic-Canic reports grants from NIH, Organogenesis Inc, Novan, and Smith & Nephew; is a Scientific Board Member for Molnlycke. In addition, Dr. Tomic-Canic has the following patents: Methods and compositions for promoting wound healing issued to Hospital for Special Surgery; GM-CSF ceosmeceutical compositions and methods of use thereof issued to NYU School of Medicine; Biological markers of chronic wound tissue and methods of using for criteria in surgical debridement pending to NYU School of Medicine; Denovo synthesis of glucocortocoid in the epidermis and it uses and applications patent pending to NYU School of Medicine; and Growth factor mediated cosmeceuticals and use of thereof to enhances skin quality patent pending to NYU School of Medicine.
H.Brem, M.Carter, T. Conner-Kerr, L.DiPietro, V.Falanga, S.Gardner, J.Harmon, W. Hazzard, P.Houghton, R. Kirsner, E.Kovacs, D.Margolis, M.Reed, K.Schmader, D.Sullivan, S.Thom, J.Walston, J.Whitney, J.Williams, S.Zieman: No conflicts to disclose
References
- 1.Margolis DJ, Bilker W, Santanna J, Baumgarten M. Venous leg ulcer: incidence and prevalence in the elderly. Journal of the American Academy of Dermatology. 2002;46(3):381–386. doi: 10.1067/mjd.2002.121739. [DOI] [PubMed] [Google Scholar]
- 2.Margolis DJ, Bilker W, Knauss J, Baumgarten M, Strom BL. The incidence and prevalence of pressure ulcers among elderly patients in general medical practice. Annals of epidemiology. 2002;12(5):321–325. doi: 10.1016/s1047-2797(01)00255-1. [DOI] [PubMed] [Google Scholar]
- 3.Bickers DR, Lim HW, Margolis D, Weinstock MA, Goodman C, Faulkner E, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. Journal of the American Academy of Dermatology. 2006;55(3):490–500. doi: 10.1016/j.jaad.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 4.Badia JG, Santos AB, Contel Segura JC, Teren CA, Gonzalez LC, Ramirez EL, et al. Predictors of mortality among elderly dependent home care patients. BMC health services research. 2013;13:316. doi: 10.1186/1472-6963-13-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hopman WM, Harrison MB, Coo H, Friedberg E, Buchanan M, VanDenKerkhof EG. Associations between chronic disease, age and physical and mental health status. Chronic diseases in Canada. 2009;29(3):108–116. [PubMed] [Google Scholar]
- 6.Redekop WK, Stolk EA, Kok E, Lovas K, Kalo Z, Busschbach JJ. Diabetic foot ulcers and amputations: estimates of health utility for use in cost-effectiveness analyses of new treatments. Diabetes & metabolism. 2004;30(6):549–556. doi: 10.1016/s1262-3636(07)70154-4. [DOI] [PubMed] [Google Scholar]
- 7.Horn SD, Fife CE, Smout RJ, Barrett RS, Thomson B. Development of a wound healing index for patients with chronic wounds. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2013;21(6):823–832. doi: 10.1111/wrr.12107. [DOI] [PubMed] [Google Scholar]
- 8.Edwards H, Finlayson K, Courtney M, Graves N, Gibb M, Parker C. Health service pathways for patients with chronic leg ulcers: identifying effective pathways for facilitation of evidence based wound care. BMC health services research. 2013;13:86. doi: 10.1186/1472-6963-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter MJ. Cost-effectiveness research in wound care: definitions, approaches, and limitations. Ostomy/wound management. 2010;56(11):48–59. [PubMed] [Google Scholar]
- 10.Harms S, Bliss DZ, Garrard J, Cunanan K, Savik K, Gurvich O, et al. Prevalence of Pressure Ulcers by Race and Ethnicity for Older Adults Admitted to Nursing Homes. Journal of gerontological nursing. 2013:1–7. doi: 10.3928/00989134-20131028-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coleman S, Gorecki C, Nelson EA, Closs SJ, Defloor T, Halfens R, et al. Patient risk factors for pressure ulcer development: systematic review. International journal of nursing studies. 2013;50(7):974–1003. doi: 10.1016/j.ijnurstu.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Yin J, Cai X, Temkin-Greener J, Mukamel DB. Association of race and sites of care with pressure ulcers in high-risk nursing home residents. JAMA : the journal of the American Medical Association. 2011;306(2):179–186. doi: 10.1001/jama.2011.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baumgarten M, Margolis D, van Doorn C, Gruber-Baldini AL, Hebel JR, Zimmerman S, et al. Black/White differences in pressure ulcer incidence in nursing home residents. Journal of the American Geriatrics Society. 2004;52(8):1293–1298. doi: 10.1111/j.1532-5415.2004.52358.x. [DOI] [PubMed] [Google Scholar]
- 14.Lanting LC, Joung IM, Mackenbach JP, Lamberts SW, Bootsma AH. Ethnic differences in mortality, end-stage complications, and quality of care among diabetic patients: a review. Diabetes care. 2005;28(9):2280–2288. doi: 10.2337/diacare.28.9.2280. [DOI] [PubMed] [Google Scholar]
- 15.Dillingham TR, Pezzin LE, Mackenzie EJ. Racial differences in the incidence of limb loss secondary to peripheral vascular disease: a population-based study. Archives of physical medicine and rehabilitation. 2002;83(9):1252–1257. doi: 10.1053/apmr.2002.34805. [DOI] [PubMed] [Google Scholar]
- 16.Lavery LA, van Houtum WH, Ashry HR, Armstrong DG, Pugh JA. Diabetes-related lower-extremity amputations disproportionately affect Blacks and Mexican Americans. Southern medical journal. 1999;92(6):593–599. doi: 10.1097/00007611-199906000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Holman KH, Henke PK, Dimick JB, Birkmeyer JD. Racial disparities in the use of revascularization before leg amputation in Medicare patients. Journal of vascular surgery. 2011;54(2):420–426. 6 e1. doi: 10.1016/j.jvs.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowe VL, Weaver FA, Lane JS, Etzioni DA. Racial and ethnic differences in patterns of treatment for acute peripheral arterial disease in the United States, 1998–2006. Journal of vascular surgery. 2010;51(4 Suppl):21S–26S. doi: 10.1016/j.jvs.2009.09.066. [DOI] [PubMed] [Google Scholar]
- 19.Regenbogen SE, Gawande AA, Lipsitz SR, Greenberg CC, Jha AK. Do differences in hospital and surgeon quality explain racial disparities in lower-extremity vascular amputations? Annals of surgery. 2009;250(3):424–431. doi: 10.1097/SLA.0b013e3181b41d53. [DOI] [PubMed] [Google Scholar]
- 20.Shaw TJ, Martin P. Wound repair at a glance. Journal of cell science. 2009;122(Pt 18):3209–3213. doi: 10.1242/jcs.031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mirza R, DiPietro LA, Koh TJ. Selective and specific macrophage ablation is detrimental to wound healing in mice. The American journal of pathology. 2009;175(6):2454–2462. doi: 10.2353/ajpath.2009.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agren MS, Steenfos HH, Dabelsteen S, Hansen JB, Dabelsteen E. Proliferation and mitogenic response to PDGF-BB of fibroblasts isolated from chronic venous leg ulcers is ulcer-age dependent. The Journal of investigative dermatology. 1999;112(4):463–469. doi: 10.1046/j.1523-1747.1999.00549.x. [DOI] [PubMed] [Google Scholar]
- 23.Kim BC, Kim HT, Park SH, Cha JS, Yufit T, Kim SJ, et al. Fibroblasts from chronic wounds show altered TGF-beta-signaling and decreased TGF-beta Type II receptor expression. Journal of cellular physiology. 2003;195(3):331–336. doi: 10.1002/jcp.10301. [DOI] [PubMed] [Google Scholar]
- 24.Falanga V, Zhou L, Yufit T. Low oxygen tension stimulates collagen synthesis and COL1A1 transcription through the action of TGF-beta1. Journal of cellular physiology. 2002;191(1):42–50. doi: 10.1002/jcp.10065. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Sarkar K, Rey S, Sebastian R, Andrikopoulou E, Marti GP, et al. Aging impairs the mobilization and homing of bone marrow-derived angiogenic cells to burn wounds. Journal of molecular medicine. 2011;89(10):985–995. doi: 10.1007/s00109-011-0754-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du J, Liu L, Lay F, Wang Q, Dou C, Zhang X, et al. Combination of HIF-1alpha gene transfection and HIF-1-activated bone marrow-derived angiogenic cell infusion improves burn wound healing in aged mice. Gene therapy. 2013;20(11):1070–1076. doi: 10.1038/gt.2013.32. [DOI] [PubMed] [Google Scholar]
- 27.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 28.Ashcroft GS, Mills SJ, Ashworth JJ. Ageing and wound healing. Biogerontology. 2002;3(6):337–345. doi: 10.1023/a:1021399228395. [DOI] [PubMed] [Google Scholar]
- 29.Ballas CB, Davidson JM. Delayed wound healing in aged rats is associated with increased collagen gel remodeling and contraction by skin fibroblasts, not with differences in apoptotic or myofibroblast cell populations. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2001;9(3):223–237. doi: 10.1046/j.1524-475x.2001.00223.x. [DOI] [PubMed] [Google Scholar]
- 30.Ashcroft GS, Horan MA, Herrick SE, Tarnuzzer RW, Schultz GS, Ferguson MW. Age-related differences in the temporal and spatial regulation of matrix metalloproteinases (MMPs) in normal skin and acute cutaneous wounds of healthy humans. Cell and tissue research. 1997;290(3):581–591. doi: 10.1007/s004410050963. [DOI] [PubMed] [Google Scholar]
- 31.Bentov I, Reed MJ. Anesthesia, microcirculation, and wound repair in aging. Anesthesiology. 2014;120(3):760–772. doi: 10.1097/ALN.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida H, Itoh S, Hara T, Sasaki Y, Kondo S, Nakagawa T, et al. A phosphodiesterase 3 inhibitor, K-134, improves hindlimb skeletal muscle circulation in rat models of peripheral arterial disease. Atherosclerosis. 2012;221(1):84–90. doi: 10.1016/j.atherosclerosis.2011.12.044. [DOI] [PubMed] [Google Scholar]
- 33.Krcma M, Cechurova D, Jankovec Z, Lacigova S, Zourek M, Rusavy Z. Effect of mild increase of physical activity on microvasculary reactivity in obese subjects with diabetes mellitus type 2. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. 2009;117(3):150–152. doi: 10.1055/s-0028-1100417. [DOI] [PubMed] [Google Scholar]
- 34.Husmann M, Willenberg T, Keo HH, Spring S, Kalodiki E, Delis KT. Integrity of venoarteriolar reflex determines level of microvascular skin flow enhancement with intermittent pneumatic compression. Journal of vascular surgery. 2008;48(6):1509–1513. doi: 10.1016/j.jvs.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 35.Scalia R. The microcirculation in adipose tissue inflammation. Reviews in endocrine & metabolic disorders. 2013;14(1):69–76. doi: 10.1007/s11154-013-9236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jadlowiec C, Brenes RA, Li X, Lv W, Protack CD, Collins MJ, et al. Stem cell therapy for critical limb ischemia: what can we learn from cell therapy for chronic wounds? Vascular. 2012;20(5):284–289. doi: 10.1258/vasc.2011.201206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roubelakis MG, Trohatou O, Roubelakis A, Mili E, Kalaitzopoulos I, Papazoglou G, et al. Platelet-Rich Plasma (PRP) Promotes Fetal Mesenchymal Stem/Stromal Cell Migration and Wound Healing Process. Stem cell reviews. 2014 doi: 10.1007/s12015-013-9494-8. [DOI] [PubMed] [Google Scholar]
- 38.Makrantonaki E, Zouboulis CC. Androgens and ageing of the skin. Current opinion in endocrinology, diabetes, and obesity. 2009;16(3):240–245. doi: 10.1097/MED.0b013e32832b71dc. [DOI] [PubMed] [Google Scholar]
- 39.Khanna S, Biswas S, Shang Y, Collard E, Azad A, Kauh C, et al. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PloS one. 2010;5(3):e9539. doi: 10.1371/journal.pone.0009539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. The Journal of clinical investigation. 2011;121(3):985–997. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swift ME, Kleinman HK, DiPietro LA. Impaired wound repair and delayed angiogenesis in aged mice. Laboratory investigation; a journal of technical methods and pathology. 1999;79(12):1479–1487. [PubMed] [Google Scholar]
- 42.Swift ME, Burns AL, Gray KL, DiPietro LA. Age-related alterations in the inflammatory response to dermal injury. The Journal of investigative dermatology. 2001;117(5):1027–1035. doi: 10.1046/j.0022-202x.2001.01539.x. [DOI] [PubMed] [Google Scholar]
- 43.Shallo H, Plackett TP, Heinrich SA, Kovacs EJ. Monocyte chemoattractant protein-1 (MCP-1) and macrophage infiltration into the skin after burn injury in aged mice. Burns : journal of the International Society for Burn Injuries. 2003;29(7):641–647. doi: 10.1016/s0305-4179(03)00070-6. [DOI] [PubMed] [Google Scholar]
- 44.Dovi JV, Szpaderska AM, DiPietro LA. Neutrophil function in the healing wound: adding insult to injury? Thrombosis and haemostasis. 2004;92(2):275–280. doi: 10.1160/TH03-11-0720. [DOI] [PubMed] [Google Scholar]
- 45.Nishio N, Okawa Y, Sakurai H, Isobe K. Neutrophil depletion delays wound repair in aged mice. Age. 2008;30(1):11–19. doi: 10.1007/s11357-007-9043-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brubaker AL, Rendon JL, Ramirez L, Choudhry MA, Kovacs EJ. Reduced neutrophil chemotaxis and infiltration contributes to delayed resolution of cutaneous wound infection with advanced age. Journal of immunology. 2013;190(4):1746–1757. doi: 10.4049/jimmunol.1201213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kurosaka M, Suzuki T, Hosono K, Kamata Y, Fukamizu A, Kitasato H, et al. Reduced angiogenesis and delay in wound healing in angiotensin II type 1a receptor-deficient mice. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2009;63(9):627–634. doi: 10.1016/j.biopha.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 48.Hao SY, Ren M, Yang C, Lin DZ, Chen LH, Zhu P, et al. Activation of skin renin-angiotensin system in diabetic rats. Endocrine. 2011;39(3):242–250. doi: 10.1007/s12020-010-9428-z. [DOI] [PubMed] [Google Scholar]
- 49.Yevdokimova N, Podpryatov S. The up-regulation of angiotensin II receptor type 1 and connective tissue growth factor are involved in high-glucose-induced fibronectin production by cultured human dermal fibroblasts. Journal of dermatological science. 2007;47(2):127–139. doi: 10.1016/j.jdermsci.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 50.Abiko M, Rodgers KE, Campeau JD, Nakamura RM, Dizerega GS. Alterations of angiotensin II Receptor levels in sutured wounds in rat skin. Journal of investigative surgery : the official journal of the Academy of Surgical Research. 1996;9(6):447–453. doi: 10.3109/08941939609025862. [DOI] [PubMed] [Google Scholar]
- 51.Burks TN, Andres-Mateos E, Marx R, Mejias R, Van Erp C, Simmers JL, et al. Losartan restores skeletal muscle remodeling and protects against disuse atrophy in sarcopenia. Science translational medicine. 2011;3(82):82ra37. doi: 10.1126/scitranslmed.3002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Margolis DJ, Hoffstad O, Thom S, Bilker W, Maldonado AR, Cohen RM, et al. The differential effect of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers with respect to foot ulcer and limb amputation in those with diabetes. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2010;18(5):445–451. doi: 10.1111/j.1524-475X.2010.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schafer M, Werner S. Oxidative stress in normal and impaired wound repair. Pharmacological research : the official journal of the Italian Pharmacological Society. 2008;58(2):165–171. doi: 10.1016/j.phrs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 54.Im MJ, Hoopes JE. Energy metabolism in healing skin wounds. The Journal of surgical research. 1970;10(10):459–464. doi: 10.1016/0022-4804(70)90070-3. [DOI] [PubMed] [Google Scholar]
- 55.Yang JH, Lee HC, Lin KJ, Wei YH. A specific 4977-bp deletion of mitochondrial DNA in human ageing skin. Archives of dermatological research. 1994;286(7):386–390. doi: 10.1007/BF00371798. [DOI] [PubMed] [Google Scholar]
- 56.Taylor R, James T. The role of oxidative stress in the development and persistence of pressure ulcers. In: Bader D, Bouten C, Colin D, Oomens C, editors. Pressure Ulcer Research. Springer Berlin Heidelberg: 2005. pp. 205–232. [Google Scholar]
- 57.Gardner SE, Hillis SL, Heilmann K, Segre JA, Grice EA. The neuropathic diabetic foot ulcer microbiome is associated with clinical factors. Diabetes. 2013;62(3):923–930. doi: 10.2337/db12-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Price LB, Liu CM, Frankel YM, Melendez JH, Aziz M, Buchhagen J, et al. Macroscale spatial variation in chronic wound microbiota: a cross-sectional study. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2011;19(1):80–88. doi: 10.1111/j.1524-475X.2010.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Han A, Zenilman JM, Melendez JH, Shirtliff ME, Agostinho A, James G, et al. The importance of a multifaceted approach to characterizing the microbial flora of chronic wounds. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2011;19(5):532–541. doi: 10.1111/j.1524-475X.2011.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hannigan GD, Hodkinson BP, McGinnis K, Tyldsley AS, Anari JB, Horan AD, et al. Culture-independent pilot study of microbiota colonizing open fractures and association with severity, mechanism, location, and complication from presentation to early outpatient follow-up. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2014;32(4):597–605. doi: 10.1002/jor.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2001;9(2):66–76. doi: 10.1046/j.1524-475x.2001.00066.x. [DOI] [PubMed] [Google Scholar]
- 62.Stojadinovic O, Pastar I, Vukelic S, Mahoney MG, Brennan D, Krzyzanowska A, et al. Deregulation of keratinocyte differentiation and activation: a hallmark of venous ulcers. Journal of cellular and molecular medicine. 2008;12(6B):2675–2690. doi: 10.1111/j.1582-4934.2008.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Charles CA, Tomic-Canic M, Vincek V, Nassiri M, Stojadinovic O, Eaglstein WH, et al. A gene signature of nonhealing venous ulcers: potential diagnostic markers. Journal of the American Academy of Dermatology. 2008;59(5):758–771. doi: 10.1016/j.jaad.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. The Journal of clinical investigation. 2007;117(5):1219–1222. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gallagher KA, Liu ZJ, Xiao M, Chen H, Goldstein LJ, Buerk DG, et al. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. The Journal of clinical investigation. 2007;117(5):1249–1259. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schultz GS, Gibson D. Measurement of biomarkers for impaired healing in fluids and tissues. In: Mani R, Romanelli M, Shukla V, editors. Measurements in wound healing. Springer; 2012. [Google Scholar]
- 67.Liu Y, Min D, Bolton T, Nube V, Twigg SM, Yue DK, et al. Increased matrix metalloproteinase-9 predicts poor wound healing in diabetic foot ulcers. Diabetes care. 2009;32(1):117–119. doi: 10.2337/dc08-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gibson D, Cullen B, Legerstee R, Harding K, Schultz G. MMPs made easy. Wounds Int. 2009;1:1–6. [Google Scholar]
- 69.Rayment EA, Upton Z, Shooter GK. Increased matrix metalloproteinase-9 (MMP-9) activity observed in chronic wound fluid is related to the clinical severity of the ulcer. The British journal of dermatology. 2008;158(5):951–961. doi: 10.1111/j.1365-2133.2008.08462.x. [DOI] [PubMed] [Google Scholar]
- 70.Ladwig GP, Robson MC, Liu R, Kuhn MA, Muir DF, Schultz GS. Ratios of activated matrix metalloproteinase-9 to tissue inhibitor of matrix metalloproteinase-1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2002;10(1):26–37. doi: 10.1046/j.1524-475x.2002.10903.x. [DOI] [PubMed] [Google Scholar]
- 71.Wolcott RD, Dowd SE. A rapid molecular method for characterising bacterial bioburden in chronic wounds. Journal of wound care. 2008;17(12):513–516. doi: 10.12968/jowc.2008.17.12.31769. [DOI] [PubMed] [Google Scholar]
- 72.Dowd SE, Wolcott RD, Kennedy J, Jones C, Cox SB. Molecular diagnostics and personalised medicine in wound care: assessment of outcomes. Journal of wound care. 2011;20(5):232, 4–9. doi: 10.12968/jowc.2011.20.5.232. [DOI] [PubMed] [Google Scholar]
- 73.Kirsner RS, Marston WA, Snyder RJ, Lee TD, Cargill DI, Slade HB. Spray-applied cell therapy with human allogeneic fibroblasts and keratinocytes for the treatment of chronic venous leg ulcers: a phase 2, multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2012;380(9846):977–985. doi: 10.1016/S0140-6736(12)60644-8. [DOI] [PubMed] [Google Scholar]
- 74.Goedkoop R, Juliet R, You PH, Daroczy J, de Roos KP, Lijnen R, et al. Wound stimulation by growth-arrested human keratinocytes and fibroblasts: HP802-247, a new-generation allogeneic tissue engineering product. Dermatology. 2010;220(2):114–120. doi: 10.1159/000277380. [DOI] [PubMed] [Google Scholar]
- 75.Kirsner RS, Marston WA, Snyder RJ, Lee TD, Cargill DI, Zhang Y, et al. Durability of healing from spray-applied cell therapy with human allogeneic fibroblasts and keratinocytes for the treatment of chronic venous leg ulcers: a 6-month follow-up. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2013;21(5):682–687. doi: 10.1111/wrr.12076. [DOI] [PubMed] [Google Scholar]
- 76.Ruttermann M, Maier-Hasselmann A, Nink-Grebe B, Burckhardt M. Local treatment of chronic wounds: in patients with peripheral vascular disease, chronic venous insufficiency, and diabetes. Deutsches Arzteblatt international. 2013;110(3):25–31. doi: 10.3238/arztebl.2013.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Othman D. Negative pressure wound therapy literature review of efficacy, cost effectiveness, and impact on patients' quality of life in chronic wound management and its implementation in the United kingdom. Plastic surgery international. 2012;2012:374–398. doi: 10.1155/2012/374398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ubbink DT, Westerbos SJ, Nelson EA, Vermeulen H. A systematic review of topical negative pressure therapy for acute and chronic wounds. The British journal of surgery. 2008;95(6):685–692. doi: 10.1002/bjs.6238. [DOI] [PubMed] [Google Scholar]
- 79.Vikatmaa P, Juutilainen V, Kuukasjarvi P, Malmivaara A. Negative pressure wound therapy: a systematic review on effectiveness and safety. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2008;36(4):438–448. doi: 10.1016/j.ejvs.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 80.Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes care. 2008;31(4):631–636. doi: 10.2337/dc07-2196. [DOI] [PubMed] [Google Scholar]
- 81.Vuerstaek JD, Vainas T, Wuite J, Nelemans P, Neumann MH, Veraart JC. State-of-the-art treatment of chronic leg ulcers: A randomized controlled trial comparing vacuum-assisted closure (V.A.C.) with modern wound dressings. Journal of vascular surgery. 2006;44(5):1029–1037. doi: 10.1016/j.jvs.2006.07.030. discussion 38. [DOI] [PubMed] [Google Scholar]
- 82.Llanos S, Danilla S, Barraza C, Armijo E, Pineros JL, Quintas M, et al. Effectiveness of negative pressure closure in the integration of split thickness skin grafts: a randomized, double-masked, controlled trial. Annals of surgery. 2006;244(5):700–705. doi: 10.1097/01.sla.0000217745.56657.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Birke-Sorensen H, Malmsjo M, Rome P, Hudson D, Krug E, Berg L, et al. Evidence-based recommendations for negative pressure wound therapy: treatment variables (pressure levels, wound filler and contact layer)--steps towards an international consensus. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2011;64(Suppl):S1–S16. doi: 10.1016/j.bjps.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 84.Cameron AR, Frith JE, Gomez GA, Yap AS, Cooper-White JJ. The effect of time-dependent deformation of viscoelastic hydrogels on myogenic induction and Rac1 activity in mesenchymal stem cells. Biomaterials. 2014;35(6):1857–1868. doi: 10.1016/j.biomaterials.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 85.Sahin I, Ozturk S, Deveci M, Ural AU, Onguru O, Isik S. Experimental assessment of the neo-vascularisation of acellular dermal matrix in the wound bed pretreated with mesenchymal stem cell under subatmospheric pressure. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2014;67(1):107–114. doi: 10.1016/j.bjps.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 86.Muller P, Langenbach A, Kaminski A, Rychly J. Modulating the actin cytoskeleton affects mechanically induced signal transduction and differentiation in mesenchymal stem cells. PloS one. 2013;8(7):e71283. doi: 10.1371/journal.pone.0071283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wiegand C, White R. Microdeformation in wound healing. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2013;21(6):793–799. doi: 10.1111/wrr.12111. [DOI] [PubMed] [Google Scholar]
- 88.Li Z, Yao SJ, Alini M, Stoddart MJ. Chondrogenesis of human bone marrow mesenchymal stem cells in fibrin-polyurethane composites is modulated by frequency and amplitude of dynamic compression and shear stress. Tissue engineering Part A. 2010;16(2):575–584. doi: 10.1089/ten.TEA.2009.0262. [DOI] [PubMed] [Google Scholar]
- 89.Wozniak MA, Kwong L, Chodniewicz D, Klemke RL, Keely PJ. R-Ras controls membrane protrusion and cell migration through the spatial regulation of Rac and Rho. Molecular biology of the cell. 2005;16(1):84–96. doi: 10.1091/mbc.E04-04-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Katsumi A, Milanini J, Kiosses WB, del Pozo MA, Kaunas R, Chien S, et al. Effects of cell tension on the small GTPase Rac. The Journal of cell biology. 2002;158(1):153–164. doi: 10.1083/jcb.200201105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.O'Reilly D, Pasricha A, Campbell K, Burke N, Assasi N, Bowen JM, et al. Hyperbaric oxygen therapy for diabetic ulcers: systematic review and meta-analysis. International journal of technology assessment in health care. 2013;29(3):269–281. doi: 10.1017/S0266462313000263. [DOI] [PubMed] [Google Scholar]
- 92.Margolis DJ, Gupta J, Hoffstad O, Papdopoulos M, Glick HA, Thom SR, et al. Lack of effectiveness of hyperbaric oxygen therapy for the treatment of diabetic foot ulcer and the prevention of amputation: a cohort study. Diabetes care. 2013;36(7):1961–1966. doi: 10.2337/dc12-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gomez CR, Knutson GJ, Clifton KB, Schreiber CA, Vuk-Pavlovic S. Age-dependent response of murine female bone marrow cells to hyperbaric oxygen. Biogerontology. 2012;13(3):287–297. doi: 10.1007/s10522-012-9373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bonomo SR, Davidson JD, Tyrone JW, Lin X, Mustoe TA. Enhancement of wound healing by hyperbaric oxygen and transforming growth factor beta3 in a new chronic wound model in aged rabbits. Archives of surgery. 2000;135(10):1148–1153. doi: 10.1001/archsurg.135.10.1148. [DOI] [PubMed] [Google Scholar]
- 95.Liu L, Rando TA. Manifestations and mechanisms of stem cell aging. The Journal of cell biology. 2011;193(2):257–266. doi: 10.1083/jcb.201010131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science. 2005;307(5710):720–724. doi: 10.1126/science.1099593. [DOI] [PubMed] [Google Scholar]
- 97.Maslov AY, Barone TA, Plunkett RJ, Pruitt SC. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24(7):1726–1733. doi: 10.1523/JNEUROSCI.4608-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Richa E, Papari M, Allen J, Martinez G, Wickrema A, Anastasi J, et al. Older age but not donor health impairs allogeneic granulocyte colony-stimulating factor (G-CSF) peripheral blood stem cell mobilization. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15(11):1394–1399. doi: 10.1016/j.bbmt.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 99.Wang TF, Wen SH, Chen RL, Lu CJ, Zheng YJ, Yang SH, et al. Factors associated with peripheral blood stem cell yield in volunteer donors mobilized with granulocyte colony-stimulating factors: the impact of donor characteristics and procedural settings. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14(11):1305–1311. doi: 10.1016/j.bbmt.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 100.Suzuya H, Watanabe T, Nakagawa R, Watanabe H, Okamoto Y, Onishi T, et al. Factors associated with granulocyte colony-stimulating factor-induced peripheral blood stem cell yield in healthy donors. Vox sanguinis. 2005;89(4):229–235. doi: 10.1111/j.1423-0410.2005.00701.x. [DOI] [PubMed] [Google Scholar]
- 101.Tecilazich F, Dinh T, Pradhan-Nabzdyk L, Leal E, Tellechea A, Kafanas A, et al. Role of endothelial progenitor cells and inflammatory cytokines in healing of diabetic foot ulcers. PloS one. 2013;8(12):e83314. doi: 10.1371/journal.pone.0083314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Koel G, Houghton PE. Electrostimulation: current status, strength of evidence Guidelines, and meta-analysis. Adv Wound Care (New Rochelle) 2014;3(2):118–126. doi: 10.1089/wound.2013.0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gyawali S, Solis L, Chong SL, Curtis C, Seres P, Kornelsen I, et al. Intermittent electrical stimulation redistributes pressure and promotes tissue oxygenation in loaded muscles of individuals with spinal cord injury. Journal of applied physiology. 2011;110(1):246–255. doi: 10.1152/japplphysiol.00661.2010. [DOI] [PubMed] [Google Scholar]
- 104.Zhao M, Bai H, Wang E, Forrester JV, McCaig CD. Electrical stimulation directly induces pre-angiogenic responses in vascular endothelial cells by signaling through VEGF receptors. Journal of cell science. 2004;117(Pt 3):397–405. doi: 10.1242/jcs.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Goldman R, Brewley B, Zhou L, Golden M. Electrotherapy reverses inframalleolar ischemia: a retrospective, observational study. Advances in skin & wound care. 2003;16(2):79–89. doi: 10.1097/00129334-200303000-00009. [DOI] [PubMed] [Google Scholar]
- 106.Junger M, Zuder D, Steins A, Hahn M, Klyscz T. Treatment of venous ulcers with low frequency pulsed current (Dermapulse): effects on cutaneous microcirculation. Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und verwandte Gebiete. 1997;48(12):897–903. doi: 10.1007/s001050050682. [DOI] [PubMed] [Google Scholar]
- 107.Herberger K, Franzke N, Blome C, Kirsten N, Augustin M. Efficacy, tolerability and patient benefit of ultrasound-assisted wound treatment versus surgical debridement: a randomized clinical study. Dermatology. 2011;222(3):244–249. doi: 10.1159/000326116. [DOI] [PubMed] [Google Scholar]
- 108.Qian Z, Sagers RD, Pitt WG. The effect of ultrasonic frequency upon enhanced killing of P. aeruginosa biofilms. Annals of biomedical engineering. 1997;25(1):69–76. doi: 10.1007/BF02738539. [DOI] [PubMed] [Google Scholar]
- 109.Breuing KH, Bayer L, Neuwalder J, Orgill DP. Early experience using low-frequency ultrasound in chronic wounds. Annals of plastic surgery. 2005;55(2):183–187. doi: 10.1097/01.sap.0000168695.20350.07. [DOI] [PubMed] [Google Scholar]
- 110.Carmen JC, Roeder BL, Nelson JL, Beckstead BL, Runyan CM, Schaalje GB, et al. Ultrasonically enhanced vancomycin activity against Staphylococcus epidermidis biofilms in vivo. Journal of biomaterials applications. 2004;18(4):237–245. doi: 10.1177/0885328204040540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Conner-Kerr T, Alston G, Stovall A, Vernon T, Winter D, Meixner J, et al. The effects of low-frequency ultrasound (35 kHz) on methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Ostomy/wound management. 2010;56(5):32–43. [PubMed] [Google Scholar]
- 112.Michailidis L, Williams CM, Bergin SM, Haines TP. Comparison of healing rate in diabetes-related foot ulcers with low frequency ultrasonic debridement versus non-surgical sharps debridement: a randomised trial protocol. Journal of foot and ankle research. 2014;7(1):1. doi: 10.1186/1757-1146-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sherman AR, Barkley M. Nutrition and wound healing. Journal of wound care. 2011;20(8):357–358. 60, 62–67. doi: 10.12968/jowc.2011.20.8.357. [DOI] [PubMed] [Google Scholar]
- 114.Williams JZ, Barbul A. Nutrition and wound healing. Critical care nursing clinics of North America. 2012;24(2):179–200. doi: 10.1016/j.ccell.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 115.Jensen GL, Compher C, Sullivan DH, Mullin GE. Recognizing malnutrition in adults: definitions and characteristics, screening, assessment, and team approach. JPEN Journal of parenteral and enteral nutrition. 2013;37(6):802–807. doi: 10.1177/0148607113492338. [DOI] [PubMed] [Google Scholar]
- 116.Legendre C, Debure C, Meaume S, Lok C, Golmard JL, Senet P. Impact of protein deficiency on venous ulcer healing. Journal of vascular surgery. 2008;48(3):688–693. doi: 10.1016/j.jvs.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 117.Castellanos VH, Litchford MD, Campbell WW. Modular protein supplements and their application to long-term care. Nutrition in clinical practice : official publication of the American Society for Parenteral and Enteral Nutrition. 2006;21(5):485–504. doi: 10.1177/0115426506021005485. [DOI] [PubMed] [Google Scholar]
- 118.Dorner B, Posthauer ME, Thomas D. National Pressure Ulcer Advisory P. The role of nutrition in pressure ulcer prevention and treatment: National Pressure Ulcer Advisory Panel white paper. Advances in skin & wound care. 2009;22(5):212–221. doi: 10.1097/01.ASW.0000350838.11854.0a. [DOI] [PubMed] [Google Scholar]
- 119.Langer G, Knerr A, Kuss O, Behrens J, Schlomer G. Nutritional interventions for preventing and treating pressure ulcers. Cochrane Database Syst Rev. 2009;3:CD003216. doi: 10.1002/14651858.CD003216. [DOI] [PubMed] [Google Scholar]
- 120.Lin JJ, Chung XJ, Yang CY, Lau HL. A meta-analysis of trials using the intention to treat principle for glutamine supplementation in critically ill patients with burn. Burns : journal of the International Society for Burn Injuries. 2013;39(4):565–570. doi: 10.1016/j.burns.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 121.Little MO. Nutrition and skin ulcers. Current opinion in clinical nutrition and metabolic care. 2013;16(1):39–49. doi: 10.1097/MCO.0b013e32835bc0a1. [DOI] [PubMed] [Google Scholar]
- 122.Desneves KJ, Todorovic BE, Cassar A, Crowe TC. Treatment with supplementary arginine, vitamin C and zinc in patients with pressure ulcers: a randomised controlled trial. Clinical nutrition. 2005;24(6):979–987. doi: 10.1016/j.clnu.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 123.Ohura T, Nakajo T, Okada S, Omura K, Adachi K. Evaluation of effects of nutrition intervention on healing of pressure ulcers and nutritional states (randomized controlled trial) Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2011;19(3):330–336. doi: 10.1111/j.1524-475X.2011.00691.x. [DOI] [PubMed] [Google Scholar]
- 124.Blass SC, Goost H, Tolba RH, Stoffel-Wagner B, Kabir K, Burger C, et al. Time to wound closure in trauma patients with disorders in wound healing is shortened by supplements containing antioxidant micronutrients and glutamine: a PRCT. Clinical nutrition. 2012;31(4):469–475. doi: 10.1016/j.clnu.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 125.Mathus-Vliegen EM. Old age, malnutrition, and pressure sores: an ill-fated alliance. The journals of gerontology Series A, Biological sciences and medical sciences. 2004;59(4):355–360. doi: 10.1093/gerona/59.4.m355. [DOI] [PubMed] [Google Scholar]
- 126.Eaglstein WH, Kirsner RS, Robson MC. Food and Drug Administration (FDA) drug approval end points for chronic cutaneous ulcer studies. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2012;20(6):793–796. doi: 10.1111/j.1524-475X.2012.00849.x. [DOI] [PubMed] [Google Scholar]
- 127.Armstrong DG, Wrobel J, Robbins JM. Guest Editorial: are diabetes-related wounds and amputations worse than cancer? International wound journal. 2007;4(4):286–287. doi: 10.1111/j.1742-481X.2007.00392.x. [DOI] [PubMed] [Google Scholar]
- 128.Eaglstein WH, Kirsner RS. Expectations for comparative effectiveness and efficacy research: with welcomed questions may come unwelcome answers. JAMA dermatology. 2013;149(1):18–19. doi: 10.1001/jamadermatol.2013.1324. [DOI] [PubMed] [Google Scholar]
- 129.Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment. A meta-analysis. Diabetes care. 1999;22(5):692–695. doi: 10.2337/diacare.22.5.692. [DOI] [PubMed] [Google Scholar]
- 130.Lantis JC, 2nd, Marston WA, Farber A, Kirsner RS, Zhang Y, Lee TD, et al. The influence of patient and wound variables on healing of venous leg ulcers in a randomized controlled trial of growth-arrested allogeneic keratinocytes and fibroblasts. Journal of vascular surgery. 2013;58(2):433–439. doi: 10.1016/j.jvs.2012.12.055. [DOI] [PubMed] [Google Scholar]
- 131.Kulkarni SR, Gohel MS, Wakely C, Minor J, Poskitt KR, Whyman MR. The Ulcerated Leg Severity Assessment score for prediction of venous leg ulcer healing. The British journal of surgery. 2007;94(2):189–193. doi: 10.1002/bjs.5597. [DOI] [PubMed] [Google Scholar]
- 132.Brem H, Tomic-Canic M, Entero H, Hanflik AM, Wang VM, Fallon JT, et al. The synergism of age and db/db genotype impairs wound healing. Experimental gerontology. 2007;42(6):523–531. doi: 10.1016/j.exger.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 133.Golinko MS, Joffe R, de Vinck D, Chandrasekaran E, Stojadinovic O, Barrientos S, et al. Surgical pathology to describe the clinical margin of debridement of chronic wounds using a wound electronic medical record. Journal of the American College of Surgeons. 2009;209(2):254–260. e1. doi: 10.1016/j.jamcollsurg.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 134.Brem H, Maggi J, Nierman D, Rolnitzky L, Bell D, Rennert R, et al. High cost of stage IV pressure ulcers. American journal of surgery. 2010;200(4):473–477. doi: 10.1016/j.amjsurg.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schiffman J, Golinko MS, Yan A, Flattau A, Tomic-Canic M, Brem H. Operative debridement of pressure ulcers. World journal of surgery. 2009;33(7):1396–1402. doi: 10.1007/s00268-009-0024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brem H, Tomic-Canic M, Tarnovskaya A, Ehrlich HP, Baskin-Bey E, Gill K, et al. Healing of elderly patients with diabetic foot ulcers, venous stasis ulcers, and pressure ulcers. Surgical technology international. 2003;11:161–167. [PubMed] [Google Scholar]
- 137.Rennert R, Golinko M, Kaplan D, Flattau A, Brem H. Standardization of wound photography using the Wound Electronic Medical Record. Advances in skin & wound care. 2009;22(1):32–38. doi: 10.1097/01.ASW.0000343718.30567.cb. [DOI] [PubMed] [Google Scholar]