Abstract
Objective
To assess the efficacy and short-term safety of levodopa as adjunctive treatment to patching for amblyopia.
Design
Randomized, placebo-controlled trial.
Participants
One hundred thirty-nine children 7 to 12 years of age with residual amblyopia resulting from strabismus, anisometropia or both combined (20/50 to 20/400) following patching.
Methods
Sixteen weeks of oral levodopa or placebo administered 3 times a day while patching the fellow eye 2 hours per day.
Main Outcome Measures
Mean change in best-corrected amblyopic-eye visual acuity at 18 weeks.
Results
At 18 weeks, amblyopic-eye visual acuity improved from randomization by an average of 5.2 letters in the levodopa group and 3.8 letters in the placebo group (difference adjusted for baseline visual acuity = +1.4 letters, 1-sided P=0.06; 2-sided 95% confidence interval = −0.4 to +3.3 letters). No serious adverse effects from levodopa were reported during treatment.
Conclusions
For children 7 to 12 years of age with residual amblyopia following patching therapy, oral levodopa while continuing to patch 2 hours per day does not produce a clinically or statistically meaningful improvement in visual acuity compared with placebo and patching.
Many children treated with patching for amblyopia have an incomplete response and are left with some reduction in visual acuity (VA) in the amblyopic eye.1–3 Recognizing the incomplete effectiveness of conventional amblyopia therapy, clinicians have sought alternatives. One such ancillary treatment is oral levodopa, which is used to supplement dopamine deficiency in brains of adults with Parkinson’s disease and children with dopamine-responsive dystonia. While there is no evidence of a deficiency of dopamine in amblyopic brains, levodopa has been used by some clinicians for amblyopia treatment since 1995 on an investigational basis.4, 5 Levodopa is converted to dopamine, which appears to play an important role in retinal function and in central visual processing.6 Improvements in VA and/or visual evoked potential amplitudes have been reported immediately following a single dose,7 a 1-week course,8 or a 7-week course4, 9, 10 of levodopa, but much of the improvement regressed after discontinuation of the drug. Studies investigating the use of levodopa as amblyopia treatment have also shown improvement in VA.4,5,7,8, 10–16 However, some participants experience partial regression after stopping the medication.4,7,8,10–12,14
A meta-analysis of 4 randomized placebo-controlled studies (110 subjects) found levodopa treatment effective with a mean improvement of 1.1 logMAR lines (95% confidence interval (CI): 0.2 to 1.9).17 The studies included in the meta-analysis had treatment durations ranging from 5 hours to 3 months (most subjects treated for less than 4 weeks), were small in size, and included participants 3 to 18 years of age undergoing initial treatment. Thus, as a result of these limitations, prior studies are inconclusive regarding the benefit of levodopa. Therefore, we conducted a randomized, placebo-controlled trial in children 7 to 12 years of age with residual amblyopia (20/50 to 20/400) following patching treatment to assess efficacy and short-term safety of levodopa as adjunctive treatment to patching.
Methods
The study was supported through a cooperative agreement with the National Eye Institute of the National Institutes of Health, Department of Health and Human Services and was conducted according to the tenets of the Declaration of Helsinki, by the Pediatric Eye Disease Investigator Group (PEDIG). The protocol and Health Insurance Portability and Accountability Act (HIPAA)-compliant informed consent forms were approved by institutional review boards, and a parent or guardian (referred to subsequently as “parent”) of each study patient gave written informed consent. Patient assent was obtained as required by institutional review boards. Study oversight was provided by an independent data and safety monitoring committee. The study is listed on www.clinicaltrials.gov, under identifier NCT 01190813, accessed 7/18/14. The complete study protocol is available on the PEDIG website (www.pedig.net, accessed 7/18/14).
Eligibility Criteria
Major eligibility criteria included 7 to 12 years of age, treatment of amblyopia with patching at least 2 hours per day for at least 12 weeks during the immediate pre-enrollment period, and no improvement of VA within 6 weeks immediately prior to enrollment. Eligible participants had amblyopic-eye VA of 20/50 to 20/400, fellow-eye VA of 20/25 or better, and the presence of strabismus and/or anisometropia meeting study-specified criteria (additional eligibility criteria are listed in Table 1, available at www.aaojournal.org). Children must not have been previously treated with levodopa.
Treatment
Participants were randomly assigned (using a permutated block design stratified by site and by baseline visual acuity) in a 2:1 ratio to three times per day use of oral levodopa 0.76 mg/kg with carbidopa 0.17 mg/kg (subsequently referred to as ‘Levodopa’) or oral placebo. Carbidopa is added to levodopa to reduce peripheral side effects. All participants had two hours of daily patching prescribed and took oral medication for 16 weeks, with a 4-day taper of the oral medication prior to the primary outcome exam two weeks later (at 18 weeks). A central pharmacy compounded the study medication based upon body weight. Levodopa and placebo were placed in identical gelatin capsules.
Follow-up
Prior to the 18-week primary outcome visit, office visits occurred at 4, 10, and 16 weeks (±1 week) after randomization and phone calls to the parent at 2, 7, and 13 weeks (±1 week) to review treatment and dosing. Following the primary outcome visit, follow up continued through 26 weeks with participants and investigators remaining masked to treatment group. If the amblyopic-eye VA had improved ≥5 letters between baseline and the 16-week visit, patching was continued and the randomized oral study medication was resumed until the 26-week visit. If improvement was <5 letters, confirmed by a retest, study medication and patching were stopped and additional treatment was at investigator discretion.
Testing Procedures and Data Collection
VA was measured in each eye (right eye first) by a study-certified VA tester using the Electronic Early Treatment of Diabetic Retinopathy Study (E-ETDRS©) visual acuity protocol.18 Ocular alignment was measured with the simultaneous prism and cover test. Stereoacuity was measured with the Randot Preschool Stereotest (Stereo Optical Co., Inc., Chicago, IL).
At each visit, the occurrence of adverse events was solicited and a symptom survey (17 items with a 5-level Likert scale for which an average score was calculated) was completed by the participant and by the parent. Neurological examinations were not performed. Treatment compliance was assessed by review of a calendar log maintained by the participant and parent documenting the amount of patching and consumption of study medication each day and by counting the remaining capsules.
Statistical Analysis
The primary outcome measure was change in amblyopic-eye VA from baseline to 18 weeks. The sample size was chosen to provide sufficient power for two secondary outcomes: the proportion with ≥10 letters improvement from baseline to 18 weeks and the proportion with ≥20/25 amblyopic-eye VA at 18 weeks. A sample size of 129 participants provided 80% power with 1-sided type I error rate of 5% to reject the hypothesis of no difference between groups if the proportion improved was 30% in the levodopa group compared with 10% in the placebo group. With 129 participants, assuming a 1-sided type I error rate of 4.85%, there was 96% power to detect a difference in mean visual acuity between treatment groups at 18 weeks adjusted for baseline and for one interim analysis for futility if the true difference was 5 letters with standard deviation of 7 letters and 82% power if the true difference was 3.75 letters. The planned sample size was increased to 138 to account for an expected 5% loss to follow up. The alpha level was set to 0.0485 for the primary analysis to adjust for alpha spending of 0.015 for one interim analysis for efficacy conducted when outcome data were available for 50% of participants.
The primary analysis was a treatment group comparison of mean VA letter scores obtained at the 18-week primary outcome exam adjusted for baseline acuity in an analysis of covariance (ANCOVA) model. A 1-sided p-value was computed from this model to test the primary hypothesis, and the 2-sided 95% CI was computed to obtain the magnitude of the treatment effect that is consistent with the data.
The primary analysis followed the “intent-to-treat” principle. For participants who did not have a visit in the ±1 week window for the primary outcome visit, data from a visit between 14 and 27 weeks after randomization were used, if available. Multiple imputation by the Monte Carlo Markov Chain method19 was used for missing 18-week VA outcomes based upon treatment group, baseline VA, and VA scores from completed follow-up visits. Alternative analyses including data only from participants who completed the 18-week exam with no imputation and adjustment for baseline covariates that were imbalanced between treatment groups (cause of amblyopia and anisometropia) yielded results similar to the primary analysis (data not shown). The primary efficacy analyses were repeated for other time points (4, 10, 16, and 26 weeks).
The treatment effect in subgroups according to baseline factors of sex, race, age, and amblyopic-eye VA at randomization was assessed by including interaction terms in the ANCOVA models. Fisher’s exact tests were used to evaluate if there were treatment group differences with respect to preplanned secondary outcomes (the proportion with ≥10 letters improvement from baseline to 18 weeks and the proportion with ≥20/25 amblyopic-eye VA at 18 weeks). The 1-sided p-values and 2-sided 95% exact CIs were computed to test the secondary hypotheses and to obtain the range of differences in proportions that were consistent with the data. It was not possible to adjust for baseline VA in these secondary analyses due to the small number of subjects meeting secondary outcome criteria.
Fisher’s exact test was used to evaluate whether there was a treatment group difference in the proportion of subjects reporting at least one adverse event. Additional treatment group comparisons included (1) change in fellow-eye VA from randomization to the 18-week visit using an ANCOVA model, adjusting for the fellow-eye VA at randomization, (2) stereoacuity at the 18-week visit using the Wilcoxon-Rank-Sum test, and (3) symptom survey scores with t-tests.
Analyses were conducted using SAS version 9.3 (SAS Institute Inc., Cary, NC).
Results
Between September 2010 and October 2013, 139 participants from 27 sites were randomly assigned to levodopa (n=90) or placebo (n=49). The average age of participants was 9.5 years; the average amblyopic-eye VA letter score was 52 (20/100+2), and the average IOD was 35 letters. Table 2 lists the baseline characteristics by treatment group.
Table 2.
Baseline Demographic and Clinical Characteristics by Treatment Group
| Levodopa N=90 |
Placebo N=49 |
|||
|---|---|---|---|---|
| N | % | N | % | |
| Sex: Female | 40 | 44% | 24 | 49% |
|
| ||||
| Race/Ethnicity | ||||
| Asian | 2 | 2% | 1 | 2% |
| Black/African American | 1 | 1% | 1 | 2% |
| Hispanic or Latino | 5 | 6% | 2 | 4% |
| White | 77 | 86% | 44 | 90% |
| Unknown/not reported | 5 | 6% | 1 | 2% |
|
| ||||
| Age at Randomization (Years) | ||||
| 7 to <9 | 39 | 43% | 21 | 43% |
| 9 to <11 | 33 | 37% | 14 | 29% |
| 11 to <13 | 18 | 20% | 14 | 29% |
| Mean (SD) Years | 9.4 (1.8) | 9.5 (1.7) | ||
|
| ||||
| Cause of Amblyopia | ||||
| Strabismus | 22 | 24% | 5 | 10% |
| Anisometropia | 20 | 22% | 16 | 33% |
| Combined Mechanism | 48 | 53% | 28 | 57% |
|
| ||||
| Patching Duration at Randomization | ||||
| 2 hours per day | 81 | 90% | 43 | 88% |
| ≥ 3 hours per day | 9 | 10% | 6 | 12% |
|
| ||||
| Visual Acuity in the Amblyopic Eye at Randomization | ||||
| 20/200 or worse (≤37 letters) | 7 | 8% | 9 | 18% |
| 20/100 to <20/200 (38–52 letters) | 34 | 38% | 13 | 27% |
| 20/80 (53–57 letters) | 15 | 17% | 8 | 16% |
| 20/63 (58–62 letters) | 20 | 22% | 6 | 12% |
| 20/50 (63–67 letters) | 14 | 16% | 13 | 27% |
| Mean (SD) letters | 52.8 (9.8) ~20/80−2 |
51.7 (11.9) ~20/100+2 |
||
|
| ||||
| Visual Acuity in the Fellow Eye at Randomization | ||||
| 20/25 (78–82 letters) | 15 | 17% | 4 | 8% |
| 20/20 (83–87 letters) | 35 | 39% | 20 | 41% |
| 20/16 (88–92 letters) | 34 | 38% | 23 | 47% |
| 20/12 (93–97 letters) | 6 | 7% | 2 | 4% |
| Mean (SD) letters | 86.7 (4.1) ~20/20+2 |
87.3 (3.5) ~20/20+2 |
||
|
| ||||
| Intraocular Visual Acuity Difference at Randomization | ||||
| Mean (SD) letters | 33.9 (10.9) ~7 lines |
35.6 (13.1) ~7 lines |
||
|
| ||||
| SE Refractive Error in Ambylopic Eye at Randomization | ||||
| Mean (SD) Diopters | +4.0 (2.6) | +4.5 (2.3) | ||
|
| ||||
| SE Refractive Error in Fellow Eye at Randomization | ||||
| Mean (SD) Diopters | +1.9 (2.0) | +1.6 (1.7) | ||
|
| ||||
| Anisometropia at Randomization | ||||
| Mean (SD) Diopters | +2.5 (2.1) | +3.1 (2.0) | ||
SD = standard deviation; SE = spherical equivalent
Visit Completion and Treatment
The 18-week primary outcome was completed by 87 participants (97%) in the levodopa group and by 45 participants (92%) in the placebo group (Figure 1, available at www.aaojournal.org). Telephone call and other visit completion rates were similar between treatment groups (Figure 1, available at www.aaojournal.org). During the 18 weeks after randomization, compliance with patching was judged by the investigator to be at least 2 hours daily for 7 days a week among 74 participants (85%) in the levodopa group and 39 participants (87%) in the placebo group. Average compliance with study medication through 16 weeks was estimated to be greater than 90% of doses in 76 participants (87%) in the levodopa group and 43 participants (93%) in the placebo group.
Efficacy Analyses
At the 18-week primary outcome visit, amblyopic-eye VA improved from randomization by an average of 5.2 letters in the levodopa group and 3.8 letters in the placebo group (difference adjusted for baseline VA = +1.4 letters, 1-sided P=0.06; 2-sided 95% CI=(−0.4 to +3.3); Table 3). The amblyopic-eye VA had changed from the 16-week visit by an average of +0.2 letter in the levodopa group and −0.3 letter in the placebo group, 2-sided P=0.52.
Table 3.
Amblyopic-Eye Visual Acuity by Treatment Group
| Amblyopic Eye | 4-week Visit | 10-week Visit | 16-week Visit | 18-week Primary Outcome Visit |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Levodopa N=88 |
Placebo N=47 |
Levodopa N=88 |
Placebo N=48 |
Levodopa N=87 |
Placebo N=46 |
Levodopa N=86* |
Placebo N=45 |
|||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| Change from Baseline | ||||||||||||||||
| 10–14 letters worse | 0 | 0 | 0 | 0 | 1 | 1% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5–9 letters worse | 6 | 7% | 2 | 4% | 4 | 5% | 1 | 2% | 1 | 1% | 0 | 0 | 2 | 2% | 1 | 2% |
| within 4 letters (±4) | 55 | 63% | 28 | 60% | 43 | 49% | 30 | 63% | 41 | 47% | 26 | 57% | 35 | 41% | 23 | 51% |
| 5–9 letters better | 23 | 26% | 15 | 32% | 30 | 34% | 8 | 17% | 30 | 34% | 10 | 22% | 36 | 42% | 19 | 42% |
| 10–14 letters better | 4 | 5% | 1 | 2% | 7 | 8% | 9 | 19% | 9 | 10% | 10 | 22% | 10 | 12% | 1 | 2% |
| ≥15 letters better | 0 | 0 | 1 | 2% | 3 | 3% | 0 | 0 | 6 | 7% | 0 | 0 | 3 | 3% | 1 | 2% |
| Mean (SD) letter change | 2.2 (4.1) | 2.5 (4.7) | 3.8 (4.9) | 3.7 (4.9) | 5.1 (6.3) | 4.2 (4.9) | 5.2 (5.3) | 3.8 (5.0) | ||||||||
|
| ||||||||||||||||
| Difference (Levodopa – Placebo) between treatment groups† | −0.3 letter | +0.1 letter | +0.9 letter | +1.4 letters | ||||||||||||
|
| ||||||||||||||||
| 1-sided P-value | 0.65 | 0.44 | 0.20 | 0.06 | ||||||||||||
|
| ||||||||||||||||
| 2-sided 95% CI for difference (letters) | (−1.9 to +1.3) | (−1.6 to +1.8) | (−1.2 to +2.9) | (−0.4 to +3.3) | ||||||||||||
|
| ||||||||||||||||
| Proportion 10 or more letters improved | 4 (5%) | 2(4%) | 10 (11%) | 9 (19%) | 15 (17%) | 10 (22%) | 13 (15%) | 2 (4%) | ||||||||
|
| ||||||||||||||||
| Treatment group difference (Levodopa – Placebo) | 0% | −7% | −5% | +11% | ||||||||||||
|
| ||||||||||||||||
| 2-sided 95% CI for difference | (−17% to +18%) | (−25% to +10%) | (−22% to +13%) | (−7% to + 28%) | ||||||||||||
SD = standard deviation; CI = confidence interval
Note: one participant completing the 18-week visit 288 days (41 weeks) post randomization is not included in the analysis as pre-specified in the analysis plan.
Positive values favor the levodopa group and negative values favor the placebo group. The treatment comparison was performed using analysis of covariance adjusting for amblyopic-eye visual acuity at randomization with multiple imputation for missing visual acuity.
At 18 weeks, no participant in either group had VA ≥20/25 (≥78 letters) in the amblyopic eye. Amblyopic-eye VA improved from baseline by ≥10 letters in 13 (15%) of 86 participants in the levodopa group and 2 (4%) of 45 participants in the placebo group (difference = +11%; 1-sided P=0.06; 2-sided 95% CI= (−7% to +28%); Table 3). However, at 16 weeks, just prior to discontinuation of study medication, the percentages were 17% versus 22%, respectively (1-sided P=0.81). In the 2-week interval, 7 participants (8%) in the levodopa group and 8 participants (18%) in the placebo group changed from success to failure for this outcome.
Adjustment for treatment group imbalances in amblyopia cause (anisometropic, strabismic, or combined) or magnitude of anisometropia (continuous) yielded a treatment effect similar to that found in the primary outcome analysis (mean difference = +1.7 letters, 1-sided P=0.03). There was no indication of a differential treatment effect in subgroups according to sex, race/ethnicity, cause of amblyopia, age at randomization, amblyopic-eye VA at randomization, or magnitude of anisometropia (Table 4, available at www.aaojournal.org).
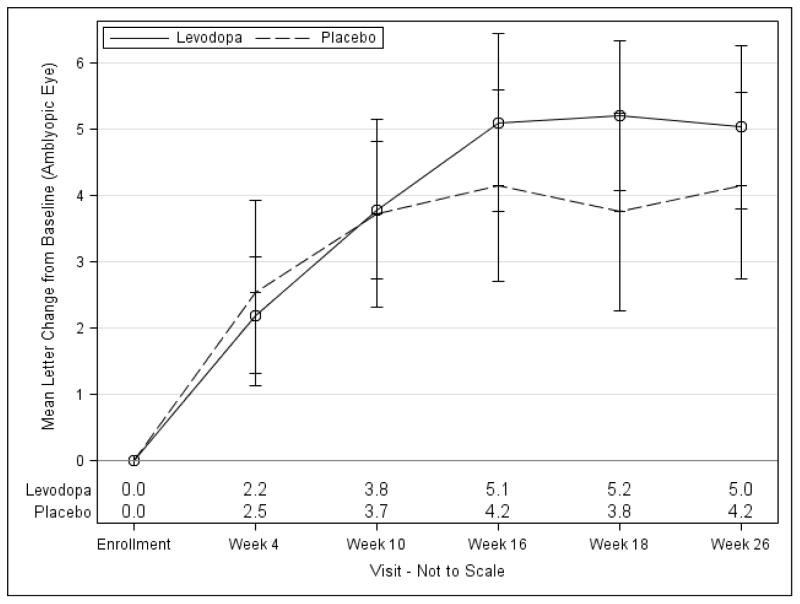
There were no differences in mean VA, adjusting for baseline VA, between levodopa and placebo groups at the 4-, 10-, and 16-week interim visits (Figure 2, Table 5, available at www.aaojournal.org).
Figure 2. Change from Baseline in Amblyopic-Eye Visual Acuity by Treatment Group*.
*The point estimates and standard errors for the mean change from baseline are shown.
Not all participants continued randomized treatment after the 18-week visit.
Stereoacuity scores were similar between treatment groups at the 18-week exam for the overall cohort (2-sided P=0.82) and when limited to participants with no history of strabismus at enrollment (2-sided P=0.43) (data not shown).
Fifty-four participants (62%) in the levodopa group and 25 participants (54%) in the placebo group improved ≥5 letters from baseline to 16 weeks (2-sided P=0.46), meeting the study criterion to continue on patching and randomized study medication. Five participants in the levodopa group and no participant in the placebo group failed to restart oral study medication, but continued patching. Among the remaining participants who did not show an improvement at the 16-week visit (33 in the levodopa group and 21 in the placebo group), study medication was not restarted; 58% and 67%, respectively, continued 2 hours of daily patching. At 26 weeks, mean treatment group difference in VA, adjusting for baseline VA, was +0.9 letter favoring the levodopa group (1-sided P=0.17; Table 6).
Table 6.
Amblyopic-Eye Visual Acuity at 26 Weeks by Treatment Group*
| Amblyopic Eye | Levodopa N=86 |
Placebo N=45 |
||
|---|---|---|---|---|
| N | % | N | % | |
| Visual Acuity | ||||
| 20/32 (73–77 letters) | 2 | 2% | 1 | 2% |
| 20/40 (68–72 letters) | 15 | 17% | 5 | 11% |
| 20/50 (63–67 letters) | 14 | 16% | 8 | 18% |
| 20/63 (58–62 letters) | 16 | 19% | 9 | 20% |
| 20/80 (53–57 letters) | 19 | 22% | 6 | 13% |
| 20/100 (48–52 letters) | 11 | 13% | 8 | 18% |
| 20/125 (43–47 letters) | 4 | 5% | 1 | 2% |
| 20/160 or worse (≤42 letters) | 5 | 6% | 7 | 16% |
| Mean (SD) letter score | 58.5 (9.3) ~20/63−1 |
55.2 (11.5) ~20/80 |
||
|
| ||||
| Change from Baseline | ||||
| 5–9 letters worse | 3 | 3% | 1 | 2% |
| within 4 letters (±4) | 36 | 42% | 23 | 51% |
| 5–9 letters better | 30 | 35% | 16 | 36% |
| 10–14 letters better | 14 | 16% | 5 | 11% |
| ≥15 letters better | 3 | 3% | 0 | 0 |
| Mean (SD) letter change | 5.0 (5.7) | 4.2 (4.7) | ||
|
| ||||
| Difference between treatment groups† | +0.9 letter | |||
|
| ||||
| 1-sided P-value | 0.17 | |||
|
| ||||
| Proportion 10 or more letters improved | 17 (20%) | 5 (11%) | ||
|
| ||||
| Treatment group difference (Levodopa – Placebo) | +9% | |||
SD = standard deviation; CI = confidence interval
All participants continued in the study regardless of whether they continued with study medication after the 18-week visit; 49 (56%) participants in the levodopa group and 24 (53%) participants in the placebo group continued study medication at the 18-week visit.
Positive values favor the levodopa group and negative values favor the placebo group. The treatment comparison was performed using analysis of covariance adjusting for amblyopic-eye visual acuity at randomization, with multiple imputation for missing 26-week visual acuity scores.
Adverse Effects
At 18 weeks, there was no significant treatment group difference in fellow-eye VA change from baseline (mean difference adjusted for baseline VA = +0.8 letter, 2-sided 95% CI= (−0.3 to +1.8), 2-sided P = 0.14) (Table 7, available at www.aaojournal.org), or the proportion of participants who developed new-onset strabismus (data not shown).
The mean number of systemic adverse events reported per participant was 1.3 in the levodopa group and 1.6 in the placebo group through 26 weeks (Table 8 and Table 9, available at www.aaojournal.org). Headache was reported at any time during the trial by 18 participants (20%) in the levodopa group and 4 participants (8%) in the placebo group (2-sided P=0.09). Nausea was reported by 6 (7%) in the levodopa group and 6 (12%) in the placebo group (2-sided P=0.34). Confusion was reported by 3 (3%) in the levodopa group and 0 in the placebo group (2-sided P=0.55). Symptom survey scores were similar between groups (Tables 10 and 11, available at www.aaojournal.org).
Table 8.
Summary of Adverse Events Reported in Randomized Trial by Treatment Group
| System Events | Ocular Events | |||
|---|---|---|---|---|
| Levodopa N=90 |
Placebo N=49 |
Levodopa N=90 |
Placebo N=49 |
|
| # of adverse events reported | 115 events | 76 events | 8 events | 12 events |
| # of participants (at least 1 event) | 53 participants | 26 participants | 3 participants | 2 participants |
| # of events/randomized participant | 1.3 | 1.6 | 0.1 | 0.2 |
|
| ||||
| # of visits | 783 visits | 417 visits | 783 visits | 417 visits |
| # of events/visit | 0.15 | 0.18 | 0.01 | 0.03 |
|
| ||||
| # of serious events | 0 events | 0 events | 0 events | 0 events |
|
| ||||
| Effect on study treatment | ||||
| No change | 108 events | 73 events | 8 events | 10 events |
| Stopped temporarily | 5 eventsb | 3 eventsc | 0 events | 2 eventsd |
| Stopped permanently | 2 eventsa | 0 events | 0 events | 0 events |
|
| ||||
| Related to study medication | ||||
| Yes | 42 events | 20 events | 0 events | 10 events |
| No | 73 events | 56 events | 8 events | 2 events |
Levodopa - Did not resume study medication after 18-week outcome visit due to fatigue (N=1). Father called in 5 weeks after 18-week outcome exam saying child has been acting funny for a couple of days and could not concentrate (N=1). Parents stopped medication before the 26-week visit.
Levodopa - Study medication was stopped temporarily due to stomach virus and vomiting for 24 hours (N=1). Study medication was stopped temporarily due to oral surgery for impacted tooth (N=1). Study medication was stopped temporarily due to vomiting, stomach pain, and fever (N=1). Study medication was stopped temporarily due to surgery on face/head to repair lacerations from hitting head when jumping off a dock (N=1). Study medication was stopped temporarily due to stomach virus (N=1).
Placebo - Study medication stopped temporarily after mother called to report frequent generalized stomach pains for 4 days, and vomiting after breakfast (N=1). Study medication stopped temporarily due to nausea and vomiting (N=1). Study medication was stopped temporarily due to “flu-like” symptoms (N=1).
Placebo - Study medication was stopped after 10 weeks when father reported participant blinking uncontrollably (one event for each eye) (N=1).
Discussion
In a randomized placebo-controlled trial, we found no meaningful benefit from adding oral levodopa to patching for the treatment of residual amblyopia in children 7 to 12 years of age. It is unlikely that a meaningful effect was missed, since the upper limit of the 2-sided 95% confidence interval for the mean difference favoring levodopa treatment was only 3.3 letters. Although a pre-specified secondary outcome found that 15% in the levodopa group and 4% in the placebo group improved ≥10 letters from baseline to 18 weeks, this difference was not observed at the 16-week visit, immediately prior to discontinuation of study medication. We believe the difference seen at 18 weeks is due to chance for several reasons. First, there is no reason to expect the amblyopic-eye VA of placebo-treated participants to deteriorate in two weeks. Second, the mean treatment group differences in VA change from baseline at 16 weeks and 18 weeks were essentially the same. Third, misclassification bias can occur when a binary outcome variable is created from a continuous variable as was done in this analysis.
About 1 in 6 children in the placebo group improved ≥10 letters during the study with continued two hours of daily patching in spite of the clinical appearance of stability at randomization. Placebo group participants and parents may have been more compliant with patching than previously since they were being monitored in a research study. Nonetheless, the observation that some participants continued to improve with patching even after apparent visual acuity stability was achieved should be considered by clinicians and parents when deciding to stop patching.20, 21
Our finding of a lack of efficacy for levodopa must be viewed in the context of the study eligibility criteria: children 7 to 12 years of age with residual amblyopia from anisometropia, strabismus, or both, after a period of treatment with patching. The strengths of our study design included randomization, a placebo control group, and masked participants, investigators, and VA testers. We had adequate sample size and power to detect a difference between groups if the true mean difference was as small as 3.75 letters, and the upper limit of the 2-sided 95% confidence interval on the observed difference was 3.3 letters, so it is unlikely the study missed detecting a clinically meaningful effect. We could identify no sources of bias or confounding that would affect our results. The ineffectiveness of levodopa therapy in our study did not appear to be from poor drug compliance or dosage. We found adherence to the protocol to be high with both patching and oral medication. In addition, we used a dosage of levodopa 0.76 mg/kg with carbidopa 0.17 mg/kg three times per day. We tested this higher dosage in a pilot study12 during which it was well tolerated and associated with VA improvement. This dosage is greater than that commonly used by many investigators (about 0.51 mg/kg/tid).11, 14, 22
Two randomized placebo-controlled studies of levodopa with patching for residual amblyopia have been conducted.5, 23 Both studies found more improvement in the levodopa group compared with the placebo group, although the studies were of one week or shorter duration and thus not completely comparable to our study of a 4-month prescribed course of drug treatment.
Regression of the benefit from levodopa has been reported to occur after cessation of levodopa in some non-controlled studies10, 14, 22 A reduction in VA was not observed in the levodopa group of our study after stopping oral medication between 16 and 18 weeks, because no treatment benefit was detected.
Levodopa was associated with no serious adverse effects reported by the participant or their parent. In particular, no dyskinesias were reported in the levodopa group. Symptom survey scores were similar between groups. Headaches and nausea have been reported as side effects of levodopa in prior amblyopia studies.4, 12 Although the study was not powered to evaluate a treatment group difference with respect to adverse events, headaches were more frequently reported in the levodopa group. The treatment group difference was not significant. The frequency of nausea was similar in the two groups.
In summary, for children 7 to 12 years of age with residual amblyopia following patching therapy, the prescription of oral levodopa while continuing to patch 2 hours per day does not produce a clinically or statistically meaningful improvement in visual acuity compared with placebo and patching.
Supplementary Material
Eligibility Criteria
Change in Amblyopic-Eye Visual Acuity at 18 Weeks According to Baseline Patient Characteristics
Amblyopic-Eye Visual Acuity by Treatment Group
Fellow-Eye Visual Acuity by Treatment Group
Adverse Events Reported in Randomized Trial by Treatment Group
Child Symptom Survey Results by Treatment Group
Cells reflect mean response score unless otherwise specified. Note: A higher number reflects a more negative response (5=Always, 4=Often, 3=Sometimes, 2=Rarely, 1=Never)
Parent Symptom Survey Results by Treatment Group
Cells reflect mean response score unless otherwise specified. Note: A higher number reflects a more negative response (5=Always, 4=Often, 3=Sometimes, 2=Rarely, 1=Never)
Flow of Participants through Study
*Of the 4 participants who dropped in the levodopa group, 1 was lost to follow up, 2 elected to withdraw, and 1 refused study treatment after randomization. Of the 4 participants who dropped in the placebo group, 2 were lost to follow up, 1 elected to withdraw, and 1 withdrew after experiencing blepharospasm which completely resolved
Acknowledgments
Financial Support: Supported through cooperative agreements from the National Eye Institute EY011751 and EY018810. The funding organization had no role in the design or conduct of this research.
Appendix
Clinical Sites
Sites are listed in order by number of subjects enrolled into the run-in phase and number randomized (number enrolled). Personnel are listed as (I) for Investigator, (C) for Coordinator, or (E) for Visual Acuity Examiner.
*Center received support utilized for this project from an unrestricted grant from Research to Prevent Blindness Inc., New York, New York.
Columbus, OH - Pediatric Ophthalmology Associates, Inc. (19)
Don L. Bremer, (I); Richard P. Golden, (I); Mary Lou McGregor, (I); David L. Rogers, (I); Gary L. Rogers, (I); Rae R. Fellows, (C); Michelle L. Hurst, (C); Meghan C. McMillin, (C); Amy J. Wagner, (C); Rich E. Cox, (E); Rebecca A. Murray, (E); Laura J. Shenberger, (E); Lisa J. Vanover, (E); Angela R. Young, (E)
Rockville, MD - Stephen R. Glaser, M.D., P.C. (12)
Stephen R. Glaser, (I); Monica M. Pacheco, (I); Laura L. Graham, (C); Noga Senderowitsch, (C); Aliza C. Shabanowitz, (C); Toba X. Sragg, (C)
The Woodlands, TX - Houston Eye Associates (12)
Aaron M. Miller, (I); Jorie L. Jackson, (C); Suzanne S. LaRiviere, (C); Maria N. Olvera, (E); Cynthia R. Ramos, (E)
West Des Moines, IA - Wolfe Clinic (10)
Donny W. Suh, (I); Jody L. Jackson, (C); Jill J. Frohwein, (C); Autumn Parrino, (C); Rhonda J. Countryman, (E); Lisa M. Fergus, (E); Susan K. Hayes, (E)
Concord, NH - Concord Eye Care P.C. (7)
Christie L. Morse, (I); Maynard B. Wheeler, (I); Melanie L. Christian, (C); Caroline C. Fang, (C); Alannah O. Price, (C)
Dallas, TX - Pediatric Ophthalmology (7)
Cynthia L. Beauchamp, (I); Angela X. Goodloe, (C)
Oklahoma City, OK - Dean A. McGee Eye Institute, University of Oklahoma (7)*
R. Michael Siatkowski, (I); Tammy Yanovitch, (I); Heather R. Miller, (C); Connie J. Dwiggins, (C); Reshial D. Ellis, (C); Sonny W. Icks, (C); Brittany L. Ross, (C); Kammerin T. White, (C); Vanessa A. Bergman, (E)
Wilmette, IL - Pediatric Eye Associates (7)
Lisa C. Verderber, (I); Deborah R. Fishman, (I); Roberta A. Forde, (C); JoAnn Spieker, (C); Sarah Ahn, (E); Paulina Rusin, (E)
Boise, ID - St Luke’s Hospital (6)
Katherine A. Lee, (I); Bonita R. Schweinler, (C); Derek Beck, (E)
Nashville, TN - Vanderbilt Eye Center (6)*
Sean P. Donahue, (I); Lisa A. Fraine, (C)
Milford, CT - Eye Physicians & Surgeons, PC (5)
Darron A. Bacal, (I); Donna Martin, (C); Kelly D. Ryan, (C)
Saint Paul, MN - Associated Eye Care (5)
Susan Schloff, (I); Rebecca A. Wolf, (C)
Erie, PA - Pediatric Ophthalmology of Erie (4)
Nicholas A. Sala, (I); Veda L. Zeto, (C); Rhonda M. Hodde, (C); Jeanine M. Romeo, (E)
Indianapolis, IN - Riley Hospital for Children (4)
Daniel E. Neely, (I); Kathryn M. Haider, (I); Michele E. Whitaker, (C); Jingyun Wang, (C)
Los Angeles, CA - Jules Stein Eye Institute at the University of California, Los Angeles (4)
Stacy L. Pineles, (I); Marianne J. Esguerra, (C); Zachary T. Fenoglio, (C); Elaine X. Ngo, (C)
Waterbury, CT - Eye Care Group, PC (4)
Andrew J. Levada, (I); Tara H. Cronin, (I); Cheryl Capobianco, (C); Nathalie M. Gintowt, (C); Susan H. Heaton, (C)
Lincoln, NE - Eye Surgical Associates (3)
Donald P. Sauberan, (I); Jody C. Hemberger, (C); Suzanne M. Abele, (C); Jenni A. Craft, (E); Dana Meves, (E); Gail Walker, (E)
Sharon, MA - Daniel M. Laby, M.D. (3)
Daniel M. Laby, (I); Heidi E. Martin, (C); Jenelle L. Mallios, (E)
Cranberry TWP, PA - Everett and Hurite Ophthalmic Association (2)
Darren L. Hoover, (I); Pamela A. Huston, (C)
Durham, NC - Duke University Eye Center (2)
Laura B. Enyedi, (I); David K. Wallace, (I); Sarah K. Jones, (C); Namita Kashyap, (E); Amanda P. Mestler, (E)
Houston, TX - Texas Children’s Hospital, Department of Ophthalmology (2)
Evelyn A. Paysse, (I); Lingkun X. Kong, (C)
Minneapolis, MN - University of Minnesota (2)*
C. Gail Summers, (I); Jill S. Anderson, (I); Erick D. Bothun, (I); Inge De Becker, (I); Ann M. Holleschau, (C); Anna I. de Melo, (E); Kathy M. Hogue, (E); Kim S. Merrill, (E)
Rochester, MN - Mayo Clinic (2)*
Jonathan M. Holmes, (I); Brian G. Mohney, (I); Rebecca A. Nielsen, (C); Chessie L. Huiting, (E); Debbie M. Priebe, (E); Emily J. Treichel, (E)
Albuquerque, NM – Family & Children’s Eye Center of New Mexico (1)
Todd A. Goldblum, (I); Angela Alfaro, (C)
Denver, CO - Denver Health Hospital and Authority Eye Clinic (1)
Miguel Paciuc-Beja, (I); Leif S. Ryman, (C); Jean Farnlof, (E)
Lancaster, PA - Family Eye Group (1)
David I. Silbert, (I); Noelle S. Matta, (C)
Lisle, IL - Progressive Eye Care (1)
Patricia L. Davis, (I); Indre M. Rudaitis, (C)
PEDIG Coordinating Center
Raymond T. Kraker, Roy W. Beck, Nicole M. Boyle, Christina M. Cagnina-Morales, Courtney L. Conner, Danielle L. Chandler, Trevano W. Dean, Quayleen Donahue, Brooke P. Fimbel, James E. Hoepner, Joseph D. Kaplon, Elizabeth L. Lazar, B. Michele Melia, Rachel V. Orlowski, Diana E. Rojas, Jennifer A. Shah
National Eye Institute – Bethesda, MD
Donald F. Everett
Amblyopia Treatment Study Steering Committee
Eileen E. Birch, Susan A. Cotter, Donald F. Everett, Nicole C. Foster (2012–13), Richard P. Golden (2012–present), Jonathan M. Holmes, Sarah K. Jones (2013–present), Raymond T. Kraker, Marjean T. Kulp, Elizabeth L. Lazar, B. Michele Melia, David B. Petersen (2011–13), Stacy Pineles (2013–present), Michael X. Repka, Donny W. Suh, Gaylord G. Ventura (2011–13), Lisa C. Verderber (2011–12), David K. Wallace
PEDIG Executive Committee
Jonathan M. Holmes (chair), William F. Astle (2013–present), Darron A. Bacal (2009–10), Roy W. Beck, Eileen E. Birch, Angela M Chen (2012–present), Melanie L. Christian (2012–present), Stephen P. Christiansen (2009–10), Susan A. Cotter (2009–present), Earl R. Crouch Jr. (2012–present), Eric R. Crouch III (2010–11, 2014–present), Sean P. Donahue (2012–present), Laura B. Enyedi (2011–13), Donald F. Everett, Darren L. Hoover (2008, 2011–13), Pamela A. Huston (2009–10), Jorie L. Jackson (2011–12), Raymond T. Kraker, Marjean T. Kulp (2010–12), Scott R. Lambert (2013–present), Katherine A. Lee (2014–present), Ruth E. Manny (2013–present), Aaron M. Miller (2011–12), David B. Petersen (2011–13), Michael X. Repka, David L. Rogers (2011–13), Benjamin H. Ticho (2010–11), David K. Wallace (2009–present)
PEDIG Data and Safety Monitoring Committee
Marie Diener-West (chair), John D. Baker, Barry Davis, Donald F. Everett, Dale L. Phelps, Stephen W. Poff, Richard A. Saunders, Lawrence Tychsen
Footnotes
An address for reprints will not be provided.
Meeting Presentations: none
Conflict of Interest: No conflicting relationship exists for any author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pediatric Eye Disease Investigator Group. Randomized trial of treatment of amblyopia in children aged 7 to 17 years. Arch Ophthalmol. 2005;123(4):437–47. doi: 10.1001/archopht.123.4.437. [DOI] [PubMed] [Google Scholar]
- 2.Pediatric Eye Disease Investigator Group. Two-year follow-up of a 6-month randomized trial of atropine vs patching for treatment of moderate amblyopia in children. Arch Ophthalmol. 2005;123(2):149–57. doi: 10.1001/archopht.123.2.149. [DOI] [PubMed] [Google Scholar]
- 3.Pediatric Eye Disease Investigator Group. Patching vs atropine to treat amblyopia in children aged 7 to 12 years: a randomized trial. Arch Ophthalmol. 2008;126(12):1634–42. doi: 10.1001/archophthalmol.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leguire LE, Walson PD, Rogers GL, et al. Levodopa/carbidopa treatment for amblyopia in older children. J Pediatr Ophthalmol Strabismus. 1995;32(3):143–51. doi: 10.3928/0191-3913-19950501-05. [DOI] [PubMed] [Google Scholar]
- 5.Procianoy E, Fuchs FD, Procianoy L, Procianoy F. The effect of increasing doses of levodopa on children with strabismic amblyopia. J AAPOS. 1999;3(6):337–40. doi: 10.1016/s1091-8531(99)70041-8. [DOI] [PubMed] [Google Scholar]
- 6.Brandies R, Yehuda S. The possible role of retinal dopaminergic system in visual performance. Neurosci Biobehav Rev. 2008;32(4):611–56. doi: 10.1016/j.neubiorev.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Leguire LE, Rogers GL, Bremer DL, et al. Levodopa and childhood amblyopia. J Pediatr Ophthalmol Strabismus. 1992;29(5):290–8. doi: 10.3928/0191-3913-19920901-08. [DOI] [PubMed] [Google Scholar]
- 8.Basmak H, Yildirim N, Erdinç O, et al. Effect of levodopa therapy on visual evoked potentials and visual acuity in amblyopia. Ophthalmologica. 1999;213(2):110–3. doi: 10.1159/000027402. [DOI] [PubMed] [Google Scholar]
- 9.Gottlob I, Charlier J, Reinecke RD. Visual acuities and scotomas after one week levodopa administration in human amblyopia. Invest Ophthalmol Vis Sci. 1992;33(9):2722–8. [PubMed] [Google Scholar]
- 10.Leguire LE, Komaromy KL, Nairus TM, Rogers GL. Long-term follow-up of L-dopa treatment in children with amblyopia. J Pediatr Ophthalmol Strabismus. 2002;39(6):326–30. doi: 10.3928/0191-3913-20021101-05. [DOI] [PubMed] [Google Scholar]
- 11.Leguire LE, Walson PD, Rogers GL, et al. Longitudinal study of levodopa/carbidopa for childhood amblyopia. J Pediatr Ophthalmol Strabismus. 1993;30(6):354–60. doi: 10.3928/0191-3913-19931101-04. [DOI] [PubMed] [Google Scholar]
- 12.Repka MX, Kraker RT, Beck RW, et al. Pilot study of levodopa dose as treatment for residual amblyopia in children aged 8 years to younger than 18 years. Arch Ophthalmol. 2010;128(9):1215–7. doi: 10.1001/archophthalmol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gottlob I, Wizov SS, Reinecke RD. Visual acuities and scotomas after 3 weeks’ levodopa administration in adult amblyopia. Graefes Arch Clin Exp Ophthalmol. 1995;233(7):407–13. doi: 10.1007/BF00180943. [DOI] [PubMed] [Google Scholar]
- 14.Mohan K, Dhankar V, Sharma A. Visual acuities after levodopa administration in amblyopia. J Pediatr Ophthalmol Strabismus. 2001;38(2):62–7. doi: 10.3928/0191-3913-20010301-05. [DOI] [PubMed] [Google Scholar]
- 15.Wu X, Liu S, Xu H, et al. A preliminary report of the effect of levodopa and carbidopa for childhood amblyopia. Yan Ke Xue Bao. 1998;14(4):238–41. [PubMed] [Google Scholar]
- 16.Leguire LE, Rogers GL, Walson PD, et al. Occlusion and levodopa-carbidopa treatment for childhood amblyopia. J AAPOS. 1998;2(5):257–64. doi: 10.1016/s1091-8531(98)90080-5. [DOI] [PubMed] [Google Scholar]
- 17.Yang X, Luo D, Liao M, et al. Efficacy and tolerance of levodopa to treat amblyopia: a systematic review and meta-analysis. Eur J Ophthalmol. 2013;23(1):19–26. doi: 10.5301/ejo.5000174. [DOI] [PubMed] [Google Scholar]
- 18.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the Early Treatment of Diabetic Retinopathy Study testing protocol. Am J Ophthalmol. 2003;135(2):194–205. doi: 10.1016/s0002-9394(02)01825-1. [DOI] [PubMed] [Google Scholar]
- 19.Little RJA, Rubin DB. Statistical Analysis with Missing Data. New York: Wiley; 1987. [Google Scholar]
- 20.Keech RV, Ottar W, Zhang L. The minimum occulsion trial for the treatment of amblyopia. Ophthalmology. 2002;109(12):2261–4. doi: 10.1016/s0161-6420(02)01282-4. [DOI] [PubMed] [Google Scholar]
- 21.Pediatric Eye Disease Investigator Group. A randomized trial to evaluate 2 hours of daily patching for strabismic and anisometropic amblyopia in children. Ophthalmology. 2006;113(6):904–12. doi: 10.1016/j.ophtha.2006.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dadeya S, Vats P, Malik KPS. Levodopa/carbidopa in the treatment of amblyopia. J Pediatr Ophthalmol Strabismus. 2009;46(2):87–90. doi: 10.3928/01913913-20090301-07. [DOI] [PubMed] [Google Scholar]
- 23.Leguire LE, Rogers GL, Bremer DL, et al. Levodopa/carbidopa for childhood amblyopia. Invest Ophthalmol Vis Sci. 1993;34(11):3090–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Eligibility Criteria
Change in Amblyopic-Eye Visual Acuity at 18 Weeks According to Baseline Patient Characteristics
Amblyopic-Eye Visual Acuity by Treatment Group
Fellow-Eye Visual Acuity by Treatment Group
Adverse Events Reported in Randomized Trial by Treatment Group
Child Symptom Survey Results by Treatment Group
Cells reflect mean response score unless otherwise specified. Note: A higher number reflects a more negative response (5=Always, 4=Often, 3=Sometimes, 2=Rarely, 1=Never)
Parent Symptom Survey Results by Treatment Group
Cells reflect mean response score unless otherwise specified. Note: A higher number reflects a more negative response (5=Always, 4=Often, 3=Sometimes, 2=Rarely, 1=Never)
Flow of Participants through Study
*Of the 4 participants who dropped in the levodopa group, 1 was lost to follow up, 2 elected to withdraw, and 1 refused study treatment after randomization. Of the 4 participants who dropped in the placebo group, 2 were lost to follow up, 1 elected to withdraw, and 1 withdrew after experiencing blepharospasm which completely resolved