Introduction
The classic teaching by Trunkey and colleagues from the late 1970s of a ‘trimodal’ distribution of death after trauma reflected deaths at the scene, followed by deaths in the emergency department (ED) immediately after trauma, and finally late deaths during the index hospitalization.[1] This distribution pattern has come into question in recent years. Since the 1966 Institute of Medicine report stating that unintended injury was the neglected disease of modern society,[2] the evolution of trauma systems and advances in imaging, minimally invasive procedures, and critical care have dramatically changed patterns of trauma mortality.[3–5] As more patients survive both initial injury and index hospitalization, there appears to be a ‘quadrimodal’ distribution of death after trauma, wherein there are deaths at the scene (peak 1), early deaths after arrival to the trauma center (peak 2), late inpatient deaths during index hospitalization (peak 3), and deaths after discharge (peak 4). Mullin and colleagues found that including 30-day post-discharge mortality for trauma patients increased the rate of injury-related deaths from 12.1 per 10,000 injured patients to 14.1 per 10,000 in their cohort of 90,048 injured patients treated in the early 1990s.[6] More recently, Davidson and colleagues reported that hospital mortality for trauma patients in Washington State decreased from 8.0% to 4.9% between 1995 and 2008, while long-term mortality increased from 4.7% to 7.4%.[7]
Meanwhile, aggressive care for patients who may be approaching the end of life has also come into question, both from the perspective of increased human suffering and in terms of costs to the healthcare system.[8, 9] When considering deaths that occur during hospitalization with those that occur after discharge, an estimated 540,000 Americans die after intensive care unit (ICU) admission each year.[10] Furthermore, 28% of total healthcare expenditures occur in the last year of life.[11] Unrealistic family expectations, provider difficulty in communication, and an overall culture of critical care have been implicated in overly aggressive care.[12–14] Thus, it is important to understand both patient and process factors associated with death after critical illness, in particular when death occurs soon after discharge, in which case the utility of the aggressiveness of care would come into question.
Several recent studies have addressed predictors of post-discharge mortality for non-traumatic causes of critical illness. These factors include age, comorbidities, early sedation requirements, development of delirium, need for mechanical ventilation, and severity of illness.[15–21] In these studies, reported post-discharge mortality after critical illness has been reported to be as high as 17% at 30 days, 26% at 180 days, 30% at 6 months, and 35% at 1 year.[15–18, 21] Furthermore, among patients requiring prolonged mechanical ventilation, 1-year mortality has been reported to be 66%.[19] Less is known about critical care resource utilization and long-term mortality in the trauma population. A 2010 study by Brattstrom and colleagues reported a 30-day mortality rate of 10.4% for critically injured patients.[20] Importantly, the effect of critical care resource utilization on long-term mortality after critical injury is unknown.
We reviewed our experience with critically ill trauma patients to determine which factors predict the fourth peak of the quadrimodal distribution of trauma mortality. We were specifically interested in which aspects of critical care resource utilization predicted long-term death as such predictors might warrant more realistic discussions of goals of care among a patient population for whom survival to discharge does not yield long term survival. We hypothesized that greater critical care resource utilization (i.e. longer ICU length of stay [LOS], tracheostomy, gastrostomy, hemodialysis, and cardiopulmonary resuscitation [CPR]) among survivors of the index hospitalization was predictive of post-discharge mortality.
Methods
This was a retrospective analysis of trauma registry data collected prospectively for all patients treated at an American College of Surgeons verified Level I trauma center in Central Massachusetts. After study approval by the institution review board, we identified all critically injured adult patients (age ≥ 18 years) enrolled in the registry from January 1, 2000 to December 31, 2010. Critical injury was indicated by an Injury Severity Score (ISS) ≥25 AND at least 1 of the following: death in the ED, death within 24 hours of admission to the ICU, or ICU admission exceeding 24 hours. We excluded patients who were discharged alive or transferred to the floor within 24 hours of admission as they would not have met clinical criteria for severe injury and/or critical illness or because an early withdrawal of care order might have influenced their outcome. Pregnant patients were also excluded as critical care considerations for a viable pregnancy may have influenced outcomes. Variables available for analysis had been prospectively entered into the trauma registry during the patients’ index hospitalization and included patient characteristics, prehospital variables, comorbidities, mechanism of injury, diagnosis, ISS, abbreviated injury scale (AIS), revised trauma score (RTS), interventions, and outcomes (i.e. total hospital LOS, ICU LOS, complications, hospital mortality, and discharge disposition).
A database was created of patients meeting the inclusion criteria during the study period whose date of death (either in the ED or in the ICU) was not within 24 hours of presentation. A single researcher (C.J.W.) blinded to hospitalization data then used the Social Security Death Index (SSDI) to identify dates of death for all patients in the database as of May 1, 2012. To measure the reliability of the SSDI for our patient population, the recorded dates of death in the registry of patients who died during the index hospitalization but after the first 24 hours were compared with those reported on the SSDI; we found 83.5% (192/230) agreement; the 16.5% disagreement may have been due to the fact that our patient identifiers included only name and date of birth (no social security number) and that most of our patient identification data is not confirmed by the patient at the time of admission due to severity of illness. The fidelity of the SSDI has been shown to be as high as 90% in studies with patient provided identifiers.[22–25] Furthermore, we could not correct potential errors in legal name documentation and dates of birth or undocumented status of patients who died. Thus, we were potentially under-estimating post-discharge mortality in our study population.
Means, standard deviations (SDs), medians and interquartile ranges (IQRs) were determined for continuous variables. Counts and percentages were determined for categorical variables. For continuous variables, differences in mean outcomes were compared using the Wilcoxon rank sum test and ANOVA, which yielded the same results. For dichotomous variables and comparisons between groups, Wald χ2 tests of association or Fisher’s exact tests were used. In addition to univariate comparisons, multivariable analysis was performed using Cox regression to determine hazards ratios (HRs) with 95% confidence intervals (CIs) for mortality across patient groups. Covariates for the model were chosen based on p-values <0.5 in the univariate analysis and clinical significance. In addition, models were also adjusted for injury severity score and revised trauma score. In the model for mortality, the LOS covariates were entered as continuous variables or dichotomized as above or below the median if maintained in the model. Covariates with p-values for Wald statistics <0.05 were considered significant predictors of mortality. All analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC).
Results
Identifying Index Survivors
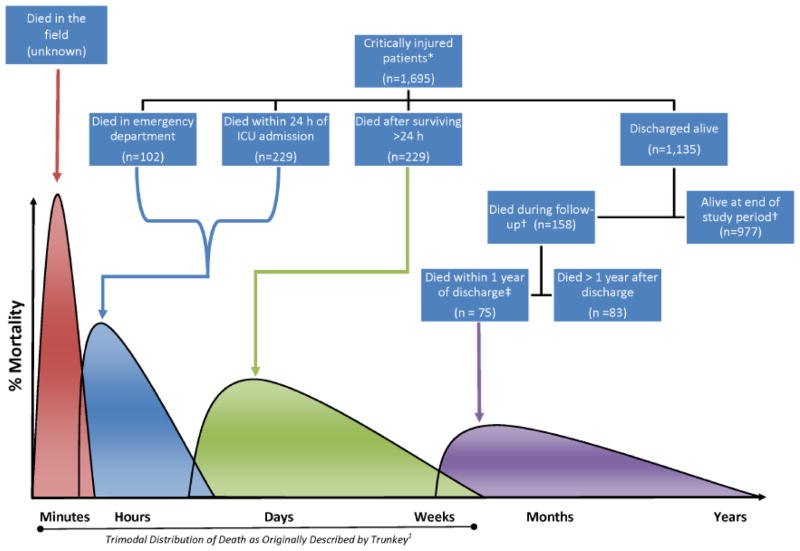
Of 21,550 patients entered in the trauma registry between January 1, 2000 and December 31, 2010, 1,695 met our inclusion criteria for critical injury because they had an ISS ≥25 and died in the ED (n = 102, 6.0%), died within 24 hours of ICU admission (n = 229, 13.5%), or survived ≥24 hours with an ICU stay of >1 day (n = 1,364, 80.5%). Figure 1 demonstrates how our patient population relates to a conceptual model quadrimodal distribution of mortality after injury; 1,135 of our cohort of 1,695 critically injured, non-pregnant, adult trauma patients (67.0%) survived to discharge. Appendix 1 compares characteristics and outcomes of patients who were characterized as early hospital deaths (died in the ED or within 24 hours of ICU admission), as deaths during index hospitalization (died after surviving the first 24 hours of ICU admission but before hospital discharge), and as index survivors (survived the index hospitalization).
Figure 1.
Mortality among critically injured* non-pregnant adult (age ≥18 years) trauma patients (2000–2010) shown relative to conceptual model of a quadrimodal distribution of death after injury.
* Injury Severity Score ≥25.
† Study follow-up closed on May 1, 2011 for a median follow-up of 62 (IQR 35, 96) months.
‡ We propose that these patients are those for whom critical injury is a terminal disease.
Appendix 1.
Demographics and injury characteristics of critically injured adult trauma patients (2000–2010) (n = 1,695)
| Early deaths* | Deaths after surviving first 24 h | Survived index hospitalization | Significant pairwise comparisons† | |
|---|---|---|---|---|
| (1) n = 331 |
(2) n = 229 |
(3) n = 1,135 |
||
| Age, median (y) | 50 (31, 72) | 60 (44, 80) | 43 (27, 59) | (1) vs. (2), (2) vs. (3) |
| Female (%) | 32 | 33 | 29 | |
| Race (%) | ||||
| White | 89 | 92 | 89 | |
| African American | 2.1 | 1.8 | 2.9 | |
| Hispanic | 6.4 | 5.7 | 5.8 | |
| Other | 3.0 | 0.4 | 2.0 | |
| Mechanism of injury (%) | (1) vs. (2) | |||
| Blunt | 91 | 97 | 96 | |
| Penetrating | 9.4 | 2.6 | 3.7 | |
| Transport method (%) | ||||
| Air | 37 | 42 | 42 | |
| Ground | 63 | 58 | 58 | |
| Patient | 0.0 | 0.0 | 0.4 | |
| Unknown | – | – | – | |
| Revised trauma score | (1) vs. (2), (2) vs. (3) | |||
| 0–2 | 32 | 0.4 | 0.3 | |
| >2–4 | 40 | 36 | 18 | |
| >4–6 | 13 | 19 | 15 | |
| >6–7 | 4.0 | 7.4 | 8.2 | |
| >7 | 11 | 37 | 58 | |
| Injury severity score | (1) vs. (2), (2) vs. (3) | |||
| 25–44 (%) | 65 | 77 | 86 | |
| 45–64 (%) | 21 | 18 | 13 | |
| >64 | 14 | 5.2 | 1.1 | |
| Days on mechanical ventilation (median days, IQR) | 1 (1, 1) | 4.5 (2, 8) | 2 (0, 11) | (1) vs. (2) |
| ICU LOS (median days, IQR) | 1 (0, 1) | 6 (3, 11) | 9 (4, 20) | (1) vs. (2), (2) vs. (3) |
| Interventions | ||||
| Tracheostomy (%) | 1.5 | 7.4 | 18 | (1) vs. (2), (2) vs. (3) |
| Gastrostromy (%) | 0.0 | 4.8 | 19 | (1) vs. (2), (2) vs. (3) |
| Hemodialysis (%) | 0.3 | 4.4 | 0.9 | (1) vs. (2), (2) vs. (3) |
| CPR (%) | 55 | 16 | 3.4 | (1) vs. (2), (2) vs. (3) |
Data are mean (standard deviation) or median (interquartile range).
Early deaths include death in emergency room and deaths within 24 hours of ICU admission.
Significant p values (<0.05/2 = 0.025) only shown where (1) vs. (2) and (2) vs. (3)
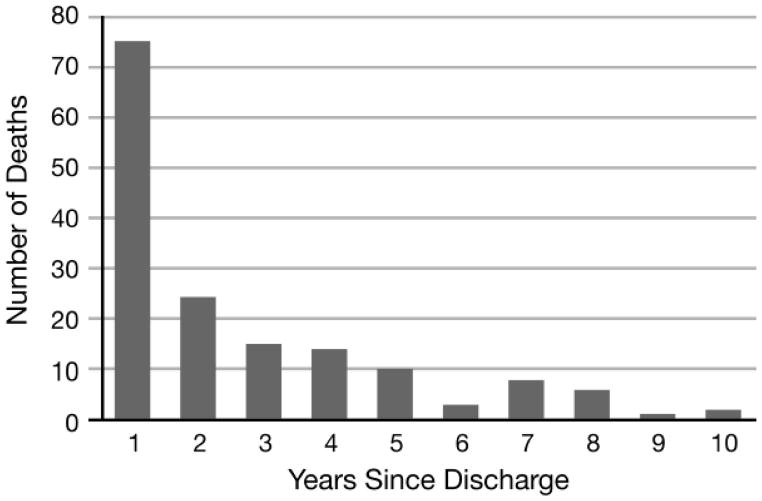
Figure 2 shows the distribution of post-discharge deaths by year of follow-up. Of the 158 index survivors who died during follow-up, 24 (15.2%) were deceased by 30 days, 31 (19.6%) by 60 days, and 40 (25.3%) by 90 days. Seventy-five (47.5%) patients died within the first year after discharge. As of May 1, 2012, 977/1,135 (86.0%) patients were still alive, with a median follow-up of 62 (IQR 35, 96) months. Thus, total overall mortality of our critically injured cohort both during and after index hospitalization was 42.4% ((331+229+158)/1,695).
Figure 2.
Distribution of deaths by decade for critically injured adult trauma patients (2000–2010) who survived index hospitalization (n = 158).
The remaining analyses and results compare index survivors who died or survived during our follow-up period.
Patient Characteristics
As shown in Table 1, index survivors who died during follow-up, compared with those who were still alive as of May 1, 2012, were more likely to be Caucasian, older at the time of initial injury, injured due to blunt trauma, having a head/neck, extremity, or external AIS >3. However, among index survivors, sex, method of transport at the time of injury, RTS, having a facial, chest, or abdomen AIS >3 were not associated with death during follow-up.
Table 1.
Demographics and injury characteristics of critically injured adult trauma patients (2000–2010) who survived index hospitalization (n = 1,135)
| Survived follow-up* | Died after discharge | p Value† | |
|---|---|---|---|
|
| |||
| n = 977 | n = 158 | ||
| Age, years, median (interquartile range) | 40 (25, 54) | 70 (52, 79) | <0.0001 |
| Female (%) | 28 | 33 | 0.20 |
| Race (%) | 0.02 | ||
| Caucasian | 88 | 94 | |
| African–American | 3.4 | 0.0 | |
| Hispanic | 6.3 | 3.2 | |
| Other | 2.0 | 2.5 | |
| Mechanism of injury (%) | 0.02 | ||
| Blunt | 96 | 99 | |
| Penetrating | 4.2 | 0.6 | |
| Transport method (%) | 0.82 | ||
| Air | 42 | 41 | |
| Ground | 57 | 59 | |
| Patient | 0.4 | 0.0 | |
| Unknown | – | – | |
| RTS (%) | 0.39 | ||
| 0–2 | 0.3 | 0.0 | |
| >2–4 | 18 | 19 | |
| >4–6 | 16 | 11 | |
| >6–7 | 8.5 | 6.5 | |
| >7 | 58 | 63 | |
| GCS on admission (%) | 0.73 | ||
| 3 | 18 | 17 | |
| 4–8 | 14 | 12 | |
| 9–12 | 9.1 | 7.7 | |
| 13–14 | 13 | 16 | |
| 15 | 46 | 47 | |
| ISS (%) | 0.49 | ||
| 25–44 | 86 | 90 | |
| 45–64 | 13 | 9.5 | |
| >64 | 1.1 | 0.6 | |
| Head/Neck AIS >3 (%) | 57 | 73 | 0.0002 |
| Face AIS >3 (%) | 1.9 | 2.0 | 0.95 |
| Chest AIS >3 (%) | 80 | 80 | 0.98 |
| Abdomen AIS >3 (%) | 39 | 31 | 0.21 |
| Extremity AIS >3 (%) | 6.0 | 3.4 | 0.01 |
| External AIS >3 (%) | 29 | 19 | 0.01 |
Median follow-up 62 (34.5, 95.5) months.
Wilcoxon Rank Sum test for comparison of means and Wald χ2 test of association for comparison of proportions (Fisher’s exact test where expected cell size <5).
GCS, Glasgow Coma Score; ISS, Injury Severity Score; AIS, Abbreviated Injury Scale; RTS: Revised Trauma Score
Resource Utilization
Resource utilization among patients who survived the index hospitalization is shown in Table 2. Patients who died after discharge had longer index hospitalization and ICU LOS and were more likely to have received both tracheostomies and gastrostomies during the index hospitalization than those who survived through the end of follow-up. However, there were no differences between these two groups in terms of days on mechanical ventilation and receipt of hemodialysis. In our original cohort, 76 patients received CPR during the index hospitalization. Since nearly half of these patients (49%, 37/76) died during the index hospitalization, only 3.4% of index survivors had undergone CPR. However, receipt of CPR was associated with mortality during the follow-up period (6.3% among those who died vs. 3.0% among those alive at end of follow-up, p = 0.03).
Table 2.
Resource utilization during index hospitalization for critically injured adult trauma patients (2000–2010) who survived index hospitalization (n = 1,135)
| Survived follow-up* | Died after discharge | ||
|---|---|---|---|
|
| |||
| n = 977 | n = 158 | p Value† | |
| Overall hospital length of stay (median days, IQR) | 17 (10, 27) | 24 (13, 38) | <0.001 |
| ICU length of stay (median days, IQR) | 8 (4, 19) | 17 (6, 29) | <0.0001 |
| Duration of mechanical ventilation (median days, IQR) | 2 (0, 10) | 3 (0, 15) | 0.87 |
| Tracheostomy (%) | 16 | 36 | <0.0001 |
| Gastrostromy (%) | 16 | 39 | <0.0001 |
| Hemodialysis (%) | 1.0 | 0.0 | 0.37 |
| Cardiopulmonary resuscitation (%) | 3.0 | 6.3 | 0.03 |
Median follow-up 62 (34.5, 95.5) months.
Wilcoxon Rank Sum test for comparison of means and Kruskal–Wallis for comparison of medians and Wald χ2 test of association for comparison of proportions (Fisher’s exact test where cell size <5).
ICU, intensive care unit.
Discharge Disposition
Table 3 shows patient discharge disposition. Patients who died during follow-up were more likely to be discharged to a rehabilitation or skilled nursing facility than those who were alive at the end of our follow-up period.
Table 3.
Discharge disposition of critically injured adult trauma patients (2000–2010) who survived index hospitalization (n = 833*)
| Discharge disposition | Survived follow-up† | Died after discharge | |
|---|---|---|---|
|
| |||
| n = 726 | n = 107 | p Value‡ | |
| Home (%) | 20 | 7.5 | 0.001 |
| Home with services (%) | 8.7 | 3.7 | 0.09 |
| Rehabilitation (%) | 63 | 76 | 0.01 |
| Skilled Nursing (%) | 5.8 | 13 | 0.01 |
| Other (%) | 2.6 | 0.0 | 0.16 |
144 patients did not have discharge disposition recorded.
Median follow-up 62 (34.5, 95.5) months.
Fisher’s exact test of association for comparison of proportions across each possible discharge dispositions; overall p Value < 0.0001.
Predictors of mortality
In multivariable analyses, age, sex, and ICU LOS were predictors of 1-year mortality after discharge for critical injury (Table 4). Age (for every 10-year increase at the time of injury), male sex, and ICU LOS >16 each conferred a nearly two-fold increased risk of death at 1 year. In models with ICU LOS included as a continuous variable, for each additional day in the ICU, there was a 1.8% increased risk of death within 1 year.
Table 4.
Predictors of post-discharge mortality for critically injured adult trauma patients (2000–2010) who survived index hospitalization (n = 1,119)
| Variable | HR (95% CI) |
|---|---|
| 1-year mortality*
| |
| ICU LOS >16 days (vs. ≤16 days) | 1.66 (1.01, 2.73) |
| Age per 10-year increase | 2.28 (1.93, 2.69) |
| Male sex (vs. female sex) | 2.45 (1.44, 4.19) |
|
| |
| Overall mortality (by May 2012)†
| |
| ICU LOS >16 days (vs. ≤16 days) | 2.22 (1.58, 3.12) |
| Age per 10-year increase | 2.06 (1.85, 2.29) |
| Male sex (vs. female sex) | 1.78 (1.24, 2.55) |
Cox proportional hazard model adjusted for age, sex, and injury severity score, revised trauma score and ICU LOS category.
Cox proportional hazard model adjusted for age, sex, ICU LOS category, injury severity score, and revised trauma score
CI, confidence intervals; GCS, Glasgow Coma Score; HR, hazard ratios; ICU, intensive care unit; LOS, length of stay; RTS, revised trauma score.
For overall mortality during follow-up, multivariable analyses again demonstrated that age, sex, and ICU LOS were all significant predictors of mortality (Table 4). Each 10-year increment in age at the time of injury increased the risk of death about two-fold. Men had about 40% increased risk of death compared with women. The risk of death was more than double for patients whose ICU LOS was >16 days compared to those with shorter ICU stays. When ICU LOS was included in the model as a continuous variable, for each additional day in the ICU there was a 2% increased risk of death within 10 years; 52.5% of patients with an initial ICU stay >16 days were dead by May 1, 2012.
Overall hospital LOS, receipt of tracheostomy, receipt of gastrostomy, and CPR were tested in our multivariable models and were not found to be associated with mortality when adjusting for other significant covariates.
Figure 3 shows Kaplan–Meier survival curves by ICU stay greater or less than 16 days. For both 1-year and overall mortality among index survivors, ISS, mechanism of injury, race, hospital LOS, resource utilization, and discharge disposition did not affect the risk of death in multivariable models.
Figure 3.
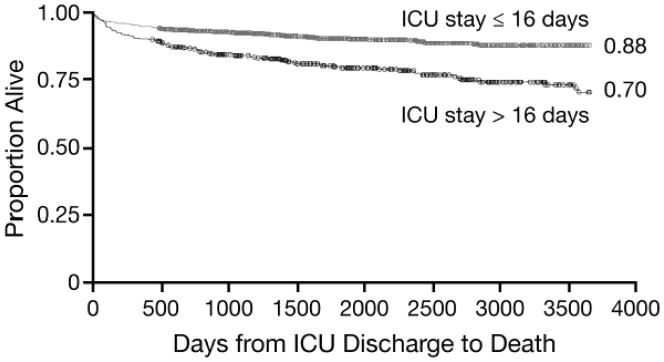
Kaplan–Meier survival curves for critically injured adult trauma patients (2000–2010) who survived index hospitalization, by ICU stay ≤16 vs. >16 days (n = 1,135).
Discussion
In this study we examined mortality patterns among critically injured patients who are at highest risk for trauma-related mortality to better understand changing patterns of death after injury. While we did not measure deaths in the field (i.e. before hospital arrival), during follow-up we demonstrated an overall mortality of 42% among critically injured, non-pregnant, adult trauma patients who survived transport at the time of injury.
The classic teaching of a trimodal distribution of death after trauma reflected deaths at the scene, deaths in the ED after trauma, and late deaths during the index hospitalization.[1] In the present study, 21% of patients died immediately in the ED or within 24 hours of ICU admission in what would be considered the second peak of trauma mortality in the trimodal distribution. Fourteen percent of patients survived the first 24 hours in the ICU but died during the index hospitalization, in what would be considered the third and final peak in the trimodal distribution. These findings are consistent with other data showing that nearly 20% of trauma patients requiring ICU admission die before discharge.[13] Importantly, of the 67% of patients who survived the index hospitalization, 14% died within a follow-up of up to 10 years, with nearly half of these deaths occurring in the first year after discharge, which we propose as the ‘fourth peak’ of death in what might be best described a the “quadrimodal distribution of trauma mortality.”
Recent studies have also questioned the teaching of three peaks of trauma-related mortality. However, these studies have suggested fewer or diminishing peaks rather than more peaks.[1, 26–29] Two studies from outside of the US have reported a single early peak: either on-scene (81% in New Zealand cohort) or during the first day after injury (47% in a Netherlands cohort), followed by a gradual decline in hospital mortality.[27, 29] Our study did not focus on prehospital deaths, and our rate of early hospital deaths—even though limited to the most severely injured—was considerably lower than in these non-US studies. However, studies from mature US trauma systems in urban settings have suggested three peaks persist albeit with a marked decline in the proportion of late deaths comprising the third peak (7.6% late deaths in a Los Angeles cohort; 10% late deaths in a Dallas cohort).[26, 28] Given our cohort of the most severely injured patients, it is not surprising that our third peak was somewhat higher. Review of this literature and our results suggests that, while few advances have been made in reducing immediate or near-immediate death after trauma, advances in management of non-immediately life-threatening injuries, improvements in resuscitation, and innovations in critical care have resulted in improved survival to hospital discharge among those who survive the first 24 hours after injury.
Still, survival to discharge has been criticized as a marker for performance evaluation in trauma. A number of studies have reported post-discharge mortality ranging from 5% to 15% depending on length of follow-up, with most deaths occurring within 1 year of discharge as was also demonstrated by our data.[7, 30–32] Thus, while we are not the first to note a fourth peak of post-injury death, in particular when focusing on the critically injured, to our knowledge we are the first to use the term “quadrimodal” to describe this phenomenon, wherein there is a ‘fourth peak’ of death in the first year after discharge. However, these studies have likely underestimated the magnitude of the fourth peak due to their focus on all injured patients. Therefore, we limited our study to only the most critically injured patients who survived to discharge, of whom 14% were dead by the close of our study period. Given the financial and human costs of critical injury and prolonged ICU stays it is important to understand the factors that may drive up resource utilization or increase human suffering after critical injury, in particular when the risk of death within 1 year remains considerable.
The 1-year overall mortality after ED arrival of our entire cohort was 39%. Since we examined only critically injured patients, it is not surprising that our cohort’s 1-year mortality exceeds that of Brattstrom’s Swedish cohort (9.3%), Laupland’s Canadian cohort (14%), Claridge’s Cleveland, OH cohort (5.4%) and Davidson’s Washington State cohort (7.2%).[7, 20, 31, 33] Studies of mixed groups of critically ill patients have found 1-year mortality rates as high as 56%.[15, 17, 21, 34] Our slightly lower mortality rate is likely explained by the fact that injured patients generally have fewer comorbidities and are younger than mixed patient populations; both age and comorbidities have been linked to higher mortality in both trauma and mixed patient populations.[15, 20, 21, 33, 35, 36] Unfortunately, our registry lacked detailed comorbidity data, but we did find that age was a significant predictor of both 1-year mortality and total mortality by the end of our follow-up. However, a study from Brattstrom’s group examining only critically injured patients found an overall 30-day mortality rate that was also markedly lower than ours (10.4% vs. 36.1%, respectively).[35] This difference may be attributable to differences in categorizing critical injury as the Brattstrom study did not use ISS as a selection criterion.
Most trauma fatalities that survive transport to a trauma center will die in the trauma bay or in the operating room; however, 20–30% of these deaths ultimately occur after a lengthy ICU stay.[37] We found that ICU stays exceeding 16 days were associated with greater likelihood of mortality during the follow-up period. Prognostication in critical illness is imprecise,[12, 38, 39] but ICU LOS has been implicated as a prognostic factor in a number of studies. In a study of patients admitted to a multidisciplinary ICU, Laupland and colleagues found 1-year mortality rates of 3% for ICU LOS <3 days, 10% for ICU LOS 3–13 days, and 8% in with ICU LOS ≥14 days.[40] In our cohort, we found that the inflection point for mortality occurred for patients who spent >16 days in the ICU during their index hospitalization, which was the median in our cohort, rather than 14 days. More than 50% of these patients were dead during the follow-up period.
Given that patients with prolonged ICU stays consume disproportionate amounts of hospital resources,[41, 42] the association between prolonged stays and long-term mortality is concerning. Stricker and colleagues found that more than half of the resources and costs of ICU care are spent on the 10% of patients whose ICU LOS is >7 days, while mortality for that group was twice as high as those with shorter stays.[42] While our study lacked specific data on costs and charges, we did find that receipt of tracheostomy and gastrostomy was associated with higher likelihood of death during the follow-up period. These interventions, though not found to be significant independent predictors of mortality in our cohort, appear to be markers for resource utilization that are more common among injured patients with prolonged ICU stays.
Ultimately, while resource utilization may be higher in patients with longer ICU stays, the investment of these resources would be expected to be worthwhile if the result was desirable for patients. In our study, patients who died during follow-up were substantially more likely to be discharged to a rehabilitation or skilled nursing facility than to home. While we lack data on functional status or quality of life at the time of death, those with the greatest limitations at time of discharge from the index hospitalization might be more vulnerable to poor outcomes. These late outcomes may range from dead (which we measured in this study), to alive but ventilator and feeding-tube dependent, to alive with functional limitations, to full return to baseline activities; a number of metrics have been created to measure these outcomes (e.g. Focus On Therapeutic Outcomes, Inc. (FOTO®); Short Form 36 Health Survey Update, SF-36®, Glasgow Outcomes Score). Unfortunately, what constitutes a desirable outcome for the patient in question is unclear. Evidence from the SUPPORT trial of medical patients suggests a disconnect between patient desires and those expressed by their proxies regarding goals for functional status and cognition after discharge.[43] Unfortunately, nearly two decades later, a substantial barrier to allocating resource utilization after critical injury remains a lack of clarity on patient health values when they cannot speak for themselves.[44] While withdrawal of care orders appears to be playing a greater role in mortality during the index hospitalization, accounting for as many as 62% of hospital deaths,[45] it is unclear what role expectations for quality and longevity of life after discharge should play in guiding the aggressiveness of care for critically injured trauma patients.
Our results suggest, however, that critically injured patients who are at risk of comprising the fourth peak for trauma mortality—those who have a long ICU stay and may require invasive procedures to sustain life, or who are discharged to another facility instead of home after the index hospitalization—may benefit from more discussion during the index hospitalization on their overall expectations for quality and longevity of life even though they are expected to survive the index hospitalization. Perhaps some critically injured trauma patients would benefit more from discharge to hospice with palliative goals rather than goals to sustain life indefinitely. Mosenthal and colleagues described a method of structured communication that reduced ICU LOS without affecting overall mortality by improving processes of end-of-life care discussions.[13] Their method may in fact improve what is currently a seemingly haphazard way of applying goals of care issues into the management of critically injured patients.[46, 47] It is possible that such a method might be tailored to address the goals of care of patients with prolonged critical illness after injury. Critically injured trauma patients may also benefit from palliative care consultation during the period of initial critical illness as is more commonly advocated across the critically ill patient population.[48, 49]
There are a number of important limitations to this study. First, it is a retrospective analysis of critically injured adult trauma patients at a single Level-I trauma center. Our results may not be generalizable to persons admitted to medical or pediatric ICU’s or at centers in different locations. Our primary outcome of interest was death after index hospitalization. Our registry lacked data on comorbidities, thus the only risk adjustment in our model was injury severity and revised trauma score. Outcomes are influenced by complications occurring during index hospitalization and our database did not record complications. We relied on the SSDI for death dates but the reliability of that resource is not 100%, in particular for patients who may lack legal residence or whose names and/or dates of birth may have been misentered into our system at the time of treatment. While the rate of these types of errors is unknown, most of our cohort would not have been able to provide verification at the time of intake due to the severity of injury. This lack of confirmation of death via the SSDI may have resulted in an under representation of long-term mortality in our cohort, biasing our results towards the null. We also do not know the ultimate cause of death that may or may not have been related to the effects of the original trauma. Finally, and perhaps most importantly, our data do not include any information on the quality of life of patients who survived index hospitalization after critical injury. This information, in addition to prediction of chances of mortality, would be key to overall goals of care discussions after devastating injury.
Despite these limitations, our findings may aid trauma surgeons and intensivists in framing overall expectations for critically injured patients, in particular those who have long ICU courses, who may fall into the quadrimodal distribution of trauma mortality. The burden of prolonged critical illness on patients and the healthcare system presents major challenges. With critical care advances, more patients are surviving the index hospitalization, hence creating the fourth peak of trauma mortality. The true meaning at the individual and societal level of ‘discharged alive’ merit both qualitative and quantitative studies on prognostication, post-discharge quality of life, and optimization of long-term outcomes. Meanwhile, it is important to approach the care of critically injured with an understanding that survival to discharge is a complex outcome without guarantee of long-term survival.
Acknowledgments
The research reported in this publication was in part supported by the University of Massachusetts Clinical Scholar Award (HPS) through the National Center for Advancing Translational Sciences of the National Institutes of Health under award numbers UL1RR031982, 1KL2RR031981-01, and UL1TR000161.
Footnotes
Conflict of Interest
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
This paper was presented as an oral presentation at the Massachusetts Chapter of the American College of Surgeons Committee on Trauma Resident’s Paper Competition on October 19, 2012 by Dr. Psoinos.
Disclosure information. Authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baker CC, Oppenheimer L, Stephens B, Lewis FR, Trunkey DD. Epidemiology of trauma deaths. Am J Surg. 1980;140(1):144–50. doi: 10.1016/0002-9610(80)90431-6. [DOI] [PubMed] [Google Scholar]
- 2.Shock CoTaCo. Accidental Death and Disability: The neglected disease of modern society. Washington, DC: Institute of Medicine; 1966. [Google Scholar]
- 3.MacKenzie EJ, Rivara FP, Jurkovich GJ, Nathens AB, Frey KP, Egleston BL, Salkever DS, Scharfstein DO. A national evaluation of the effect of trauma-center care on mortality. N Engl J Med. 2006;354(4):366–78. doi: 10.1056/NEJMsa052049. [DOI] [PubMed] [Google Scholar]
- 4.Mann NC, Mullins RJ, MacKenzie EJ, Jurkovich GJ, Mock CN. Systematic review of published evidence regarding trauma system effectiveness. J Trauma. 1999;47(3 Suppl):S25–33. doi: 10.1097/00005373-199909001-00007. Epub 1999/09/25. [DOI] [PubMed] [Google Scholar]
- 5.Celso B, Tepas J, Langland-Orban B, Pracht E, Papa L, Lottenberg L, Flint L. A systematic review and meta-analysis comparing outcome of severely injured patients treated in trauma centers following the establishment of trauma systems. J Trauma. 2006;60(2):371–8. doi: 10.1097/01.ta.0000197916.99629.eb. [DOI] [PubMed] [Google Scholar]
- 6.Mullins RJ, Mann NC, Hedges JR, Worrall W, Helfand M, Zechnich AD, Jurkovich GJ. Adequacy of hospital discharge status as a measure of outcome among injured patients. JAMA. 1998;279(21):1727–31. doi: 10.1001/jama.279.21.1727. [DOI] [PubMed] [Google Scholar]
- 7.Davidson GH, Hamlat CA, Rivara FP, Koepsell TD, Jurkovich GJ, Arbabi S. Long-term survival of adult trauma patients. JAMA. 2011;305(10):1001–7. doi: 10.1001/jama.2011.259. [DOI] [PubMed] [Google Scholar]
- 8.A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT) The SUPPORT Principal Investigators. JAMA. 1995;274(20):1591–8. [PubMed] [Google Scholar]
- 9.Pronovost P, Angus DC. Economics of end-of-life care in the intensive care unit. Crit Care Med. 2001;29(2 Suppl):N46–51. doi: 10.1097/00003246-200102001-00009. [DOI] [PubMed] [Google Scholar]
- 10.Angus DC, Barnato AE, Linde-Zwirble WT, Weissfeld LA, Watson RS, Rickert T, Rubenfeld GD. Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med. 2004;32(3):638–43. doi: 10.1097/01.ccm.0000114816.62331.08. [DOI] [PubMed] [Google Scholar]
- 11.Calfo S, Smith J, Zezza M. Last Year of Life Study. Washington, DC: 2008. [Google Scholar]
- 12.Nelson JE, Angus DC, Weissfeld LA, Puntillo KA, Danis M, Deal D, Levy MM, Cook DJ. End-of-life care for the critically ill: A national intensive care unit survey. Crit Care Med. 2006;34(10):2547–53. doi: 10.1097/01.CCM.0000239233.63425.1D. [DOI] [PubMed] [Google Scholar]
- 13.Mosenthal AC, Murphy PA, Barker LK, Lavery R, Retano A, Livingston DH. Changing the culture around end-of-life care in the trauma intensive care unit. J Trauma. 2008;64(6):1587–93. doi: 10.1097/TA.0b013e318174f112. [DOI] [PubMed] [Google Scholar]
- 14.Covinsky KE, Fuller JD, Yaffe K, Johnston CB, Hamel MB, Lynn J, Teno JM, Phillips RS. Communication and decision-making in seriously ill patients: findings of the SUPPORT project. The Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. J Am Geriatr Soc. 2000;48(5 Suppl):S187–93. doi: 10.1111/j.1532-5415.2000.tb03131.x. [DOI] [PubMed] [Google Scholar]
- 15.Sligl WI, Eurich DT, Marrie TJ, Majumdar SR. Age still matters: prognosticating short- and long-term mortality for critically ill patients with pneumonia. Crit Care Med. 2010;38(11):2126–32. doi: 10.1097/CCM.0b013e3181eedaeb. [DOI] [PubMed] [Google Scholar]
- 16.Shehabi Y, Bellomo R, Reade MC, Bailey M, Bass F, Howe B, McArthur C, Seppelt IM, Webb S, Weisbrodt L. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186(8):724–31. doi: 10.1164/rccm.201203-0522OC. [DOI] [PubMed] [Google Scholar]
- 17.Rockwood K, Noseworthy TW, Gibney RT, Konopad E, Shustack A, Stollery D, Johnston R, Grace M. One-year outcome of elderly and young patients admitted to intensive care units. Crit Care Med. 1993;21(5):687–91. doi: 10.1097/00003246-199305000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, Jr, Inouye SK, Bernard GR, Dittus RS. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–62. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 19.Chelluri L, Im KA, Belle SH, Schulz R, Rotondi AJ, Donahoe MP, Sirio CA, Mendelsohn AB, Pinsky MR. Long-term mortality and quality of life after prolonged mechanical ventilation. Crit Care Med. 2004;32(1):61–9. doi: 10.1097/01.CCM.0000098029.65347.F9. [DOI] [PubMed] [Google Scholar]
- 20.Brattstrom O, Larsson E, Granath F, Riddez L, Bell M, Oldner A. Time dependent influence of host factors on outcome after trauma. Eur J Epidemiol. 2012;27(3):233–41. doi: 10.1007/s10654-012-9651-4. [DOI] [PubMed] [Google Scholar]
- 21.Brinkman S, de Jonge E, Abu-Hanna A, Arbous MS, de Lange DW, de Keizer NF. Mortality after hospital discharge in ICU patients. Crit Care Med. 2013;41(5):1229–36. doi: 10.1097/CCM.0b013e31827ca4e1. [DOI] [PubMed] [Google Scholar]
- 22.Davis KB, Fisher L, Gillespie MJ, Pettinger M. A test of the National Death Index using the Coronary Artery Surgery Study (CASS) Controlled clinical trials. 1985;6(3):179–91. doi: 10.1016/0197-2456(85)90001-7. [DOI] [PubMed] [Google Scholar]
- 23.Hermansen SW, Leitzmann MF, Schatzkin A. The impact on National Death Index ascertainment of limiting submissions to Social Security Administration Death Master File matches in epidemiologic studies of mortality. American journal of epidemiology. 2009;169(7):901–8. doi: 10.1093/aje/kwn404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wojcik NC, Huebner WW, Jorgensen G. Strategies for using the National Death Index and the Social Security Administration for death ascertainment in large occupational cohort mortality studies. American journal of epidemiology. 2010;172(4):469–77. doi: 10.1093/aje/kwq130. [DOI] [PubMed] [Google Scholar]
- 25.Curb JD, Ford CE, Pressel S, Palmer M, Babcock C, Hawkins CM. Ascertainment of vital status through the National Death Index and the Social Security Administration. American journal of epidemiology. 1985;121(5):754–66. doi: 10.1093/aje/121.5.754. [DOI] [PubMed] [Google Scholar]
- 26.Gunst M, Ghaemmaghami V, Gruszecki A, Urban J, Frankel H, Shafi S. Changing epidemiology of trauma deaths leads to a bimodal distribution. Proc (Bayl Univ Med Cent) 2010;23(4):349–54. doi: 10.1080/08998280.2010.11928649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Knegt C, Meylaerts SA, Leenen LP. Applicability of the trimodal distribution of trauma deaths in a Level I trauma centre in the Netherlands with a population of mainly blunt trauma. Injury. 2008;39(9):993–1000. doi: 10.1016/j.injury.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 28.Demetriades D, Kimbrell B, Salim A, Velmahos G, Rhee P, Preston C, Gruzinski G, Chan L. Trauma deaths in a mature urban trauma system: is “trimodal” distribution a valid concept? J Am Coll Surg. 2005;201(3):343–8. doi: 10.1016/j.jamcollsurg.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Pang JM, Civil I, Ng A, Adams D, Koelmeyer T. Is the trimodal pattern of death after trauma a dated concept in the 21st century? Trauma deaths in Auckland 2004. Injury. 2008;39(1):102–6. doi: 10.1016/j.injury.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 30.Skaga NO, Eken T, Jones JM, Steen PA. Different definitions of patient outcome: consequences for performance analysis in trauma. Injury. 2008;39(5):612–22. doi: 10.1016/j.injury.2007.11.426. [DOI] [PubMed] [Google Scholar]
- 31.Claridge JA, Leukhardt WH, Golob JF, McCoy AM, Malangoni MA. Moving beyond traditional measurement of mortality after injury: evaluation of risks for late death. J Am Coll Surg. 2010;210(5):788–94. 94–6. doi: 10.1016/j.jamcollsurg.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 32.Probst C, Zelle BA, Sittaro NA, Lohse R, Krettek C, Pape HC. Late death after multiple severe trauma: when does it occur and what are the causes? J Trauma. 2009;66(4):1212–7. doi: 10.1097/TA.0b013e318197b97c. [DOI] [PubMed] [Google Scholar]
- 33.Laupland KB, Svenson LW, Grant V, Ball CG, Mercado M, Kirkpatrick AW. Long-term mortality outcome of victims of major trauma. Injury. 2010;41(1):69–72. doi: 10.1016/j.injury.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Short TG, Buckley TA, Rowbottom MY, Wong E, Oh TE. Long-term outcome and functional health status following intensive care in Hong Kong. Crit Care Med. 1999;27(1):51–7. doi: 10.1097/00003246-199901000-00026. Epub 1999/02/06. [DOI] [PubMed] [Google Scholar]
- 35.Brattstrom O, Granath F, Rossi P, Oldner A. Early predictors of morbidity and mortality in trauma patients treated in the intensive care unit. Acta Anaesthesiol Scand. 2010;54(8):1007–17. doi: 10.1111/j.1399-6576.2010.02266.x. [DOI] [PubMed] [Google Scholar]
- 36.Azoulay E, Adrie C, De Lassence A, Pochard F, Moreau D, Thiery G, Cheval C, Moine P, Garrouste-Orgeas M, Alberti C, Cohen Y, Timsit JF. Determinants of postintensive care unit mortality: a prospective multicenter study. Crit Care Med. 2003;31(2):428–32. doi: 10.1097/01.CCM.0000048622.01013.88. [DOI] [PubMed] [Google Scholar]
- 37.Mosenthal AC, Murphy PA. Trauma care and palliative care: time to integrate the two? J Am Coll Surg. 2003;197(3):509–16. doi: 10.1016/S1072-7515(03)00651-3. [DOI] [PubMed] [Google Scholar]
- 38.Carson SS, Bach PB. Predicting mortality in patients suffering from prolonged critical illness: an assessment of four severity-of-illness measures. Chest. 2001;120(3):928–33. doi: 10.1378/chest.120.3.928. [DOI] [PubMed] [Google Scholar]
- 39.Rue M, Quintana S, Alvarez M, Artigas A. Daily assessment of severity of illness and mortality prediction for individual patients. Crit Care Med. 2001;29(1):45–50. doi: 10.1097/00003246-200101000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Laupland KB, Kirkpatrick AW, Kortbeek JB, Zuege DJ. Long-term mortality outcome associated with prolonged admission to the ICU. Chest. 2006;129(4):954–9. doi: 10.1378/chest.129.4.954. [DOI] [PubMed] [Google Scholar]
- 41.Arabi Y, Venkatesh S, Haddad S, Al Shimemeri A, Al Malik S. A prospective study of prolonged stay in the intensive care unit: predictors and impact on resource utilization. Int J Qual Health Care. 2002;14(5):403–10. doi: 10.1093/intqhc/14.5.403. [DOI] [PubMed] [Google Scholar]
- 42.Stricker K, Rothen HU, Takala J. Resource use in the ICU: short- vs. long-term patients. Acta Anaesthesiol Scand. 2003;47(5):508–15. doi: 10.1034/j.1399-6576.2003.00083.x. [DOI] [PubMed] [Google Scholar]
- 43.Tsevat J, Cook EF, Green ML, Matchar DB, Dawson NV, Broste SK, Wu AW, Phillips RS, Oye RK, Goldman L. Health values of the seriously ill. SUPPORT investigators. Ann Intern Med. 1995;122(7):514–20. doi: 10.7326/0003-4819-122-7-199504010-00007. [DOI] [PubMed] [Google Scholar]
- 44.Weaver JL, Bradley CT, Brasel KJ. Family engagement regarding the critically ill patient. Surg Clin North Am. 2012;92(6):1637–47. doi: 10.1016/j.suc.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 45.Franklin GA, Cannon RW, Smith JW, Harbrecht BG, Miller FB, Richardson JD. Impact of withdrawal of care and futile care on trauma mortality. Surgery. 2011;150(4):854–60. doi: 10.1016/j.surg.2011.07.065. [DOI] [PubMed] [Google Scholar]
- 46.Cooper Z, Rivara FP, Wang J, MacKenzie EJ, Jurkovich GJ. Withdrawal of life-sustaining therapy in injured patients: variations between trauma centers and nontrauma centers. J Trauma. doi: 10.1097/TA.0b013e31819ea047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nathens AB, Rivara FP, Wang J, Mackenzie EJ, Jurkovich GJ. Variation in the rates of do not resuscitate orders after major trauma and the impact of intensive care unit environment. J Trauma. 2008;64(1):81–8. doi: 10.1097/TA.0b013e31815dd4d7. discussion 8–91. [DOI] [PubMed] [Google Scholar]
- 48.O’Mahony S, McHenry J, Blank AE, Snow D, Eti Karakas S, Santoro G, Selwyn P, Kvetan V. Preliminary report of the integration of a palliative care team into an intensive care unit. Palliat Med. 2010;24(2):154–65. doi: 10.1177/0269216309346540. [DOI] [PubMed] [Google Scholar]
- 49.Campbell ML. Palliative care consultation in the intensive care unit. Crit Care Med. 2006;34(11 Suppl):S355–8. doi: 10.1097/01.CCM.0000237248.16818.E5. [DOI] [PubMed] [Google Scholar]