Abstract
Objective
To assess how frequently pediatric practitioners perform latent tuberculosis infection (LTBI) screening according to guidelines. We hypothesized that screening occurs less frequently among children whose parents do not speak English as the primary language.
Study design
We conducted a retrospective cohort study of patients attending well-child visits in an urban academic pediatric primary care clinic from 4/1/2012–3/31/2013. We assessed documentation of three LTBI screening components and tested the association between parent primary language and tuberculin skin test (TST) placement and documentation of results.
Results
During the study period, 387 of 9,143 (4%) children had no documentation of screening question responses. Of the other 8,756 children, 831 (10%) were identified as high-risk for LTBI. Of these, 514 (62%) did not have documented TST administration in the appropriate time frame. Thirty-nine of 213 (18%) children who had a TST placed did not have documented results. Multivariable regression showed that parent language was not associated with TST placement or documentation of results, but non-Hispanic Black children were more likely not to have a documented test result (aOR 2.12, 95% CI 1.07–4.19, P=0.03) when adjusting for age, sex, parent primary language, insurance status, day of the week and study year of TST placement.
Conclusions
Parent primary language was not associated with LTBI testing. However, we found substantial gaps in TST placement and documentation of TST result among high-risk children, the latter of which was associated with race/ethnicity. Targeted quality improvement efforts should focus on developing processes to ensure complete screening in high-risk children.
Keywords: Tuberculin skin test, Mycobacterium
Tuberculosis in children remains a public health concern in the United States (US) and internationally. According to the World Health Organization, in 2012 over 74,000 children died from tuberculosis worldwide.1 Despite an overall decrease in annual incidence in the US, pediatric tuberculosis case rates among Hispanic, Black, and foreign-born children remain disproportionately high.2, 3 Child or parent country of birth are among the most important risk factors for pediatric tuberculosis in the US as 31% of children diagnosed with tuberculosis from 2008 to 2010 were foreign born, and 66% of all US-born children diagnosed with tuberculosis had at least one foreign-born parent.3
Treatment of individuals with latent tuberculosis infection (LTBI) remains an important strategy for prevention of LTBI reactivation and reduction of the burden of active tuberculosis among populations in low-incidence countries.4–7 In 2004, the American Academy of Pediatrics (AAP) updated its recommendations for a targeted LTBI screening approach.7–9 To identify children at highest risk for LTBI, the AAP recommends four screening questions be asked at well-child visits at least once annually.10, 11 Based on the most important risk factors for LTBI, these screening questions identify children who subsequently require tuberculin skin test (TST) placement and reading for the diagnosis of LTBI.
Despite the most recent recommendations from the AAP, pediatric tuberculosis screening rates are low in many practices.12–14 Therefore, we sought to better characterize the frequency of adherence to the AAP recommendations at each step of the screening process among children at a large academic pediatric primary care institution in the US. We also sought to identify patient-specific factors that may contribute to any identified gaps in adherence to screening and testing recommendations. Recognizing that non-English speakers often have difficulty accessing health care and that children of foreign-born parents are at higher risk for LTBI,15, 16 we hypothesized that parent primary language other than English would be associated with less frequent adherence to AAP recommended LTBI screening.
METHODS
We performed a retrospective cohort study, utilizing electronic medical record review to test the association between parent primary language and incomplete documentation of LTBI screening. The pediatric primary care clinic from which we sampled serves a broad range of racial and ethnic minority patients with over 40 languages spoken by patients and families. Additionally, 72% of the patients were insured by TennCare, the state’s form of Medicaid. The clinic used tuberculosis skin tests (TSTs) exclusively for LTBI screening during the study period. The clinic was not affected by the US Tubersol shortage documented in April 2013.17 The Vanderbilt University Institutional Review Board approved the study and granted a waiver of informed consent.
We included all children between the ages of one and 18 years who had a well-child visit in the clinic between April 1, 2012 and March 31, 2013. We limited the cohort to well-child visits because our primary objective was to study screening practices of asymptomatic individuals.
Documented Components of LTBI Screening
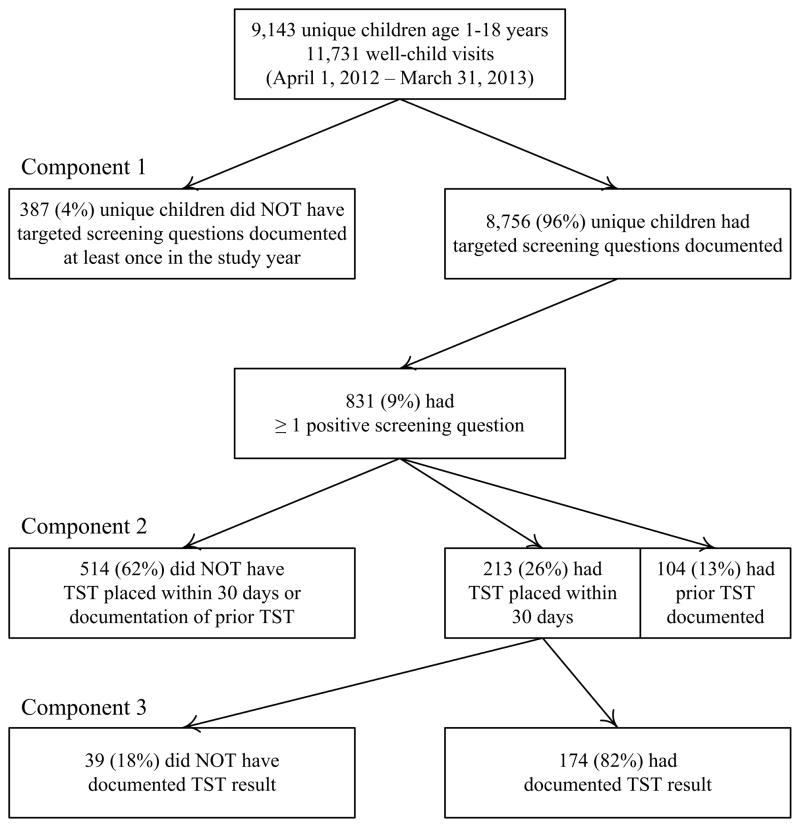
We evaluated three components of targeted LTBI screening by determining if the following were documented in the electronic medical record (Figure; available at www.jpeds.com).
Figure.
Components of LTBI screening included documentation of: (1) identification of high-risk children at annual well-child visits; (2) placement of a TST within 30 days for those at risk (or previous TST documented); and (3) results of TSTs that were placed. Percentage sums exceeding 100% due to rounding.
Identification of high-risk children through screening questions
Since March 2011, the pediatric practitioners in the clinic have used an electronic template used during well-child checks that has a specifically designed feature to facilitate provider documentation of LTBI screening question responses. The template includes a slightly modified version of the AAP recommended tuberculosis screening questions (Table I) with “Yes,” “No,” and “Unsure” response options, and a free text field following the statement “Date of child’s most recent PPD [purified protein derivative] and results.” We considered a child to have been screened appropriately if well-child visit documentation noted one of three conditions: (1) a “No” response to all four screening questions; (2) a “Yes” response to any of the four questions; or (3) any associated comment in the free text field indicating completed screening, even if none of the questions were answered. Children who had multiple well-child visits during the study year needed to have documentation of LTBI screening questions at least once to be considered appropriately screened in accordance with annual screening recommendations. Every child who had a “Yes” response to at least one of the four screening questions was designated high-risk for LTBI.
TABLE 1.
Responses to Recommended Screening Questions for Pediatric Latent Tuberculosis Infection in the United States*
| Question | Yes (% of 831) |
|---|---|
| Has a family member or contact had tuberculosis disease? | 128 (15%) |
| Has a family member had a positive tuberculin skin test result? | 293 (35%) |
| Was your child born in a high-risk country†? | 279 (34%) |
| Has your child traveled (had contact with resident populations) to a high-risk country† for more than one week? | 425 (51%) |
These questions are listed in the template of the provider well-child visit note at the study clinic. They have been adapted from AAP10 and RedBook11 guidelines.
The clinic template does not specifically list high-risk countries. The AAP recommendations consider countries in Africa, Asia, Latin America, or Eastern Europe to be high-risk countries.10
Placement of a TST within 30 days for those at high-risk (or documentation of a previous TST)
We subsequently assessed whether children identified as high-risk had a TST placed within 30 days following the well-child visit at which they were screened. High-risk children who did not have a TST placed within 30 days were considered to have care consistent with AAP recommendations if: 1) they had a TST result recorded in the 365 days preceding the date of the well-child visit at which they were identified as high-risk, or 2) their only positive screening question was birth in a high-risk country, and they had documentation of a TST result at any time in the past. The results and follow-up of children with prior TST results were beyond the scope of the current study.
Results of TSTs that were placed
Care consistent with AAP recommendations was defined as documentation of a TST result that occurred between two and three days after the TST was placed. For those patients who had multiple TSTs placed during the study period, only the first TST was included to preserve independence of observations. We did not evaluate the size of induration reported for TST results. As a pre-specified secondary analysis, in order to expand the sample size of children who had a TST placed, we identified all children (1–18 years old) who had a TST placed between April 1, 2010 and March 31, 2013 in association with a primary care clinic visit, regardless of the purpose of their clinic visit (i.e., not limited to well-child clinic visits). We did not use this extended study period for the first two components because the implementation of the screening questions in the electronic templates did not occur until March 2011. Finally, we reviewed the charts of all children who had positive TST results to see if a health care provider noted the positive result, a referral to the health department was made, and a chest x-ray was performed.
Parent Primary Language
Parent primary language was self-reported by parents at registration for pediatric visits and was categorized as English, Spanish, Arabic, or other. Parent primary language was selected as the primary predictor variable because it approximates foreign-born status and was readily available in the electronic medical record. In 2010, approximately 85% of the foreign-born population in the US spoke a non-English language at home, and approximately 10% of the US-born population spoke a non-English language at home.18
Covariates
For the primary analysis, we selected a limited set of covariates including patient age, sex, and race/ethnicity (Hispanic; non-Hispanic Black; non-Hispanic White; and other non-Hispanic). We assessed race/ethnicity because tuberculosis disproportionately affects racial and ethnic minorities in the US, and racial and ethnic disparities exist in access to health care among pediatric patients.3, 19, 20 We were limited in our ability to extract further covariates for the primary analysis due to the mechanism of data query from the electronic medical record. For the secondary analysis, we were also able to evaluate patient insurance status (TennCare, Private Insurance, or Self-Pay) and the following clinic characteristics: provider type (Attending, Resident, or Nurse Practitioner), clinic volume on day of TST placement (continuous), day of the week the TST was placed (Monday through Saturday), time of day the TST was placed (AM or PM), and the study year during which the TST was placed (4/1/10–3/31/11, 4/1/11–3/31/12, or 4/1/12–3/31/13).
Statistical Analyses
Participant characteristics were summarized using median and interquartile range for continuous variables and proportions for categorical variables. LTBI screening-related differences in demographic and clinic characteristics were evaluated using the χ2 test for categorical variables and the Wilcoxon rank-sum test for continuous variables.
We used a multivariable logistic regression model to evaluate the association between parent primary language and TST placement among high-risk children. Due to the low number of children who did not have documented TST results in the main analysis, we used the secondary analysis for a more robust multivariable logistic regression model to evaluate the association of parent primary language with documented TST results.
We used multiple imputation techniques with one hundred imputation data sets to perform a sensitivity analysis that included children with missing covariates. All analyses were performed using Stata, version 12.1 (Stata Corporation, College Station, TX, USA). All P values were two-sided and P≤0.05 was considered significant.
RESULTS
Documented Components of LTBI Screening
During the study period, 9,143 children ages 1–18 were seen at 11,731 well-child visits. Of these children, 4,417 (48%) were female, 3,932 (43%) were Black, 2,418 (26%) were Hispanic or Latino. 2,842 (31%) of the parents spoke a primary language other than English, including 1,815 (20%) who spoke Spanish and 605 (7%) who spoke Arabic. Providers noted that 279 (3%) children were born in a high-risk country. Of the 9,143 unique children seen at well-child visits, 8,756 (96%) had LTBI screening questions asked at least once during the study period (Figure).
TST Placement
Of the 8,756 children for whom LTBI screening questions were asked, 831 (9%) had a “Yes” response to at least one of the four screening questions and were considered high-risk for LTBI. Of these, 213 (26%) children had a TST placed within 30 days, and 104 (13%) children had documentation of prior TST results (317 total children who were considered to have appropriate testing). The remaining 514 (62%) children at risk for LTBI did not have a TST placed according to AAP recommendations (Percentage sums exceed 100% due to rounding), 59 of which had a TST placed more than 30 days after being identified as high-risk. Children who did not have a TST placed were significantly older than those who did have a TST placed (6.0 years versus 5.3 years; P=0.03), but did not significantly differ based on sex, race/ethnicity, or parent primary language (Table II). Travel to a high-risk country was the screening question most frequently answered “Yes” (51%), and having a family member or contact with tuberculosis disease was least frequently answered “Yes” (15%) (Table I).
TABLE 2.
Characteristics of Children at High Risk for Latent Tuberculosis Infection, by Tuberculin Skin Test Placement Status
| TST placed (n = 317) | No TST placed (n = 514) | P Value | |
|---|---|---|---|
| Age, median (IQR) years | 5.3 (2.6, 8.8) | 6.0 (3.2, 9.3) | 0.03 |
| Male Sex, n (%) | 156 (49) | 270 (53) | 0.35 |
| Race/Ethnicity*, n (%) | 0.26 | ||
| Hispanic | 97 (33) | 135 (29) | |
| Black, Non-Hispanic | 55 (19) | 109 (23) | |
| White, Non-Hispanic | 109 (37) | 162 (35) | |
| Other | 32 (11) | 63 (13) | |
| Parent Primary Language, n (%) | 0.06 | ||
| English | 132 (42) | 245 (48) | |
| Spanish | 69 (22) | 96 (19) | |
| Arabic | 84 (26) | 105 (20) | |
| Other | 32 (10) | 68 (13) |
TST, tuberculin skin test; IQR, interquartile range.
n = 762
Documentation of TST Results
Of the 213 children who had a TST placed within 30 days of their well-child visit, 39 (18%) did not have a documented test result. The secondary analysis, which included all TST placements between April 1, 2010 and March 31, 2013, showed that 181 of 805 (22%) children did not have documentation of a TST result within 72 hours of placement. Children who did not have a documented TST result significantly differed from children who did have a documented TST result with regard to race/ethnicity, parent primary language, and year of TST placement (Table III).
TABLE 3.
Characteristics of Children Who had Tuberculin Skin Tests Placed, by Tuberculin Skin Test Result Documentation Status (data from secondary analysis April 1, 2010 – March 31, 2013
| TST Result Documented (n = 624) | No TST Result Documented (n = 181) | Total n | P Value | |
|---|---|---|---|---|
| Age, median (IQR), years | 5.4 (2.9, 9.1) | 5.0 (2.5, 8.2) | 805 | 0.16 |
| Male Sex, n (%) | 321 (51) | 99 (55) | 805 | 0.44 |
| Race/Ethnicity, n (column% / row%) | 736 | <0.001 | ||
| Hispanic | 204 (35/80) | 50 (31/20) | 254 | |
| Black, Non-Hispanic | 98 (17/64) | 56 (35/36) | 154 | |
| White, Non-Hispanic | 199 (35/84) | 38 (24/16) | 237 | |
| Other | 74 (13/81) | 17 (11/19) | 91 | |
| Parent Primary Language, n (column% / row%) | 805 | 0.05 | ||
| English | 261 (42/73) | 95 (52/27) | 356 | 0.01* |
| Spanish | 159 (26/80) | 41 (23/20) | 200 | 0.44* |
| Arabic | 139 (22/84) | 27 (15/16) | 166 | 0.03* |
| Other | 65 (10/78) | 18 (10/22) | 83 | 0.85* |
| Type of Insurance, n (column% / row%) | 805 | 0.16 | ||
| TennCare | 489 (78/77) | 145 (80/23) | 634 | |
| Private | 126 (20/81) | 30 (17/19) | 156 | |
| Self-Pay | 9 (1/60) | 6 (3/40) | 15 | |
| Type of Provider, n (column% / row%) | 805 | 0.43 | ||
| Resident | 389 (62/77) | 119 (66/23) | 508 | |
| Attending | 185 (30/80) | 45 (25/20) | 230 | |
| Nurse Practitioner | 50 (8/75) | 17 (9/25) | 67 | |
| Clinic Volume, median (IQR) | 180 (159, 200) | 180 (162, 194) | 805 | 0.85 |
| Time of Day TST Placed, n (column% / row%) | 779 | 0.09 | ||
| Morning | 237 (39/82) | 53 (32/18) | 290 | |
| Afternoon | 374 (61/76) | 115 (68/24) | 489 | |
| Day of Week TST Placed, n (column% / row%) | 805 | 0.38 | ||
| Monday | 193 (31/81) | 45 (25/19) | 238 | |
| Tuesday | 148 (24/75) | 49 (27/25) | 197 | |
| Wednesday | 150 (24/76) | 47 (26/24) | 197 | |
| Thursday | 46 (7/71) | 19 (11/29) | 65 | |
| Friday | 79 (13/80) | 20 (11/20) | 99 | |
| Saturday | 8 (1/89) | 1 (1/11) | 9 | |
| Year TST Placed, n (column% / row%) | 805 | <0.001 | ||
| 1 (4/1/10–3/31/11) | 147 (24/74) | 51 (28/26) | 198 | |
| 2 (4/1/11–3/31/12) | 200 (32/72) | 79 (44/28) | 279 | |
| 3 (4/1/12–3/31/13) | 277 (44/84) | 51 (28/16) | 328 |
TST, tuberculin skin test; IQR, interquartile range.
P value represents comparison between row parent language and all other parent languages, e.g., English vs non-English, Spanish vs non-Spanish, etc.
Positive TSTs
Among the 624 children who had a documented TST result in the secondary analysis, 43 (7%) had a positive TST, all of whom had documented follow-up by a provider. Of 41 children with a positive TST who did not have a prior diagnosis of tuberculosis or a prior positive TST, 40 (98%) were referred to the local health department for further evaluation and care. The one child who was not referred to the local health department had a documented negative chest x-ray.
Parent Primary Language and LTBI Screening
TST Placement
Parent primary languages other than English were not significantly associated with placement of a TST in a multivariable logistic regression model adjusted for age, sex, and race/ethnicity (Table IV; available at www.jpeds.com). Age was the only factor with a significant association; older children were more likely not to have a TST placed (adjusted Odds Ratio [aOR] 1.05 per year of age, 95% Confidence Interval [CI] 1.01–1.09, P=0.02)
TABLE 4.
Factors Associated with Not Having a Tuberculin Skin Test Placed, Multivariable Logistic Regression
| Factor | aOR | 95% CI | P Value |
|---|---|---|---|
| Age | 1.05 | 1.01–1.09 | 0.02 |
| Male sex | 1.17 | 0.88–1.55 | 0.29 |
| Race/Ethnicity (compared to Hispanic) | |||
| Black, non-Hispanic | 1.34 | 0.77–2.33 | 0.30 |
| White, non-Hispanic | 1.28 | 0.73–2.26 | 0.38 |
| Other, non-Hispanic | 1.33 | 0.68–2.61 | 0.40 |
| Parent language (compared to English) | |||
| Spanish | 0.90 | 0.53–1.52 | 0.68 |
| Arabic | 0.66 | 0.42–1.03 | 0.07 |
| Other | 1.12 | 0.65–1.93 | 0.68 |
aOR, adjusted odds ratio; CI, confidence interval
Documentation of TST Results
Parent primary language was also not associated with documentation of TST results in a multivariable logistic regression model adjusted for age, sex, race/ethnicity, insurance status, day of the week that TST was placed, and study year that TST was placed (Table V; available at www.jpeds.com). Non-Hispanic Black children, however, were more likely not to have a documented TST result (aOR 2.17, 95% CI 1.10–4.30, P=0.03) than children of other race/ethnicity categories. Additionally, younger children (aOR 0.95 per year of age, 95% CI 0.90–1.00, P=0.04) and children with TST placement in the third study year (aOR 0.51, 95% CI 0.32–0.83, P=0.007) were less likely to be missing a documented TST result.
TABLE 5.
Factors Associated with Not Having a Tuberculin Skin Test Result Documented, Multivariable Logistic Regression
| Factor | aOR | 95% CI | P Value |
|---|---|---|---|
| Age | 0.95 | 0.90–1.00 | 0.04 |
| Male sex | 1.16 | 0.80–1.67 | 0.44 |
| Race/Ethnicity (compared to Hispanic) | |||
| Black, non-Hispanic | 2.12 | 1.07–4.19 | 0.03 |
| White, non-Hispanic | 0.79 | 0.39–1.62 | 0.52 |
| Other, non-Hispanic | 0.94 | 0.42–2.15 | 0.89 |
| Parent language (compared to English) | |||
| Spanish | 0.82 | 0.41–1.61 | 0.56 |
| Arabic | 0.65 | 0.35–1.20 | 0.17 |
| Other | 0.53 | 0.25–1.09 | 0.08 |
| Day of the Week (compared to Monday) | |||
| Tuesday | 1.33 | 0.81–2.17 | 0.26 |
| Wednesday | 1.11 | 0.66–1.86 | 0.69 |
| Thursday | 1.37 | 0.68–2.79 | 0.38 |
| Friday | 0.95 | 0.51–1.79 | 0.88 |
| Saturday | 0.62 | 0.07–5.25 | 0.66 |
| Insurance (compared to Private) | |||
| Self-pay | 2.59 | 0.80–8.38 | 0.11 |
| TennCare | 1.23 | 0.75–2.02 | 0.42 |
| Study Year (compared to 4/1/10–3/31/11) | |||
| 4/1/11–3/31/12 | 1.11 | 0.71–1.76 | 0.64 |
| 4/1/12–3/31/13 | 0.51 | 0.32–0.83 | 0.01 |
aOR, adjusted odds ratio; CI, confidence interval
Of the 805 children in the expanded analysis, 69 (9%) were missing combined race and ethnicity data. When we used multiple imputation techniques to predict missing data, the regression model had similar findings, except that age was no longer significantly associated with documentation of TST results.
DISCUSSION
Our results identify gaps at each stage of LTBI screening in a large, urban academic medical center. Most notably, 62% of children who were identified as high-risk by LTBI screening questions did not have a TST placed; and 18–22% of children did not have a documented result within 72 hours of TST placement. Additionally, recommended screening questions were not documented at least once in the study year for 4% of children. However, we did not find the hypothesized association between parent primary language and placement of TST or documentation of TST results in adjusted analyses.
Few prior studies have quantified gaps at each step of pediatric tuberculosis screening. The results from this study indicate that the largest gap occurs in placement of a TST after a patient is identified as high-risk by LTBI screening questions (only 38% of children had either a TST placed within 30 days of positive screening or documentation of a prior TST). This is likely because if a parent will not be able to arrange the child’s return in 48 to 72 hours for test interpretation, providers at the clinic often ask the parent to return to clinic another time to have it placed. We were unable to assess whether parents were instructed to return later if a TST was not immediately placed. It is not clear why non-Hispanic Black children were the least likely to have a documented TST result, though it may indicate that parents of these children were the least likely to return for reading of the TST. These data suggest that use of an interferon-gamma release assay, which does not require a patient to return to have the results read, may warrant further investigation as a cost-effective screening approach for families for whom the use of a TST is not feasible. Furthermore, the results of an interferon-gamma release assay could be automatically entered into the patient electronic medical record, avoiding separate documentation of results required for the TST. Currently, lack of data comparing the performance of interferon-gamma release assays and TSTs and difficulties drawing enough blood in small children require caution when considering the use of interferon-gamma release assays in children aged < 5 years.21
The gaps in the targeted LTBI screening approach that we identified demonstrate the need for provider and clinic-specific quality improvement strategies. Previous studies have shown some promise in improving screening and testing rates using quality improvement approaches. A state-wide quality improvement initiative in North Carolina reported a baseline tuberculosis risk assessment and testing rate of 18% among two year olds, which improved to 39% with practice-specific quality improvement projects directed at the practice providers and staff.14 In contrast, other studies reported limited improvements in the percent of children who had their TST read by coordinating with school nurses, and no meaningful improvements with measures to improve parent knowledge or provide transportation back to clinic.13, 22 One clinic-specific step for improvement in our study clinic could be clarifying high-risk countries in the provider template.
Between 2008 and 2010 only 25% of pediatric tuberculosis cases in the US had no known international connection.3 According to one study that surveyed 20 US sites, US-born children with foreign-born parents had six times higher tuberculosis rates than US-born children with US-born parents.16 Because the clinic in our study does not reliably capture information about country of birth for children or their parents, we considered parent primary language a factor that could potentially approximate parent foreign birth, though this has not been studied before. We hypothesized that factors such as cultural or language barriers, difficulties returning to clinic, and stigma associated with tuberculosis could have contributed to incomplete LTBI screening.23–25 In the adjusted models, parent language was not significantly associated with adherence to LTBI screening recommendations. However, children whose parents speak primarily Arabic had more completed LTBI screenings in unadjusted models and approached significance in one of the adjusted models. Although not statistically significant, these results were counter to our hypothesis and suggest that parent language was not a significant barrier to thorough LTBI screening.
Rather than identifying an association between parent language and documentation of adherence with AAP recommended LTBI screening practices, we report uniformly distributed screening rates. Compared with previous studies, our findings show substantial improvement in LTBI screening. We report that 96% of children had documentation of LTBI screening questions, likely due to the use of electronic forms that incorporate these questions. In a previous survey of 520 pediatric primary care providers, 85% reported screening for tuberculosis risk factors. However, there was significant variation in the providers’ knowledge of the AAP screening recommendations and screening implementation.12 One study published in 2001 reported a completed tuberculosis-screening rate between 43–50% in an urban, academic pediatric practice.13 Despite improvements in overall screening since the release of updated AAP recommendations in 2004, incomplete documentation of pediatric LTBI screening continues to occur. We did find that children who had a TST placed in the last year of the secondary analysis were more likely to have documentation of TST results compared with the first year. This may suggest a temporal trend from ongoing clinic improvement efforts, potentially from the incorporation of the screening questions in well-child visit note templates in March 2011, or it may be related to other unmeasured factors.
The 2004 AAP recommendations were based on data from multiple case-control and large cohort studies that identified potential risk factors for child tuberculosis.26, 27 We found that 7% of children with completed TSTs had a positive result. Identification of more specific screening algorithms would ideally allow for even more targeted screening and be more cost effective. If rates of drug-resistant tuberculosis continue to rise, screening strategies that identify high-risk countries for drug-resistant LTBI may also be needed.28
Our study had several limitations. First, if providers documented LTBI screening, TST placement, or TST result data in areas of the medical record other than those designed for these purposes, we would not have captured their responses. Providers routinely use the clinic note template, so we expect that we captured most of the risk assessment that truly occurred. Second, among children identified as high-risk for LTBI who did not have placement of a TST, we do not have data to indicate why providers did not adhere to recommendations. Third, we were unable to identify patients who had a TST result recorded outside of the primary care clinic. It is a reasonable professional expectation, however, for the ordering provider or clinic to be made aware of and properly document a test result. Finally, because the local health department manages LTBI treatment, follow-up data were not available for children who were referred to the health department for a positive TST.
Further studies to identify barriers to each component of LTBI screening, particularly testing children who have been identified as high risk for LTBI, could lead to targeted strategies to optimize completed care.
Acknowledgments
Funded by the National Institute of Allergy and Infectious Diseases/National Institutes of Health (K24 A1065298, K08 AI 106420).
We thank Kathryn Carlson, MD (funded by Vanderbilt Division of Academic General Pediatrics Quality Improvement Fund and Vanderbilt Katherine A. Dodd Faculty Scholars Program), for her work implementing LTBI screening questions in the well-child visit templates in the electronic medical record.
Abbreviations
- aOR
Adjusted Odds Ratio
- AAP
American Academy of Pediatrics
- LTBI
Latent tuberculosis infection
- TST
Tuberculin skin test
Footnotes
Portions of the study were presented as an abstract at the Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting, Vancouver, BC, Canada, May 5, 2014.
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization. Global Tuberculosis Report 2013. World Health Organization; 2013. WHO/HTM/TB/2013.11. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Epidemiology of Pediatric Tuberculosis in the United States, 1993–2010. Atlanta: updated December 30, 2013; cited December 30, 2013. Available from: http://www.cdc.gov/tb/publications/slidesets/pediatricTB/default.htm. [Google Scholar]
- 3.Winston CA, Menzies HJ. Pediatric and adolescent tuberculosis in the United States, 2008–2010. Pediatrics. 2012;130:e1425–32. doi: 10.1542/peds.2012-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pareek M, Watson JP, Ormerod LP, Kon OM, Woltmann G, White PJ, et al. Screening of immigrants in the UK for imported latent tuberculosis: a multicentre cohort study and cost-effectiveness analysis. Lancet Infect Dis. 2011;11:435–44. doi: 10.1016/S1473-3099(11)70069-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruz AT, Mandalakas AM, Starke JR. Childhood tuberculosis: a preventable disease not being prevented. Pediatrics. 2012;130:e1672–3. doi: 10.1542/peds.2012-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Morbidity and mortality weekly report; Centers for Disease Control. 2000;49:1–51. [PubMed] [Google Scholar]
- 7.Pediatric Tuberculosis Collaborative Group. Targeted Tuberculin Skin Testing and Treatment of Latent Tuberculosis Infection in Children and Adolescents. Pediatrics. 2004;114:1175–201. [Google Scholar]
- 8.American Academy of Pediatrics Committee on Infectious Diseases. Screening for tuberculosis in infants and children. Pediatrics. 1994;93:131–4. [PubMed] [Google Scholar]
- 9.American Academy of Pediatrics Committee on Infectious Diseases. Update on tuberculosis skin testing of children. Pediatrics. 1996;97:282–4. [PubMed] [Google Scholar]
- 10.Pediatric Tuberculosis Collaborative Group. Targeted Tuberculin Skin Testing and Treatment of Latent Tuberculosis Infection in Children and Adolescents. Pediatrics. 2004;114:1175–201. [Google Scholar]
- 11.American Academy of Pediatrics. Tuberculosis. In: Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red Book: 2012 Report of the Committee on Infectious Diseases. 29. Elk Grove Village, IL: American Academy of Pediatrics; 2012. [Google Scholar]
- 12.Lazar CM, Sosa L, Lobato MN. Practices and policies of providers testing school-aged children for tuberculosis, Connecticut, 2008. J Community Health. 2010;35:495–9. doi: 10.1007/s10900-009-9218-9. [DOI] [PubMed] [Google Scholar]
- 13.DeLago CW, Spector ND, Moughan B, Moran MM, Kersten H, Smals L. Collaboration with school nurses: improving the effectiveness of tuberculosis screening. Arch Pediatr Adolesc Med. 2001;155:1369–73. doi: 10.1001/archpedi.155.12.1369. [DOI] [PubMed] [Google Scholar]
- 14.Shaw JS, Wasserman RC, Barry S, Delaney T, Duncan P, Davis W, et al. Statewide quality improvement outreach improves preventive services for young children. Pediatrics. 2006;118:e1039–47. doi: 10.1542/peds.2005-2699. [DOI] [PubMed] [Google Scholar]
- 15.Flores G, Tomany-Korman SC. The language spoken at home and disparities in medical and dental health, access to care, and use of services in US children. Pediatrics. 2008;121:e1703–14. doi: 10.1542/peds.2007-2906. [DOI] [PubMed] [Google Scholar]
- 16.Pang J, Teeter LD, Katz DJ, Davidow AL, Miranda W, Wall K, et al. Epidemiology of tuberculosis in young children in the United States. Pediatrics. 2014;133:e494–504. doi: 10.1542/peds.2013-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. National shortage of purified-protein derivative tuberculin products. MMWR Morb Mortal Wkly Rep. 2013;62:312. [PMC free article] [PubMed] [Google Scholar]
- 18.US Census Bureau. American Community Survey Reports. May, 2012. The Foreign-Born Population in the United States: 2010. [Google Scholar]
- 19.Centers for Disease Control and Prevention. Reported Tuberculosis in the United States. Atlanta, GA: U.S. Department of Health and Human Services, CDC; 2012. [Google Scholar]
- 20.Flores G, Tomany-Korman SC. Racial and ethnic disparities in medical and dental health, access to care, and use of services in US children. Pediatrics. 2008;121:e286–98. doi: 10.1542/peds.2007-1243. [DOI] [PubMed] [Google Scholar]
- 21.Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K, et al. Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010;59:1–25. [PubMed] [Google Scholar]
- 22.Cheng TL, Ottolini MC, Baumhaft K, Brasseux C, Wolf MD, Scheidt PC. Strategies to increase adherence with tuberculosis test reading in a high-risk population. Pediatrics. 1997;100:210–3. doi: 10.1542/peds.100.2.210. [DOI] [PubMed] [Google Scholar]
- 23.Cheng EM, Chen A, Cunningham W. Primary language and receipt of recommended health care among Hispanics in the United States. J Gen Intern Med. 2007;22 (Suppl 2):283–8. doi: 10.1007/s11606-007-0346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naish J, Brown J, Denton B. Intercultural consultations: investigation of factors that deter non-English speaking women from attending their general practitioners for cervical screening. BMJ. 1994;309:1126–8. [PMC free article] [PubMed] [Google Scholar]
- 25.Tardin A, Dominice Dao M, Ninet B, Janssens JP. Tuberculosis cluster in an immigrant community: case identification issues and a transcultural perspective. Trop Med Int Health. 2009;14:995–1002. doi: 10.1111/j.1365-3156.2009.02325.x. [DOI] [PubMed] [Google Scholar]
- 26.Froehlich H, Ackerson LM, Morozumi PA Pediatric Tuberculosis Study Group of Kaiser Permanente NC. Targeted testing of children for tuberculosis: validation of a risk assessment questionnaire. Pediatrics. 2001;107:E54. doi: 10.1542/peds.107.4.e54. [DOI] [PubMed] [Google Scholar]
- 27.Ozuah PO, Ozuah TP, Stein RE, Burton W, Mulvihill M. Evaluation of a risk assessment questionnaire used to target tuberculin skin testing in children. JAMA. 2001;285:451–3. doi: 10.1001/jama.285.4.451. [DOI] [PubMed] [Google Scholar]
- 28.Finnell SM, Christenson JC, Downs SM. Latent tuberculosis infection in children: a call for revised treatment guidelines. Pediatrics. 2009;123:816–22. doi: 10.1542/peds.2008-0433. [DOI] [PubMed] [Google Scholar]