Abstract
Objective
Higher parity is associated with increased subclinical cardiovascular disease (CVD) in mid-life and older women, and with increased CVD risk overall. The relationship between parity, subclinical CVD, and infertility in overweight and obese young women has been infrequently evaluated.
Methods
Reproductive histories were obtained in 191 (73%) overweight and obese (BMI 25 – 39.9 kg/m2) young women participating in a weight loss trial. Baseline carotid intima-media thickness (IMT) and inter-adventitial diameter (IAD) were assessed via B-mode ultrasound. Linear regression was used to estimate the relationship between parity and carotid measures, adjusted for demographic, cardiovascular and reproductive risk factors.
Results
Nulliparous women (n=70, age 34.9 ± 7.1) had increased common carotid IAD (.230 mm, SE .08, P = .003) and mean CCA IMT (.031 mm, SE .01, P = .007) compared with parous women (n=102, age 39.5 ± 4.9), persisting after adjustment for age, race, and CVD risk factors. No other reproductive factors were statistically significantly associated.
Conclusions
Nulliparity is associated with markers of less healthy carotid arteries in a sample of disease-free, overweight or obese 25-45 year-old women. This may represent a beneficial effect of pregnancy or indicate overall better health in overweight/obese women capable of childbearing.
Keywords: obesity, cardiovascular risk, women
Introduction
Nearly two-thirds of US women of childbearing age are at elevated risk for cardiovascular disease (CVD) because they are overweight or obese,(1) but little is known about how reproductive factors influence CVD risk in this population. Studies demonstrate a relationship between parity (number of births after 20 weeks gestation)(2) and CVD risk in the general population. A cohort study of more than a million Swedish women demonstrated a J-shaped relationship, with lowest CVD risk in women with 2 births. Women of lower parity had a 10% increased risk, and women of parity 5+ had a 50% increased risk.(3) This population, however, was leaner and more homogeneous than the US population. Understanding the relationship between parity and CVD risk in overweight women may lead to better screening and interventions to decrease excess risk of high or low parity in this already high risk group.
Changes in inter-adventitial diameter (IAD) and intima-media thickness (IMT) of the carotid arteries serve as markers of vascular aging and can be measured reliably and non-invasively using high frequency B-mode ultrasound. Multiple studies have demonstrated that increased IAD and thicker IMT are associated with traditional CVD risk factors and ultimately with cardiovascular events,(4, 5) and they are often used as surrogate markers for CVD risk. Several investigations have examined the relationship between parity and IMT with inconsistent results. In a prospective study of women of childbearing age, each birth over a 6 year period was associated with a 7.5 ± 3.2 μm increase in mean IMT of the common carotid artery (CCA).(6) Cross-sectional studies have largely shown increased CCA IMT with increased parity in midlife(7, 8) and younger women;(9) one study also showed increased CCA IMT in nulliparous women.(8)
Thus, increases in CCA IMT with parity may reflect a potential mechanism linking parity to CVD, but many questions remain unanswered. Women in the aforementioned studies were relatively lean and not representative of the current US population. They were primarily mid-life or older. The existing studies did not evaluate the effect of infertility on CVD risk, ignoring a potentially significant confounding factor. In the majority of studies, the CCA, not other segments of the carotid artery, was assessed. IMT measured in different segments of the carotid artery have different CVD risk factor associations and may reflect varying pathophysiology, thus providing further insight into the underlying CVD mechanisms involved.(10) Body mass index is most closely related to CCA IMT, while glucose metabolism is more related to bulb and ICA IMT.(10) Furthermore, none of the studies assessed parity's relationship with IAD, itself an independent CVD risk factor and marker of vascular remodeling and aging.(5, 11)
The purpose of this analysis is to explore the relationship between parity and structure of the common and internal carotid arteries and the carotid bulb in a US population of overweight or obese women of reproductive age without history of fertility problems. We hypothesized that parity would be positively related to IMT and IAD in the CCA.
Methods
SAVE Study Design and Population
This study is a secondary analysis of data from the Slow Adverse Vascular Effects (SAVE) clinical trial (NCT00366990). Methods for participant recruitment and intervention in the SAVE trial have been previously reported.(12) Briefly, SAVE is a randomized controlled trial examining effects of weight loss, physical activity, and sodium reduction on vascular health. Participants (N = 349) were 25-45 year-old women and men from Allegheny County, PA, who were physically inactive and overweight to class II obese (BMI 25 – 39.9). Exclusions were: 1) diabetes; 2) hypertension; 3) current use of cholesterol-lowering, antipsychotic, or vasoactive medicines; 4) underlying inflammatory conditions; 5) known atherosclerotic disease; and 6) pregnancy or breastfeeding. No other requirements about reproductive history were included. All participants signed an informed consent document approved by the University of Pittsburgh Institutional Review Board. SAVE enrolled 290 women between June 2007 and February 2009; 191 (66%) completed the reproductive history and 19 reported infertility, leaving 172 (59%) women for the analytic sample for this study.
Carotid Artery Measures
Carotid ultrasounds were performed at the University of Pittsburgh Ultrasound Research Laboratory (Pittsburgh, PA) using high resolution B-mode ultrasound (Siemans, Malvern, PA). This analysis uses the baseline values. The carotid arteries were imaged bilaterally at end diastole with participants supine. IMT, the distance from the media- adventitial interface to the intima-lumen interface, was measured bilaterally in four locations: the near and far walls of the common carotid artery 1 cm proximal to the carotid bulb, the far wall of the carotid bulb, and the far wall of the internal carotid artery (ICA) for the first 1 cm distal to the flow divider. Mean and maximum values were calculated for each carotid segment and the mean and maximum of the eight readings identified. Inter-adventitial diameter was measured as the distance from the adventitial-medial interface of the near wall of the common carotid artery to the medial -adventitial interface of the far wall. Reproducibility of IMT was excellent with an intraclass correlation coefficient of ≥0.82 between sonographers and ≥ 0.97 between readers.(12) A semi-automated reading program (AMS system) allowed the reading to be done by computer. (13)
Reproductive Histories
Starting June 1, 2009, all women participants were given reproductive history forms to complete. Studies have demonstrated high reliability and validity for maternal recall of pregnancy-related events.(14, 15)Participants reporting they had ever been pregnant then completed a form for each pregnancy. They designated each birth outcome as a live birth, tubal/ectopic pregnancy, abortion, miscarriage (fetus born before 20 weeks or 5 months gestation), stillbirth (baby lost after 20 weeks or 5 months gestation) or current pregnancy. Parity was calculated as the sum of live (n=257) and stillbirths (n=6). Women who reported a pregnancy since their baseline visit were coded with their parity at baseline.
Each pregnancy form asked for date of pregnancy outcome, birth weight, length of gestation, presence of specific pregnancy complications, and breastfeeding duration. Women were coded as having had any pregnancy complication if they reported a stillbirth, preeclampsia or gestational hypertension in any pregnancy, or a birth weight of < 2500 grams or gestational age of less than 37 weeks for any singleton pregnancy. Women were designated as having a history of infertility (n=19) if they answered yes to either: “Have you ever had a period of 12 months when you could not get pregnant although you would have liked to get pregnant” or “Have you ever taken any fertility medication to help you get pregnant?” Women reported usual menstrual cycle length and whether they had menstruated in the past 12 months.
Demographic, Physical, and Laboratory Measures
Participants provided self-reported information at baseline regarding age, gender, race, and smoking status. Staff measured height, weight and blood pressure using standardized protocols. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared.
Laboratory assays were performed on fasting serum samples at the Heinz Laboratory at the University of Pittsburgh (Pittsburgh, PA). Total cholesterol, HDL(c), LDL(c), and glucose were determined using standard laboratory procedures.
Statistical Analysis
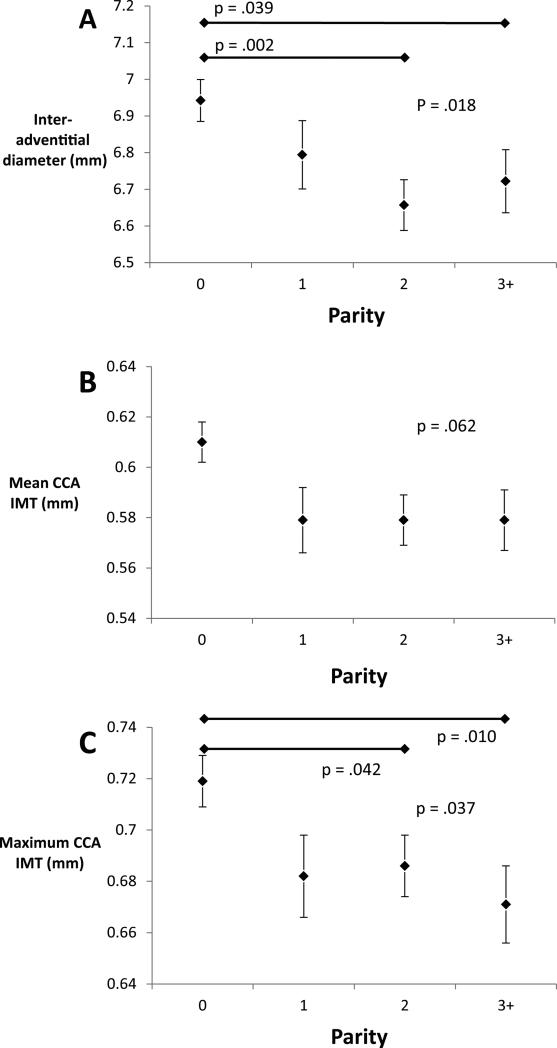
Normally distributed variables are presented as mean ± SD and categorical variables as percentages. Characteristics of women who did and did not participate in the reproductive study were compared. Due to small numbers of women of high parity (parity 4 n=8, parity 5 n=1), women of parity 3 or greater were analyzed together. As only 5 women reported a race other than white or black, women were classified as black or non-black for the analysis. Because of significant difference in age and racial composition of the different groups, age and race-adjusted means for the carotid measures were calculated. When analysis of variance testing detected a significant difference among these means pair-wise comparisons were done. Inspection of these results demonstrated that IAD and CCA IMT were greatest in nulliparous women and approximately equal in women with parity 1, 2 and 3+ (Figure 1). For this reason we compared nulliparous to parous women for the remaining analyses.
Figure 1.
Age and race-adjusted carotid artery measures by parity.
Multivariable linear regression was used to estimate the relationship between parity and the carotid measures. Covariates were considered a priori according to known factors associated with the carotid measures and CVD, and retained in the model if they were statistically significant predictors and affected the parameter estimates for any of the outcomes. The first model was adjusted for age, race, and educational achievement, our chosen measure of socioeconomic status. The second model included adjustment for those demographics plus baseline cardiovascular risk factors: BMI, current smoking, use of alcohol, average systolic blood pressure, fasting glucose and non-HDL cholesterol concentration. Educational achievement and non-HDL cholesterol concentration did not meet inclusion criteria; education was maintained a priori and non-HDL cholesterol was dropped from the model to maintain precision. A third model was considered including all of the previous covariates with reproductive factors: menopausal status, regularity of menstrual periods, breastfeeding, and the composite variable for pregnancy complications. None of these met the criteria for inclusion in the final model. Thus, the main model included parity, age, race, education, BMI, current smoking, alcohol use, systolic blood pressure and fasting glucose level. We explored interactions between BMI category (25-29.9, 30-34.9, 35+) and parity and between race and parity, and also adjusted the IMT models for IAD.
A separate analysis was done for parous women to estimate the effects of levels of parity (1, 2 or 3+) on carotid measures using the main model as with additional factors unique to parous women: age at first birth and time since last birth. These factors were not significant predictors and results are not presented here.
A sensitivity analysis to test for potential residual confounding based on infertility history considered a model including all women in SAVE to test the effect of known infertility on the carotid measures, controlling for parity, demographic and cardiovascular risk factors.
All statistical analyses were performed using SAS 9.2 or 9.3 (SAS Institute, Cary, NC) with significance level set at p = 0.05.
Results
Participants and non-participants in the reproductive study were largely comparable at baseline (Supplemental Table 1). Participants had a lower BMI (32.2 vs. 33.7, p = 0.01) and higher total (207.2 vs. 193.9, p = 0.01) and LDL (125.2 vs. 116.3, p = 0.03) cholesterol. For participants, average age increased with parity, from 34.9 years old for nulliparas to 40.7 for women of parity 3+ (Table 1). Black women were over-represented in the group with parity of 1 (46%, vs. ≤ 14% in the other groups). Nulliparous women were more likely to regularly consume alcohol. By study design all women were overweight or obese, and mean BMI of 32 did not differ significantly by parity.
TABLE 1.
Baseline characteristics of participants in SAVE reproductive substudy by parity
| Nulliparous n = 70 | Parity 1 n = 26 | Parity 2 n = 45 | Parity 3+ n=31 | Overall P Value | Nulliparous vs. Parous P Value | |
|---|---|---|---|---|---|---|
| Age (yr) | 34.9 ± 7.1 | 37.5 ± 6.1 | 39.8 ± 4.6 | 40.7 ± 3.8 | <.000 | <.000 |
| Black race | 10 (14.3) | 12 (46.2) | 7 (15.6) | 5 (16.1) | 0.004 | 0.135 |
| College graduate | 58 (82.9) | 14 (53.9) | 28 (66.6) | 18 (58.0) | 0.162 | 0.014 |
| Body mass index (kg/m2) | 32.5 ± 4.5 | 32.3 ± 4.1 | 32.1 ± 3.4 | 31.8 ± 3.1 | 0.889 | 0.550 |
| Alcohol consumption > 1/month | 41 (58.6) | 9 (34.6) | 14 (31.1) | 14 (45.2) | 0.020 | 0.004 |
| Average systolic blood pressure | 112.4 ± 9.8 | 112.6 ± 8.8 | 112.2 ± 9.9 | 112.3 ± 9.9 | 0.999 | 0.959 |
| Current smoking | 9 (12.9) | 3 (11.5) | 3 (6.7) | 0 (0.0) | 0.174 | 0.112 |
| Fasting glucose (mg/dl) | 97.1 ± 8.7 | 97.2 ± 8.1 | 97.2 ± 8.6 | 97.6 ± 7.3 | 0.995 | 0.863 |
| Total cholesterol (mg/dl) | 205.3 ± 38.9 | 207.1 ± 38.5 | 198.6 ± 35.7 | 213.1 ± 42.8 | 0.452 | 0.977 |
| LDL (mg/dl) | 118.1 ± 35.8 | 129.3 ± 36.4 | 120.9 ± 28.1 | 133.8 ± 37.4 | 0.142 | 0.098 |
| Triglycerides (mg/dl) | 138.8 ± 76.8 | 116.4 ± 134.6 | 113.0 ± 60.0 | 128.7 ± 53.3 | 0.353 | 0.110 |
| HDL (mg/dl) | 60.0 ± 15.2 | 55.1 ± 14.9 | 55.1 ± 11.4 | 53.7 ± 11.5 | 0.091 | 0.012 |
| Reproductive History | ||||||
| Irregular menses | 5 (7.1) | 0 (0.0) | 2 (4.4) | 2 (6.5) | 0.552 | 0.351 |
| Post-menopause | 4 (5.7) | 3 (11.5) | 2 (4.4) | 7 (22.6) | 0.030 | 0.180 |
| Any pregnancy complication | - | 8 (30.8) | 14 (31.1) | 13 (41.9) | 0.563 | |
| Ever breastfed | - | 16 (61.5) | 39 (86.7) | 26 (83.9) | 0.032 | |
| Age at first birth (yr) | - | 25.5 ± 5.9 | 26.2 ± 4.7 | 24.7 ± 4.5 | 0.457 | |
| Time since last birth (yr) | - | 11.6 ± 6.9 | 9.0 ± 6.1 | 7.7 ± 3.6 | 0.040 | |
Values shown are means ± SDs or frequency counts (with percentages).
P values from analysis of variance or chi-square test, as appropriate.
Among traditional CVD risk factors, only HDL(c) differed by parity and was highest in nulliparous women. Nulliparous women tended to be current smokers compared to women of parity 3+ (12.9% vs 0.0%). Average systolic blood pressure, fasting glucose, total cholesterol, LDL(c), and triglycerides were similar among all groups.
For reproductive factors, history of irregular menstrual periods was similar in each group. Among parous women, age at first birth and rate of reporting any pregnancy complication were similar. Women of higher parity were more likely to have ever breastfed and to be post-menopausal, and had a shorter time since their last birth.
Nulliparous women had the highest values for IAD, average CCA IMT, maximum CCA IMT, average IMT and maximum IMT (Table 2). For the 3 measures in the CCA, nulliparous women had the highest values and values for the 3 levels of parity are roughly equivalent. When comparing the adjusted means for nulliparous vs. parous women these results were statistically significant. Patterns were less obvious for the other carotid measures. There was no clear pattern for internal carotid artery IMT. Bulb IMT, however, was significantly greater for nulliparous women and for women with 3 or more births than for women with 2 births.
TABLE 2.
Age and race-adjusted mean carotid measures by parity in women without infertility history.
| Nulliparous n = 70 | Parity 1 n = 26 | Parity 2 n = 45 | Parity 3+ N = 31 | P value | |
|---|---|---|---|---|---|
| Carotid Measure (mm) | |||||
| Inter-adventitial Diameter | 6.942 (.057) | 6.794 (.093) | 6.657 (.069)* | 6.722 (.086)† | 0.018 |
| Average CCA IMT | .610 (.008) | .579 (.013) | .579 (.010) | .577 (.012) | 0.062 |
| Maximum CCA IMT | .719 (.010) | .682 (.016) | .686 (.012)* | .671 (.015)* | 0.037 |
| Average ICA IMT | .547 (.016) | .575 (.027) | .518 (.020) | .567 (.024) | 0.254 |
| Average bulb IMT | .688 (.016) | .672 (.025) | .627 (.019)† | .713 (.023)‡ | 0.018 |
| Average IMT | .613 (.009) | .601 (.015) | .576 (.011) | .606 (.014) | 0.084 |
| Maximum IMT | .770 (.013) | .748 (.021) | .724 (.016) | .756 (.019) | 0.167 |
Values shown are means (standard error).
Different from nulliparous at p <.01
Different from nulliparous at p <.05
Different from parity 2 at p <.01
P values from analysis of variance.
Relationship between having ever given birth and the carotid measures is presented in Table 3. After adjustment for demographics and cardiovascular risk factors nulliparous women had IAD 0.19 mm larger (p=0.014), average CCA IMT 0.03 mm thicker (p=0.009) and maximum CCA IMT 0.04 mm thicker (p= 0 .004) than parous women. Average bulb IMT, average ICA IMT, average IMT, and maximum IMT were also greater in nulliparous women, but these differences do not reach significance. There were no differences in average ICA IMT based on parity. Adjustment for IAD attenuated the differences in IMT.
TABLE 3.
Carotid measures for parous vs. nulliparous women. Regression coefficients represent change from a baseline parity of 0.
| Age & Race adjusted | Model 11 | Model 22 | ||||
|---|---|---|---|---|---|---|
| Carotid Measure (mm) | ß (SE) | P value | ß (SE) | P value | ß (SE) | P value |
| Inter-adventitial Diameter | −.230 (.077) | .0032 | −.185 (.074) | .0139 | - | - |
| Average CCA IMT | −.031 (.011) | .0067 | −.029 (.011) | .0086 | −.019 (.010) | .0695 |
| Maximum CCA IMT | −.039 (.014) | .0050 | −.038 (.013) | .0039 | −.029 (.013) | .0255 |
| Average ICA IMT | .000 (.022) | .9889 | −.010 (.022) | .6475 | −.004 (.022) | .8489 |
| Average bulb IMT | −.024 (.021) | .2589 | −.015 (.021) | .4851 | −.010 (.022) | .6370 |
| Average IMT | −.022 (.017) | .0801 | −.021 (.012) | .0813 | −.013 (.012) | .2663 |
| Maximum IMT | −.030 (.017) | .0811 | −.029 (.017) | .0798 | −.022 (.017) | .1879 |
Model 1 represents the simultaneous effects of parity, age, race, BMI, education, current smoking, use of alcohol, average systolic blood pressure, and fasting glucose level on the carotid measures.
Model 1 with inter-adventitial diameter.
P values represent significance of regression coefficient for parity.
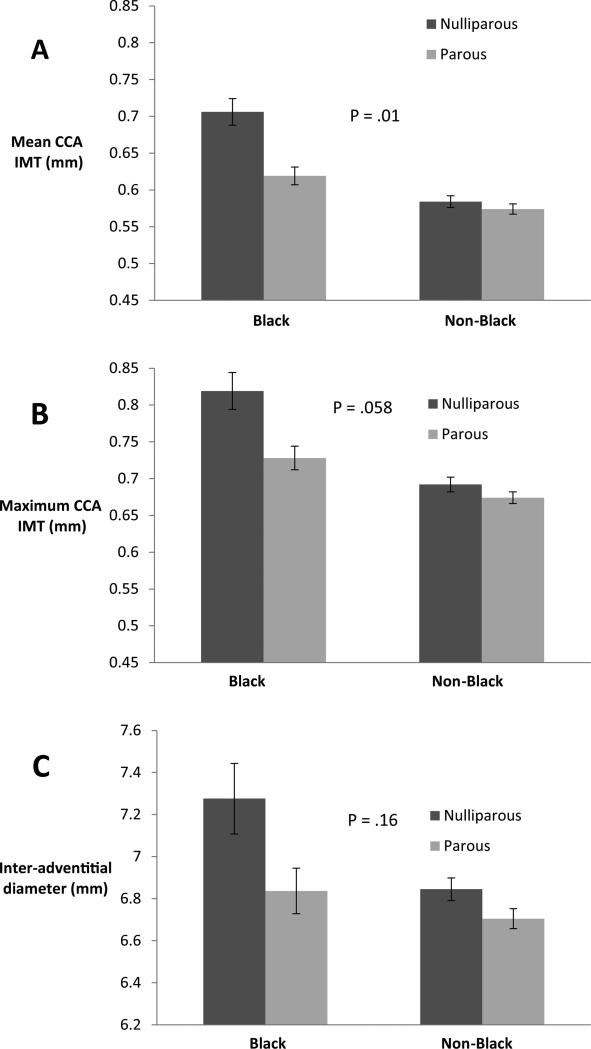
There was no significant interaction between BMI and nulliparity. In an adjusted model the interaction term for black race and parity was statistically significant for mean CCA IMT (p=0.01) and borderline significant for maximum CCA IMT (p=0.058). Figure 2 shows that black women had thicker mean and maximum CCA IMT than did non-black women, and the difference between IMT for nulliparous and parous women appeared greater for blacks than for non-blacks.
Figure 2.
Interaction between race and parity, adjusting for age, body mass index, smoking, alcohol use, systolic blood pressure and fasting glucose levels. P values are for race x parity interaction.
In the sensitivity analysis including the 19 women with a known infertility history, infertility was not a significant predictor of any carotid outcome, although the effect size was similar to the effect size of nulliparity for mean and maximum CCA IMT (Supplemental Table 2).
Discussion
This analysis demonstrates that in a sample of disease-free overweight and obese young women without known infertility history, nulliparity is associated with less healthy markers of subclinical CVD even after accounting for traditional CVD risk factors. Nulliparous women had greater IAD and mean and maximum CCA IMT compared to parous women, and thicker bulb IMT compared to women with 2 births. Increased IAD seemed to mediate some, but not all of the difference in IMT measures. This might describe a first birth effect – a one-time change occurring after first birth but not repeated after subsequent births - as seen with decreased HDL-c and systolic blood pressure after a first birth.(16, 17) Or it may represent the left peak of a J-shaped relationship, similar to the J-shaped relationship seen between parity and cardiovascular risk. The small number of women of higher parity makes it difficult to discern if CCA IMT or IAD might increase with higher numbers of births.
Our results contradict findings of some studies. In the Rotterdam Study, among women aged 55-99 years old, there was a trend towards increased mean and maximum CCA IMT with increased parity, significant even after adjustment for demographics and cardiovascular risk factors.(7) The Study of Health in Pomerania detected a U-shaped association between CCA IMT and parity in women aged 45-79 years old. After adjustment for demographic, cardiovascular risk, and reproductive factors, greatest mean and maximum CCA IMT were in women with parity of 0 or 3 or greater.(8) Different rates of nulliparity (20% in the Rotterdam Study and 8.5% in the Study of Health in Pomerania) may explain the contradiction. The authors of the Pomerania study suggest that in the Rotterdam Study many women were nulliparous by choice, while in their population most nulliparous women had true infertility. Women with infertility may be at higher risk for CVD because of hormonal conditions such as polycystic ovary syndrome that can lead to both infertility and increased CVD risk factors.(18) Our study addressed this issue by removing women with known infertility from the analysis.
These studies looked at mid-life and older women, as opposed to the younger women in our study. It is possible that pregnancy has differing effects on vasculature at different times. Our study may show short term positive effects on vasculature from the increased hormone levels and cardiac output of pregnancy, while studies in older women may reflect negative long-term effects of pregnancy on lipid levels, glucose metabolism, and weight and body fat. Women in these studies were also leaner than women in our analysis, with average BMI's of about 28 and 27, compared to the average BMI of 32 in our study. This suggests that the effects of pregnancy on the vasculature might differ in women who are and are not obese.
Our results also differ from those of the only study done in women of childbearing age. The Cardiovascular Risk in Young Finns Study followed 1005 women aged 24-39 years old for 6 years and found that mean CCA IMT increased 0.0075 mm for each birth occurring during that time period, after adjusting for demographic and cardiovascular risk factors.(6) The authors speculated this increase may be a result of atherogenic metabolic changes in pregnancy. The women in the studies differ substantially; women in the Young Finns study were substantially leaner, with average BMI of about 24. It is possible that pregnancy has different net effects on vascular health depending on women's risk factor profiles, including BMI.(9)
Our finding that carotid bulb IMT is not associated with nulliparity is consistent with the findings of Kharazmi et al. They studied 746 Finnish women between the ages of 45 and 74 years old and found no relationship between parity and the mean of the IMT in the CCA and the carotid bulb, after adjustment for demographic and cardiovascular risk factors.(19) We found different patterns of change in the CCA and bulb, suggesting they should not be analyzed together. This is supported by various population-based studies that found that bulb IMT is related to true atherosclerosis, while CCA IMT is more a marker of vascular aging and adaptation.(10)
One possible explanation for increased CCA IMT and inter-adventitial diameter in nulliparous women is potential residual confounding from fertility status. While we removed all women reporting infertility from the sample, some nulliparous women may have never attempted pregnancy and could have unidentified fertility problems. If these women had greater IMT and IAD, the increased values we found in nulliparous women may be from the influence of unidentified infertility, not nulliparity. In our model comparing women with and without known fertility problems the effect size of infertility was very similar to the effect size of nulliparity for mean and maximum CCA IMT. This suggests that some of the nulliparous women may have unknown infertility, and that infertility-related problems may be the cause of the less healthy arterial parameters.
Another possible explanation is that these results represent a beneficial effect of pregnancy on the vasculature. Several studies of midlife women demonstrate that higher levels of estrogen are associated with decreased IMT and/IAD.(20, 21, 22, 23) Perhaps we are seeing a lasting positive effect from increased pregnancy hormones assessed an average of 9 years after pregnancy.
Two findings relating to health equity deserve further study. Lower educational attainment was associated with thicker average and maximum IMT after adjustment for age and race, but not after adjustment for CVD risk factors. This suggests that CVD risk factors may mediate a relationship between less education and CVD, and merits thorough exploration in a larger study with a more diverse population. In our study, as in others, (4, 24, 25) black women had worse measures of subclinical CVD than non-black women; our study adds that differences between nulliparous and parous women were greater for black women, and indeed, may only be present in black women. This suggests that overweight, black, nulliparous women might represent a group at particularly elevated risk for CVD, and should be explored further.
This analysis is one of the only studies of the relationship between parity and vascular health in overweight and obese young women and it uses well-validated, reliable measures of IMT and inter-adventitial diameter. Limitations include small sample size and reliance on self-report pregnancy histories. Lack of healthy weight participants makes aspects of the results difficult to interpret; a life course epidemiologic study would be necessary to determine the full trajectory of the relationship between reproductive events and changes in adiposity and markers of vascular remodeling. The consideration of multiple IMT outcomes may increase the risk of Type 1 error, and P-values should be considered in this light. The study selection criteria are both a strength and a limitation. By including only overweight or obese women who are non-diabetic and normotensive the study limits the generalizability of the results and also limits its ability to determine fully whether pregnancy's effects on BMI, glucose metabolism, and blood pressure mediate pregnancy's effect on the vasculature. At the same time, by focusing on a uniform group of women, the study is able to limit confounding factors and focus on the effects of the pregnancy itself.
Pregnancy affects cardiovascular health in many ways and the ability to achieve successful pregnancy is a marker of good health. This study demonstrates that nulliparous overweight and obese young women show poorer vascular health compared to parous women, as measured by greater CCA IMT and inter-adventitial diameter. Nulliparous overweight and obese women, particularly black women, may be at increased risk for CVD even if they are normotensive and non-diabetic, and may particularly benefit from risk reduction efforts.
Supplementary Material
What is already known about this subject:
Cardiovascular disease risk is increased for women with more than 2 births.
Increased inter-adventitial diameter and thicker intima-media thickness of the carotid artery are associated with traditional CVD risk factors and cardiovascular events.
Studies suggest that increases in common carotid artery intima-media thickness with parity (number of births) may reflect a potential mechanism linking parity to cardiovascular disease.
What this study adds:
Healthy nulliparous overweight and obese young women show poorer vascular health compared to parous women, as measured by greater common carotid artery intima-media thickness and inter-adventitial diameter.
Both absolute measures of subclinical cardiovascular disease and relative differences between nulliparous and parous women were greater for black women, suggesting that overweight, black nulliparous women might represent a group at particularly elevated risk for cardiovascular disease.
ACKNOWLEDGEMENTS
The SAVE Trial is funded by NHLBI grant R01 HL077525-01A2. NN was supported by NICHD grant T32HD0055162-04 and NHBLI grant T32HL083825 to the University of Pittsburgh.
NN analyzed the data, CM designed the ancillary study, EB-M and KS-T served as principal trial investigators, PT assisted with analysis, and NN, JC, EB-M, CM, JR, PT and KS-T wrote the manuscript.
Footnotes
COMPETING INTERESTS:
The authors have no competing interests.
REFERENCES
- 1.Weight Gain During Pregnancy: Reexamining the Guidelines. National Academy of Sciences; Washington DC: 2009. [PubMed] [Google Scholar]
- 2.Creinin MD, Simhan HN. Can we communicate gravidity and parity better? Obstetrics and gynecology. 2009;113:709–711. doi: 10.1097/AOG.0b013e3181988f8f. [DOI] [PubMed] [Google Scholar]
- 3.Parikh NI, Cnattingius S, Dickman PW, Mittleman MA, Ludvigsson JF, Ingelsson E. Parity and risk of later-life maternal cardiovascular disease. American heart journal. 2010;159:215–221. e216. doi: 10.1016/j.ahj.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 4.Polak JF, Wong Q, Johnson WC, Bluemke DA, Harrington A, O'Leary DH, et al. Associations of cardiovascular risk factors, carotid intima-media thickness and left ventricular mass with inter- adventitial diameters of the common carotid artery: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2011 doi: 10.1016/j.atherosclerosis.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eigenbrodt ML, Sukhija R, Rose KM, Tracy RE, Couper DJ, Evans GW, et al. Common carotid artery wall thickness and external diameter as predictors of prevalent and incident cardiac events in a large population study. CardiovascUltrasound. 2007;5:11:11. doi: 10.1186/1476-7120-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skilton MR, Bonnet F, Begg LM, Juonala M, Kahonen M, Lehtimaki T, et al. Childbearing, child- rearing, cardiovascular risk factors, and progression of carotid intima-media thickness: the Cardiovascular Risk in Young Finns study. Stroke. 2010;41:1332–1337. doi: 10.1161/STROKEAHA.110.579219. [DOI] [PubMed] [Google Scholar]
- 7.Humphries KH, Westendorp IC, Bots ML, Spinelli JJ, Carere RG, Hofman A, et al. Parity and carotid artery atherosclerosis in elderly women: The Rotterdam Study. Stroke. 2001;32:2259–2264. doi: 10.1161/hs1001.097224. [DOI] [PubMed] [Google Scholar]
- 8.Wolff B, Volzke H, Robinson D, Schwahn C, Ludemann J, Kessler C, et al. Relation of parity with common carotid intima-media thickness among women of the Study of Health in Pomerania. Stroke. 2005;36:938–943. doi: 10.1161/01.STR.0000162712.27799.20. [DOI] [PubMed] [Google Scholar]
- 9.Skilton MR, Serusclat A, Begg LM, Moulin P, Bonnet F. Parity and carotid atherosclerosis in men and women: insights into the roles of childbearing and child-rearing. Stroke; a journal of cerebral circulation. 2009;40:1152–1157. doi: 10.1161/STROKEAHA.108.535807. [DOI] [PubMed] [Google Scholar]
- 10.Mackinnon AD, Jerrard-Dunne P, Sitzer M, Buehler A, von Kegler S, Markus HS. Rates and determinants of site-specific progression of carotid artery intima-media thickness: the carotid atherosclerosis progression study. Stroke; a journal of cerebral circulation. 2004;35:2150–2154. doi: 10.1161/01.STR.0000136720.21095.f3. [DOI] [PubMed] [Google Scholar]
- 11.Pries AR, Reglin B, Secomb TW. Remodeling of blood vessels: responses of diameter and wall thickness to hemodynamic and metabolic stimuli. Hypertension. 2005;46:725–731. doi: 10.1161/01.HYP.0000184428.16429.be. [DOI] [PubMed] [Google Scholar]
- 12.Njoroge JN, El Khoudary SR, Fried LF, Barinas-Mitchell E, Sutton-Tyrrell K. High urinary sodium is associated with increased carotid intima-media thickness in normotensive overweight and obese adults. American journal of hypertension. 2011;24:70–76. doi: 10.1038/ajh.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wendelhag I, Gustavsson T, Suurkula M, Berglund G, Wikstrand J. Ultrasound measurement of wall thickness in the carotid artery: fundamental principles and description of a computerized analysing system. Clinical physiology (Oxford, England) 1991;11:565–577. doi: 10.1111/j.1475-097x.1991.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 14.Tomeo CA, Rich-Edwards JW, Michels KB, Berkey CS, Hunter DJ, Frazier AL, et al. Reproducibility and validity of maternal recall of pregnancy-related events. Epidemiology. 1999;10:774–777. [PubMed] [Google Scholar]
- 15.McCormick MC, Brooks-Gunn J. Concurrent child health status and maternal recall of events in infancy. Pediatrics. 1999;104:1176–1181. [PubMed] [Google Scholar]
- 16.Gunderson EP, Chiang V, Lewis CE, Catov J, Quesenberry CP, Jr., Sidney S, et al. Long-term blood pressure changes measured from before to after pregnancy relative to nonparous women. ObstetGynecol. 2008;112:1294–1302. doi: 10.1097/AOG.0b013e31818da09b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gunderson EP, Lewis CE, Murtaugh MA, Quesenberry CP, Smith West D, Sidney S. Long-term plasma lipid changes associated with a first birth: the Coronary Artery Risk Development in Young Adults study. American journal of epidemiology. 2004;159:1028–1039. doi: 10.1093/aje/kwh146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Health and fertility in World Health Organization group 2 anovulatory women. Human reproduction update. 2012;18:586–599. doi: 10.1093/humupd/dms019. [DOI] [PubMed] [Google Scholar]
- 19.Kharazmi E, Moilanen L, Fallah M, Kaaja R, Kattainen A, Kahonen M, et al. Reproductive history and carotid intima-media thickness. Acta ObstetGynecolScand. 2007;86:995–1002. doi: 10.1080/00016340701464374. [DOI] [PubMed] [Google Scholar]
- 20.Wildman RP, Colvin AB, Powell LH, Matthews KA, Everson-Rose SA, Hollenberg S, et al. Associations of endogenous sex hormones with the vasculature in menopausal women: the Study of Women's Health Across the Nation (SWAN). Menopause. 2008;15:414–421. doi: 10.1097/gme.0b013e318154b6f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jonas HA, Kronmal RA, Psaty BM, Manolio TA, Meilahn EN, Tell GS, et al. Current estrogen progestin and estrogen replacement therapy in elderly women: association with carotid atherosclerosis. CHS Collaborative Research Group. Cardiovascular Health Study. Annals of epidemiology. 1996;6:314–323. doi: 10.1016/s1047-2797(96)00048-8. [DOI] [PubMed] [Google Scholar]
- 22.Liang YL, Teede H, Shiel LM, Thomas A, Craven R, Sachithanandan N, et al. Effects of oestrogen and progesterone on age-related changes in arteries of postmenopausal women. Clinical and experimental pharmacology & physiology. 1997;24:457–459. doi: 10.1111/j.1440-1681.1997.tb01225.x. [DOI] [PubMed] [Google Scholar]
- 23.Muscelli E, Kozakova M, Flyvbjerg A, Kyriakopoulou K, Astiarraga BD, Glintborg D, et al. The effect of menopause on carotid artery remodeling, insulin sensitivity, and plasma adiponectin in healthy women. AmJHypertens. 2009;22:364–370. doi: 10.1038/ajh.2009.16. [DOI] [PubMed] [Google Scholar]
- 24.D'Agostino RB, Jr., Burke G, O'Leary D, Rewers M, Selby J, Savage PJ, et al. Ethnic differences in carotid wall thickness. The Insulin Resistance Atherosclerosis Study. Stroke; a journal of cerebral circulation. 1996;27:1744–1749. doi: 10.1161/01.str.27.10.1744. [DOI] [PubMed] [Google Scholar]
- 25.Freedman BI, Hsu FC, Langefeld CD, Rich SS, Herrington DM, Carr JJ, et al. The impact of ethnicity and sex on subclinical cardiovascular disease: the Diabetes Heart Study. Diabetologia. 2005;48:2511–2518. doi: 10.1007/s00125-005-0017-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.