Abstract
GOPC (FIG/PIST/CAL) is a PDZ-domain scaffolding protein that regulates the trafficking of a wide array of proteins, including small GTPases, receptors, and cell surface molecules such as cadherin 23 and CFTR. In Madin-Darby Canine Kidney (MDCK) cells, we find that GOPC localizes to the Trans-Golgi Network (TGN), but not the cis or trans Golgi cisternae. There is also colocalization with the early endosome Rab GTPase Rab5 and a TGN/endosome marker Rab14, but not with Rab11, a marker of recycling endosomes. There is no localization of GOPC to the lateral membranes or tight junctions. We find that knockdown of GOPC in MDCK cells results in decreased transepithelial resistance and increased paracellular flux. This may be due to compromised trafficking of tight junction components from the TGN, as GOPC knockdown cells have decreased lateral labeling of the tight junction protein claudin-1 and decreased protein levels of claudin-2. GOPC may mediate trafficking of newly synthesized tight junction proteins from the TGN to the cell surface or recycling of these proteins from specialized endosomal compartments.
Keywords: GOPC, tight junction, endosomes, trans-Golgi network
Introduction
The sorting and targeting of cell surface receptors and channels is a regulated process that requires the coordinated activity of cellular machinery in subcellular compartments, including the trans-Golgi network and endosomes. PDZ-domain proteins have been implicated in the polarized and specialized trafficking of proteins to the apical plasma membrane and junctional region (Gee et al., 2009; Ohno, 2001). GOPC (Golgi-associated PDZ and coiled-coil motif-containing protein), also known as PIST (PDZ domain protein interacting specifically with TC10), FIG (fused in glioblastoma), and CAL (CFTR-associated ligand) is a peripheral membrane PDZ-domain protein. While first identified as interacting with the small GTPase TC10, the receptor Frizzled, and syntaxin 6 (Charest et al., 2001; Neudauer et al., 2001; Yao et al., 2001), subsequent studies have found that it controls the trafficking of many integral membrane proteins, including cell surface receptors (Cuadra et al., 2004; Hassel et al., 2003; Meiffren et al., 2010; Wawrzak et al., 2009; Yao et al., 2001; Yue et al., 2002; Zhang et al., 2008), ion channels and pumps (Cheng et al., 2002; Gentzsch et al., 2003; Goellner et al., 2003; Herrmann et al., 2013), and adhesion molecules (Xu et al., 2010). It also interacts with the Golgi cisternae protein Golgin160 (Hicks et al., 2006; Hicks and Machamer, 2005). Its domain structure consists of an N-terminal region with two coiled-coil domains and a C-terminal PDZ domain (Charest et al., 2001; Neudauer et al., 2001; Yao et al., 2001). This structure allows this protein to interact with both cargo and trafficking machinery. Interestingly, GOPC promotes the lysosomal targeting of CFTR and Muc3 but promotes cell surface targeting of receptors such as Frizzled (Cheng et al., 2010a; Cheng et al., 2002; Cheng et al., 2004; Cushing et al., 2010; Pelaseyed and Hansson, 2011; Yao et al., 2001). The enhanced lysosomal targeting of CFTR and Muc3 may relate to the fact that GOPC interacts with the autophagy machinery, and is required for development of autophagic vacuoles after activation of the cell surface antigen CD46 (Meiffren et al., 2010; Richetta et al., 2013).
Epithelial cells form a barrier between the outside world and the interior of the organism. To establish this barrier, the cells develop discrete apical and basolateral membrane domains separated by specialized junctions (Apodaca et al., 2012; Rodriguez-Boulan and Macara, 2014). The formation of tight junctions requires targeted trafficking to the specialized plasma membrane domain at the apical pole of the cell. This domain is not static, and tight junction proteins continuously cycle through endosomal compartments to maintain epithelial integrity (Ivanov et al., 2005; Marchiando et al., 2010; Marzesco et al., 2002; Morimoto et al., 2005; Shen, 2012). GOPC has been reported to reside at the adherens junction and regulate the targeting of a specialized apical cadherin, cadherin-23 (Ito et al., 2006; Xu et al., 2010). However, it is not known if GOPC impacts the structure or assembly of tight junctions. Here, we show that knockdown of GOPC results in decreased transepithelial resistance, increased paracellular permeability, decreased claudin-1 at the tight junction, and decreased protein levels of claudin-2. These results suggest that GOPC regulates tight junction structure in epithelial cells, perhaps through modulation of trafficking of a subset of tight junction proteins from either the TGN or through specialize endosomes.
Materials and Methods
Materials
Cell culture reagents were obtained from Mediatech Inc, Manassas, VA. The following antibodies were used: rabbit anti-GOPC (Millipore, Billerica, MA), anti-E-Cadherin (Cell Signaling, Danvers, MA), mouse anti-EEA1 (BD Biosciences, San Jose, CA), mouse anti-TGN38 (ABR Affinity Bioreagents, Golden, CO). Rat anti-E-Cadherin, mouse anti-G58K, mouse anti-GM130 and mouse anti-β-actin were from Sigma (Saint Louis, MO). Rabbit and mouse anti-claudin-1, rabbit and mouse anti-claudin-2, rabbit and mouse anti-occludin, mouse anti-claudin-4, mouse anti-ZO-1 were from Invitrogen (Grand Island, NY). Mouse anti-JAM-A was a gift from Dr. Charles Parkos, Emory University. AlexaFluor secondary antibodies were also from Invitrogen. All other chemicals and reagents were from Sigma-Aldrich unless otherwise specified.
Plasmid /Lentivirus Constructs
pLKO-shRNAs for GOPC were a gift from the Broad Institute, Harvard Medical School. The following sequence was used for the knockdown of GOPC: CTGGAGAAGGAGTTCGACAAA. There is one nucleotide difference between this sequence and the canine sequence at the 3’ end. Knockdown was confirmed and quantified by immunoblotting using anti-GOPC antibody (see below). Plasmids encoding galactosyltransferase-CFP and Rab11-GFP were from Addgene (Cambridge, MA). Plasmids encoding Rab14-GFP were described previously (Kitt et al., 2008).
Cell culture
MDCK II cells were used in all experiments. Cells were cultured in DMEM-High glucose (Mediatech) supplemented with 10% FBS (Atlanta Biologicals, Lawrenceville, GA), 1% nonessential amino acids (Mediatech) and 1% Pen/Strept/L-Glut (Sigma) under 5% CO2 at 37 °C.
For transient transfection, 25kD linear polyethylenimine (PEI, Fisher Scientific, Waltham, MA) was used as a DNA carrier. Briefly, PEI (1mg/ml stock) and DNA were mixed at a ratio of 4:1 in OPTI-MEM to a final concentration of 1µg/ml DNA and 4µg/ml of PEI. After incubation at room temperature for 10 minutes, the mixture was added to cells for 16 hours, followed by replacement with normal growth meda. Cells were fixed 8 hours later.
For lentiviral transduction, cells were infected with virus in the presence of 6ug/ml polybrene (Sigma-Aldrich) overnight followed by selection in growth medium containing 2µg/mL puromycin. Controls were transduced with empty vector.
For TER measurements, cells were plated at a density of 1.3×105cells/cm2 on filter inserts (Corning, NY, #3460) and cultured for 4–7 days. TER was measured with a Millicell ERS-2 epithelial volt-ohm meter (Millipore). TER measurements are expressed as Ω•cm2. TER measurements were performed in triplicate and values are expressed as mean ± SEM. Significance was determined using Student’s T-test.
For fluoroscein-dextran flux assays, cells were plated on permeable supports and grown for 4–5 days. Cells were rinsed three times with HBSS and equilibrated for 30 minutes at 37°C. 4kd fluoroscei n dextran (1mg/ml, Sigma), dissolved in HBSS, was added to the apical chamber. Filters were incubated for 3 hours and the basolateral medium was collected. Flux was measured using a Thermo Electron Varioskan Flash fluorimeter. Fluxes were calculated using the apparent permeability coefficient (Papp) equation: Papp=(Δ CxV/ Δ t)/A×C0, where Papp is the apparent permeability (cm/s), ΔC is the change of 4kD FL-Dextran concentration (mM) in the basolateral medium, V is the volume of the basolateral medium (mL), Δt is the change in time (seconds), A is the surface area of the membrane (cm2), and C0 is the initial concentration (mM) in the apical chamber (Van Itallie et al., 2008). Values were averaged and significance determined using a Student’s T-test.
Immunofluorescent labeling and fluorescence microscopy
Cells were cultured on sterilized coverslips or Corning Costar (Sigma) filters. For imaging cells on filters, cells were plated at confluent density (2×105 cells/cm2) and cultured for 3–7 days. For immunofluorescent labeling, cells were fixed in 4% formaldehyde freshly prepared from paraformaldehyde in PBS, pH 7.4, or in 100% methanol at −20°C, permeabilized/blocked with 0.2% saponin/PBS, pH 7.4 and incubated in primary antibodies overnight at 4°C followed by incubation with fluorescent secondary antibodies in the same buffer. Images were acquired using an Olympus Fluoview confocal microscope with a 60× (NA1.4) oil immersion objective. Excitation wavelengths of 488, 568 and/or 633 nm were used for simultaneous two or three-channel recording. Images were processed and merged using Adobe Photoshop software (Adobe Systems) or ImageJ (Schneider et al., 2012). Quantification of pixel intensity was performed using NIH ImageJ. Briefly, cells labeled for claudin-1 or occludin, together with GOPC were imaged and cells within the field marked as GOPC positive or negative. Plot profiles across the lateral membrane were generated and maximum intensities were averaged. Identical imaging and processing parameters were used within an experiment to allow comparison of intensity and labeling patterns. Data are represented as mean ± SEM. Statistical significance was determined using the Student’s T-test.
Western Blot Analysis
Cells were lysed in 0.025M Tris, pH 7.4, 0.15M NaCl, 0.001M EDTA, 1% NP-40 and protein concentrations were determined with the Pierce protein assay reagent. Samples were then solubilized in LiCor (Lincoln, NE) sample buffer with 10% β-mercaptoethanol to a concentration of 1µg/µl. Blots were blocked with LiCor blocking buffer, then probed with primary antibodies diluted in 0.05% Tween/LiCor blocking buffer. Secondary antibodies were rabbit or mouse IR-Dye 680 or 800 in blocking buffer. Membranes were imaged using a LiCor Odyssey scanner. Boxes were manually placed around each band of interest, which returned near-infrared fluorescent values of raw intensity with intra-lane background subtracted using Odyssey 3.0 analytical software (LiCor). The fluorescence value for each protein of interest was normalized to the in-lane value of β-actin, and this normalized ratio from duplicate or triplicate lanes was averaged. Data were analyzed using Student’s T-test. Measures were considered significant when p<0.05. Error bars are SEM.
Results
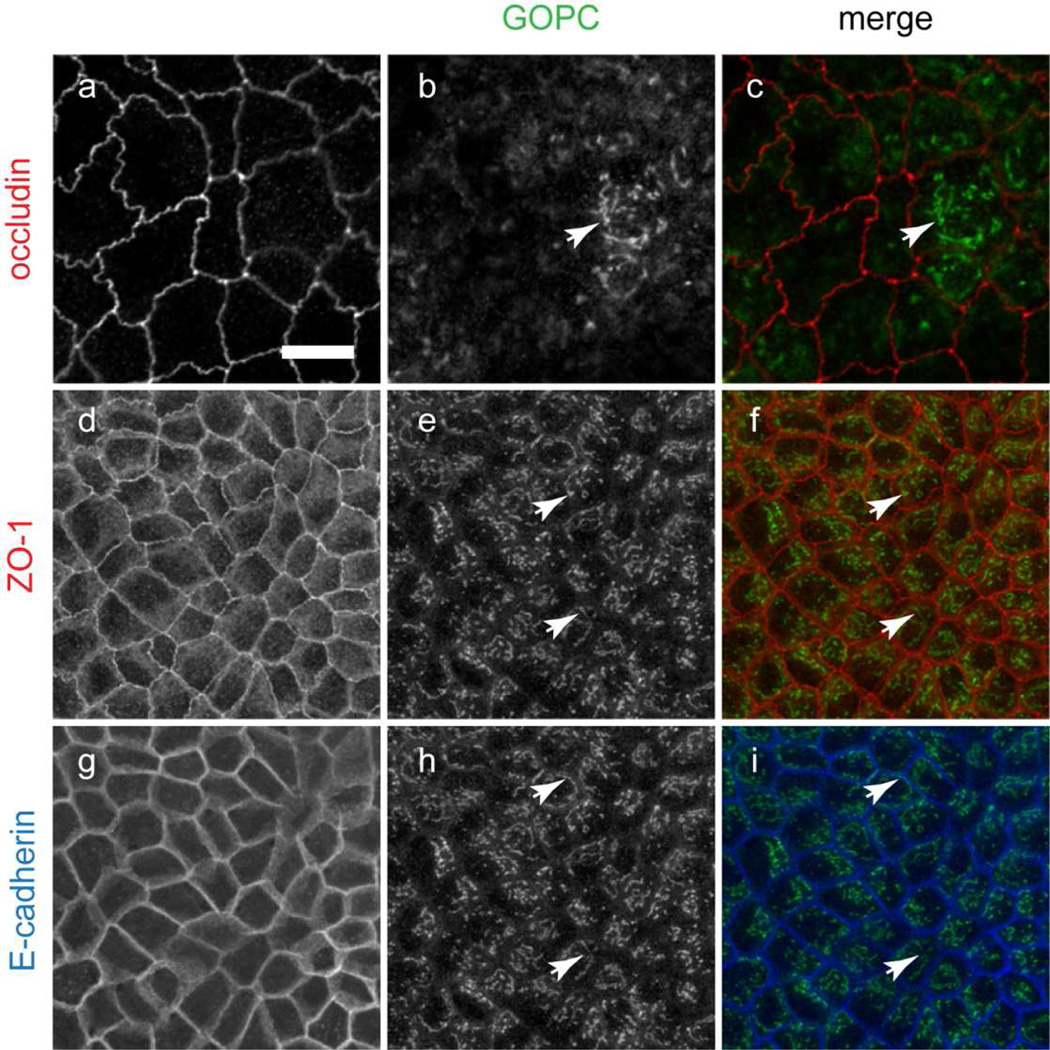
GOPC localization and functional studies have suggested diverse subcellular distributions, and it localizes to the Golgi apparatus, in cytoplasmic tubules and, in epithelial cells, at the adherens junctions (Chen et al., 2012; Cheng et al., 2002; Gentzsch et al., 2003; Hicks and Machamer, 2005; Ito et al., 2006; Yao et al., 2001). To further define the subcellular compartments in epithelial cells where GOPC is localized, we double-labeled MDCK cells grown on permeable supports with antibodies against GOPC and tight junction (occludin and ZO-1) or adherens junctions (E-cadherin) markers. As shown in Figure 1, GOPC localizes to puncta within the cytoplasm. However GOPC was not found at the tight or adherens junctions using a variety of fixations and labeling conditions (Figure 1 and data not shown). This contrasts a previously reported localization that found GOPC localized to adherens junctions (Ito et al., 2006). This difference may be due to antibody specificity differences.
Figure 1.
MDCK cells grown on Transwell filters were fixed and double or triple-labeled for the tight junction markers occludin (a–c) and ZO-1 (d–f) and the adherens junction marker E-cadherin (g–i), together with GOPC. GOPC is localized to puncta and cisternal structures in the cytoplasm (arrows), but does not colocalize with tight junction or adherens junction markers. ZO-1 and E-cadherin labeling are the same optical section but imaged with distinct fluorescence filters. Scale bar, 20µm. Representative images are from at least 5 experiments.
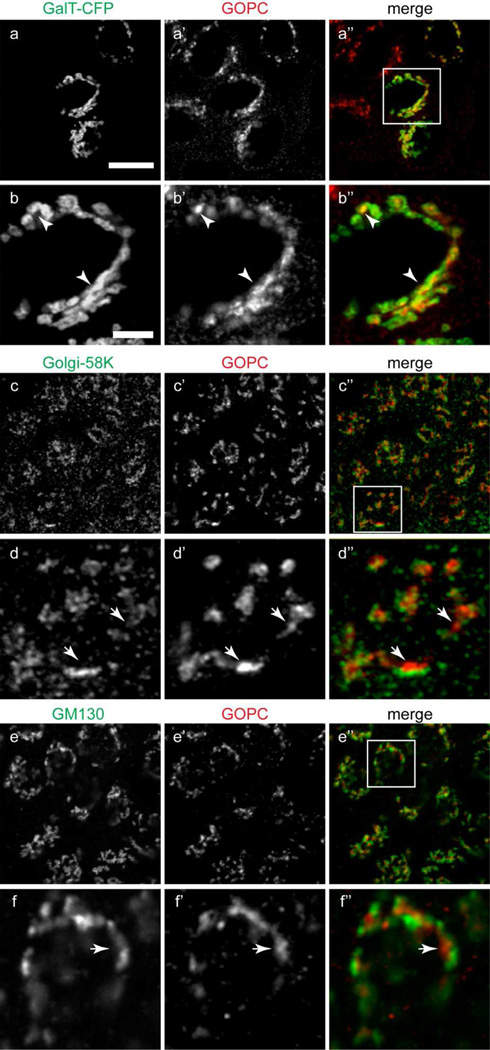
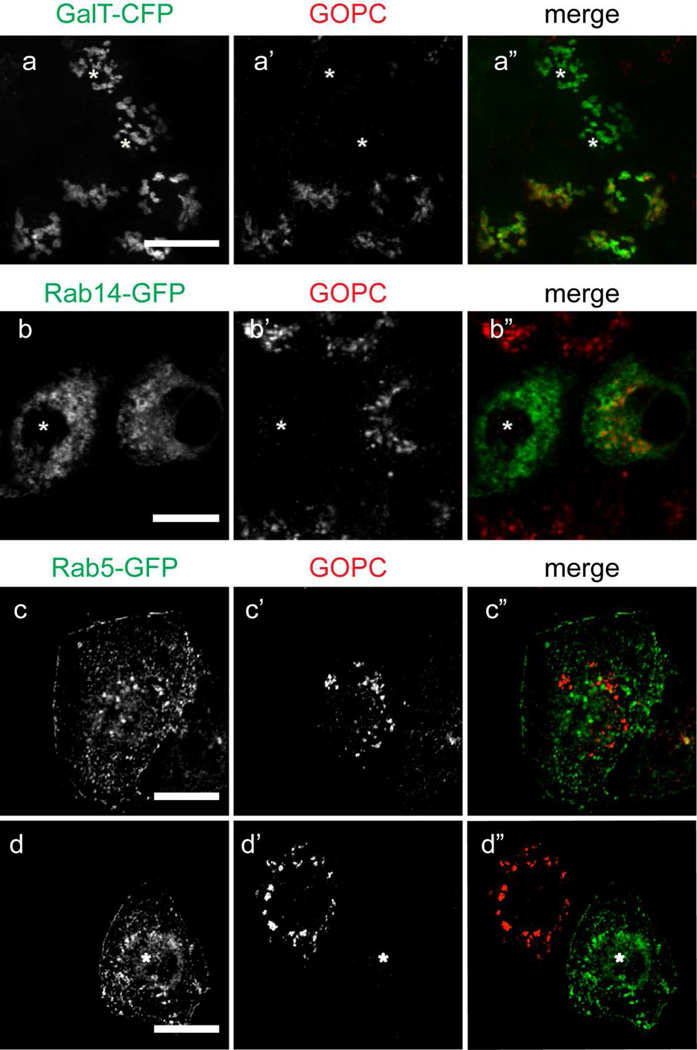
GOPC is ubiquitously expressed (Cheng et al., 2002), and is implicated in the control of protein trafficking from the Golgi apparatus to the plasma membrane or lysosomes. To assess its localization in epithelial cells, MDCK cells were double-labeled with anti-GOPC and markers of the Golgi complex. Because of antibody incompatibility, the Trans-Golgi network was labeled by expression of Galactosyltransferase-CFP. As shown in Figure 2a–b”, there is extensive colocalization of GOPC and the TGN marker galactyosyltransferase. To ensure that this overlap was not due to overexpression of the TGN enzyme, we also double-labeled NRK cells with antibodies against the endogenous TGN marker, TGN38 and GOPC. As shown in Figure S1, there is extensive overlap of the labels. The medial Golgi was marked with antibody against Golgi 58K (Figure 2 c–d”), and the cis-Golgi was marked using antibody against the Golgi matrix protein GM130 (Figure 2e–f”). However, GOPC did not colocalize with medial or cis-Golgi markers (Figure 2d” and f”), suggesting that GOPC functions predominantly in the final sorting step of protein trafficking in these cells.
Figure 2.
(a–b”) MDCK cells transfected with the TGN-marker enzyme galactosyltransferase-CFP (a), fixed and labeled for GOPC (a’). b-b” show the inset region in image a”. There is extensive overlap of the markers (b-b”, arrowheads). (c–d”) Cells double-labeled with antibodies against the trans-Golgi cisternae marker Golgi-58K (c) and GOPC (c’). d-d” show the inset area in image c”. The labeling is in adjacent cisternae (d-d”, arrows). (e–f”) Cells double-labeled with antibodies against the medial Golgi marker GM130 (e) and GOPC (e’). f-f” show the inset area in image e”. The merged image shows no overlap of the markers (f-f”, arrows). Scale bar top panel, 20µm, bottom panel, 1µm. Representative images are from at least 4 experiments.
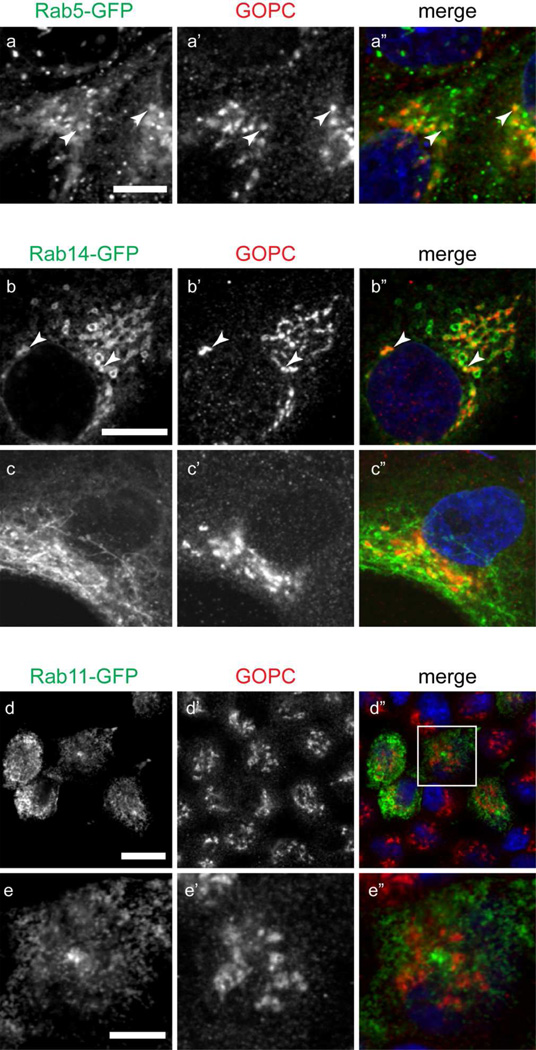
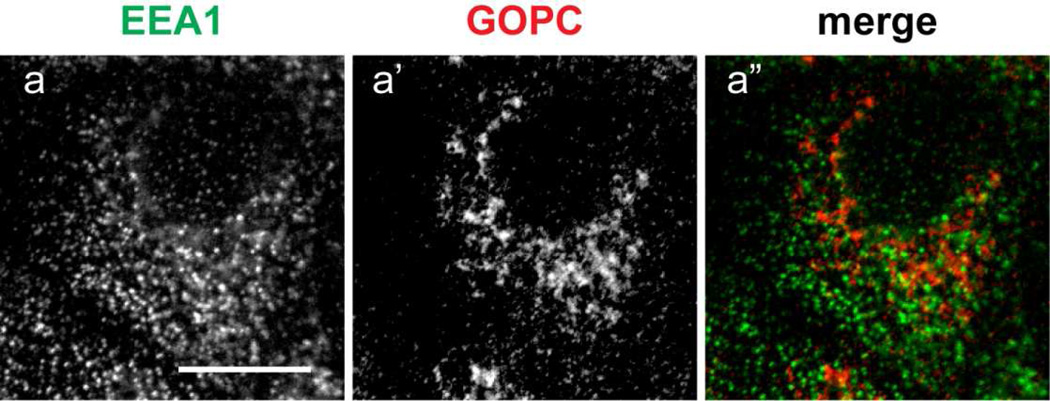
GOPC has also been implicated in endosomal regulation of cell surface receptors, generation of the sperm acrosome (a specialized endosomal compartment), and in autophagy (Cuadra et al., 2004; Meiffren et al., 2010; Wente et al., 2005; Yao et al., 2002; Yue et al., 2002). To determine if GOPC localizes to endosomal compartments, cells were transfected with plasmids expressing the early endosomal marker Rab5-GFP, the TGN/specialized endosome marker Rab14-GFP, and the recycling endosome marker Rab11-GFP. As shown in Figure 3, there is overlap of GOPC labeling with Rab5-GFP (Figure 3a-a”) and Rab14-GFP (Figure 3b-b”). However, no colocalization with Rab11-GFP is observed (Figure 3d–e”), suggesting that GOPC acts through early and specialized endosomes, but not within the recycling endosomal compartment. Interestingly, GOPC has been shown to disperse from the membrane after Brefeldin A (BFA) treatment in lung epithelial cells (Cheng et al., 2002). In MDCK cells, BFA causes tubulation of the endosomal compartment, but not the trans-Golgi network (Hunziker et al., 1991). When cells transfected with Rab14-GFP were incubated with BFA, followed by fixation and labeling for GOPC, we observe tubulation of Rab14-GFP but not GOPC (Figure 3c-c”). This suggests that colocalization of Rab14-GFP and GOPC seen in untreated cells occurs primarily in the TGN. Finally, the early endosomal antigen EEA1 has been shown to localize to some, but not all, early endosomes in MDCK cells (Wilson et al., 2000). Interestingly, double labeling of cells with EEA1 and GOPC resulted in no colocalization of these markers (Figure 4).
Figure 3.
MDCK cells transfected with (a) Rab5-GFP, (b) Rab14-GFP, or (d) Rab11-GFP were fixed and labeled for GOPC (a’, b’, c’, d’). There is colocalization of GOPC with Rab5-GFP (a-a”, arrowheads) and Rab14-GFP (b-b”, arrowheads). Treatment of cells with Brefeldin A results in tubulation of Rab14 (c). However, GOPC distribution is unaffected (c’). (d-d”) There is no overlap of GOPC with Rab11-GFP. e-e” show the inset in image d”. Scale bars, top panels, 20µm, bottom panel, 4µm. Representative images are from at least 3 experiments.
Figure 4.
MDCK cells were fixed and labeled for the early endosomal antigen EEA1 (a) and GOPC (a’). The merged image shows that there is no overlap of the labels (a”). Scale bar, 10µm.
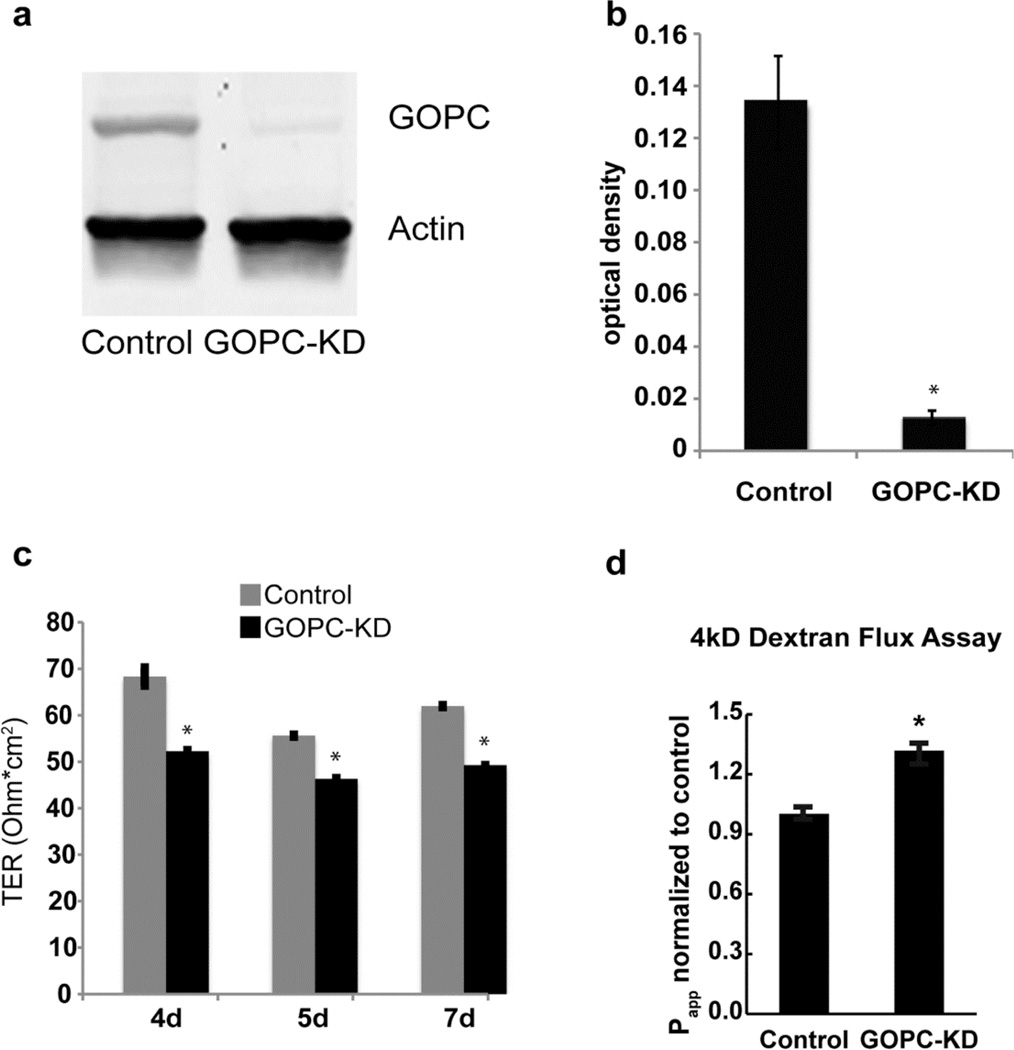
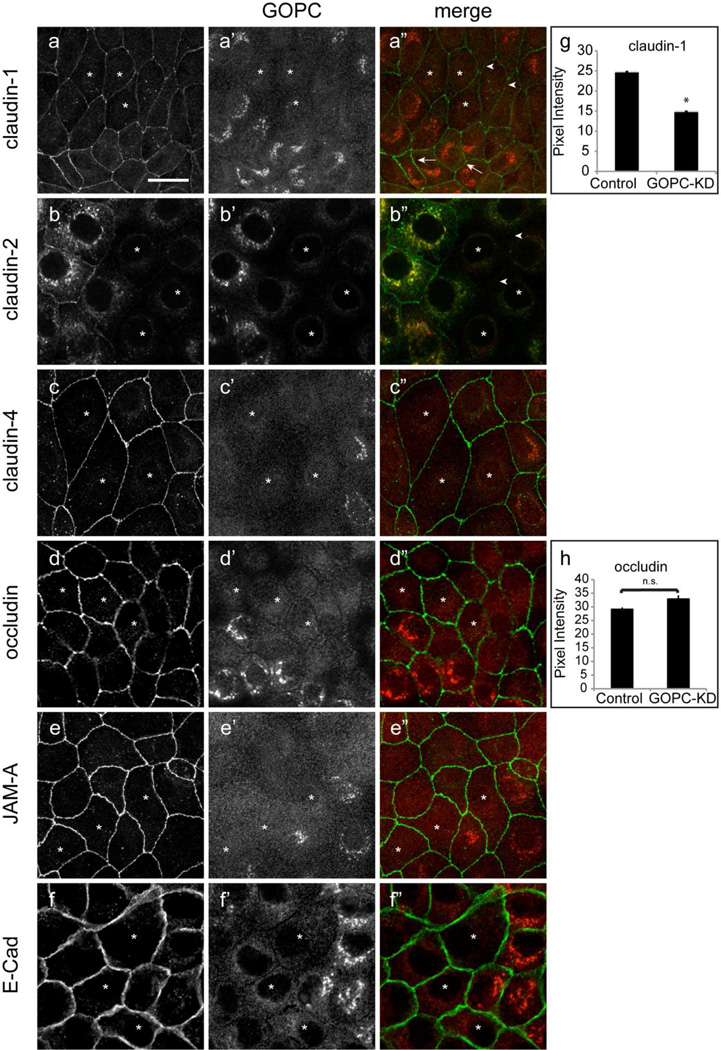
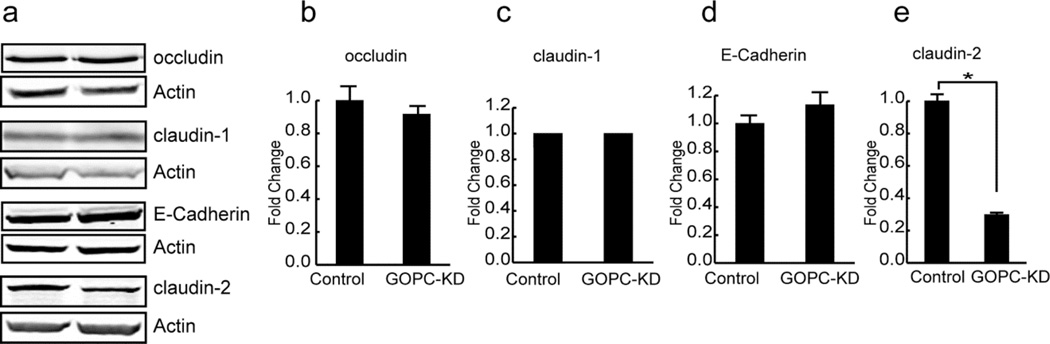
To determine the role of GOPC in the regulation of membrane traffic in epithelial cells, GOPC was knocked down in MDCK cells using a lentiviral vector followed by selection with puromycin to establish stable cell lines (Figure 5a and b). MDCK cells form polarized monolayers when grown on permeable supports, and we tested the ability of GOPC-knockdown cells to form a tight monolayer. As shown in Figure 5c, knockdown of GOPC resulted in a small but significant decrease in the transepithelial resistance of these cells. To determine if the TER decrease resulted from increased paracellular permeability, we assessed permeability using fluorescein-dextran in a flux assay (Figure 5d). GOPC-KD cells showed significantly higher paracellular permeability than control cells, consistent with the decrease in TER. To test if tight junction structure is altered, we labeled knockdown cells with antibodies against the tight junction proteins claudin- 1, 2, and 4, occludin, JAM-A, and E-cadherin, together with antibodies against GOPC. GOPC knockdown cells had decreased lateral labeling of claudin- 1 and 2, but labeling with other markers was unaffected (Figure 6a-f”). To quantify this, we measured the pixel intensity of claudin-1 and occludin at the lateral membranes using NIH ImageJ. As shown in Figure 6g, cells that were knocked down for GOPC had decreased pixel intensity of claudin-1 labeling at the intercellular junctions. However, occludin labeling was unaffected by GOPC knockdown (Figure 6h). To determine if the change in claudin-1 labeling at the membrane was due to decreased expression of claudin-1, we quantified the levels of tight junction and adherens junction proteins using immunoblotting. Western blots of lysates from control and KD cell lines showed that there is no change in total protein levels of occludin, claudin-1, or E-cadherin (Figure 7a, b, c and d), suggesting that the loss of claudin-1 from the junctions is due to a change in the intracellular trafficking of this protein. Interestingly, there was a significant decrease in the protein levels of the leaky claudin, claudin-2 (Figure 7a and e). Because loss of claudin-2 normally results in increased TER, this may mean that the moderate decrease in TER observed with GOPC knockdown is due to the opposing effects of changes in claudin-1 trafficking and the decrease of claudin-2 protein levels.
Figure 5.
MDCK cells knocked down for GOPC expression exhibit decreased transepithelial resistance. Western blotting (a) and quantification (b) demonstrates significant knockdown. (c) Transepithelial resistance is significantly reduced with GOPC knockdown. Cells were plated on filters and TER was measured on the days after plating as indicated. (d) 4kD Fluoroscein-Dextran was added to the apical media and samples were collected from bottom chambers after a 3 hr incubation. *p< 0.01, n=3.
Figure 6.
Knockdown of GOPC results in reduced claudin-1 at the tight junction. GOPC knockdown cells were double labeled for GOPC (a’, b’, c’, d’, e’, f’) and claudin-1 (a), claudin-2 (b), claudin-4(c), occludin (d), JAM-A (e), and E-cadherin (f). Knockdown of GOPC results in decreased claudin-1 at the lateral membrane (a”, arrowheads). Normal lateral membrane claudin-1 labeling is observed in adjacent cells that express GOPC (a”, arrows). Claudin-2 is also absent from cells knocked down for GOPC (b”, arrowheads). However, claudin-4, occludin, JAM-A, and E-cadherin labeling is unaffected by GOPC knockdown (c”, d”, e”, f”). Knockdown cells are indicated with (*). Scale bar, 20µm. (g,h) NIH-ImageJ quantification of pixel intensity of claudin-1 (n=52) (g) and occludin (n=37) labeling (h) at the lateral membrane after GOPC knockdown. *p < 0.01.
Figure 7.
Quantification of tight junction protein expression in GOPC knockdown cells. (a) Lysates from control and GOPC knockdown cells were immunoblotted for the indicated tight and adherens junction proteins. Actin is used as a loading control. (b–e) Most junction proteins are unchanged (b–d), but there is significantly less claudin-2 in GOPC knockdown cells (e). * p< 0.01, n=3.
We next tested whether knockdown of GOPC disrupts the morphology of the Trans-Golgi Network or endosomes. Knockdown cells expressing galactosyl-transferase-CFP, Rab14-GFP, and Rab5-GFP were labeled with antibodies against GOPC. As shown in Figure 8, the localization of all of these markers is unaffected by knockdown of GOPC when compared to cells expressing GOPC. These results suggest that GOPC does not play a structural role in these compartments but rather functions to regulate trafficking through interaction with effectors.
Figure 8.
(a-a”) GOPC-KD cells transfected with galactosyltransferase-CFP (a) were fixed and labeled for GOPC (a’). Knockdown cells are indicated with an asterisk. GOPC knockdown does not change the distribution of galactosyltransferase-CFP when compared with adjacent cells that have not been knocked down for GOPC (a”). (b-b”) GOPC-KD cells were transfected with Rab14-GFP (b) and labeled for GOPC (b’). There is no change in the distribution of Rab14-GFP after GOPC knockdown (b”). (c–d”) GOPC-KD cells were transfected with Rab5-GFP (c, d). GOPC knockdown does not change the distribution of Rab5 when compared with control cells (c”, d”). Knockdown cells are indicated with (*). Scale bars, 10µm. Representative images are from at least 3 experiments.
Discussion
Scaffolding proteins provide a framework for the regulated targeting of membrane proteins within the cell. The fact that GOPC is ubiquitously expressed and interacts with and regulates a large number of integral membrane proteins suggests that it serves a “housekeeping” role for a subset of cell surface proteins (Cheng et al., 2002). We find that GOPC regulates the subcellular distribution (claudin-1) and protein level (claudin-2) of two tight junction proteins. Since these claudins mediate opposing ion fluxes, i.e., decreased claudin-1 results in increased permeability whereas decreased claudin-2 results in decreased permeability (Van Itallie and Anderson, 2006), we observe only a small net decrease in transepithelial resistance. In contrast, movement across the leak pathway is regulated by occludin (Buschmann et al., 2013). We do observe an increase in paracellular movement of 4kd dextran, suggesting that the leak pathway is also impacted by GOPC knockdown. However, we do not observe changes in the levels or distribution of other tight junctions proteins such as occludin or the adherens junction protein E-cadherin. These findings are consistent with others showing that GOPC regulates the trafficking of a variety of ion channels, receptors, and mucins in epithelial and non-epithelial cells (Cheng et al., 2010a; Cheng et al., 2002; Cheng et al., 2004; Cheng et al., 2010b; Cuadra et al., 2004; Gee et al., 2009; Hassel et al., 2003; Hicks and Machamer, 2005; Pelaseyed and Hansson, 2011; Wente et al., 2005; Yao et al., 2001; Zhang et al., 2008).
Our finding that depletion of GOPC results in decreased claudin-2 protein expression suggests that GOPC positively regulates claudin-2 through inhibition of ubiquitination or by acting as a chaperone. In other cells, overexpression of GOPC enhanced the expression of mGluR5a and is thought to act as a chaperone to deliver the receptor Frizzled to the cell surface. Interestingly, GOPC colocalizes with Rab14, and Rab14 knockdown also results in decreased claudin-2 expression (Lu et al., 2014). Whether this similar phenotype is due to the same pathway remains to be tested. In contrast, the PDZ-dependent interaction of GOPC with CFTR and several other ion channels is well documented, and this interaction results in decreased cell surface expression and increased targeting to lysosomes.
We find that GOPC localizes to both the TGN and a subset of endosomal compartments. The subcellular site where GOPC acts to control the expression of membrane proteins remains incompletely understood. It has been implicated in endocytic down-regulation of receptors and channels from the plasma membrane, endosomal sorting and recycling, and direct targeting from the TGN to lysosomes (Cheng et al., 2005; He et al., 2004; Wente et al., 2005; Xu et al., 2010) both through direct interaction (as in the case of CFTR) or indirect interaction with other effectors. For example, a neuronal form of GOPC, nPIST, regulates AMPA receptor clustering on surface of hippocampal neurons through interaction with the AMPA receptor-binding partner stargazin (Cuadra et al., 2004). In addition, GOPC regulates autophagy induced by activation of the pathogen receptor CD46 (Joubert et al., 2009). Our finding that some GOPC is localized to Rab5-positive structures indicates that GOPC can traffic to endosomal compartments and may impact the recycling of receptors and channels.
Early reports in MDCK cells placed GOPC at the adherens junctions (Ito et al., 2006). We do not find GOPC at the lateral membrane/junctional region, which may be explained by antibody differences. Nonetheless, GOPC depletion did decrease transepithelial resistance as well as the surface distribution of claudin-1 and the protein level of claudin-2, indicating that that it regulates the trafficking of these molecules. This may occur from the endosomal compartment or at the level of the TGN, as trafficking between both the endosomes and the Golgi apparatus has been show to impact the integrity of both tight and adherens junctions (Desclozeaux et al., 2008; Ivanov et al., 2004; Lock and Stow, 2005; Lu et al., 2014; Marchiando et al., 2010; Naydenov et al., 2012; Regan-Klapisz et al., 2009). Claudins contain C-terminal PDZ binding motifs, however, these domains may not be required for localization at junctions, although deletion of a larger portion of the C-terminus results in mistargeting (Ruffer and Gerke, 2004). It may be that GOPC binds to a larger portion of the cytoplasmic domain to direct claudin trafficking. Alternatively, the interaction of GOPC with other regulators of junctional assembly or vesicular pH may indirectly impact the trafficking of these proteins.
Supplementary Material
Supplemental Figure 1. Normal rat kidney (NRK) cells labeled for TGN38 (a) and GOPC (a’). Merged image shows that endogenous TGN38 co-localizes with GOPC (a”).
Acknowledgements
We would like to thank members of the Wilson laboratory for helpful discussions and the Delamere and Simon laboratories for assistance with the dextran flux assays. We also thank Dr. Charles Parkos for antibody against JAM-A and the Broad Institute for shRNA against GOPC. This work was supported by NIH RO1 DK84047 (to JMW).
Literature cited
- Apodaca G, Gallo LI, Bryant DM. Role of membrane traffic in the generation of epithelial cell asymmetry. Nat Cell Biol. 2012;14:1235–1243. doi: 10.1038/ncb2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschmann MM, Shen L, Rajapakse H, Raleigh DR, Wang Y, Wang Y, Lingaraju A, Zha J, Abbott E, McAuley EM, Breskin LA, Wu L, Anderson K, Turner JR, Weber CR. Occludin OCEL-domain interactions are required for maintenance and regulation of the tight junction barrier to macromolecular flux. Mol Biol Cell. 2013;24:3056–3068. doi: 10.1091/mbc.E12-09-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charest A, Lane K, McMahon K, Housman DE. Association of a novel PDZ domain-containing peripheral Golgi protein with the Q-SNARE (Q-soluble N-ethylmaleimide-sensitive fusion protein (NSF) attachment protein receptor) protein syntaxin 6. J Biol Chem. 2001;276:29456–29465. doi: 10.1074/jbc.M104137200. [DOI] [PubMed] [Google Scholar]
- Chen A, Gossling EK, Witkowski L, Bhindi A, Bauch C, Roussy G, Sarret P, Kreienkamp HJ, Stroh T. Regional and subcellular distribution of the receptor-targeting protein PIST in the rat central nervous system. J Comp Neurol. 2012;520:889–913. doi: 10.1002/cne.22774. [DOI] [PubMed] [Google Scholar]
- Cheng J, Cebotaru V, Cebotaru L, Guggino WB. Syntaxin 6 and CAL mediate the degradation of the cystic fibrosis transmembrane conductance regulator. Mol Biol Cell. 2010a;21:1178–1187. doi: 10.1091/mbc.E09-03-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Moyer BD, Milewski M, Loffing J, Ikeda M, Mickle JE, Cutting GR, Li M, Stanton BA, Guggino WB. A Golgi-associated PDZ domain protein modulates cystic fibrosis transmembrane regulator plasma membrane expression. J Biol Chem. 2002;277:3520–3529. doi: 10.1074/jbc.M110177200. [DOI] [PubMed] [Google Scholar]
- Cheng J, Wang H, Guggino WB. Modulation of mature cystic fibrosis transmembrane regulator protein by the PDZ domain protein CAL. J Biol Chem. 2004;279:1892–1898. doi: 10.1074/jbc.M308640200. [DOI] [PubMed] [Google Scholar]
- Cheng J, Wang H, Guggino WB. Regulation of cystic fibrosis transmembrane regulator trafficking and protein expression by a Rho family small GTPase TC10. J Biol Chem. 2005;280:3731–3739. doi: 10.1074/jbc.M410026200. [DOI] [PubMed] [Google Scholar]
- Cheng S, Zhang J, Zhu P, Ma Y, Xiong Y, Sun L, Xu J, Zhang H, He J. The PDZ domain protein CAL interacts with mGluR5a and modulates receptor expression. J Neurochem. 2010b;112:588–598. doi: 10.1111/j.1471-4159.2009.06454.x. [DOI] [PubMed] [Google Scholar]
- Cuadra AE, Kuo SH, Kawasaki Y, Bredt DS, Chetkovich DM. AMPA receptor synaptic targeting regulated by stargazin interactions with the Golgi-resident PDZ protein nPIST. J Neurosci. 2004;24:7491–7502. doi: 10.1523/JNEUROSCI.1255-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing PR, Vouilleme L, Pellegrini M, Boisguerin P, Madden DR. A stabilizing influence: CAL PDZ inhibition extends the half-life of DeltaF508-CFTR. Angew Chem Int Ed Engl. 2010;49:9907–9911. doi: 10.1002/anie.201005585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desclozeaux M, Venturato J, Wylie FG, Kay JG, Joseph SR, Le HT, Stow JL. Active Rab11 and functional recycling endosome are required for E-cadherin trafficking and lumen formation during epithelial morphogenesis. Am J Physiol Cell Physiol. 2008;295:C545–C556. doi: 10.1152/ajpcell.00097.2008. [DOI] [PubMed] [Google Scholar]
- Gee HY, Park HW, Kim KH, Lee MG. PDZ-based adaptor proteins in epithelial anion transport and VIP receptor regulation. J Med Invest. 2009;56(Suppl):302–305. doi: 10.2152/jmi.56.302. [DOI] [PubMed] [Google Scholar]
- Gentzsch M, Cui L, Mengos A, Chang XB, Chen JH, Riordan JR. The PDZ-binding chloride channel ClC-3B localizes to the Golgi and associates with cystic fibrosis transmembrane conductance regulator-interacting PDZ proteins. J Biol Chem. 2003;278:6440–6449. doi: 10.1074/jbc.M211050200. [DOI] [PubMed] [Google Scholar]
- Goellner GM, DeMarco SJ, Strehler EE. Characterization of PISP, a novel single-PDZ protein that binds to all plasma membrane Ca2+ATPase b-splice variants. Ann N Y Acad Sci. 2003;986:461–471. doi: 10.1111/j.1749-6632.2003.tb07230.x. [DOI] [PubMed] [Google Scholar]
- Hassel B, Schreff M, Stube EM, Blaich U, Schumacher S. CALEB/NGC interacts with the Golgi-associated protein PIST. J Biol Chem. 2003;278:40136–40143. doi: 10.1074/jbc.M305577200. [DOI] [PubMed] [Google Scholar]
- He J, Bellini M, Xu J, Castleberry AM, Hall RA. Interaction with cystic fibrosis transmembrane conductance regulator-associated ligand (CAL) inhibits beta1-adrenergic receptor surface expression. J Biol Chem. 2004;279:50190–50196. doi: 10.1074/jbc.M404876200. [DOI] [PubMed] [Google Scholar]
- Herrmann S, Ninkovic M, Kohl T, Pardo LA. PIST (GOPC) modulates the oncogenic voltage-gated potassium channel KV10.1. Front Physiol. 2013;4:201. doi: 10.3389/fphys.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks SW, Horn TA, McCaffery JM, Zuckerman DM, Machamer CE. Golgin-160 Promotes Cell Surface Expression of the Beta-1 Adrenergic Receptor. Traffic. 2006;7:1666–1677. doi: 10.1111/j.1600-0854.2006.00504.x. [DOI] [PubMed] [Google Scholar]
- Hicks SW, Machamer CE. Isoform-specific interaction of golgin-160 with the Golgi-associated protein PIST. J Biol Chem. 2005;280:28944–28951. doi: 10.1074/jbc.M504937200. [DOI] [PubMed] [Google Scholar]
- Hunziker W, Whitney JA, Mellman I. Selective inhibition of transcytosis by brefeldin A in MDCK cells. Cell. 1991;67:617–627. doi: 10.1016/0092-8674(91)90535-7. [DOI] [PubMed] [Google Scholar]
- Ito H, Iwamoto I, Morishita R, Nozawa Y, Asano T, Nagata K. Identification of a PDZ protein, PIST, as a binding partner for Rho effector Rhotekin: biochemical and cell-biological characterization of Rhotekin-PIST interaction. Biochem J. 2006;397:389–398. doi: 10.1042/BJ20052015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov AI, Nusrat A, Parkos CA. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol Biol Cell. 2004;15:176–188. doi: 10.1091/mbc.E03-05-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov AI, Nusrat A, Parkos CA. Endocytosis of the apical junctional complex: mechanisms and possible roles in regulation of epithelial barriers. Bioessays. 2005;27:356–365. doi: 10.1002/bies.20203. [DOI] [PubMed] [Google Scholar]
- Joubert PE, Meiffren G, Gregoire IP, Pontini G, Richetta C, Flacher M, Azocar O, Vidalain PO, Vidal M, Lotteau V, Codogno P, Rabourdin-Combe C, Faure M. Autophagy induction by the pathogen receptor CD46. Cell Host Microbe. 2009;6:354–366. doi: 10.1016/j.chom.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Kitt KN, Hernandez-Deviez D, Ballantyne SD, Spiliotis ET, Casanova JE, Wilson JM. Rab14 regulates apical targeting in polarized epithelial cells. Traffic. 2008;9:1218–1231. doi: 10.1111/j.1600-0854.2008.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock JG, Stow JL. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol Biol Cell. 2005;16:1744–1755. doi: 10.1091/mbc.E04-10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Johnson DL, Stewart L, Waite K, Elliott D, Wilson JM. Rab14 regulation of claudin-2 trafficking modulates epithelial permeability and lumen morphogenesis. Mol Biol Cell. 2014;25:1744–1754. doi: 10.1091/mbc.E13-12-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiando AM, Shen L, Graham WV, Weber CR, Schwarz BT, Austin JR, 2nd, Raleigh DR, Guan Y, Watson AJ, Montrose MH, Turner JR. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol. 2010;189:111–126. doi: 10.1083/jcb.200902153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzesco AM, Dunia I, Pandjaitan R, Recouvreur M, Dauzonne D, Benedetti EL, Louvard D, Zahraoui A. The small GTPase Rab13 regulates assembly of functional tight junctions in epithelial cells. Mol Biol Cell. 2002;13:1819–1831. doi: 10.1091/mbc.02-02-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiffren G, Joubert PE, Gregoire IP, Codogno P, Rabourdin-Combe C, Faure M. Pathogen recognition by the cell surface receptor CD46 induces autophagy. Autophagy. 2010;6:299–300. doi: 10.4161/auto.6.2.11132. [DOI] [PubMed] [Google Scholar]
- Morimoto S, Nishimura N, Terai T, Manabe S, Yamamoto Y, Shinahara W, Miyake H, Tashiro S, Shimada M, Sasaki T. Rab13 mediates the continuous endocytic recycling of occludin to the cell surface. J Biol Chem. 2005;280:2220–2228. doi: 10.1074/jbc.M406906200. [DOI] [PubMed] [Google Scholar]
- Naydenov NG, Brown B, Harris G, Dohn MR, Morales VM, Baranwal S, Reynolds AB, Ivanov AI. A membrane fusion protein alphaSNAP is a novel regulator of epithelial apical junctions. PLoS One. 2012;7:e34320. doi: 10.1371/journal.pone.0034320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neudauer CL, Joberty G, Macara IG. PIST: a novel PDZ/coiled-coil domain binding partner for the rho-family GTPase TC10. Biochem Biophys Res Commun. 2001;280:541–547. doi: 10.1006/bbrc.2000.4160. [DOI] [PubMed] [Google Scholar]
- Ohno S. Intercellular junctions and cellular polarity: the PAR-aPKC complex, a conserved core cassette playing fundamental roles in cell polarity. Curr Opin Cell Biol. 2001;13:641–648. doi: 10.1016/s0955-0674(00)00264-7. [DOI] [PubMed] [Google Scholar]
- Pelaseyed T, Hansson GC. CFTR anion channel modulates expression of human transmembrane mucin MUC3 through the PDZ protein GOPC. J Cell Sci. 2011;124:3074–3083. doi: 10.1242/jcs.076943. [DOI] [PubMed] [Google Scholar]
- Regan-Klapisz E, Krouwer V, Langelaar-Makkinje M, Nallan L, Gelb M, Gerritsen H, Verkleij AJ, Post JA. Golgi-associated cPLA2alpha regulates endothelial cell-cell junction integrity by controlling the trafficking of transmembrane junction proteins. Mol Biol Cell. 2009;20:4225–4234. doi: 10.1091/mbc.E08-02-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richetta C, Gregoire IP, Verlhac P, Azocar O, Baguet J, Flacher M, Tangy F, Rabourdin-Combe C, Faure M. Sustained autophagy contributes to measles virus infectivity. PLoS Pathog. 2013;9:e1003599. doi: 10.1371/journal.ppat.1003599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Macara IG. Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Biol. 2014;15:225–242. doi: 10.1038/nrm3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffer C, Gerke V. The C-terminal cytoplasmic tail of claudins 1 and 5 but not its PDZ-binding motif is required for apical localization at epithelial and endothelial tight junctions. Eur J Cell Biol. 2004;83:135–144. doi: 10.1078/0171-9335-00366. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Meth. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L. Tight junctions on the move: molecular mechanisms for epithelial barrier regulation. Ann N Y Acad Sci. 2012;1258:9–18. doi: 10.1111/j.1749-6632.2012.06613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–429. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- Van Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, Proctor W, Colegio OR, Anderson JM. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci. 2008;121:298–305. doi: 10.1242/jcs.021485. [DOI] [PubMed] [Google Scholar]
- Wawrzak D, Luyten A, Lambaerts K, Zimmermann P. Frizzled-PDZ scaffold interactions in the control of Wnt signaling. Adv Enzyme Regul. 2009;49:98–106. doi: 10.1016/j.advenzreg.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Wente W, Stroh T, Beaudet A, Richter D, Kreienkamp HJ. Interactions with PDZ domain proteins PIST/GOPC and PDZK1 regulate intracellular sorting of the somatostatin receptor subtype 5. J Biol Chem. 2005;280:32419–32425. doi: 10.1074/jbc.M507198200. [DOI] [PubMed] [Google Scholar]
- Wilson JM, de Hoop M, Zorzi N, Toh BH, Dotti CG, Parton RG. EEA1, a tethering protein of the early sorting endosome, shows a polarized distribution in hippocampal neurons, epithelial cells, and fibroblasts. Mol Biol Cell. 2000;11:2657–2671. doi: 10.1091/mbc.11.8.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Oshima K, Heller S. PIST regulates the intracellular trafficking and plasma membrane expression of cadherin 23. BMC Cell Biol. 2010;11:80. doi: 10.1186/1471-2121-11-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao R, Ito C, Natsume Y, Sugitani Y, Yamanaka H, Kuretake S, Yanagida K, Sato A, Toshimori K, Noda T. Lack of acrosome formation in mice lacking a Golgi protein, GOPC. Proc Natl Acad Sci U S A. 2002;99:11211–11216. doi: 10.1073/pnas.162027899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao R, Maeda T, Takada S, Noda T. Identification of a PDZ domain containing Golgi protein, GOPC, as an interaction partner of frizzled. Biochem Biophys Res Commun. 2001;286:771–778. doi: 10.1006/bbrc.2001.5430. [DOI] [PubMed] [Google Scholar]
- Yue Z, Horton A, Bravin M, DeJager PL, Selimi F, Heintz N. A novel protein complex linking the delta 2 glutamate receptor and autophagy: implications for neurodegeneration in lurcher mice. Neuron. 2002;35:921–933. doi: 10.1016/s0896-6273(02)00861-9. [DOI] [PubMed] [Google Scholar]
- Zhang J, Cheng S, Xiong Y, Ma Y, Luo D, Jeromin A, Zhang H, He J. A novel association of mGluR1a with the PDZ scaffold protein CAL modulates receptor activity. FEBS Lett. 2008;582:4117–4124. doi: 10.1016/j.febslet.2008.10.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Normal rat kidney (NRK) cells labeled for TGN38 (a) and GOPC (a’). Merged image shows that endogenous TGN38 co-localizes with GOPC (a”).