Abstract
Bronchopulmonary dysplasia (BPD) is the most common respiratory consequence of premature birth and contributes to significant short- and long-term morbidity, mortality, and resource utilization. Initially defined as a radiographic, clinical, and histopathologic entity, the chronic lung disease known as BPD has evolved as obstetrical and neonatal care have improved the survival of lower gestational age infants. Now, definitions based on need for supplemental oxygen at 28 days and or 36 weeks provide a useful reference point in the NICU, but are no longer based on histopathologic findings, and are neither designed to predict longer-term respiratory consequences nor to study the evolution of a multifactorial disease. The aims of this review are to critically examine the evolution of the diagnosis of BPD and the challenges inherent to current classifications. We found that the increasing use of respiratory support strategies that administer ambient air without supplemental oxygen confounds oxygen-based definitions of BPD. Furthermore, lack of reproducible, genetic, biochemical, and physiologic biomarkers limits the ability to identify impending BPD for early intervention, quantify disease severity for standardized classification and approaches, and reliably predict the long-term outcomes. More comprehensive, multidisciplinary approaches to overcome these challenges involve longitudinal observation of extremely preterm infants, not only those with BPD, using genetic, environmental, physiologic and clinical data as well as large databases of patient samples. The Prematurity and Respiratory Outcomes Program (PROP) will provide such a framework to address these challenges through high-resolution characterization of both NICU and post-NICU discharge outcomes.
Keywords: prematurity, bronchopulmonary dysplasia, chronic lung disease
Approximately one out of every nine live births in the United States and Europe occurs at less than 37 completed weeks of gestation. These infants are at risk for significant respiratory morbidity especially in the first year of life 1, 2. Many return to pediatricians, pulmonologists, and emergency departments with respiratory symptoms, typically recurrent or chronic wheeze and/or cough, poor growth and an excess of upper and lower respiratory tract infections 3. The frequency of these signs of respiratory dysfunction correlates inversely with gestational age and weight at birth, displays a male predominance4 and is more common in those who meet criteria for the diagnosis of bronchopulmonary dysplasia (BPD). Depending on differences in definition, the racial/ethnic population and clinical management variables, approximately 35-45% of infants born before 28 weeks of gestation develop BPD of varying severity and are at risk for long-term multi-organ system consequences 5. Even those without BPD exhibit respiratory limitations at school age and into adulthood 2, 6, 7. The aims of this review are to critically examine the evolution of the diagnosis of BPD and the challenges inherent to current classifications. Based on these, we propose a more comprehensive approach to understanding, diagnosing and researching the larger problem of respiratory disease of prematurity.
An ongoing debate among neonatologist and pulmonary specialists is the disconnect between the lung disease or respiratory morbidities of preterm infants and the clinical disease classified as BPD. Some infants with BPD do not appear to develop long-term respiratory sequelae, while others without BPD or oxygen requirement at 36 weeks exhibit respiratory morbidities in infancy 8 or even limitations at school age and adulthood, 2, 6, 7 perhaps related to other contributors such as sex, socioeconomic conditions, genetic/atopic contributions and microbial burden. Among these infants, the annual socioeconomic costs of BPD are estimated at well over $2.5 billion in the United States, second only to asthma, and far greater than cystic fibrosis 9. A California survey study of discharge summaries indicated that about 15% of preterm infants required at least one re-hospitalization within the first year of life, most commonly with acute respiratory disease. The highest rate of readmission (31%) was in infants born at less than 25 weeks of gestation, whereas the largest volume of readmissions was infants born at 35 weeks of gestation with a total cost of readmission exceeding $90 million 10. Studies of adolescents demonstrate persistent, and in some cases deteriorating, chronic obstructive pulmonary disease/dysfunction in children born prematurely, suggesting that the socioeconomic and public health impact of preterm birth extends into adulthood 11. A study based on the Nationwide Inpatient Sample (1993-2006, including more than 9.5 million neonatal hospitalizations) demonstrated a significant decrease in the incidence of diagnosed BPD yet increases in initial hospitalization charges and length of stay for patients with BPD, despite controlling for increases in the incidence of very low birth weight infants (VLBW) 12. Therefore, the costs of lung disease of prematurity are likely to continue rising.
Evolution of the diagnosis of BPD
Although BPD is the most common chronic respiratory consequence of prematurity, its relative incidence fluctuates with evolving diagnostic criteria along with changes in delivery room and NICU practice, degree of prematurity, and survival. BPD was initially described in 1967 by Northway 13 as a clinical and pathological diagnosis in preterm infants who received mechanical ventilation and high concentrations of inspired oxygen for more than 150 hours. In this report, mean gestational age of survivors was 34 vs. 31 weeks for those who died, representing a significantly different population than the survivors of today. The study focused on radiological, pathological and clinical findings such as heterogeneous opacification of airspaces, fibrosis, inflammation, and non-homogeneous inflation of alveoli resulting in dysplastic changes, and prolonged need for respiratory support or death.
In 1979, Tooley suggested that a significant predictor of long term lung disease in preterm infants might be the requirement for oxygen supplementation at 28 days after birth 14, and in 1988, Shennan, in a single center study, suggested that oxygen use at 36 weeks postmenstrual age (PMA) was an even stronger predictor for persistent pulmonary disease in the first 2 years of life, especially for those born at less than 30 weeks of gestation, and hence a more clinically useful indicator of the disease BPD 15. Abnormal pulmonary findings at 2-year follow-up were defined as death, use of supplemental oxygen at 40 weeks, >1 hospitalization, wheezing requiring medication or growth failure. When the diagnosis of BPD is based on oxygen supplementation at 36 weeks PMA, up to 35-45% of infants born at <29 weeks of gestation qualify for the diagnosis 5. Although the Shennan report set the standard for identifying infants with BPD, the study was conducted at a time when NICU care differed markedly from current practices. Antenatal corticosteroids, surfactant replacement, nasal continuous positive airway pressure (CPAP) and humidified high flow cannula were not part of clinical care in the early 1980's and the study did not include infants born at less than 25 weeks of gestation.
In 1999, Alan Jobe published the term “new BPD” to highlight the histopathological failure of alveolarization and vascular development that was being observed in animal models and in human autopsy specimens following preterm delivery in the post-surfactant and “gentle ventilation” era 16. An NICHD/NHLBI/ORD Workshop further strengthened the concept of a “new BPD” characterized more by developmental arrest than by inflammation and fibrosis 17. The Workshop recommended recognizing four degrees of BPD severity in infants born at <32 weeks: NONE (<28 days of oxygen support); MILD (oxygen or respiratory support at >28d but on room air at 36 weeks PMA); MODERATE (<30% oxygen at 36 weeks); SEVERE (>30% oxygen or positive pressure support at 36 weeks). This is frequently referred to as the “NIH consensus” definition of BPD. The need for a standardized test to confirm oxygen requirement was noted. Considerations of “effective” FiO2 by noninvasive delivery systems, and of the appropriate classification of infants treated with nasal cannula with 21% oxygen, were not yet addressed by this definition.
Testing for BPD
Meanwhile, in the 1990's, the NICHD Neonatal Research Network (NRN) was concerned by the wide variations in the incidence of BPD reported across sites within their network. In order to develop more inter-institutional consistency, Walsh et al. published a “physiological” definition of BPD in 2003 5. This definition included criteria for an oxygen reduction test or “room air challenge” to standardize the determination of oxygen requirement at 36 weeks. For the room air challenge, those receiving positive pressure or >30% oxygen were assigned the diagnosis of BPD without further testing. Those receiving less support underwent a timed stepwise reduction to room air. Outcomes of the room-air challenge were “no BPD” if saturations remained ≥88% in room air for 60 minutes or “BPD” (room air saturation <88%). Results of the room air challenge were compared to oxygen prescription based on clinical practice style. A modification of the Walsh oxygen reduction test (saturation limit at 90% with 30 minutes in room air) has remained the standard physiologic test for determining oxygen requirement and the basis for assigning a diagnosis of BPD18 later alos associated with an increase risk of cognitive impairment 19. The new categories of BPD and the room air challenge test also resulted in some confusion with regards to clinical practice, prompting an inclusive grading system that incorporated both the new standard and physiologic definitions 20. However, these systems have yet to be universally adopted. A recent systematic review of clinical trials for therapeutic agents to prevent BPD highlighted that primary outcomes of trials were often confounded by the inconsistencies in BPD definition used 21. Of the 47 RCTs published between 1992 and 2013 that were evaluated by the authors, 45% still used oxygen requirement at 28 days, 71% oxygen at 36 weeks and only 6% used the physiologic definition despite the more objective nature of the challenge test and the reduction in inter-institutional variation in BPD diagnosis.
The grading system has also been questioned due to its poor positive predictive value for long-term respiratory outcomes. In 2005, Ehrenkranz sought to validate the various working definitions of BPD based on 18-22 month outcomes, including use of pulmonary medications, rehospitalization for pulmonary causes, receipt of respiratory syncytial virus prophylaxis, and neurodevelopmental abnormalities 22. Data were collected for 3848 infants (birth weight ≤1000 g, GA <32 weeks, alive at 36 weeks PMA) entered into the NRN Very Low Birth Weight (VLBW) Infant Registry (1995 – 1999) and linked to data from the Network Extremely Low Birth Weight (ELBW) Follow-up Program of neurodevelopmental and health assessments at 18 to 22 months’ corrected age. Using the Workshop BPD definition, 27% and 30% of infants who met criteria for “no BPD” and “mild BPD,” respectively, required pulmonary medication or hospitalization for pulmonary causes, or had neurodevelopmental impairment in that follow-up interval. Conversely, only 47% of those categorized as having severe BPD required pulmonary medication and 39% required rehospitalization. A retrospective analysis of respiratory outcomes data factoring in the room air challenge showed that the positive predictive value of the new classification plus testing at 36 weeks would only result in a positive predictive value of 49% at best for poor long-term respiratory outcome23. Additional studies of respiratory outcomes of preterm infants at 8 to 10 years of age similarly identified persistent abnormalities such as reduced forced vital capacity, forced expired volume at 1 second (FEV1) and diffusion capacity per alveolar volume, as compared to term controls, independent of the diagnosis of BPD 24, 25, while others have confirmed that the severity of BPD as assigned by the NIH consensus predicts pulmonary function abnormalities in childhood 26. Although classification of BPD by NIH Workshop criteria was significantly associated with short-term outcomes, the prognostic value for adverse outcome at 18-22 months based simply on degree of respiratory support at 36 weeks is somewhat limited and ignores other confounders, such as respiratory infections, that may occur in the interval between 36 weeks and the outcome time point of interest and further contribute to respiratory morbidity. 27, 28.
Challenges in characterization and prediction of BPD
An incisive commentary by Lefkowitz and Rosenberg in 2008 pointed out profound gaps in the logic between association of a disease now characterized by alveolar growth inhibition with oxygen requirement at 36 weeks and poor long-term respiratory outcomes 23. The authors pointed out that there is little statistical or mechanistic evidence to hypothesize that a physiologic challenge as described above can differentiate between presence or absence of alveolar growth. To understand the links between BPD and respiratory outcomes, they highlighted the importance of studying the pathology and natural history of the disease, its signs and characteristics independent of treatment and its long-term effects.
Mechanistic inferences are currently imprecise
The gaps in our understanding of chronic and long-term respiratory sequelae arising from preterm birth are exacerbated by a relative paucity of clinically derived and relevant biospecimens and assessments that can be utilized to predict outcome and/or identify mechanisms contributing to disease pathogenesis (Table 1). Current histopathology used to characterize and understand mechanisms of BPD is based on autopsy or lung biopsy specimens from the most severely affected children 29 and lacks direct relevance for evaluating progression of the disease in less affected infants. Similarly, animal models attempt to mimic the preconceived inciting events that lead to disrupted development and repair 30, 31, 32, 33. These models have allowed tremendous advances in understanding the progression of disease, influence of exposures and response to various therapies 34, 35, 36, 37, 38. However, animal models can have imperfect correspondence with human infant development. While extremes of interventions often give a clearer profile of response to interventions, some animal models use conditions inconsistent with current clinical care (e.g. use of 100% oxygen). Therefore, to continue using animal models for future therapeutic research, it is imperative to obtain enough human data to validate these essential translational models. As a case in point, large studies have examined early markers of inflammatory status such as pro-inflammatory cytokines, chemokines or oxidative stress modulators and mediators 39, 40. These studies have allowed the refinement of animal models of disease for BPD. Some biomarker panels such as those from tracheal aspirates, may even hold promise in predicting which infants will later develop BPD 41 consistent with the concept of pro/anti-inflammatory cytokine imbalance in BPD 42. However, at our current level of understanding, these biomarkers cannot routinely be used to guide clinical care.
Table 1.
Challenges to characterizing lung disease of prematurity
| Phenotype characterization | Lack of access to tissue at various stages of disease |
| Lack of severity biomarkers | |
| Interaction of development/maturation and disease evolution | |
| Physiologic function in disease | Lack of high-resolution, non-invasive assessment of lung structure |
| Differences in baseline function vs. “challenge” tests | |
| Timing of testing and predictive value | |
| Response to therapeutic intervention | Variability of respiratory support |
| Lack of data on medication efficacy | |
| Choice of short vs. long term functional gains | |
| Respiratory outcomes | Lack of relevance to health status and healthcare needs |
| Lack of genetic and epigenetic information on infants/families | |
| Incomplete knowledge of the contribution of respiratory or systemic infections to outcomes | |
| Difficulty in performing infant pulmonary function tests and/or in obtaining high resolution imaging studies | |
| Lack of information on medication usage after discharge | |
| Contribution of respiratory or systemic infections | |
| Lack of standardized examinations for preterm infants |
With higher resolution characterization of phenotype, these and other markers may yet prove relevant in understanding and characterizing the severity and progression of lung disease in preterm infants: the timing and source of biospecimens, as well as the best panel of biomarkers, still need to be refined and tested. Recent comprehensive reviews suggest the need for large sample size studies of biospecimens from multiple sources (such as tracheal aspirates, urine, saliva and blood) in order to start to understand the normal continuum of lung development to better define the multifactorial etiologies and processes of BPD 43. Finally, biomarkers need to be considered within the context of gene-environment interactions, adding to the complexity of mechanistic inferences. BPD appears to have a heritable component, but contrary to earlier assumptions, it now appears most likely to be polygenic 44. Just as important, there may be a genetic predisposition of preterm infants to later respiratory viral infections and respiratory morbidities, unrelated to genetic characteristics associated with poor lung function at discharge from the NICU. Therefore candidate-gene approaches may be insufficient and large genome-wide association studies will prove more valuable in understanding the role of genes, environment and evolution of BPD 45, 46.
Technological advances change the precision of existing definitions
While still meaningful, the current operational definition of BPD lacks precision needed to provide mechanistic insight, quantify severity of disease, or to predict disparate long-term respiratory consequences (Table 1. Challenges by category). A definition based mostly on the need for supplemental oxygen is less relevant in current practice conditions, as neonatologists strive to avoid excessive oxygen exposure and its presumed oxidant stress. This management approach has resulted in ventilatory strategies that provide flow or positive pressure with ambient air alone. Recent meta-analysis of randomized controlled trials of non-invasive respiratory support of preterm infants showed a reduction in BPD of borderline significance but additional survival without BPD of one infant for every 25 babies treated with CPAP in the delivery room instead of intubation 47. High-flow nasal cannulas are now ubiquitous in NICUs and non-invasive ventilation can be delivered through ventilators or by multiple other forms of CPAP. A summary of studies comparing nasal intermittent positive pressure ventilation and of high flow nasal cannula suggests that there is yet insufficient evidence to definitively recommend use of these therapies to reduce chronic lung disease 48. These advances, respiratory support without intubation and with little or no oxygen, have yet to be reflected in the algorithms of existing BPD definitions.
Timing is everything, or maybe not.
The PMA of 36 weeks represents a feasible and convenient endpoint for lung disease testing, as most extremely preterm infants are still in NICUs at this age and can be evaluated in a controlled hospital setting. At this late preterm stage, neonatal lungs are in the early alveolar phase of development and thus a need for supplemental oxygen may reflect ventilation and perfusion abnormalities; these could be related to immature alveolar and vascular development, to chronic lung disease or to a combination of the two. The pathophysiology prompting treatment with supplemental oxygen at this point may be related not only to lung injury, but also to immature neurorespiratory control mechanisms, airway obstruction, airway hyperreactivity, or pulmonary hypertension 49, 50, 51. Even as apnea becomes less frequent, intermittent hypoxia may continue 52. The apparent pulmonary phenotype in these cases is actually a function of the treatment, not necessarily an indication of respiratory failure. Essential developmental changes occur between 36 and 44 weeks including maturation of neurorespiratory control, and increasing chest wall stability such that testing later than 40 weeks may reflect persistent lung disease more accurately 49, 53. To date, the accuracy at predicting respiratory outcomes based on oxygen requirement at 36 weeks is similar to that at 37 weeks, and not substantially improved at 40 weeks 54 supporting the possible dissociation between surrogate measure and clinical disease. Further, it was recently confirmed that lung disease in very preterm infants represents a pathophysiological continuum that may have lasting effects independent of the current 36-week, oxygen-based definitions 55. This continuum needs to be evaluated in its entirety to provide more precise clinical surrogates of disease severity and to monitor the effect of interventions in improving outcomes.
Infant respiratory outcomes present unique difficulties for quantitation
A history of BPD is associated with lower lung function indices in infancy 56, 57, 58. However, even preterm infants with minimal oxygen requirement at discharge demonstrate airflow obstruction that persists and does not “catch up” to normal controls in the first 2 years of life 59. In addition, these children have increased rates of wheezing, asthma, and respiratory illness-related hospitalizations 60. Even young adults born at <28 weeks of gestation, with or without the diagnosis of BPD, demonstrate limitations in exercise capacity and have pulmonary function indices not unlike those of chronic obstructive pulmonary disease. Therefore, more sensitive endpoints of respiratory function during infancy may unmask subclinical disease and serve as markers of disrupted lung growth and repair 6, 7. A comprehensive physical examination of the respiratory system, such as the Respiratory Distress Assessment Instrument (RDAI), quantifies responses to interventions in infants with wheezing 61. While this system has good validity and inter-observer reliability, it has not been adapted to track the respiratory courses of infants born preterm. In addition, objective, validated and sensitive outcome measures of lung disease beyond the neonatal period are lacking. As a consequence, respiratory morbidity during the first year of life is poorly understood, especially in children with less overt symptoms. Even infants with no oxygen requirement at discharge can have physiologic abnormalities such as gas trapping or airflow obstruction measurable on infant pulmonary function tests (iPFTs) 56, 57, 62, 63. iPFTs can replicate lung function tests performed in older children and adults, but may require sedation, are time and labor intensive and only available at specialized centers 64. These tests are however essential to characterizing the lung disease of preterm infants after discharge especially given the increased airway resistance these infants develop after viral infections 65.
Due to a paucity of sensitive outcome measures and lack of detailed respiratory phenotyping during the first year of life, the contribution of practice variation among NICUs, including differing modalities of respiratory support, medication use and nutritional interventions, to the incidence and severity of lung disease in preterm infants has not been fully elucidated. Given the difficulty inherent in evaluating the infant and toddler, this lack of understanding is not unique to the preterm infant. Large scale initiatives have been actively promoted in other diseases that impact the young, such as cystic fibrosis 66. Understanding the pathology and mechanisms that contribute to outcomes in the context of current NICU care is essential. Even more important, however, is the identification of predictive markers for long-term outcomes and possible therapeutic targets.
A comprehensive approach to studying respiratory consequences of prematurity: the Prematurity and Respiratory Outcomes Program (PROP)
In recognition of the challenges outlined in this review, the NHLBI developed the PROP as a multidisciplinary initiative involving pediatric pulmonologists, neonatologists, pharmacists, and basic scientists to promote a broad, collaborative and innovative approach for understanding the respiratory consequences of preterm birth. The central hypothesis of PROP is that, in survivors of extreme prematurity to 36 weeks PMA, specific biologic, physiologic and clinical data obtained during the initial hospitalization will predict respiratory morbidity as defined by respiratory health care utilization and respiratory symptoms between discharge and 1 year corrected age. These data composites may then provide surrogate endpoints for future trials of prevention and therapy of respiratory diseases in preterm infants (Table 2). To test this hypothesis, a series of aims addresses questions ranging from mechanisms and evolution of disease to the influence of clinical care and to long-term respiratory outcomes. To maximize the characterization of BPD, the overarching hypothesis is tested by the multicenter study while various mechanistic hypotheses are tested at the single-site level.
Table 2.
Comprehensive characterization of lung disease of prematurity
| Role in BPD characterization | Constructs | Procedure |
|---|---|---|
| Mechanistic links | ||
| Maternal, Heritable and Environmental Factors | Aggregates of maternal disease states during pregnancy, heritable pulmonary and immunologic conditions, environmental stressors | Demographic questionnaire |
| Biomarkers | Immunologic, anti-oxidant function, genetic variation, microbiome, hormonal milieu, nitric oxide metabolites, markers of inflammation | Patient samples |
| Definition precision | ||
| Quantitative measures | Non invasive physiological assessments | Room air challenge, respiratory rate, tidal breathing patterns and oxygenation with sleep |
| Biological characterization | Biorepository | Patient samples (tracheal aspirates, urine, stool, DNA) |
| Medical practices | Medication use | Inhaled steroids, diuretics, bronchodilators, systemic steroids, methylxanthines |
| Respiratory support | Ventilation, positive pressure, supplemental oxygen and air flow | |
| Respiratory outcomes | ||
| Morbidity measures | Standardized exams | Targeted standardized physical exams |
| Functional assessments | Infant pulmonary function tests | |
| Respiratory symptoms | Parent questionnaires | |
| Resource utilization | Emergency room use, hospitalizations, supplemental oxygen, ventilator support, medication use | Parent questionnaires |
Mechanistic relationships are investigated by creating a biospecimen repository of DNA from participants and their parents, and by obtaining tracheal aspirate and urine samples from infants at pre-specified postnatal ages. Other site-specific biomarkers are also measured from urine and plasma. A multi-center core database containing prospectively collected, standardized, clinical data will then allow testing for associations between these clinical parameters and novel, putative biomarkers with the goal of identifying mechanisms of causation of respiratory disease in preterm infants. Mechanistic pathways under investigation will include inflammation, oxidative stress, immune cell function, nitric oxide synthesis and signaling, surfactant composition, and host-microbiome interaction.
Pathobiology is characterized at the molecular (eg. mRNA profiling), biochemical (biomarker assays), cellular (immune, type II and neuroendocrine), and genetic (polymorphisms) levels. The data should allow a more detailed exploration of the complex interactions between genetic predisposition and environmental factors. The role of gene-environment interactions in respiratory outcomes of prematurity is also considered. Investigation of possible disruptive genetic variants in pathways mediating development of structural and regulatory processes will inform the susceptibility to adverse pulmonary outcomes in the face of immature lungs.
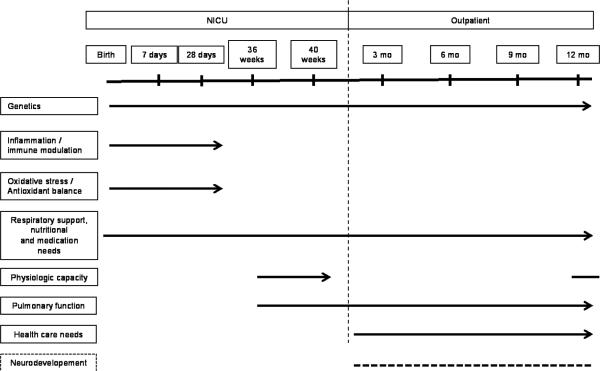
Timing of outcomes and measurements is distributed along a continuum (Figure 1) and not limited to the hospital stay or 36 weeks. For example, respiratory status is recorded daily during hospitalization and is assessed by room air challenge and respiratory inductance plethysmography (RIP) before discharge plus quarterly after discharge by questionnaire and at 1 year by exam and pulmonary function studies (in a subpopulation of infants). The physiology of respiratory disease is investigated until 1 year of age using a series of quantitative respiratory assessments performed prior to NICU discharge as well as infant pulmonary function testing in a subset of infants to identify associations between standard measurements of lung function at 1 year and the quantitative and qualitative assessments of respiratory function and morbidity between 36 weeks PMA and 1 year of age. Functional capacity of preterm lungs at near term using RIP is related to physical exams. A detailed characterization of practice variations investigates the contribution to institution-specific respiratory outcomes. In particular, detailed data are collected on respiratory medication exposures in extremely preterm infants from birth through 1 year of age in order to understand the variability of current prescribing practice and its relationship to respiratory morbidity. Longer-term respiratory morbidities composites have been created to provide a qualitative and quantitative approach. Serial reports of respiratory symptoms, medications, hospitalizations and dependence on technology during the first year of life are complemented by results of infant pulmonary function testing, and a standardized physical exam at 1 year corrected age. The new examination uses some elements of the RDAI, but incorporates other elements that reflect the effects of chronic respiratory impairment. The impact of respiratory disease in preterm infants is also considered in proposed longer-term studies of this cohort, including neurodevelopmental assessment to school-age.
Figure 1.
Continuum of evaluation in lung disease of prematurity. In addition to clinical and medication data information, a series of biospecimens obtained during the NICU hospitalization can provide valuable evidence and mechanisms for disease. Physiologic testing of pulmonary function, and physical and observational assessments of pulmonary function, technology and health care utilization must occur both in the NICU and at 1 year corrected age to address a continuum of function. Neurodevelopmental outcomes are indirectly but importantly linked with lung disease of prematurity and are therefore designated by dotted lines.
Future Directions
As investigative technology progresses, a more refined characterization of the adverse pulmonary outcomes of prematurity is likely to emerge taking into account that BPD is a systemic “disease”, the consequence of multiple organ systems being affected by premature birth. The Molecular Atlas of Lung Development Program (LungMAP, www.lungmap.net) will provide insight into the mechanisms of structural and functional lung development while systems biology approaches will unmask gene networks in multiple organ systems that intersect and contribute to lung development and repair. Magnetic resonance imaging with or without hyperpolarized helium will become a clinical tool to define at high resolution structural and functional characteristics that can further refine the phenotype. New funding mechanisms focused on the use of genomics, transcriptomics, proteomics and metabolomics in existing patient cohorts to elucidate biomarkers and genetic variants are especially relevant to the study of respiratory consequences of prematurity. Finally, the evolving understanding of the microbiome on health and disease will provide insights into pre- and peri-natal maternal-fetal determinants of premature birth and its consequences.
CONCLUSION
Initially a radiographic and pathologic diagnosis, the clinical context in which BPD has been defined has evolved. Survival at earlier gestational ages has improved, surfactant and antenatal corticosteroids have become standard of care, and the use of non-invasive respiratory support has become more widespread. The need for supplemental oxygen at 36 weeks has provided a conceptual framework for short-term, NICU-based outcomes, however the consequences of prematurity and the attendant respiratory outcomes have implications well beyond the neonatal period. As practice evolves and the mechanisms that influence respiratory outcomes are clarified, new paradigms for describing, investigating, stratifying, and ultimately preventing or treating these respiratory consequences of prematurity will be necessary. New study designs, PROP among others, have the opportunity to shift paradigms, by using high resolution respiratory phenotyping that is independent of current short-term definitions, by integrating longer-term outcomes, acquiring biological specimens, forming multidisciplinary research teams and elucidating the mechanisms that influence respiratory outcomes of premature newborns.
Supplementary Material
Acknowledgements
Supported by U01 HL101794 to B Schmidt, U01 HL101456 to JL Aschner, U01 HL101798 to PL Ballard and RL Keller, U01 HL101813 to GS Pryhuber, R Ryan and T Mariani, U01 HL101465 to A Hamvas and T Ferkol, U01 HL101800 to AH Jobe and CA Chougnet, and 5R01HL105702 to CM Cotton, SD Davis and JA Voynow. In addition to the Principal Investigators, the authors would like to acknowledge the following PROP Investigators for input into the manuscript: James Kemp, MD and Clement Ren, MD. The authors would also like to acknowledge Carol J. Blaisdell, MD of NHLBI for her guidance and review of the manuscript and Lynn Taussig for his contributions as Chair of the PROP Steering Committee.
References
- 1.Martin JA, Hamilton BE, Ventura SJ, Osterman MJK, Mathews TJ. Births: Final Data for 2011. In: Services USDoHaH, editor. National Vital Statistics Reports. National Center of Health Statistics; Hyattsville, MD: 2013. pp. 1–69. [PubMed] [Google Scholar]
- 2.Vom Hove M, Prenzel F, Uhlig HH, Robel-Tillig E. Pulmonary outcome in former preterm, very low birth weight children with bronchopulmonary dysplasia: a case-control follow-up at school age. J Pediatr. 2014;164(1):40–45. e44. doi: 10.1016/j.jpeds.2013.07.045. [DOI] [PubMed] [Google Scholar]
- 3.Halterman JS, Lynch KA, Conn KM, Hernandez TE, Perry TT, Stevens TP. Environmental exposures and respiratory morbidity among very low birth weight infants at 1 year of life. Arch Dis Child. 2009;94(1):28–32. doi: 10.1136/adc.2008.137349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peacock JL, Marston L, Marlow N, Calvert SA, Greenough A. Neonatal and infant outcome in boys and girls born very prematurely. Pediatr Res. 2012;71(3):305–310. doi: 10.1038/pr.2011.50. [DOI] [PubMed] [Google Scholar]
- 5.Walsh MC, Wilson-Costello D, Zadell A, Newman N, Fanaroff A. Safety, reliability, and validity of a physiologic definition of bronchopulmonary dysplasia. J Perinatol. 2003;23(6):451–456. doi: 10.1038/sj.jp.7210963. [DOI] [PubMed] [Google Scholar]
- 6.Lovering AT, Elliott JE, Laurie SS, Beasley KM, Gust CE, Mangum TS, et al. Ventilatory and sensory responses in adult survivors of preterm birth and bronchopulmonary dysplasia with reuced exercise capacity. Ann Am Thorac Soc. 2014;11(10):1528–1537. doi: 10.1513/AnnalsATS.201312-466OC. [DOI] [PubMed] [Google Scholar]
- 7.Clemm HH, Vollsaeter M, Roksund OD, Eide GE, Markestad T, Halvorsen T. Exercise capacity after extremely preterm birth. Development from adolescence to adulthood. Ann Am Thorac Soc. 2014;11(4):537–545. doi: 10.1513/AnnalsATS.201309-311OC. [DOI] [PubMed] [Google Scholar]
- 8.Greenough A, Limb E, Marston L, Marlow N, Calvert S, Peacock J. Risk factors for respiratory morbidity in infancy after very premature birth. Arch Dis Child Fetal Neonatal Ed. 2005;90(4):F320–323. doi: 10.1136/adc.2004.062018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NHLBI, Division of Lung Diseases and Office of Prevention Education and Control. 1998 Publication No. 98-4081. [Google Scholar]
- 10.Underwood MA, Danielsen B, Gilbert WM. Cost, causes and rates of rehospitalization of preterm infants. J Perinatol. 2007;27:614–619. doi: 10.1038/sj.jp.7211801. [DOI] [PubMed] [Google Scholar]
- 11.Doyle LW, Faber B, Callanan C, Freezer N, Ford GW, Davis NM. Bronchopulmonary dysplasia in very low birth weight subjects and lung function in late adolescence. Pediatrics. 2006;118(1):108–113. doi: 10.1542/peds.2005-2522. [DOI] [PubMed] [Google Scholar]
- 12.Stroustrup A, Trasande L. Epidemiological Characteristics and Resource Use in Neonates With Bronchopulmonary Dysplasia: 1993–2006. Pediatrics. 2010126(2):291–297. doi: 10.1542/peds.2009-3456. [DOI] [PubMed] [Google Scholar]
- 13.Northway WH, Jr., Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967;276(7):357–368. doi: 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- 14.Tooley WH. Epidemiology of bronchopulmonary dysplasia. J Pediatr. 1979;95(5 Pt 2):851–858. doi: 10.1016/s0022-3476(79)80451-5. [DOI] [PubMed] [Google Scholar]
- 15.Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics. 1988;82(4):527–532. [PubMed] [Google Scholar]
- 16.Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res. 1999;46(6):641–643. doi: 10.1203/00006450-199912000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 18.Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 2004;114(5):1305–1311. doi: 10.1542/peds.2004-0204. [DOI] [PubMed] [Google Scholar]
- 19.Natarajan G, Pappas A, Shankaran S, Kendrick DE, Das A, Higgins RD, et al. Outcomes of extremely low birth weight infants with bronchopulmonary dysplasia: impact of the physiologic definition. Early Hum Dev. 2012;88(7):509–515. doi: 10.1016/j.earlhumdev.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan RM. A new look at bronchopulmonary dysplasia classification. J Perinatol. 2006;26(4):207–209. doi: 10.1038/sj.jp.7211449. [DOI] [PubMed] [Google Scholar]
- 21.Beam KS, Aliaga S, Ahlfeld SK, Cohen-Wolkowiez M, Smith PB, Laughon MM. A systematic review of randomized controlled trials for the prevention of bronchopulmonary dysplasia in infants. J Perinatol. 2014;34(9):705–710. doi: 10.1038/jp.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116(6):1353–1360. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 23.Lefkowitz W, Rosenberg SH. Bronchopulmonary dysplasia: pathway from disease to long-term outcome. J Perinatol. 2008;28(12):837–840. doi: 10.1038/jp.2008.110. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan E, Bar-Yishay E, Prais D, Klinger G, Mei-Zahav M, Mussaffi H, et al. Encouraging pulmonary outcome for surviving, neurologically intact, extremely premature infants in the postsurfactant era. Chest. 2012;142(3):725–733. doi: 10.1378/chest.11-1562. [DOI] [PubMed] [Google Scholar]
- 25.Cazzato S, Ridolfi L, Bernardi F, Faldella G, Bertelli L. Lung function outcome at school age in very low birth weight children. Pediatr Pulmonol. 2013;48(8):830–837. doi: 10.1002/ppul.22676. [DOI] [PubMed] [Google Scholar]
- 26.Landry JS, Chan T, Lands L, Menzies D. Long-term impact of bronchopulmonary dysplasia on pulmonary function. Can Respir J. 2011;18(5):265–270. doi: 10.1155/2011/547948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drysdale SB, Alcazar M, Wilson T, Smith M, Zuckerman M, Lauinger IL, et al. Respiratory outcome of prematurely born infants following human rhinovirus A and C infections. Eur J Pediatr. 2014;173(7):913–919. doi: 10.1007/s00431-014-2262-1. [DOI] [PubMed] [Google Scholar]
- 28.Drysdale SB, Lo J, Prendergast M, Alcazar M, Wilson T, Zuckerman M, et al. Lung function of preterm infants before and after viral infections. Eur J Pediatr. 2014;173(11):1497–1504. doi: 10.1007/s00431-014-2343-1. [DOI] [PubMed] [Google Scholar]
- 29.Bhattacharya S, Go D, Krenitsky DL, Huyck HL, Solleti SK, Lunger VA, et al. Genome-wide transcriptional profiling reveals connective tissue mast cell accumulation in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2012;186(4):349–358. doi: 10.1164/rccm.201203-0406OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Reilly M, Harding R, Sozo F. Altered small airways in aged mice following neonatal exposure to hyperoxic gas. Neonatology. 2014;105(1):39–45. doi: 10.1159/000355641. [DOI] [PubMed] [Google Scholar]
- 31.Albertine KH. Progress in understanding the pathogenesis of BPD using the baboon and sheep models. Semin Perinatol. 2013;37(2):60–68. doi: 10.1053/j.semperi.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collins JJ, Kunzmann S, Kuypers E, Kemp MW, Speer CP, Newnham JP, et al. Antenatal glucocorticoids counteract LPS changes in TGF-beta pathway and caveolin-1 in ovine fetal lung. Am J Physiol Lung Cell Mol Physiol. 2013;304(6):L438–444. doi: 10.1152/ajplung.00251.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maniscalco WM, Watkins RH, Roper JM, Staversky R, O'Reilly MA. Hyperoxic ventilated premature baboons have increased p53, oxidant DNA damage and decreased VEGF expression. Pediatr Res. 2005;58(3):549–556. doi: 10.1203/01.pdr.0000176923.79584.f7. [DOI] [PubMed] [Google Scholar]
- 34.Backstrom E, Hogmalm A, Lappalainen U, Bry K. Developmental stage is a major determinant of lung injury in a murine model of bronchopulmonary dysplasia. Pediatr Res. 2011;69(4):312–318. doi: 10.1203/PDR.0b013e31820bcb2a. [DOI] [PubMed] [Google Scholar]
- 35.Choo-Wing R, Syed MA, Harijith A, Bowen B, Pryhuber G, Janer C, et al. Hyperoxia and interferon-gamma-induced injury in developing lungs occur via cyclooxygenase-2 and the endoplasmic reticulum stress-dependent pathway. Am J Respir Cell Mol Biol. 2013;48(6):749–757. doi: 10.1165/rcmb.2012-0381OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madurga A, Mizikova I, Ruiz-Camp J, Morty RE. Recent advances in late lung development and the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2013;305(12):L893–905. doi: 10.1152/ajplung.00267.2013. [DOI] [PubMed] [Google Scholar]
- 37.Hilgendorff A, Reiss I, Ehrhardt H, Eickelberg O, Alvira CM. Chronic lung disease in the preterm infant. Lessons learned from animal models. Am J Respir Cell Mol Biol. 2014;50(2):233–245. doi: 10.1165/rcmb.2013-0014TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Reilly MA, Marr SH, Yee M, McGrath-Morrow SA, Lawrence BP. Neonatal hyperoxia enhances the inflammatory response in adult mice infected with influenza A virus. Am J Respir Crit Care Med. 2008;177(10):1103–1110. doi: 10.1164/rccm.200712-1839OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bose CL, Dammann CE, Laughon MM. Bronchopulmonary dysplasia and inflammatory biomarkers in the premature neonate. Arch Dis Child Fetal Neonatal Ed. 2008;93(6):F455–461. doi: 10.1136/adc.2007.121327. [DOI] [PubMed] [Google Scholar]
- 40.Ambalavanan N, Carlo WA, D'Angio CT, McDonald SA, Das A, Schendel D, et al. Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics. 2009;123(4):1132–1141. doi: 10.1542/peds.2008-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneibel KR, Fitzpatrick AM, Ping XD, Brown LA, Gauthier TW. Inflammatory mediator patterns in tracheal aspirate and their association with bronchopulmonary dysplasia in very low birth weight neonates. J Perinatol. 2013;33(5):383–387. doi: 10.1038/jp.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Speer CP. Pulmonary inflammation and bronchopulmonary dysplasia. J Perinatol. 2006;26(Suppl 1):S57–62. doi: 10.1038/sj.jp.7211476. discussion S63-54. [DOI] [PubMed] [Google Scholar]
- 43.Bhandari A, Bhandari V. Biomarkers in bronchopulmonary dysplasia. Paediatr Respir Rev. 2013;14(3):173–179. doi: 10.1016/j.prrv.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 44.Bhandari A, Bhandari V. Pitfalls, problems, and progress in bronchopulmonary dysplasia. Pediatrics. 2009;123(6):1562–1573. doi: 10.1542/peds.2008-1962. [DOI] [PubMed] [Google Scholar]
- 45.Drysdale SB, Prendergast M, Alcazar M, Wilson T, Smith M, Zuckerman M, et al. Genetic predisposition of RSV infection-related respiratory morbidity in preterm infants. Eur J Pediatr. 2014;173(7):905–912. doi: 10.1007/s00431-014-2263-0. [DOI] [PubMed] [Google Scholar]
- 46.Hadchouel A, Franco-Montoya ML, Delacourt C. Altered lung development in bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol. 2014;100(3):158–167. doi: 10.1002/bdra.23237. [DOI] [PubMed] [Google Scholar]
- 47.Schmolzer GM, Kumar M, Pichler G, Aziz K, O'Reilly M, Cheung PY. Noninvasive versus invasive respiratory support in preterm infants at birth: systematic review and meta-analysis. BMJ. 2013;347:f5980. doi: 10.1136/bmj.f5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhandari V. The potential of non-invasive ventilation to decrease BPD. Semin Perinatol. 2013;37(2):108–114. doi: 10.1053/j.semperi.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 49.Carroll JL, Agarwal A. Development of ventilatory control in infants. Paediatr Respir Rev. 2010;11(4):199–207. doi: 10.1016/j.prrv.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 50.Bates ML, Pillers DA, Palta M, Farrell ET, Eldridge MW. Ventilatory control in infants, children, and adults with bronchopulmonary dysplasia. Respir Physiol Neurobiol. 2013;189(2):329–337. doi: 10.1016/j.resp.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim DH, Kim HS, Choi CW, Kim EK, Kim BI, Choi JH. Risk factors for pulmonary artery hypertension in preterm infants with moderate or severe bronchopulmonary dysplasia. Neonatology. 2012;101(1):40–46. doi: 10.1159/000327891. [DOI] [PubMed] [Google Scholar]
- 52.Rhein LM, Dobson NR, Darnall RA, Corwin MJ, Heeren TC, Poets CF, et al. Effects of caffeine on intermittent hypoxia in infants born prematurely: a randomized clinical trial. JAMA Pediatr. 2014;168(3):250–257. doi: 10.1001/jamapediatrics.2013.4371. [DOI] [PubMed] [Google Scholar]
- 53.Heldt GP. Development of stability of the respiratory system in preterm infants. J Appl Physiol (1985) 1988;65(1):441–444. doi: 10.1152/jappl.1988.65.1.441. [DOI] [PubMed] [Google Scholar]
- 54.Davis PG, Thorpe K, Roberts R, Schmidt B, Doyle LW, Kirpalani H, et al. Evaluating “old” definitions for the “new” bronchopulmonary dysplasia. J Pediatr. 2002;140(5):555–560. doi: 10.1067/mpd.2002.123291. [DOI] [PubMed] [Google Scholar]
- 55.Hjalmarson O, Brynjarsson H, Nilsson S, Sandberg KL. Persisting hypoxaemia is an insufficient measure of adverse lung function in very immature infants. Arch Dis Child Fetal Neonatal Ed. 2014;99:F257–262. doi: 10.1136/archdischild-2013-304625. [DOI] [PubMed] [Google Scholar]
- 56.Bhandari A, Panitch HB. Pulmonary outcomes in bronchopulmonary dysplasia. Semin Perinatol. 2006;30(4):219–226. doi: 10.1053/j.semperi.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 57.Robin B, Kim YJ, Huth J, Klocksieben J, Torres M, Tepper RS, et al. Pulmonary function in bronchopulmonary dysplasia. Pediatr Pulmonol. 2004;37(3):236–242. doi: 10.1002/ppul.10424. [DOI] [PubMed] [Google Scholar]
- 58.Sanchez-Solis M, Garcia-Marcos L, Bosch-Gimenez V, Perez-Fernandez V, Pastor-Vivero MD, Mondejar-Lopez P. Lung function among infants born preterm, with or without bronchopulmonary dysplasia. Pediatr Pulmonol. 2012;47(7):674–681. doi: 10.1002/ppul.21609. [DOI] [PubMed] [Google Scholar]
- 59.Friedrich L, Pitrez PM, Stein RT, Goldani M, Tepper R, Jones MH. Growth rate of lung function in healthy preterm infants. Am J Respir Crit Care Med. 2007;176(12):1269–1273. doi: 10.1164/rccm.200703-476OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Been JV, Lugtenberg MJ, Smets E, van Schayck CP, Kramer BW, Mommers M, et al. Preterm birth and childhood wheezing disorders: a systematic review and meta-analysis. PLoS Med. 2014;11(1):e1001596. doi: 10.1371/journal.pmed.1001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schuh S, Coates AL, Binnie R, Allin T, Goia C, Corey M, et al. Efficacy of oral dexamethasone in outpatients with acute bronchiolitis. J Pediatr. 2002;140(1):27–32. doi: 10.1067/mpd.2002.120271. [DOI] [PubMed] [Google Scholar]
- 62.Hoo AF, Dezateux C, Henschen M, Costeloe K, Stocks J. Development of airway function in infancy after preterm delivery. J Pediatr. 2002;141(5):652–658. doi: 10.1067/mpd.2002.128114. [DOI] [PubMed] [Google Scholar]
- 63.Friedrich L, Stein RT, Pitrez PM, Corso AL, Jones MH. Reduced lung function in healthy preterm infants in the first months of life. Am J Respir Crit Care Med. 2006;173(4):442–447. doi: 10.1164/rccm.200503-444OC. [DOI] [PubMed] [Google Scholar]
- 64.American Thoracic S, European Respiratory S. ATS/ERS statement: raised volume forced expirations in infants: guidelines for current practice. Am J Respir Crit Care Med. 2005;172(11):1463–1471. doi: 10.1164/rccm.200408-1141ST. [DOI] [PubMed] [Google Scholar]
- 65.Broughton S, Sylvester KP, Fox G, Zuckerman M, Smith M, Milner AD, et al. Lung function in prematurely born infants after viral lower respiratory tract infections. Pediatr Infect Dis J. 2007;26(11):1019–1024. doi: 10.1097/INF.0b013e318126bbb9. [DOI] [PubMed] [Google Scholar]
- 66.Davis SD, Rosenfeld M, Kerby GS, Brumback L, Kloster MH, Acton JD, et al. Multicenter evaluation of infant lung function tests as cystic fibrosis clinical trial endpoints. Am J Respir Crit Care Med. 2010;182(11):1387–1397. doi: 10.1164/rccm.200908-1236OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.