Abstract
Objective
Preclinical studies indicate that oxytocin is anorexigenic and has beneficial metabolic effects. Oxytocin effects on nutrition and metabolism in humans are not well defined. We hypothesized that oxytocin would reduce caloric intake and appetite, and alter levels of appetite-regulating hormones. We also explored metabolic effects of oxytocin.
Methods
We performed a randomized, placebo-controlled crossover study of single-dose intranasal oxytocin (24 IU) in 25 fasting healthy men. After oxytocin/placebo, subjects selected breakfast from a menu, and were given double portions. Caloric content of food consumed was measured. Visual analogue scales were used to assess appetite and blood was drawn for appetite-regulating hormones, insulin, and glucose before and after oxytocin/placebo. Indirect calorimetry assessed resting energy expenditure (REE) and substrate utilization.
Results
Oxytocin reduced caloric intake with a preferential effect on fat intake and increased levels of the anorexigenic hormone cholecystokinin without affecting appetite or other appetite-regulating hormones. There was no effect of oxytocin on REE. Oxytocin resulted in a shift from carbohydrate to fat utilization and improved insulin sensitivity.
Conclusions
Intranasal oxytocin reduces caloric intake and has beneficial metabolic effects in men without concerning side effects. The efficacy and safety of sustained oxytocin administration in the treatment of obesity warrants investigation.
Keywords: Oxytocin, obesity, nutrition, glucose homeostasis
Introduction
Obesity is a major public health concern, affecting more than one-third of the U.S. adult population and associated with significant morbidity, including higher rates of diabetes and increased mortality(1). Although complications from obesity may be reversible with weight loss, meaningful weight reduction is difficult to achieve. There are few treatment options available to supplement lifestyle modifications. Bariatric surgery is effective, but invasive and associated with complications(2). FDA-approved medications result in modest weight loss, but this effect is not sustained, and the drugs are associated with significant side effects(3). There is a need for effective, tolerable therapies for obesity.
In animal models, oxytocin, a peptide hormone primarily produced in the supraoptic (SON) and paraventricular (PVN) hypothalamic nuclei, is anorexigenic(4, 5). Administration of oxytocin to rats reduces caloric intake, and this effect is reversed by first giving an oxytocin antagonist(4, 5). Oxytocin induces weight loss in rodents, and this effect may be related to increased energy expenditure and lipolyis in addition to appetite suppression(6, 7, 8, 9, 10, 11). Preclinical studies indicate that oxytocin has positive metabolic effects(11, 12). The role of oxytocin in regulating nutrition and metabolism in humans is only beginning to be understood (12, 13, 14).
Oxytocin administered intranasally has central actions with minimal side effects, and is appealing as a potential targeted therapy for obesity(15). We performed a randomized, double-blind, placebo-controlled crossover study investigating oxytocin effects on caloric intake. We hypothesized that a single dose of intranasal oxytocin would decrease food intake at a subsequent meal. We also predicted that oxytocin would reduce appetite and modulate appetite-regulating hormone levels. Finally, we explored metabolic effects of oxytocin, hypothesizing that oxytocin would result in an increase in resting energy expenditure (REE), fat utilization and insulin sensitivity.
Methods and Procedures
Subjects
We studied 25 healthy men, 18-45 years old: 13 normal-weight (NW) and 12 overweight/obese men (OB) recruited from the community. Body mass index (BMI) was 18.5-24.9 for NW and 25-40 for OB. Subjects were required to have had a stable weight for three months and to eat breakfast ≥4 times/week.
Subjects were excluded for psychiatric disease, psychotropic medications, eating disorder history by structured clinical interview for DSM disorders-IV (SCID), excessive exercise within three months (running>25 miles or exercising >10 hours in any one week), diabetes, substance abuse, anemia, gastrointestinal surgery, cardiovascular disease, untreated thyroid disease, and smoking.
Protocol
This study was approved by Partners Human Research Committee. Written informed consent was obtained. Subjects were admitted to the Massachusetts General Hospital (MGH) Clinical Research Center (CRC) for outpatient screening and two main study visits. The first and second main study visits took place one to eight weeks apart.
At the screen, height and weight were measured, blood was drawn, and a history and physical exam were performed. BMI was obtained [weight (kg)/height (m2). Exercise patterns were assessed by history and the Paffenbarger activity assessment. A food diary was provided to subjects to track food intake during the 72 hours before the first main study visit. The disordered eating modules of the SCID were administered by trained study personnel. Subjects were advised to avoid alcohol and strenuous exercise for 24 hours and to fast for 12 hours before main visits.
Figure 1 shows the study schema. At main study visits, weight and BMI were assessed. The food diary from the first visit was collected and reviewed with the subject, and a second diary was provided for documentation of food intake in the 72 hours prior to the second visit. A copy of the food diary collected at the first visit was returned to the subject, who was asked to replicate food intake from the 72 hours prior to the first visit in the 72 hours prior to the second visit. Food records were reviewed by a dietician. Medical history was updated. Intranasal oxytocin (Syntocinon, Novartis) (24 IU) or placebo (same inactive ingredients and packaging) was self-administered (3 sprays per nostril) under the supervision of a nurse practitioner at approximately 07:30am. The research pharmacy randomized the visit order in which subjects received drug or placebo. Subjects and study personnel were blinded to the randomization. An intravenous catheter was placed and blood was drawn immediately prior and 15, 30, and 55 minutes after oxytocin/placebo. Vital signs were obtained immediately prior and 15 minutes after oxytocin/placebo. Heart rate and blood pressure were repeated at 55 and 120 minutes post-oxytocin/placebo. Appetite was assessed using visual analogue scales (VAS) before and 55 minutes after oxytocin/placebo. Subjects were offered a menu (Supplemental Figure 1) 15 minutes after oxytocin/placebo and told to order whatever amount of food they thought they could eat. Indirect calorimetry was performed (VMAX Encore 29 metabolic cart, Viasys Healthcare, Carefusion; San Diego, CA) to assess fasting REE and respiratory quotient (RQ) 30 minutes post-oxytocin/placebo. Substrate utilization was calculated: carbohydrate oxidation (g/min) = 4.21*VCO2-2.962*VO2-0.4*n; fat oxidation (g/min) = 1.695*VO2- 1.701*VCO2-1.77*n, where n represents nitrogen excretion from protein oxidation (estimated at 135 mg*kg*min−1)(16). Sixty minutes after oxytocin/placebo, subjects were offered two of every food item ordered and given 30 minutes to eat, followed by quantification of nutrient intake. Subjects ate the meal in a private room without disruption. Side effects were systematically assessed at the end of each visit.
Figure 1.
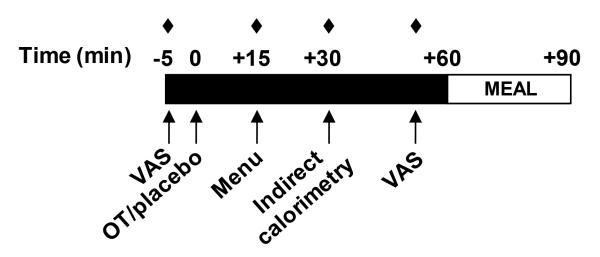
Study schema. VAS, visual analogue scales. OT, oxytocin. Filled in diamonds, blood draws.
Bionutrition measures
The menu was created using ProNutra (Viocare; Princeton, NJ) and managed as an individualized weighed diet. Each item had a known weight and remaining food was weighed back. Nutrient analysis investigated the difference between prepared and consumed total calories and fat, carbohydrate, and protein content. VAS, a reliable method to assess appetite(17), were administered fasting before and after oxytocin/placebo. Subjects were asked to make a mark on a 100 mm line with extremes on either end indicating how they felt at that moment. Scores were calculated by measuring the distance from the left side of the line. The following were assessed: hunger, desire to eat preferred foods, satisfaction, fullness, quantity one could eat, desire for specific types of foods (sweet, salty, savory, and fatty), and nausea.
Biochemical analysis
Plasma glucose was measured by LabCorp. Serum and plasma samples were stored at −80° C. Triglycerides were measured on the ARCHITECT c System™ [ci8200, Abbott Diagnostics Division, Abbott Park IL] by the glycerol phosphate oxidase assay using ACS grade glycerol calibrators traceable to gravimeteric reference methods [inter-assay coefficients of variation (CV) for controls 1.5-2.5%]. Oxytocin was measured in unextracted serum by ELISA (Assay Designs, Inc., Ann Arbor, MI). A new (2013) polyclonal rabbit antibody has increased specificity to non-human mesotocin and Arg8-vasotocin, but otherwise showed no specificity differences. Calibration curve statistics and in-house quality controls (QC) were monitored. The detection limit was 12.5 pg/mL. In house QCs had a mean of 152 and 338 pg/mL, respectively and inter-assay CV of 15 and 18%, respectively. Active serum cholecystokinin (CCK) peptide levels were measured at baseline and 30 and 55 min after oxytocin/placebo using an ELISA (Abnova, Walnut, CA; intra-assay and inter-assay CVs <10% and <15%, respectively; detection limit 12.5 pg/mL). Plasma for ghrelin was collected in tubes containing 10mg/mL PMSF and 1N HCL. Active ghrelin was measured using an ELISA (EMD Millipore, Billerica, MA; inter-assay CV<12%, detection limit 10 pg/mL). Serum insulin was measured using a chemiluminescence immunoassay (Roche Diagnostics, Indianapolis, IN; inter-assay CV <3%, detection limit 0.2 uU/mL). HOMA-IR was calculated: Insulin (uU/mL) × Glucose (mmol/L)/22.5. Serum leptin was measured using an ELISA (EMD Millipore; inter-assay CV<9%, detection limit 0.25 ng/mL). Serum PYY was measured using an electrochemiluminescent assay (Meso Scale Discovery, Gaithersburg, MD; inter-assay CV <12%, detection limit 70 pg/mL).
Data analysis
The crossover study was analyzed using an analysis of variance with a period, treatment and patient effect and a baseline value as covariate when available. We used the same model to test for a group (NW, OB)-treatment interaction to determine whether the treatment effect was different in NW vs. OB. We used a repeated measures analysis of variance with the terms above, and time, for analyzing those measures which were measured repeatedly during each visit. The treatment effect that is calculated by this model is essentially the mean difference between outcomes for each treatment averaged over the time points corrected for possible covariate, period, subject, and missing value effects.
Results
Subjects
Mean age was 27.1±1.5 (mean±SEM) years old. NW were younger than OB (23.9±1.9 vs. 30.6±2.0 yrs, p=0.02). Mean weight and BMI were 80.7±17.8 kg and 26.1±1.2 kg/m2, and were lower in NW than OB (weight: 67.4±2.2 vs. 95.2±3.9 kg, p<0.0001; BMI: 21.2±0.3 vs. 31.5±1.2 kg/m2, p<0.0001). Subjects ate breakfast 5.6±0.2 days/week on average, and this did not differ by group [5.8±0.3 (NW) vs. 5.3±0.4 (OB) days, p=0.3]. Across groups, mean exercise assessed by history was 3.4±0.6 hours per week, and vigorous activity assessed by Paffenbarger was 5.2±0.9 hours per week. Reported activity levels did not differ by group [exercise/week: 5.8±0.3 (NW) vs. 5.3±0.4 (OB) hours, p=0.9; vigorous activity/week: 5.2±0.9 (NW) vs. 5.3±1.0 (OB) hours, p=1.0]. Recorded food intake in the 72 hours leading up to oxytocin and placebo visits did not differ within subjects (Table 1).
Table 1. Energy intake and expenditure.
| Oxytocin | Placebo | Least Squared Mean Difference |
p-value | |
|---|---|---|---|---|
| Food diary (kcal/24 hr in 72 hrs prior to study visit) | ||||
| Total calories (kcal) | 1930±56 | 1920±58 | 11±80 | 0.9 |
| Protein (g) | 73.6±2.2 | 81.9±2.3 | −2.3±3.2 | 0.5 |
| Carbohydrate (g) | 226.8±7.9 | 226.9±8.2 | −0.1±11.3 | 1.0 |
| Fat (g) | 77.6±2.5 | 74.6±2.6 | 3.0±3.6 | 0.4 |
| Fasting indirect calorimetry | ||||
| Resting energy expenditure (kcal) | 1484±22 | 1479±22 | 5.8±31.2 | 0.9 |
| RQ | 0.79±0.01 | 0.82±0.02 | −0.03±0.01 | 0.02 |
| Fat utilization (g/min) | 0.07±0.01 | 0.06±0.01 | 0.01±0.00 | 0.04 |
| Carbohydrate utilization (g/min) | 0.08±0.01 | 0.11±0.01 | −0.02±0.01 | 0.03 |
| Meal | ||||
| Total calories ordered (kcal) | 853±27 | 878±27 | −25.1±37.6 | 0.5 |
| Total calories consumed (kcal) | 1153±36 | 1275±36 | −122±51 | 0.03 |
| Protein ordered (g) | 24.5±1.3 | 25.7±1.3 | −1.2±1.8 | 0.5 |
| Protein consumed (g) | 34.9±1.5 | 38.8±1.5 | −3.9±2.2 | 0.08 |
| Carbohydrate ordered (g) | 119.0±5.3 | 115.7±5.3 | 3.3±7.5 | 0.7 |
| Carbohydrates consumed (g) | 148.4±6.6 | 155.6±6.6 | −7.2±9.4 | 0.4 |
| Fat ordered (g) | 31.7±1.7 | 35.5±1.7 | −3.8±2.4 | 0.1 |
| Fat consumed (g) | 47.3±2.7 | 56.0±2.7 | −8.7±3.8 | 0.03¥ |
Mean±SEM
Bold typeface indicates p-value <0.05
, no longer significant after controlling for multiple comparisons
Caloric intake and appetite
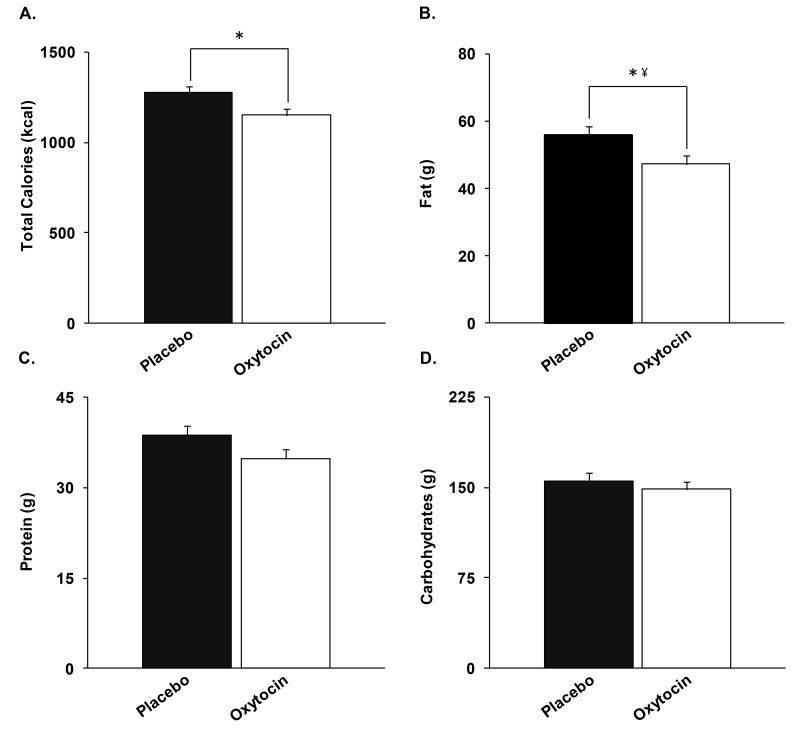
Table 1 shows oxytocin effects on energy intake. Oxytocin did not impact caloric content of food ordered from the menu. Oxytocin reduced the total caloric intake by 122±51 kcal (p=0.03) (Figure 2). Group (NW vs. OB) did not affect results. Oxytocin reduced fat intake by 8.7±3.8 g (p=0.03; NS after controlling for multiple comparisons). Protein (−3.9±2.2 g) and carbohydrate (−7.2±9.4 g) intake were reduced, but this was not statistically significant. Oxytocin did not significantly impact subjective appetite measures.
Figure 2.
Compared to placebo, oxytocin reduced A.) total caloric intake by 122±51 kcal, B.) fat intake by 8.7±3.8 g, C.) protein intake by 3.9±2.2 g, and D.) carbohydrate content by 7.2±9.4g. *, p=0.03. ¥, no longer significant after controlling for multiple comparisons.
Indirect calorimetry and triglyceride levels
Table 1 shows indirect calorimetry results. While REE was not changed by oxytocin administration, RQ was reduced (−0.03±0.01, p=0.02). Oxytocin reduced carbohydrate utilization (−0.02±0.01, p=0.03) and increased fat utilization (0.01±0.00, p=0.04). There was a trend toward reduction of triglyceride levels following oxytocin (−1.8 mg/dL, p=0.07) (Table 2).
Table 2.
Treatment effect of oxytocin on fasting levels of hormones, triglycerides and glucose
| Mean Baseline Value |
Treatment Effect Estimate |
P-value* | |
|---|---|---|---|
| Insulin (uU/mL) | 7.0±0.9 | −1.7±0.8 | 0.04 |
| Glucose (mg/dL) | 84.6±1.2 | −0.1±0.5 | 0.9 |
| HOMA-IR | 1.5±0.2 | −0.4±0.2 | 0.04 |
| Triglycerides (mg/dL ) | 103.1±7.4 | −1.8±1.0 | 0.07 |
| Oxytocin (pg/mL) | 965.0±53.7 | −32.5±20.7 | 0.1 |
| CCK (pg/mL) | 72.5±11.0 | 10.9±3.1 | 0.002 |
| Leptin (ng/mL) | 3.7±0.5 | −0.1±0.2 | 0.5 |
| Active Ghrelin (pg/mL) | 92.2±6.1 | 14.4±12.1 | 0.2 |
| PYY (pg/mL) | 61.9±5.2 | 0.6±2.5 | 0.8 |
Mean±SEM
Bold typeface indicates p-value <0.05
p-value, overall repeated measures
Fasting appetite hormones
Table 2 presents appetite regulating hormones. Oxytocin resulted in increased levels of CCK (10.9±3.1 pg/mL, p=0.002). However, the increase in CCK levels were not related to caloric intake. There was no treatment effect on oxytocin (p=0.1), leptin (p=0.5), ghrelin (p=0.2) or PYY (p=0.8) levels.
Glucose homeostasis
Table 2 shows fasting insulin and glucose levels and HOMA-IR. Oxytocin reduced average insulin levels (−1.7±0.8 uU/mL, p=0.04) and HOMA-IR (−0.4, p=0.04) over the time points but had no effect on glucose levels. At baseline, HOMA-IR was higher in OB than NW (2.1±0.4 vs. 0.9±0.1, p=0.04). There was no significant difference in treatment effect on OB vs. NW.
Safety and tolerability
Oxytocin did not affect blood pressure or heart rate. There was a slight decrease in temperature 15 minutes after administration (−0.3 °F, p=0.006). There were few adverse events in the study, none of which was severe (Table 3), with no difference between oxytocin and placebo conditions.
Table 3.
Adverse events
| Oxytocin | Placebo | |||||
|---|---|---|---|---|---|---|
| Mild | Moderate | Total | Mild | Moderate | Total | |
| Dizziness | 1 | 0 | 1 | 0 | 0 | 0 |
| Drowsiness | 5 | 1 | 6 | 4 | 0 | 4 |
| Dry oropharynx | 1 | 0 | 1 | 1 | 0 | 1 |
| Nasal irritation/rhinorrhea | 0 | 0 | 0 | 0 | 0 | 0 |
| Abdominal pain | 0 | 1 | 1 | 0 | 0 | 0 |
| Anxiety | 0 | 0 | 0 | 0 | 0 | 0 |
| Headache | 0 | 0 | 0 | 0 | 0 | 0 |
Discussion
In a study of men across a spectrum of weights, we report that a single dose of intranasal oxytocin reduces total caloric intake at a subsequent meal. The effect size of 122 kcal may be clinically relevant, as this would amount to 366 kcal/day or about 4 kg over twelve weeks and more than 17 kg over a year if the effect of oxytocin is equivalent over three meals per day and sustained over time. To put this into context, currently FDA-approved medications for obesity lead to 3.2-8.9 kg weight loss at one year(3). Further research will be important to establish whether the reduction in nutritional intake continues and leads to weight loss with sustained intranasal oxytocin.
In animal models, oxytocin administration reduces food intake and an oxytocin antagonist reverses this effect(4, 5). Heterozygous mice for single-minded 1, a gene responsible for hyperphagic obesity in humans, have low levels of hypothalamic oxytocin mRNA, and administration of oxytocin in these animals reverses excessive food intake and weight gain(18). Whether oxytocin is anorexigenic in humans has not been well established. Borg reported that in 10 healthy adults, 40 mU/min IV oxytocin, but not 20 mU/min or 80 mU/min, reduced satiety without affecting volume of intake of a liquid meal(13). In contrast, our study used intranasal oxytocin which may have central in addition to peripheral effects(19). An investigation by Ott et al. in 20 healthy men did not find an acute effect of intranasal oxytocin on caloric intake at a breakfast buffet. Possible explanations for the differences in our results include differences in food intake leading up to study visits (our subjects were asked to consume the same caloric content in the 72 hours preceding each of their two visits) or anticipatory effects (our subjects selected food from a menu prior to the meal). Although Ott’s study did not show an effect of oxytocin on breakfast intake, it did demonstrate reduced postprandial consumption of a snack, particularly chocolate cookies, suggesting an effect on reward-driven feeding(14). Neither our study nor Ott’s identified an effect of oxytocin on subjective appetite(14). Side effects, such as nausea, did not differ between oxytocin and placebo in our study, and therefore do not explain oxytocin’s effect on food intake. Further studies will be important to define the mechanisms underlying oxytocin’s reduction of caloric intake.
Several food motivation pathways, including those involving leptin, alpha-MSH and AgRP, converge on oxytocin neurons(20, 21, 22). Parvocellular neurons of the PVN extend to the caudal brainstem, where they regulate autonomic control of feeding(23, 24). Oxytocin is also released by dendrites of magnocellular neurons of the PVN and SON, and may reduce food intake in part via diffusion to the ventromedial nucleus, a satiety-regulating brain region rich in oxytocin receptors(25). Axonal release of oxytocin from the PVN may also have effects on hedonic food consumption via dopaminergic mesolimbic neurons in the ventral tegmental area (VTA)(25, 26, 27). In rats, acute administration of oxytocin into the VTA suppresses sucrose intake(28). Furthermore, Blevins et al. have demonstrated that chronic administration of oxytocin reduces consumption of fructose-sweetened beverage in obese nonhuman primates(11). Oxytocin may also reduce food intake by inhibiting gastric emptying. Studies in rats demonstrate that intraperitoneal oxytocin increases levels of the peripheral satiety hormone CCK and inhibits gastric emptying; these effects are blocked by administration of a CCK-1 receptor antagonist(29, 30). We found that intranasal oxytocin resulted in an increase in serum CCK levels preceding suppression of food intake. However, it is not clear whether CCK is a mediator of oxytocin effects as we did not find a relationship between change in CCK levels and food consumption. Whether oxytocin has indirect effects on food intake mediated by other hormones is unknown. Although another study found that intravenous oxytocin reduced total ghrelin levels, ours did not find an effect of intranasal oxytocin on levels of acylated ghrelin, the active form of ghrelin with established orexigenic properties(31). The discrepancy in findings may be due to differences in oxytocin dosage or mode of administration, or the form of ghrelin measured. We found no effect of oxytocin on levels of leptin or PYY, indicating that oxytocin’s suppression of caloric intake is not mediated by changes in these hormones.
We did not find an increase in serum oxytocin levels in the oxytocin vs. placebo condition. Given the short half life of oxytocin in blood (approximately 3-5 minutes), it is possible that we did not capture a brief increase in peripheral levels at the sampled timepoints. Alternatively, intranasal oxytocin effects may be central, and not reflected in peripheral levels. Although central oxytocin in some cases may stimulate peripheral oxytocin secretion (7), there is evidence that stimulation of oxytocin secretion in appetite pathways is accompanied by inhibition of peripheral secretion of oxytocin via the posterior pituitary(22). It is therefore possible that the nonsignificant reduction in peripheral oxytocin levels we found in response to intranasal oxytocin in the context of food anticipation was due to oxytocinergic activity in central appetite pathways.
In addition to its role in food intake, oxytocin has been implicated in the regulation of energy metabolism. Oxytocin and oxytocin receptor knockout mice develop obesity and reduced thermogenesis without a change in nutrient intake(6, 32). In an investigation of rats receiving a high fat diet, central oxytocin attenuated weight gain without changing food intake(7). In another study of diet induced obesity in rats, systemic oxytocin reduced caloric intake and body weight without changing REE, indicating that oxytocin blocked the effect of weight loss to reduce energy expenditure(33). Chronic subcutaneous administration of oxytocin to obese nonhuman primates increased energy expenditure in the dark cycle by 14% (11). These studies suggest that oxytocin promotes energy expenditure. A small study of obese men and women found that intranasal oxytocin four times per day led to weight loss of nearly 20 lbs over eight weeks(12). Although this pilot study was limited by small size and, despite randomization, the groups were not well-matched for age or baseline weight, the weight loss was striking(12). There were no assessments of appetite, food intake, energy expenditure or activity levels, so the mechanisms of oxytocin effects on weight over time are unclear. Neither our study nor Ott’s identified an effect of a single dose of oxytocin on energy expenditure(14). Whether chronic administration of oxytocin increases energy expenditure remains a key question.
Although REE did not differ after oxytocin (vs. placebo) in our study, RQ decreased, consistent with a change in substrate utilization. A study of chronic oxytocin infusion in mice similarly reported a reduction in RQ(34). Importantly, in our study, there was no difference in caloric intake in the preceding 72 hours in oxytocin or placebo conditions, and subjects abstained from eating, drinking (other than water), and strenous exercise before visits. No subjects were smokers or had respiratory illnesses. Therefore, the RQ difference likely reflects a shift in substrate utilization toward fatty acid oxidation. It has been previously shown that adipocytes and fat progenitor cells have receptors for oxytocin(35, 36). In vitro and in vivo experiments have demonstrated that oxytocin inhibits adipogenesis and promotes lipolytic pathways and fatty acid oxidation(7, 37). In a study of obese nonhuman primates, chronic peripheral administration of oxytocin increased markers of lipolysis(11). Our finding of a trend toward reduced triglycerides following oxytocin administration is consistent with a possible lipolytic effect.
Preclinical research indicates that oxytocin may be involved in glucose homeostasis. Oxytocin knockout mice develop insulin resistance and glucose intolerance(38). In obese mice, oxytocin reduces fasting insulin secretion and insulin resistance and improves glucose tolerance prior to a change in weight(12). Peripheral oxytocin administration in rats increases insulin-sensitive glucose transporter 4 mRNA in fat, suggesting a mechanism for increasing insulin sensitivity(39). Ott et al. showed that intranasal oxytocin reduced postprandial glucose but not insulin levels in healthy men(14). Our finding in men that a single dose of oxytocin reduces fasting insulin secretion and HOMA-IR without affecting glucose levels supports the concept that oxytocin may improve insulin sensitivity independent of its effects on weight. However, Zhang et al. did not show effects of sustained intranasal oxytocin on fasting insulin or glucose levels in a small study of nondiabetic obese men and women(12). Further research will be important to examine the effects of oxytocin on glucose tolerance in humans, particularly diabetics.
This study is limited by a relatively small sample size. It is possible that with more subjects, we may have detected significant differences in appetite, types of calories consumed (e.g., protein or carbohydrate), or REE. Due to potential effects of estrogen and progesterone on oxytocin secretion and receptor distribution, this study focused exclusively on men. Future studies will need to examine oxytocin effects on eating behavior in females, as oxytocin has gender-specific effects(40). We have demonstrated an acute effect of oxytocin on food intake, substrate utilization, and insulin sensitivity. Whether chronic oxytocin administration would be useful in promoting and maintaining weight loss and improved glucose metabolism in obesity is unknown, and warrants investigation.
In summary, in a study of healthy men, we found that a single dose of intranasal oxytocin reduced caloric intake at a breakfast meal without changing subjective appetite or appetite-regulating hormones leptin, ghrelin or PYY. Although oxytocin resulted in a rise in serum CCK levels preceding the oxytocin-induced suppression of food intake, it is not clear from this study whether CCK is a mediator of oxytocin effects. Oxytocin reduced the RQ, indicating a shift in substrate utilization to fatty acid oxidation. Finally, oxytocin reduced fasting insulin and HOMA-IR without changing glucose levels, indicating improved insulin sensitivity. Further investigation of sustained oxytocin safety and effects on caloric intake and metabolism will be important.
Supplementary Material
What is known
Oxytocin is a peptide hormone produced in the hypothalamus.
In animal models, oxytocin reduces caloric intake and has positive metabolic effects.
In a small study of humans, intranasal oxytocin over eight weeks led to weight loss.
Intranasal oxytocin has central actions with minimal side effects.
What does this study add
A single dose of intranasal oxytocin reduces caloric intake in men without effects on subjective hunger or satiety, or mediation by established endocrine regulators of food intake.
In addition, oxytocin reduces the fasting respiratory quotient in men, consistent with a shift in substrate utilization toward fatty acid oxidation.
Oxytocin also reduces fasting insulin levels in men without affecting glucose levels, indicating improved insulin sensitivity.
Acknowledgements
EAL designed and oversaw the protocol, analyzed and interpreted data and wrote the manuscript. DAM, RLD, CJT carried out experiments. TMH was involved in study design, experiments, and data interpretation. DAS analyzed the data. All authors reviewed, edited and approved the manuscript. Sources of support: NIH K23 MH092560, 1UL1 TR001102-01, 2P30DK046200; MGH Claflin Distinguished Scholar Award. We thank the MGH CRC staff and study participants.
Footnotes
Conflict of interest: The authors declare no conflicts of interest.
Supplementary information is available at the Obesity website.
Clinical Trial Registration Number: NCT01513499
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009-2010. NCHS data brief. 2012:1–8. [PubMed] [Google Scholar]
- 2.Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. Bmj. 2013;347:f5934. doi: 10.1136/bmj.f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA: the journal of the American Medical Association. 2014;311:74–86. doi: 10.1001/jama.2013.281361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arletti R, Benelli A, Bertolini A. Influence of oxytocin on feeding behavior in the rat. Peptides. 1989;10:89–93. doi: 10.1016/0196-9781(89)90082-x. [DOI] [PubMed] [Google Scholar]
- 5.Olson BR, Drutarosky MD, Chow MS, Hruby VJ, Stricker EM, Verbalis JG. Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats. Peptides. 1991;12:113–118. doi: 10.1016/0196-9781(91)90176-p. [DOI] [PubMed] [Google Scholar]
- 6.Kasahara Y, Takayanagi Y, Kawada T, Itoi K, Nishimori K. Impaired thermoregulatory ability of oxytocin-deficient mice during cold-exposure. Bioscience, biotechnology, and biochemistry. 2007;71:3122–3126. doi: 10.1271/bbb.70498. [DOI] [PubMed] [Google Scholar]
- 7.Deblon N, Veyrat-Durebex C, Bourgoin L, Caillon A, Bussier AL, Petrosino S, et al. Mechanisms of the anti-obesity effects of oxytocin in diet-induced obese rats. PloS one. 2011;6:e25565. doi: 10.1371/journal.pone.0025565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noble EE, Billington CJ, Kotz CM, Wang C. Oxytocin in the ventromedial hypothalamic nucleus reduces feeding and acutely increases energy expenditure. American journal of physiology Regulatory, integrative and comparative physiology. 2014;307:R737–745. doi: 10.1152/ajpregu.00118.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang G, Bai H, Zhang H, Dean C, Wu Q, Li J, et al. Neuropeptide exocytosis involving synaptotagmin-4 and oxytocin in hypothalamic programming of body weight and energy balance. Neuron. 2011;69:523–535. doi: 10.1016/j.neuron.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang G, Cai D. Circadian intervention of obesity development via resting-stage feeding manipulation or oxytocin treatment. American journal of physiology Endocrinology and metabolism. 2011;301:E1004–1012. doi: 10.1152/ajpendo.00196.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blevins JE, Graham JL, Morton GJ, Bales KL, Schwartz MW, Baskin DG, et al. Chronic oxytocin administration inhibits food intake, increases energy expenditure, and produces weight loss in fructose-fed obese rhesus monkeys. American journal of physiology Regulatory, integrative and comparative physiology. 2014 doi: 10.1152/ajpregu.00441.2014. ajpregu 00441 02014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, Wu C, Chen Q, Chen X, Xu Z, Wu J, et al. Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models. PloS one. 2013;8:e61477. doi: 10.1371/journal.pone.0061477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borg J, Simren M, Ohlsson B. Oxytocin reduces satiety scores without affecting the volume of nutrient intake or gastric emptying rate in healthy subjects. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2011;23:56–61, e55. doi: 10.1111/j.1365-2982.2010.01599.x. [DOI] [PubMed] [Google Scholar]
- 14.Ott V, Finlayson G, Lehnert H, Heitmann B, Heinrichs M, Born J, et al. Oxytocin reduces reward-driven food intake in humans. Diabetes. 2013;62:3418–3425. doi: 10.2337/db13-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacDonald E, Dadds MR, Brennan JL, Williams K, Levy F, Cauchi AJ. A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology. 2011;36:1114–1126. doi: 10.1016/j.psyneuen.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Gagnon DD, Rintamaki H, Gagnon SS, Cheung SS, Herzig KH, Porvari K, et al. Cold exposure enhances fat utilization but not non-esterified fatty acids, glycerol or catecholamines availability during submaximal walking and running. Frontiers in physiology. 2013;4:99. doi: 10.3389/fphys.2013.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 18.Kublaoui BM, Gemelli T, Tolson KP, Wang Y, Zinn AR. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Molecular endocrinology. 2008;22:1723–1734. doi: 10.1210/me.2008-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Striepens N, Kendrick KM, Hanking V, Landgraf R, Wullner U, Maier W, et al. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Scientific reports. 2013;3:3440. doi: 10.1038/srep03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blevins JE, Schwartz MW, Baskin DG. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. American journal of physiology Regulatory, integrative and comparative physiology. 2004;287:R87–96. doi: 10.1152/ajpregu.00604.2003. [DOI] [PubMed] [Google Scholar]
- 22.Sabatier N, Caquineau C, Dayanithi G, Bull P, Douglas AJ, Guan XM, et al. Alpha-melanocyte-stimulating hormone stimulates oxytocin release from the dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypophysis. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:10351–10358. doi: 10.1523/JNEUROSCI.23-32-10351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rinaman L. Oxytocinergic inputs to the nucleus of the solitary tract and dorsal motor nucleus of the vagus in neonatal rats. The Journal of comparative neurology. 1998;399:101–109. doi: 10.1002/(sici)1096-9861(19980914)399:1<101::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 24.McCann MJ, Rogers RC. Oxytocin excites gastric-related neurones in rat dorsal vagal complex. J Physiol. 1990;428:95–108. doi: 10.1113/jphysiol.1990.sp018202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabatier N, Leng G, Menzies J. Oxytocin, feeding, and satiety. Frontiers in endocrinology. 2013;4:35. doi: 10.3389/fendo.2013.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sofroniew MV. Projections from vasopressin, oxytocin, and neurophysin neurons to neural targets in the rat and human. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 1980;28:475–478. doi: 10.1177/28.5.7381192. [DOI] [PubMed] [Google Scholar]
- 27.Qi J, Yang JY, Song M, Li Y, Wang F, Wu CF. Inhibition by oxytocin of methamphetamine-induced hyperactivity related to dopamine turnover in the mesolimbic region in mice. Naunyn-Schmiedeberg’s archives of pharmacology. 2008;376:441–448. doi: 10.1007/s00210-007-0245-8. [DOI] [PubMed] [Google Scholar]
- 28.Mullis K, Kay K, Williams DL. Oxytocin action in the ventral tegmental area affects sucrose intake. Brain research. 2013;1513:85–91. doi: 10.1016/j.brainres.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu CL, Hung CR, Chang FY, Pau KY, Wang PS. Pharmacological effects of oxytocin on gastric emptying and intestinal transit of a non-nutritive liquid meal in female rats. Naunyn-Schmiedeberg’s archives of pharmacology. 2003;367:406–413. doi: 10.1007/s00210-003-0690-y. [DOI] [PubMed] [Google Scholar]
- 30.Wu CL, Doong ML, Wang PS. Involvement of cholecystokinin receptor in the inhibition of gastrointestinal motility by oxytocin in ovariectomized rats. European journal of pharmacology. 2008;580:407–415. doi: 10.1016/j.ejphar.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 31.Vila G, Riedl M, Resl M, van der Lely AJ, Hofland LJ, Clodi M, et al. Systemic administration of oxytocin reduces basal and lipopolysaccharide-induced ghrelin levels in healthy men. The Journal of endocrinology. 2009;203:175–179. doi: 10.1677/JOE-09-0227. [DOI] [PubMed] [Google Scholar]
- 32.Takayanagi Y, Kasahara Y, Onaka T, Takahashi N, Kawada T, Nishimori K. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport. 2008;19:951–955. doi: 10.1097/WNR.0b013e3283021ca9. [DOI] [PubMed] [Google Scholar]
- 33.Morton GJ, Thatcher BS, Reidelberger RD, Ogimoto K, Wolden-Hanson T, Baskin DG, et al. Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats. American journal of physiology Endocrinology and metabolism. 2012;302:E134–144. doi: 10.1152/ajpendo.00296.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maejima Y, Iwasaki Y, Yamahara Y, Kodaira M, Sedbazar U, Yada T. Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging. 2011;3:1169–1177. doi: 10.18632/aging.100408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gould BR, Zingg HH. Mapping oxytocin receptor gene expression in the mouse brain and mammary gland using an oxytocin receptor-LacZ reporter mouse. Neuroscience. 2003;122:155–167. doi: 10.1016/s0306-4522(03)00283-5. [DOI] [PubMed] [Google Scholar]
- 36.Schaffler A, Binart N, Scholmerich J, Buchler C. Hypothesis paper Brain talks with fat--evidence for a hypothalamic-pituitary-adipose axis? Neuropeptides. 2005;39:363–367. doi: 10.1016/j.npep.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Elabd C, Basillais A, Beaupied H, Breuil V, Wagner N, Scheideler M, et al. Oxytocin controls differentiation of human mesenchymal stem cells and reverses osteoporosis. Stem cells. 2008;26:2399–2407. doi: 10.1634/stemcells.2008-0127. [DOI] [PubMed] [Google Scholar]
- 38.Camerino C. Low sympathetic tone and obese phenotype in oxytocin-deficient mice. Obesity. 2009;17:980–984. doi: 10.1038/oby.2009.12. [DOI] [PubMed] [Google Scholar]
- 39.Eckertova M, Ondrejcakova M, Krskova K, Zorad S, Jezova D. Subchronic treatment of rats with oxytocin results in improved adipocyte differentiation and increased gene expression of factors involved in adipogenesis. British journal of pharmacology. 2011;162:452–463. doi: 10.1111/j.1476-5381.2010.01037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carter CS, Boone EM, Pournajafi-Nazarloo H, Bales KL. Consequences of early experiences and exposure to oxytocin and vasopressin are sexually dimorphic. Developmental neuroscience. 2009;31:332–341. doi: 10.1159/000216544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.