Abstract
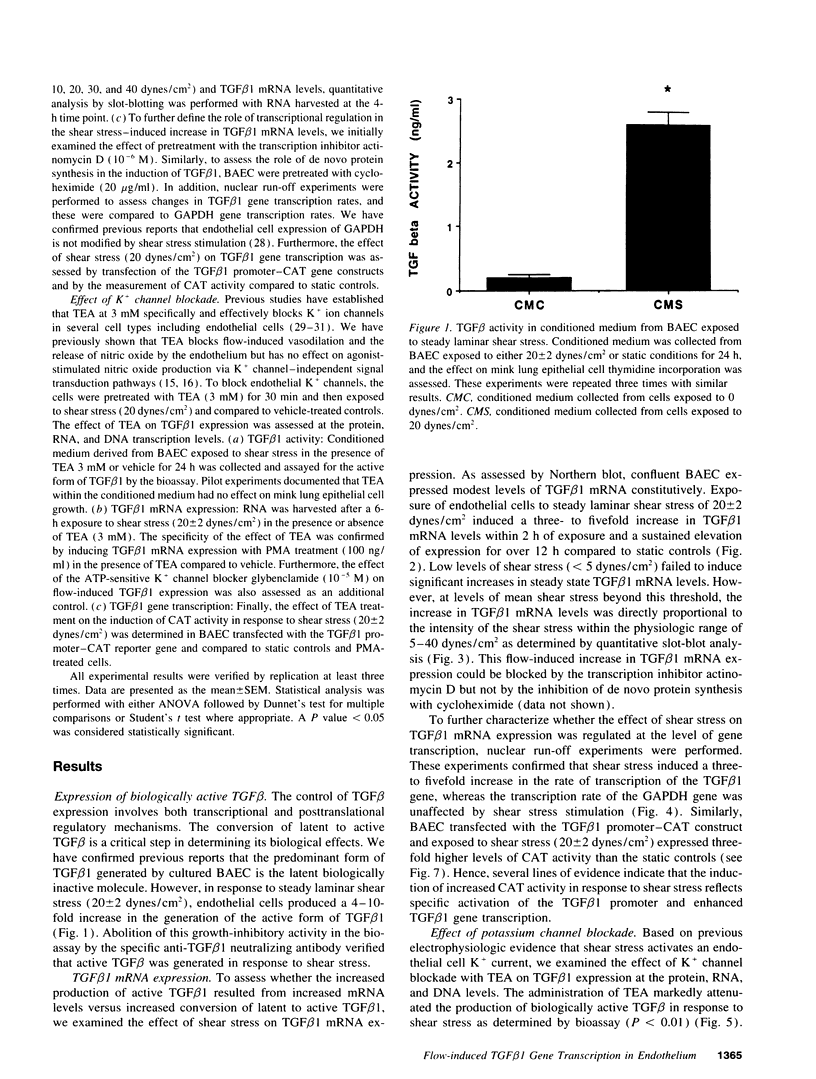
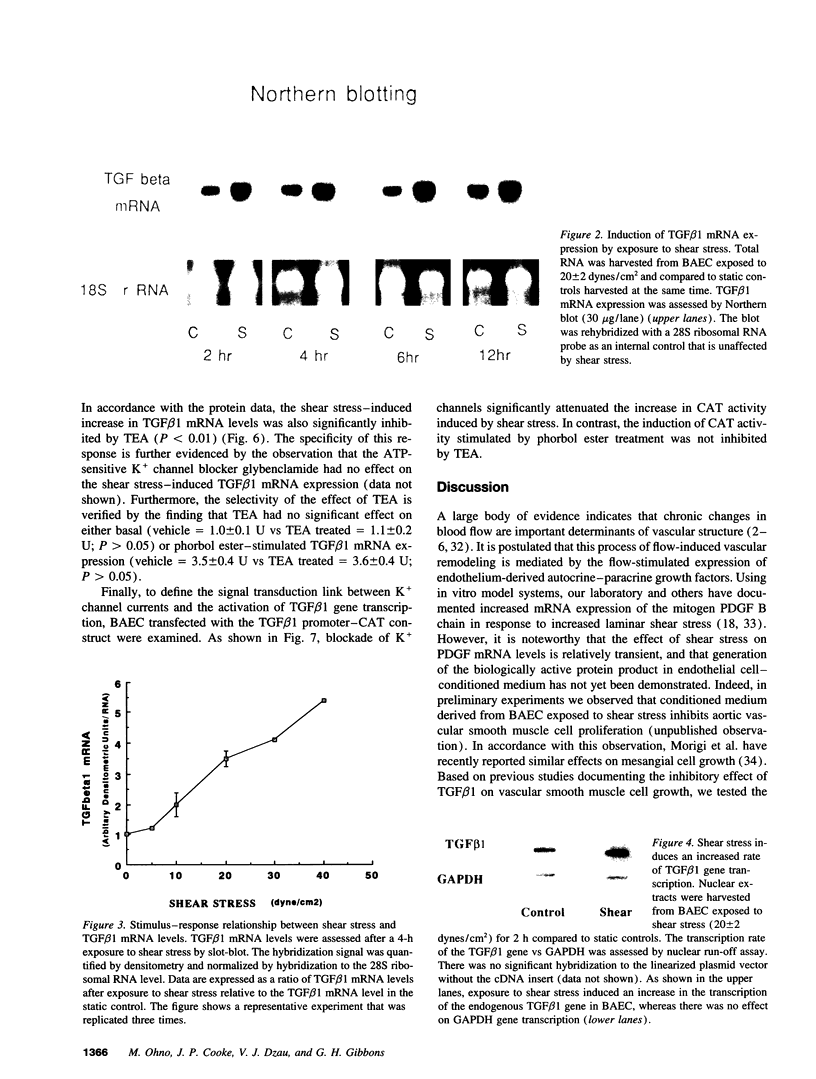
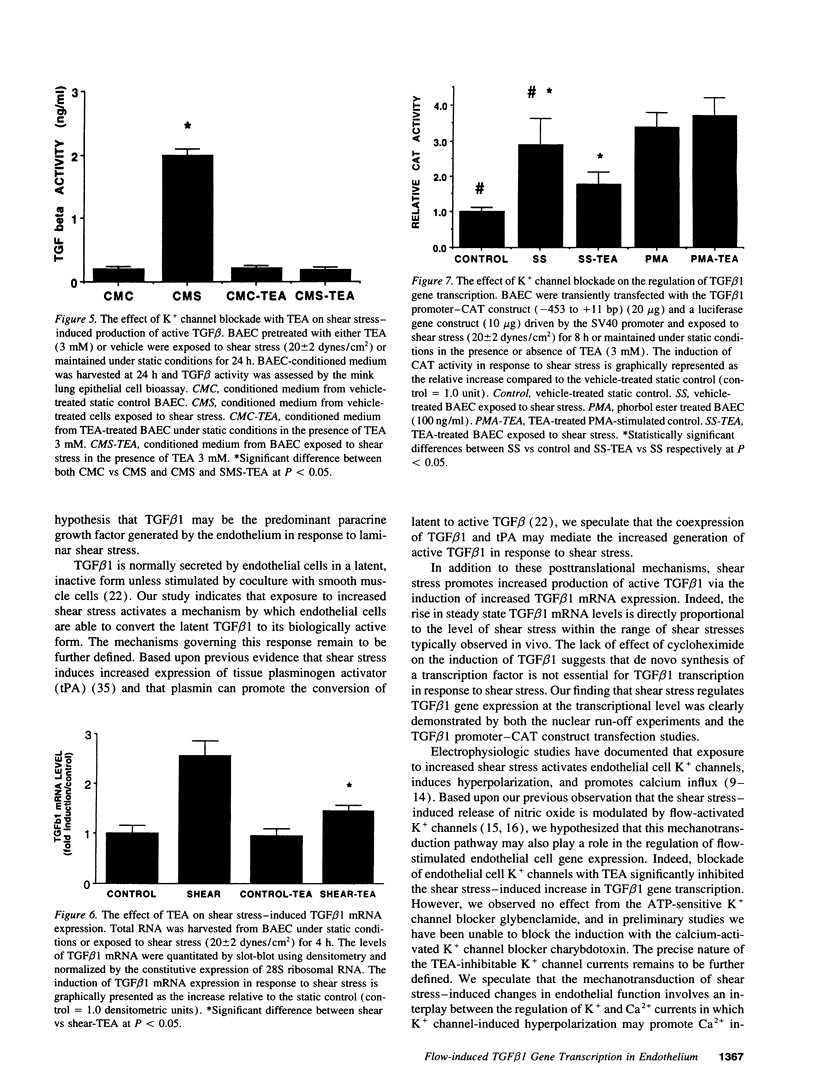
The endothelium has the capacity to modulate vascular structure in response to hemodynamic stimuli. We tested the hypothesis that exposure of the endothelium to increased laminar shear stress induces the expression of TGF beta 1 via a signal transduction pathway modulated by K+ channel currents. Although TGF beta 1 is normally secreted in a latent, inactive form, exposure of cultured endothelial cells to steady laminar shear stress (20 dynes/cm2) induced increased generation of biologically active TGF beta 1. This increase in active TGF beta 1 was associated with a sustained increase in TGF beta 1 mRNA expression within 2 h of stimulation. TGF beta 1 mRNA levels increased in direct proportion to the intensity of the shear stress within the physiologic range. The effect of shear stress on TGF beta 1 mRNA expression was regulated at the transcriptional level as defined by nuclear run-off studies and transient transfection of a TGF beta 1 promoter-reporter gene construct. Blockade of endothelial K+ channels with tetraethylammonium significantly inhibited: activation of TGF beta 1 gene transcription; increase in steady state mRNA levels; and generation of active TGF beta 1 in response to shear stress. These data suggest that endothelial K+ channels and autocrine-paracrine TGF beta 1 may be involved in the mechanotransduction mechanisms mediating flow-induced vascular remodeling.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonelli-Orlidge A., Saunders K. B., Smith S. R., D'Amore P. A. An activated form of transforming growth factor beta is produced by cocultures of endothelial cells and pericytes. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4544–4548. doi: 10.1073/pnas.86.12.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett G., Horiuchi M., Paul M., Pratt R. E., Nakamura N., Dzau V. J. Identification of a negative regulatory element involved in tissue-specific expression of mouse renin genes. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):885–889. doi: 10.1073/pnas.89.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolari S. R., Dewey C. F., Jr, Gimbrone M. A., Jr Apparatus for subjecting living cells to fluid shear stress. Rev Sci Instrum. 1982 Dec;53(12):1851–1854. doi: 10.1063/1.1136909. [DOI] [PubMed] [Google Scholar]
- Chen G. F., Cheung D. W. Characterization of acetylcholine-induced membrane hyperpolarization in endothelial cells. Circ Res. 1992 Feb;70(2):257–263. doi: 10.1161/01.res.70.2.257. [DOI] [PubMed] [Google Scholar]
- Cook N. S. The pharmacology of potassium channels and their therapeutic potential. Trends Pharmacol Sci. 1988 Jan;9(1):21–28. doi: 10.1016/0165-6147(88)90238-6. [DOI] [PubMed] [Google Scholar]
- Cooke J. P., Rossitch E., Jr, Andon N. A., Loscalzo J., Dzau V. J. Flow activates an endothelial potassium channel to release an endogenous nitrovasodilator. J Clin Invest. 1991 Nov;88(5):1663–1671. doi: 10.1172/JCI115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielpour D., Dart L. L., Flanders K. C., Roberts A. B., Sporn M. B. Immunodetection and quantitation of the two forms of transforming growth factor-beta (TGF-beta 1 and TGF-beta 2) secreted by cells in culture. J Cell Physiol. 1989 Jan;138(1):79–86. doi: 10.1002/jcp.1041380112. [DOI] [PubMed] [Google Scholar]
- Diamond S. L., Eskin S. G., McIntire L. V. Fluid flow stimulates tissue plasminogen activator secretion by cultured human endothelial cells. Science. 1989 Mar 17;243(4897):1483–1485. doi: 10.1126/science.2467379. [DOI] [PubMed] [Google Scholar]
- Dull R. O., Davies P. F. Flow modulation of agonist (ATP)-response (Ca2+) coupling in vascular endothelial cells. Am J Physiol. 1991 Jul;261(1 Pt 2):H149–H154. doi: 10.1152/ajpheart.1991.261.1.H149. [DOI] [PubMed] [Google Scholar]
- Geiser A. G., Kim S. J., Roberts A. B., Sporn M. B. Characterization of the mouse transforming growth factor-beta 1 promoter and activation by the Ha-ras oncogene. Mol Cell Biol. 1991 Jan;11(1):84–92. doi: 10.1128/mcb.11.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons G. H., Pratt R. E., Dzau V. J. Vascular smooth muscle cell hypertrophy vs. hyperplasia. Autocrine transforming growth factor-beta 1 expression determines growth response to angiotensin II. J Clin Invest. 1992 Aug;90(2):456–461. doi: 10.1172/JCI115881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson C. M., Diaz L., Kandarpa K., Sacks F. M., Pasternak R. C., Sandor T., Feldman C., Stone P. H. Relation of vessel wall shear stress to atherosclerosis progression in human coronary arteries. Arterioscler Thromb. 1993 Feb;13(2):310–315. doi: 10.1161/01.atv.13.2.310. [DOI] [PubMed] [Google Scholar]
- Glagov S., Zarins C., Giddens D. P., Ku D. N. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch Pathol Lab Med. 1988 Oct;112(10):1018–1031. [PubMed] [Google Scholar]
- Hsieh H. J., Li N. Q., Frangos J. A. Shear stress increases endothelial platelet-derived growth factor mRNA levels. Am J Physiol. 1991 Feb;260(2 Pt 2):H642–H646. doi: 10.1152/ajpheart.1991.260.2.H642. [DOI] [PubMed] [Google Scholar]
- Kamiya A., Togawa T. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am J Physiol. 1980 Jul;239(1):H14–H21. doi: 10.1152/ajpheart.1980.239.1.H14. [DOI] [PubMed] [Google Scholar]
- Keski-Oja J., Lyons R. M., Moses H. L. Immunodetection and modulation of cellular growth with antibodies against native transforming growth factor-beta 1. Cancer Res. 1987 Dec 15;47(24 Pt 1):6451–6458. [PubMed] [Google Scholar]
- Kim S. J., Glick A., Sporn M. B., Roberts A. B. Characterization of the promoter region of the human transforming growth factor-beta 1 gene. J Biol Chem. 1989 Jan 5;264(1):402–408. [PubMed] [Google Scholar]
- Kraiss L. W., Kirkman T. R., Kohler T. R., Zierler B., Clowes A. W. Shear stress regulates smooth muscle proliferation and neointimal thickening in porous polytetrafluoroethylene grafts. Arterioscler Thromb. 1991 Nov-Dec;11(6):1844–1852. doi: 10.1161/01.atv.11.6.1844. [DOI] [PubMed] [Google Scholar]
- Langille B. L., O'Donnell F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science. 1986 Jan 24;231(4736):405–407. doi: 10.1126/science.3941904. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Calcium influx into endothelial cells and formation of endothelium-derived relaxing factor is controlled by the membrane potential. Pflugers Arch. 1990 May;416(3):305–311. doi: 10.1007/BF00392067. [DOI] [PubMed] [Google Scholar]
- Malek A. M., Gibbons G. H., Dzau V. J., Izumo S. Fluid shear stress differentially modulates expression of genes encoding basic fibroblast growth factor and platelet-derived growth factor B chain in vascular endothelium. J Clin Invest. 1993 Oct;92(4):2013–2021. doi: 10.1172/JCI116796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey T. A., Falcone D. J., Brayton C. F., Agarwal L. A., Welt F. G., Weksler B. B. Transforming growth factor-beta activity is potentiated by heparin via dissociation of the transforming growth factor-beta/alpha 2-macroglobulin inactive complex. J Cell Biol. 1989 Jul;109(1):441–448. doi: 10.1083/jcb.109.1.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo M., Eskin S. G., Schilling W. P. Flow-induced changes in Ca2+ signaling of vascular endothelial cells: effect of shear stress and ATP. Am J Physiol. 1991 May;260(5 Pt 2):H1698–H1707. doi: 10.1152/ajpheart.1991.260.5.H1698. [DOI] [PubMed] [Google Scholar]
- Morigi M., Zoja C., Figliuzzi M., Remuzzi G., Remuzzi A. Supernatant of endothelial cells exposed to laminar flow inhibits mesangial cell proliferation. Am J Physiol. 1993 Apr;264(4 Pt 1):C1080–C1083. doi: 10.1152/ajpcell.1993.264.4.C1080. [DOI] [PubMed] [Google Scholar]
- Nakache M., Gaub H. E. Hydrodynamic hyperpolarization of endothelial cells. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1841–1843. doi: 10.1073/pnas.85.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M., Gibbons G. H., Dzau V. J., Cooke J. P. Shear stress elevates endothelial cGMP. Role of a potassium channel and G protein coupling. Circulation. 1993 Jul;88(1):193–197. doi: 10.1161/01.cir.88.1.193. [DOI] [PubMed] [Google Scholar]
- Olesen S. P., Clapham D. E., Davies P. F. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 1988 Jan 14;331(6152):168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- Owens G. K., Geisterfer A. A., Yang Y. W., Komoriya A. Transforming growth factor-beta-induced growth inhibition and cellular hypertrophy in cultured vascular smooth muscle cells. J Cell Biol. 1988 Aug;107(2):771–780. doi: 10.1083/jcb.107.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick N., Collins T., Atkinson W., Bonthron D. T., Dewey C. F., Jr, Gimbrone M. A., Jr Platelet-derived growth factor B chain promoter contains a cis-acting fluid shear-stress-responsive element. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4591–4595. doi: 10.1073/pnas.90.10.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubanyi G. M., Freay A. D., Kauser K., Johns A., Harder D. R. Mechanoreception by the endothelium: mediators and mechanisms of pressure- and flow-induced vascular responses. Blood Vessels. 1990;27(2-5):246–257. doi: 10.1159/000158816. [DOI] [PubMed] [Google Scholar]
- Rusko J., Tanzi F., van Breemen C., Adams D. J. Calcium-activated potassium channels in native endothelial cells from rabbit aorta: conductance, Ca2+ sensitivity and block. J Physiol. 1992 Sep;455:601–621. doi: 10.1113/jphysiol.1992.sp019318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Rifkin D. B. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989 Jul;109(1):309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J., Luscinskas F. W., Connolly A., Dewey C. F., Jr, Gimbrone M. A., Jr Fluid shear stress modulates cytosolic free calcium in vascular endothelial cells. Am J Physiol. 1992 Feb;262(2 Pt 1):C384–C390. doi: 10.1152/ajpcell.1992.262.2.C384. [DOI] [PubMed] [Google Scholar]





