INTRODUCTION
Chronic subdural hematoma (CSDH) is one of the most commonly encountered entities in neurosurgery practice. The incidence rate of CSDH has been reported to be as high as 13.1 cases per 100000 inhabitants24). It is common in the elderly, with the highest incidence rate in people older than 70 years17,18,23).
Surgical management is the preferred treatment for CSDH, which includes twist-drill drainage, burr-hole trephination, craniotomy with capsulectomy, and subduro-peritoneal shunt. Among them, burr-hole trephination is widely performed because of its less invasiveness and relative simplicity15). However, the incidence rate of recurrent CSDH is reported to range from 2 to 37%4,7,11,12,14,16,21,22,24). Furthermore, the crucial risk factors are still debatable despite the various elements that may be associated with the recurrence of CSDH. Thus, the goal of this study is to determine the predictors for recurrence of CSDH after burr-hole trephination.
MATERIALS AND METHODS
Patients' population
The authors retrospectively reviewed the medical records as well as the pre and postoperative computed tomography (CT) scans of the 182 patients who underwent burr-hole trephination from January 2008 to December 2012. There were 131 male (71.9%) and 51 (28.1%) female in this study, ranging in age from 24 to 94 years old (median age : 68.12 years old). The patients were divided into two groups according to the recurrence of CSDH. The clinical and radiological factors were compared between the recurrence group and the no recurrence group. In this study, 35 patients with bilateral CSDH were excluded in analyzing the CT density, the width of hematoma, the degree of air collection, the number of burr hole, and the drainage tube.
Clinical and radiological evaluations
The recurrence of CSDH was defined as a subsequent increase in hematoma volume in the subdural space for which reoperation was required because of newly developed symptoms21).
Hematoma type was classified into four types according to their density on CT scan : homogeneous, laminar, separate, and trabecular type14). Brain atrophy was divided into three stages : none or mild atrophy, definite atrophy, such as dilated sulci, and severe atrophy, such as widely dilated sulci and subdural space10,17). Air collection was categorized into two groups according to the subdural air found on immediate postoperative CT scan : none or mild, such as residual air bubble, and definite, such as total replacement with air17). The midline displacement of the septum pellucidum and pineal body were measured at the level of foramen of Monro on the CT scans taken before and after surgery. The cut-off values for the midline displacement were defined based on previous reports10,16,19). Follow-up was for at least 1 year.
Surgical procedure and management
All patients underwent one or two burr-hole trephination with closed-system drainage under general anesthesia. After dural incision, the outer hematoma membrane was opened, and irrigation was performed with lactated Ringer solution. A silicon tube was inserted into the cavity of the hematoma and connected to a closed drainage system. All patients were kept in the supine position and enough fluid was supplied to promote brain expansion. The closed-system drainage tube was usually removed after 2-3 days, when the drainage became negligible.
Statistical analysis
A univariate analysis was performed with Pearson's chi-square test and Student t-test to assess the relationship between each factor and the recurrence of CSDH. A multivariate analysis was performed using a logistic regression model. The relationship between the variables and the recurrence of CSDH are presented based on the 95% confidence interval and the odd ratio (OR). The statistical significance was set at p<0.05.
RESULTS
Patients' characteristics are summarized in Table 1. Reoperation was performed in 25 patients (13.7%) because of the symptomatic recurrence of CSDH. The demographic data and history of head trauma were not significantly associated with recurrence of CSDH. Underlying medical diseases, antithrombotic usage, and smoking or alcohol consumption were also not related to the recurrence of CSDH.
Table 1. Characteristics in 182 patients with chronic subdural hematomas.
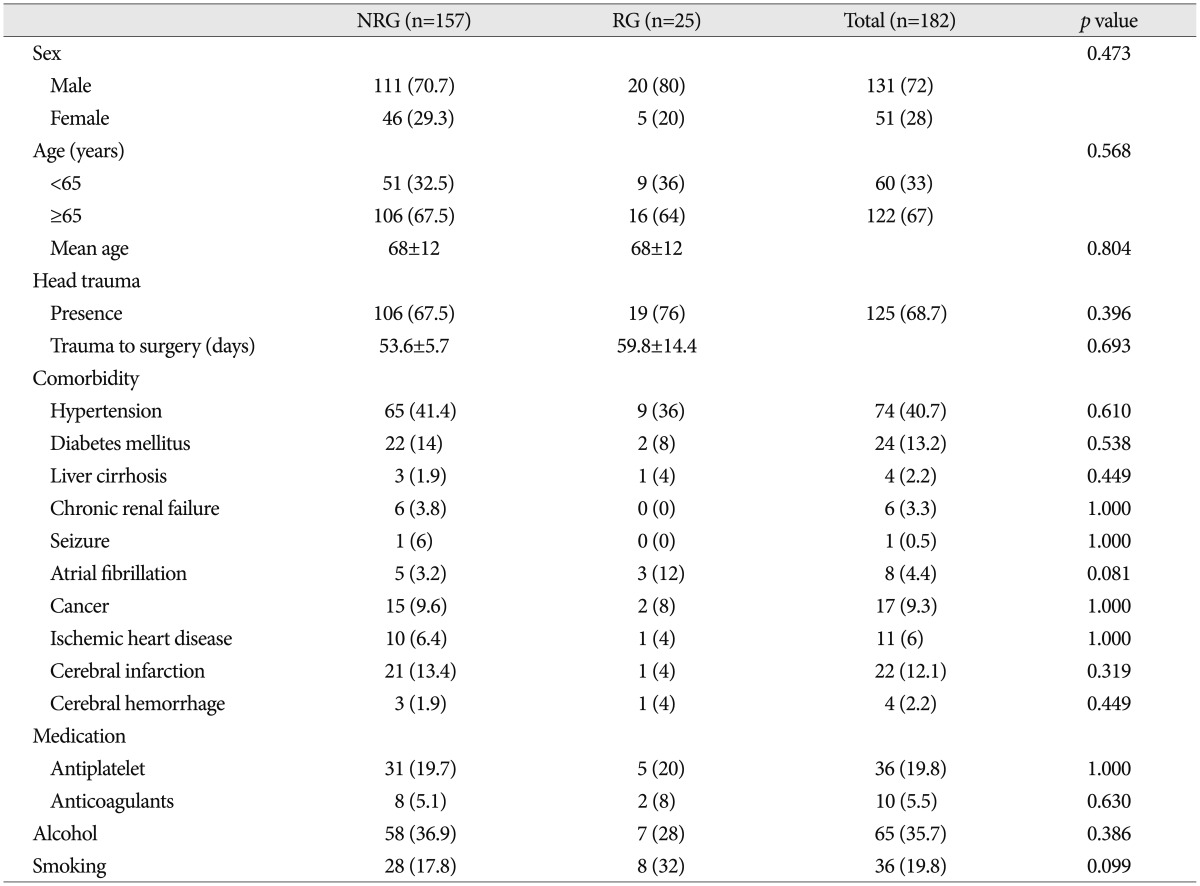
NRG : no recurrence group, RG : recurrence group
Clinical manifestations between the recurrence and the no recurrence group are shown in Table 2. The incidence of recurrence in patients with hemiparesis (18.8%) was higher than that in those without hemiparesis (7.4%) with statistical significance (p=0.026). However, any other clinical factors were not related to the recurrence of CSDH.
Table 2. Relationship between clinical presentation and recurrence of chronic subdural hematoma.
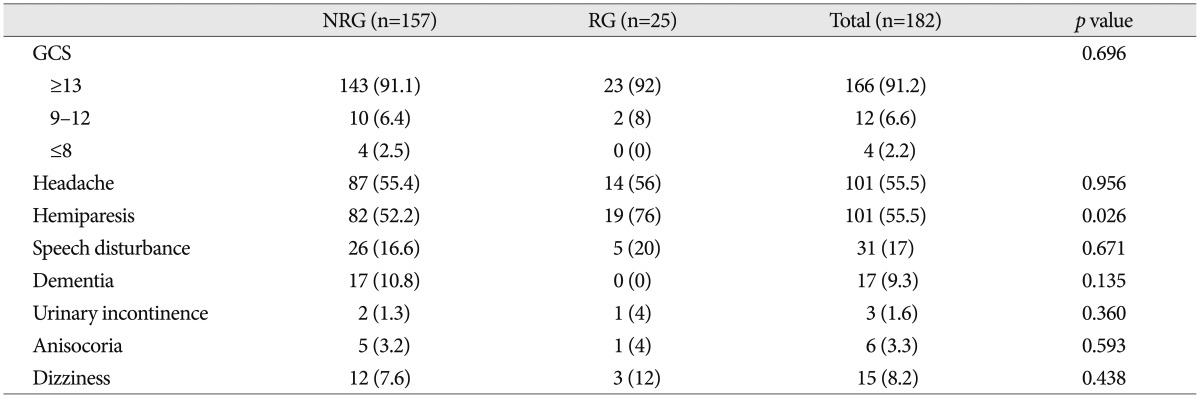
NRG : no recurrence group, RG : recurrence group, GCS : Glasgow Coma Scale
Comparison of radiological factors between the two groups is demonstrated in Table 3. In patients with unilateral CSDH, the incidence of recurrence (16.3%) was higher than that in patients with bilateral CSDH (2.9%). Although it did not get the statistical significance (p=0.052), unilateral CSDH tended to be associated with recurrence of CSDH. In patient with midline displacement of more than 10 mm before surgery, the incidence of recurrence rate (27.3%) was higher than that in patients with midline displacement of less than 10 mm (6.0%). The relationship between the preoperative midline displacement and the recurrence of CSDH was statistically significant (p=0.000). Immediate postoperative midline displacement of more than 10 mm was also associated with the recurrence of CSDH (31.3% vs. 7.7%; p=0.000). Other radiological parameters did not have statistical meaning to affect recurrent CSDH.
Table 3. Summary of perioperative CT findings in recurrence group and no recurrence group.
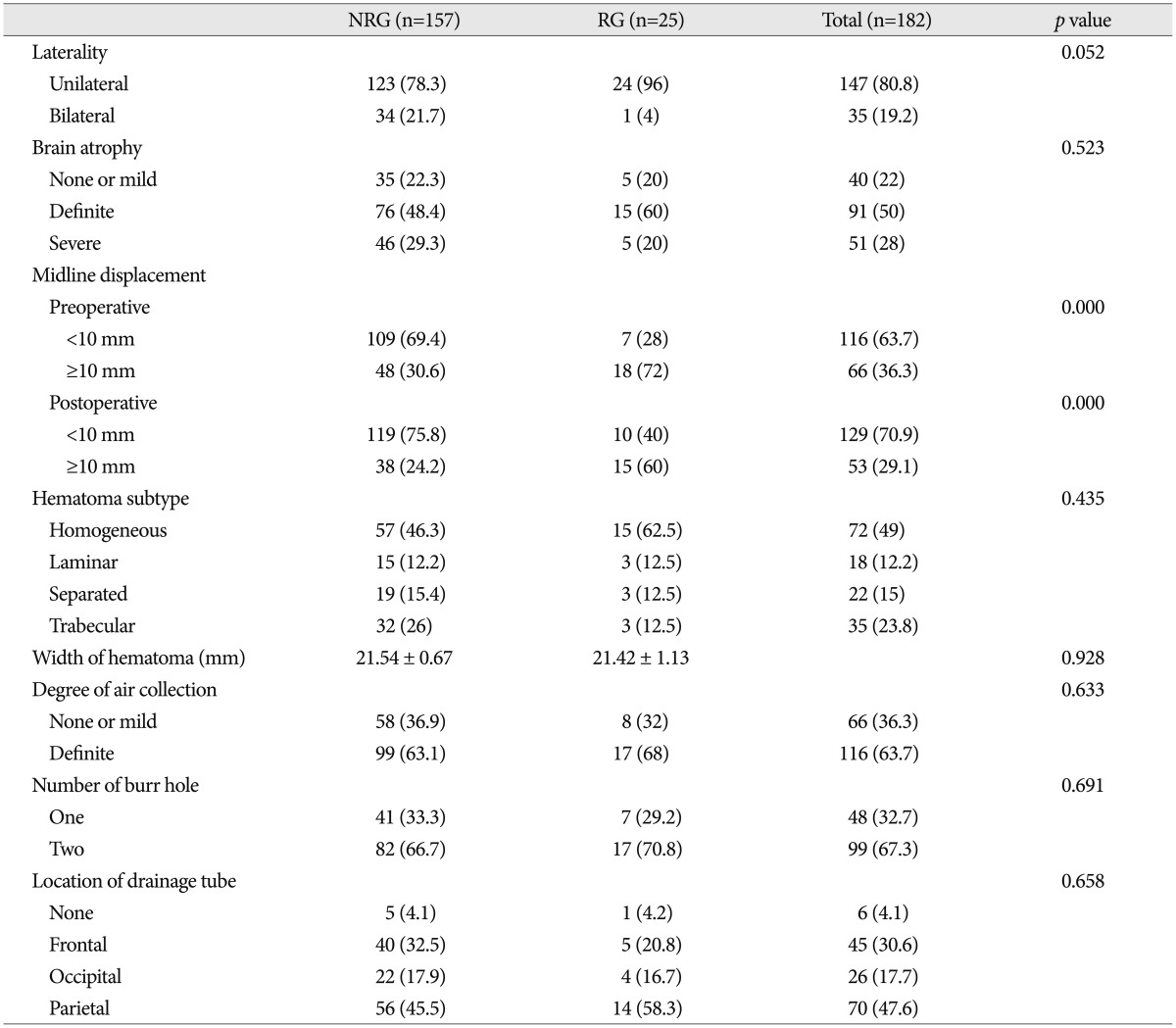
NRG : no recurrence group, RG : recurrence group
In summary, univariate analysis clarified that hemiparesis (p=0.026), pre (p=0.000) and postoperative (p=0.000) midline displacement of more than 10 mm were risk factors for recurrence of CSDH. The preoperative midline displacement of more than 10 mm (OR, 5.7; 95%; p=0.000) was the only independent risk factor for the recurrence of CSDH by multivariate logistic regression analysis (Table 4).
Table 4. Results of univariate and multivariate analysis of variables related to the recurrence of chronic subdural hematoma.
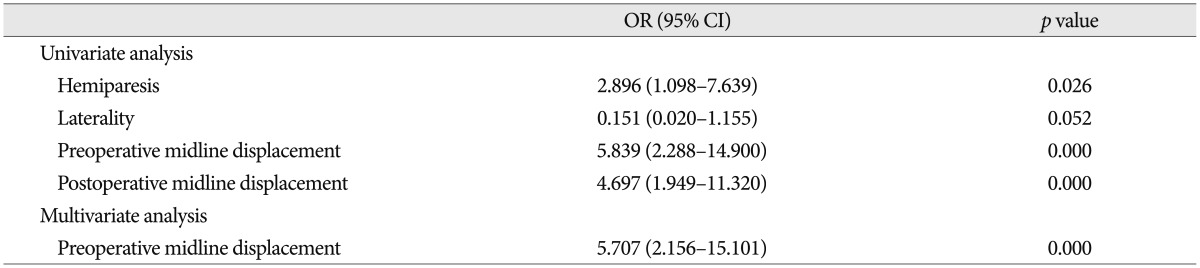
OR : odds ratio, CI : confidence interval
DISCUSSION
CSDH is defined as a watery or xanthochromic fluid collection under dura mater. The mechanism of CSDH is a pathophysiologic process that begins as a local inflammatory reaction of the dura mater to injury or external stimuli, such as blood or CSF. This process causes the neovascularization of the outer membrane of CSDH and vascular hyperperrmeability. Exudation through the microcapillaries in the outer membrane of CSDH plays an important role in the enlargement of CSDHs1).
The reported recurrence rates of CSDH range from 2 to 37%, and this study showed a recurrence rate of 13.7%. In the previous study, a few factors for the recurrence of CSDH have been reported, such as advanced age, bleeding tendency, brain atrophy, alcohol abuse, as well as bilateral CSDH, hematoma density, diabetes mellitus, arachnoid cyst, postoperative posture, postoperative subdural air accumulation, inflammatory cytokines, and some technical aspects of surgery3,5,13,23). However, the crucial risk factors are debatable until now. In this study, the recurrence of CSDH was correlated with the following variables : 1) midline displacement of more than 10 mm and 2) clinical presentation of hemiparesis.
Midline displacement
The authors found that the pre and postoperative midline displacement of more than 10 mm were the predictors for the recurrence of CSDH. In the series reported, the recurrence rate was significantly higher when the postoperative midline displacement was more than 5 mm compared to less displacement2,19). In patients with CSDH, it is reasonable to evaluate the pre and postoperative midline displacement because it shows that hemorrhage has filled up the potential space in the cranium and has exerted compression on the brain tissue. Fukuhara and coworkers showed that advanced age, brain atrophy, large amount of hematoma, and prolonged compressed parenchyma influenced brain elasticity2,6). Brain with high elastance tends to reexpand poorly, and poor reexpansion of the brain may lead to the persistence of postoperative midline displacement2,6). A prolonged postoperative midline displacement may cause impaired adhesion between the inner and outer neomembranes, thus facilitating postoperative recurrence2,15).
Hemiparesis
CSDH is asymptomatic in a large number of patients, but it may also cause high intracranial pressure that results in coma. Among these extreme states, nearly every constellation of speech, sensorimotor, neuropsychiatric, or mood disturbances may occur24). In the present study, hemiparesis and headache are the most common preoperative symptoms in CSDH, and the incidence of recurrent CSDH in patients with hemiparesis is higher than that in those without hemiparesis. The cause of hemiparesis in CSDH was reported to be the reduced local cerebral blood flow in the rolandic cortex or in deep structures, including the thalamus8,20). When the hematoma thickness increased beyond spatial compensation, both the superficial and deep brain structures shifted and deformed, and hemiparesis occurred in relation to the degree of midline displacement9). In other words, based on the aggravation of the midline displacement, hemiparesis occurred.
Laterality
In previous studies, hematoma laterality was not associated with the recurrence of CSDH5,23). Inconsistent with previous studies, our study show that the incidence of recurrence in patients with unilateral CSDH (16.3%) was higher than that in patients with bilateral CSDH (2.9%). Although it did not get the statistical significance (p=0.052) because of the lack of the number of cases, unilateral CSDH tended to be associated with recurrence of CSDH. As such, further study is necessary to clarify the association between the laterality of hematoma and the recurrence of CSDH.
CONCLUSION
The present study suggested that patients who show hemiparesis or pre and postoperative midline displacement of more than 10 mm could go through recurrent CSDH and shall undergo further surgical management. Therefore, the clinical and radiological surveillance is essential for the patients who have moderate midline displacement or motor weakness.
References
- 1.Abouzari M, Rashidi A, Rezaii J, Esfandiari K, Asadollahi M, Aleali H, et al. The role of postoperative patient posture in the recurrence of traumatic chronic subdural hematoma after burr-hole surgery. Neurosurgery. 2007;61:794–797. doi: 10.1227/01.NEU.0000298908.94129.67. discussion 797. [DOI] [PubMed] [Google Scholar]
- 2.Chon KH, Lee JM, Koh EJ, Choi HY. Independent predictors for recurrence of chronic subdural hematoma. Acta Neurochir(Wien) 2012;154:1541–1548. doi: 10.1007/s00701-012-1399-9. [DOI] [PubMed] [Google Scholar]
- 3.El-Kadi H, Miele VJ, Kaufman HH. Prognosis of chronic subdural hematomas. Neurosurg Clin N Am. 2000;11:553–567. [PubMed] [Google Scholar]
- 4.Ernestus RI, Beldzinski P, Lanfermann H, Klug N. Chronic subdural hematoma : surgical treatment and outcome in 104 patients. Surg Neurol. 1997;48:220–225. doi: 10.1016/s0090-3019(97)80031-6. [DOI] [PubMed] [Google Scholar]
- 5.Foelholm R, Waltimo O. Epidemiology of chronic subdural haematoma. Acta Neurochir(Wien) 1975;32:247–250. doi: 10.1007/BF01405457. [DOI] [PubMed] [Google Scholar]
- 6.Fukuhara T, Gotoh M, Asari S, Ohmoto T, Akioka T. The relationship between brain surface elastance and brain reexpansion after evacuation of chronic subdural hematoma. Surg Neurol. 1996;45:570–574. doi: 10.1016/0090-3019(95)00471-8. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton MG, Frizzell JB, Tranmer BI. Chronic subdural hematoma : the role for craniotomy reevaluated. Neurosurgery. 1993;33:67–72. doi: 10.1227/00006123-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Ikeda K, Ito H, Yamashita J. Relation of regional cerebral blood flow to hemiparesis in chronic subdural hematoma. Surg Neurol. 1990;33:87–95. doi: 10.1016/0090-3019(90)90017-j. [DOI] [PubMed] [Google Scholar]
- 9.Inao S, Kawai T, Kabeya R, Sugimoto T, Yamamoto M, Hata N, et al. Relation between brain displacement and local cerebral blood flow in patients with chronic subdural haematoma. J Neurol Neurosurg Psychiatry. 2001;71:741–746. doi: 10.1136/jnnp.71.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko BS, Lee JK, Seo BR, Moon SJ, Kim JH, Kim SH. Clinical analysis of risk factors related to recurrent chronic subdural hematoma. J Korean Neurosurg Soc. 2008;43:11–15. doi: 10.3340/jkns.2008.43.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotwica Z, Brzeziński J. Chronic subdural haematoma treated by burr holes and closed system drainage : personal experience in 131 patients. Br J Neurosurg. 1991;5:461–465. doi: 10.3109/02688699108998474. [DOI] [PubMed] [Google Scholar]
- 12.Kwon TH, Park YK, Lim DJ, Cho TH, Chung YG, Chung HS, et al. Chronic subdural hematoma : evaluation of the clinical significance of postoperative drainage volume. J Neurosurg. 2000;93:796–799. doi: 10.3171/jns.2000.93.5.0796. [DOI] [PubMed] [Google Scholar]
- 13.Markwalder TM. Chronic subdural hematomas : a review. J Neurosurg. 1981;54:637–645. doi: 10.3171/jns.1981.54.5.0637. [DOI] [PubMed] [Google Scholar]
- 14.Nakaguchi H, Tanishima T, Yoshimasu N. Factors in the natural history of chronic subdural hematomas that influence their postoperative recurrence. J Neurosurg. 2001;95:256–262. doi: 10.3171/jns.2001.95.2.0256. [DOI] [PubMed] [Google Scholar]
- 15.Nakaguchi H, Tanishima T, Yoshimasu N. Relationship between drainage catheter location and postoperative recurrence of chronic subdural hematoma after burr-hole irrigation and closed-system drainage. J Neurosurg. 2000;93:791–795. doi: 10.3171/jns.2000.93.5.0791. [DOI] [PubMed] [Google Scholar]
- 16.Ohba S, Kinoshita Y, Nakagawa T, Murakami H. The risk factors for recurrence of chronic subdural hematoma. Neurosurg Rev. 2013;36:145–149. doi: 10.1007/s10143-012-0396-z. discussion 149-150. [DOI] [PubMed] [Google Scholar]
- 17.Oishi M, Toyama M, Tamatani S, Kitazawa T, Saito M. Clinical factors of recurrent chronic subdural hematoma. Neurol Med Chir(Tokyo) 2001;41:382–386. doi: 10.2176/nmc.41.382. [DOI] [PubMed] [Google Scholar]
- 18.Okada Y, Akai T, Okamoto K, Iida T, Takata H, Iizuka H. A comparative study of the treatment of chronic subdural hematoma--burr hole drainage versus burr hole irrigation. Surg Neurol. 2002;57:405–409. doi: 10.1016/s0090-3019(02)00720-6. discussion 410. [DOI] [PubMed] [Google Scholar]
- 19.Stanisic M, Lund-Johansen M, Mahesparan R. Treatment of chronic subdural hematoma by burr-hole craniostomy in adults : influence of some factors on postoperative recurrence. Acta Neurochir (Wien) 2005;147:1249–1256. doi: 10.1007/s00701-005-0616-1. discussion 1256-1257. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka A, Yoshinaga S, Kimura M. Xenon-enhanced computed tomographic measurement of cerebral blood flow in patients with chronic subdural hematomas. Neurosurgery. 1990;27:554–561. doi: 10.1097/00006123-199010000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Torihashi K, Sadamasa N, Yoshida K, Narumi O, Chin M, Yamagata S. Independent predictors for recurrence of chronic subdural hematoma : a review of 343 consecutive surgical cases. Neurosurgery. 2008;63:1125–1129. doi: 10.1227/01.NEU.0000335782.60059.17. discussion 1129. [DOI] [PubMed] [Google Scholar]
- 22.Wakai S, Hashimoto K, Watanabe N, Inoh S, Ochiai C, Nagai M. Efficacy of closed-system drainage in treating chronic subdural hematoma : a prospective comparative study. Neurosurgery. 1990;26:771–773. doi: 10.1097/00006123-199005000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto H, Hirashima Y, Hamada H, Hayashi N, Origasa H, Endo S. Independent predictors of recurrence of chronic subdural hematoma : results of multivariate analysis performed using a logistic regression model. J Neurosurg. 2003;98:1217–1221. doi: 10.3171/jns.2003.98.6.1217. [DOI] [PubMed] [Google Scholar]
- 24.Youmans JR, Winn HR. Youmans Neurological Surgery. ed 6. New York: W.B. Saunders; 2011. pp. 532–543. [Google Scholar]


