Abstract
Objective
To explore racial differences in newborn telomere length (TL) and the effect moderation of the sex of the infant while establishing the methodology for the use of newborn blood spots for telomere length analyses.
Study design
Pregnant mothers were recruited from the Greater New Orleans area. TL was determined using MMQ-PCR on DNA extracted from infant blood spots. Demographic data and other covariates were obtained via maternal report prior to infant birth. Birth outcome data were obtained from medical records and maternal report.
Results
Black infants weighed significantly less than white infants at birth, and had significantly longer TL than White infants (p=0.0134), with the strongest effect observed in Black female infants. No significant differences in gestational age were present.
Conclusions
Significant racial differences in TL were present at birth in this sample, even after controlling for a range of birth outcomes and demographic factors. As longer initial TL is predictive of more rapid TL attrition across the life course, these findings provide evidence that, even at birth, biological vulnerability to early life stress may differ by race and sex.
Keywords: telomere length, newborn, race, health disparities
Telomeres normally shorten with age in somatic tissues and, in general, telomere length (TL) generally is correlated between different tissues, particularly in youth1-3 Aging and multiple other factors influence TL, including oxidative stress, DNA damage, DNA repair mechanisms, and genetic factors.4 TL also has been associated with both psychosocial stressors (exposure to violence, neighborhood disorder, racial discrimination), as well as chronic diseases (obesity, cardiovascular disease, diabetes).5-9 These diseases, associated with both early adversity and the aging process,10 have significantly higher rates in Blacks, particularly women, than in other races.11, 12
Racial differences in TL have been demonstrated in adults and adolescents,.13, 14 Baseline TL is longer in Blacks. TL attrition, an indicator of cellular aging predicted by initial TL, is also significantly greater in Blacks. This suggests that biological processes associated with aging may underlie persistent health disparities.6, 11, 13 Chae et al reported that racism was a significant predictor of shorter TL in Black adults, suggesting that not only do racial differences exist but that exposure to racism may contribute to part of the observed racial differences in TL.5 Thus, the determination of the earliest developmental time point at which racial differences in TL exist is salient.15
Shorter infant TL has been associated with a range of negative birth outcomes including preterm rupture of membranes, gestational diabetes, prenatal exposure to anti-retroviral therapy in HIV exposed infants, and maternal stressors during pregnancy.16-19 Studies have also demonstrated shorter placental TL of infants with intrauterine growth restriction (IUGR).20 Only one previous study, designed to examine cross tissue correlation of TL, presented any data on the association between race and TL at birth. 21 In this study, TL was not significantly different between Black and White infants. Although newborn TL was significantly associated with both maternal age and infant birth weight, analyses did not account for these associations or potential moderation of racial differences by gestational age or infant sex. Given consistent evidence of both sex differences in TL,22, 23 and racial differences in birth weight, these factors are likely critical covariates when examining racial differences in newborn TL.
One study has demonstrated a significant correlation between TL from dried blood spots and whole blood obtained via venipuncture, suggesting that blood spot DNA is a practical alternative DNA source. 25 This study examined the feasibility of newborn blood spots as a source for newborn TL analyses, and explored racial differences in newborn TL in a prospective cohort of infants. Based on existing data in adults, we hypothesized that racial differences would be found, with Blacks having longer TL than Whites. Further, as our previous work and that of others has demonstrated sex differences, 7, 22, 23 we examined whether the sex of the infant moderated racial differences. The established evidence that the rate of TL attrition is proportional to baseline TL coupled with the increasing evidence linking TL to negative health outcomes, suggest that evaluation of racial differences in infant TL may provide novel insight into the early biological evidence of health disparities.
Methods
Data were collected on 71 infants recruited from New Orleans, Louisiana as part of a larger longitudinal study examining the effect of cumulative maternal life course and prenatal stress on infant TL and early childhood development. Pregnant mothers, age 18 to 41, were recruited from prenatal and Women, Infant and Children (WIC) clinics, as well as from other on-going studies of maternal health and pregnancy outcomes at Tulane University. Recruitment areas were identified using the community identification process, a mapping method to record epidemiological indicators of the prevalence and incidence of community stressors and other selected social and health conditions. Mothers were excluded if they were less than 18 years of age or expecting multiple infants. Only English speaking mothers were recruited. Mothers provided information about multiple levels of their and their infant's social ecology (ie, household and neighborhood) using an interview-assisted computer survey administered face-to-face at the research site or at prenatal clinics (Questionnaire Development System, QDS, Nova Research, Bethesda, MD). Oral responses were recorded onto the computer by trained interviewers. This study was approved by the Tulane University Institutional Review Board.
Newborn DNA was extracted from newborn blood spots. Maternal consent was obtained during pregnancy. Genomic DNA was isolated according to the manufacturer's recommended protocol included in the Purelink Genomic DNA Mini kit (Invitrogen) and eluted in 70 μL of nuclease-free water. The resulting DNA samples were then stored at − 20 °C. Control DNA utilized for TL assay was obtained from pooled dried blood spot extracted DNA.
All DNA samples were evaluated for dsDNA integrity and concentration with Qubit (Invitrogen, Carlsbad, CA, USA), for purity with Nanodrop-2000 (Thermo Scientific, Waltham, MA, USA), and DNA integrity via agarose gel electrophoresis. The average relative TL as represented by the telomere repeat copy number to single gene (albumin) copy number (T/S) ratio, was determined using monochrome multiplex quantitative real-time PCR (MMQ-PCR) and standard methods in our laboratory 7, 26. A 10μl DNA sample, containing ~0.1-0.5ng of DNA diluted in pure water, was briefly combined with 15μl of PCR mixture, for a final volume of 25μl per reaction. The PCR reaction consisted of 0.75X Sybr Green I (Invitrogen, Carlsbad, CA, USA), 1X Gene Amp Buffer II (Applied Biosystems, Foster City, CA, USA), 0.8mM dNTPs, 10mM MgCl2, 3mM DTT, 1M Betaine, 2.5U AmpliTaq Gold polymerase (Applied Biosystems, Foster City, CA, USA), 0.9μM telg primer (ACACTAAGGTTTGGGTTTGGGTTTGGGTTTGGGTTAGTGT), 0.9μM telc primer (TGTTAGGTATCCCTATCCCTATCCCTATCCCTATCCCTAACA), 0.6μM albd2 primer (GCGGGCCCGCGTGGCGGAGCGAGGCCGgaaaagcatggtcgcctgt) and 0.6μM albu2 primer (GCCTCGCTCCGGGAGCGCCGCGCGGCCaaatgctgcacagaatccttg). All samples are performed in triplicate with a 7-point standard curve (0.0313ng to 2ng) with DNA extracted from pooled control blood spot cards. Each plate was duplicated with all samples in a different well position. All plates were run with a standard curve obtained from the same pooled control blood DNA sample to eliminate possible additional sources of interplate variability. Intraplate and interplate coefficients of variations were calculated for uniformity of TL estimates (CV≤5%). The slope of the standard curve for both the telomere and albumin reactions was used to calculate the T/S ratio for each sample. PCR efficiency criteria for telomere and albumin reactions are between 90-110% and paired-plates were not allowed to differ by more than 10% or they were repeated. Coefficients of variations (CV) were calculated within each triplicate (CV criteria ≤6%) and between plates (CV criteria ≤10%). Samples with unacceptably high CVs (>6% intra-assay or > 10% inter-assay CV) were removed from analysis or repeated (N = 5) and did not differ on any demographic or other birth outcome factors from samples that amplified appropriately. Bloodspot TL ratio was determined by the average T/S ratio of the triplicates from both plates (e.g. the average of six different assays of each individual). Children without valid bloodspot TL data did not significantly differ from children with bloodspot TL data on study measures.
Infant birth outcome data was obtained from medical records: sex, birthdate, birth weight, and gestational age. Additional covariates were obtained during the prenatal maternal interview-assisted computer survey and included parental age when the infant was conceived, maternal race, and maternal highest level of education as a proxy for socioeconomic status.
Statistical analyses
Descriptive, bivariate, and multivariate analyses were conducted using SAS 9.3. Univariate analyses included examination of central tendency measures and frequencies. Bivariate analyses were performed to determine initial association between infant TL and race, as well as between race and TL potential confounders. Bivariate analyses included Spearman and Pearson correlations, Likelihood Ratio chi-square or t-test where appropriate, as well as non-parametric equivalents when necessary. Multiple linear regression analyses, controlling for relevant covariates, was performed. A variable was considered a potential confounder and kept in the multivariate model if there was a 10% or more change in estimate between race and infant TL. Variables were also included if there were established evidence in previous studies of confounding in relation to TL and telomere length dynamics. Given the differential impact of stressors on TL among children in previous studies, we also examined effect modification by the sex of the infant in the race-TL relation 7, 22.
Results
71 infant blood spots were obtained; however, TL was not determined in 5 infants due to failure in quality control. The five infants excluded did not differ in any covariate from the subjects included in the final analyses. Characteristics of the sample by race are presented in Table I. Approximately 64% of the sample was Black and 47% of the infants were female. Black mothers reported significantly lower educational achievement, and they weighed significantly less at birth.
Table 1.
Demographics
| Race | |||
|---|---|---|---|
| Black N=42 | White N=24 | ||
| N (%) or mean (SD) | N (%) or mean (SD) | p-value | |
| Sex of Infant | |||
| Male | 23 (55) | 12 (50) | 0.09 |
| Female | 19 (45) | 12 (50) | 0.28 |
| Maternal Education | <0.01* | ||
| Less than high school | 7 (17) | 3 (13) | |
| High school graduate or GED | 13 (31) | 3 (13) | |
| Some college or vocational training | 17 (40) | 5 (21) | |
| College or professional degree | 4 (10) | 13 (54) | |
| Maternal age at conception (years) | 26.3 (4.4) | 28.5( 6.1) | 0.09 |
| Paternal age at conception (years) | 30.8 (7.1) | 30.8 (5.89) | 1.00 |
| Birth weight (grams) | 3143 (436) | 3443 (438) | 0.01* |
| Gestational age (weeks) | 38.8 (1.3) | 39.1 (1.4) | 0.40 |
Note.
P-value based on Likelihood Ratio Chi-square or t-test where appropriate
Infant TL was normally distributed with a mean TL of 1.77 and standard deviation of .34 (range 2.72 to 1.12). No significant associations were found between infant TL and maternal or paternal age, sex of the infant, maternal education, infant birth weight or gestational age. Significant associations were observed between select covariates, including education and gestational age where mothers with higher education have infants born at later gestational ages (rho=0.364, p= 0.0024; Table II). As expected birth weight and gestational age were highly correlated (rho=0.394, p= 0.0009), as were maternal and paternal age (rho=0.636, p < 0.0001).
Table 2.
Bivariate Correlation between Key Covariates
| Maternal age | Race | Maternal education | Birth Weight | Gestational age | Paternal age | |
|---|---|---|---|---|---|---|
| Maternal age | ----- | 0.17 | 0.24 | 0.02 | 0.03 | 0.64*** |
| Race | ----- | 0.37** | 0.33** | 0.11 | 0.03 | |
| Maternal education | ----- | 0.29* | 0.35** | 0.13 | ||
| Birth Weight | ----- | 0.38** | −0.02 | |||
| Gestational age | ----- | 0.04 | ||||
| Paternal age | ----- |
Spearman correlations:
p< .05
p<.01
p<.001
Race was not significantly correlated with gestational age or maternal or paternal age. However significant differences by race were found for maternal education (rho=0.322, p=0.0079) and birth weight, (rho=0.320, p= 0.0078) with Black mothers having significantly lower educational achievement and Black infants weighing significantly less at birth.
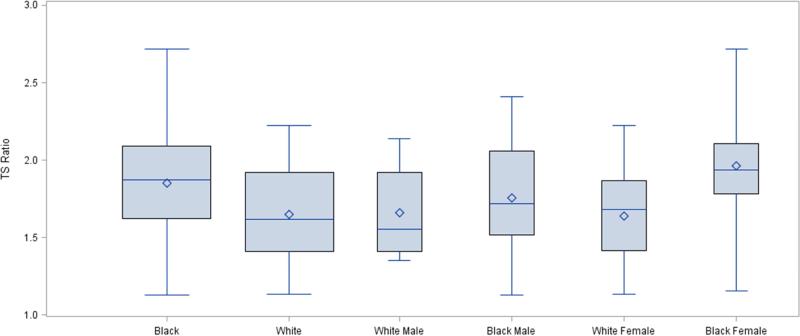
Infant TL was significantly longer in Black compared with White infants (1.8 vs. 1.6, p=0.0134; Figure). Although only at trend level, results reveal potential moderation by sex (interaction term β= -0.2303, p = 0.148). Post-hoc pairwise analyses revealed that Black female infant TL was longer than both male and female White infant TL ((t=-2.61, p=0.014), and (t=-2.68, p=0.012)). Further, Black female infant TL was significantly longer than Black male infant TL (t=-2.04, p=0.048). In White infants, no sex differences were noted in TL.
Figure 1.
Average Infant Telomere Length (TS Ratio) by Infant Race
The final multivariate results are presented in Table III. Even after controlling for key covariates, racial differences in infant TL remained, with Blacks exhibiting significantly longer TL compared with White infants (β = 0.2021, SE=0.1001; t = 2.02, p=0.048).
Table 3.
Final Multivariate Results – Racial Differences in Infant Telomere Length (N=64)
| Parameter | Estimate | Standard Error | Pr > |t| | 95% Confidence Limits | |
|---|---|---|---|---|---|
| Intercept | 1.64 | 0.49 | 0.00 | 0.66 | 2.62 |
| Race | |||||
| Black | 0.20 | 0.10 | 0.05 | 0.00 | 0.40 |
| White | Referent | ||||
| Maternal age at conception (years) | 0.01 | 0.01 | 0.20 | −0.01 | 0.04 |
| Maternal Education | |||||
| Less than high school | 0.14 | 0.16 | 0.37 | −0.17 | 0.46 |
| High school graduate or GED | −0.11 | 0.13 | 0.41 | −0.36 | 0.15 |
| Some college or vocational training | 0.09 | 0.14 | 0.52 | −0.18 | 0.36 |
| College or professional degree | Referent | ||||
| Birth weight (grams) | 0.00 | 0.00 | 0.34 | 0.00 | 0.00 |
| Sex of Infant | |||||
| Male | −0.09 | 0.08 | 0.28 | −0.26 | 0.08 |
| Female | Referent | ||||
| Paternal age at conception (years) | 0.00 | 0.01 | 0.77 | 0.02 | 0.01 |
Discussion
There are significant racial differences in newborn TL that are moderated by infant sex. This is consistent with adult studies. 27, 28 In agreement with our previous studies, and larger meta analyses indicating sex differences in TL, the sex of the infant moderated the association between race and TL. 7, 22, 29 Pairwise analysis demonstrated no significant sex differences in TL in Whites. However, Black female TL was significantly longer than all other groups. Our findings of longer TL in females matches data in adult studies that suggest females have longer TL across the life course. 30
TL, measured from infant cord blood and placental tissue, has been associated with gestational diabetes, premature rupture of amniotic membranes, and IUGR- all conditions linked to increased oxidative stress and inflammatory processes.17, 19, 20 As TL has independently been associated with both oxidative stress and inflammation31, 32 these findings suggest mechanistic pathways relating early life experiences and TL. Consistent with other studies we did not detect a direct association between TL and gestational age or birth weight,33 although rapid TL shortening may exist earlier in fetal development.34 These results also fit with animal studies that suggest TL in offspring are influenced by both genetic and maternal factors. 35
It is important to note that maternal education, parental age, and gestational age did not significantly alter our findings. Our results differ from the study by Okuda et al, who did not detect racial differences in newborn TL. 21 Notably, although not statistically significant, TL measurement was slightly longer in Black newborns compared with White in both cord blood (10.98kb vs 10.92kb) and umbilical artery tissue (10.99 vs 10.89kb). Similar to our study, infants in their study did not have significant differences in gestational age, but did differ in mean birth weight by race, however TL analyses did not control for racial differences in birth weight. This study, and other studies in newborns, have utilized DNA extracted from umbilical cord blood for TL determination. In addition to practical considerations related to obtaining cord blood, the use of umbilical cord blood for telomere length determination raises methodological concerns related to potential confounding of TL measurement by the possible presence of maternal DNA and variable amounts and types of fetal stem cells.21, 34, 36 The failure to include critical covariates (eg, maternal age, parental age at conception, sex of the infant, and birth weight), combined with the differing sources of DNA, may explain the differing results in the current study and the study by Okuda et al.
Our study utilized DNA obtained from newborn blood spot cards for TL measurement. As such, dried blood spot DNA appears to be an appropriate alternative to cord blood for measuring newborn TL. Our findings are consistent with a previous study in adults testing the correlation between TL determined from DNA extracted from venipuncture with DNA from blood taken from a finger stick and stored on blood spot cards.25 That study also noted that no difference in TL were found after utilizing six different spots on a single blood card, indicating that sampling methodology did not alter TL measurement. TL determined from the blood spot card was highly correlated with DNA obtained from blood from the same individual utilizing a finger prick , indicating that clotting and aspects of the blood spot card themselves did not significantly influence TL measurement. 25 Thus, TL determination from blood spot cards may represent a cost effective and informative source of DNA for larger epidemiologic studies as well as studies in infants. Future studies should carefully examine the impact of blood spot storage conditions on TL measurement. Although the qPCR methodology may be more robust than Southern analyses of TL to DNA degradation, significant DNA degradation or impurities in the DNA sample can influence TL measurement and caution about the integrity of the extracted DNA is warranted given the potential for wide intra-assay variability. 37, 38
There are important limitations to this study. It is a small and relatively homogenous convenience sample, making it less generalizable. We also acknowledge potential over-fitting of the model due to the number of covariates in relation to the sample size, however it is notable that a direct correlation between race and infant TL existed. Further, all included variables met either statistical criterion for confounders or were included due to extant evidence from molecular genetics work in relation to TL and/or previous studies of TL in other populations. We were not able to correct for white blood cell count or cell type differences in these samples. However, in a previous study of telomere length in adults, white blood cell count did not impact findings13 and one study of neutrophil counts in 30,354 newborns did not detect any association between race/ethnicity and white blood cell count at birth.39 In the current study, sex differences were not found within the White subjects. Our ability to evaluate sex differences within the White racial group may have been influenced by sample size. However, LTL measured by Southern blot analyses in the Bogalusa Heart study, appeared to have greater sex differences within African American subjects (7.95 (female) vs. 7.81 (male)) then within White subjects (7.32 (female) vs. 7.28 (male)), in part consistent with our current results.13 Larger studies that are adequately powered to assess both racial and sex differences, as well as span the life course, are needed. Failure to detect sex differences early in development within one racial group would have unique implications for research related to both health disparities and sex differences. The use of self-reported measures, with the potential for recall and social desirability biases, is an additional limitation. Lastly, medical complications during pregnancy were not collected, limiting the ability to control for these factors in our analyses.
Despite heightened awareness, health disparities in birth outcomes, as well other health and behavioral outcomes (e.g., cardiovascular disease, diabetes) across the life course, persist.40 The failure to achieve the goals of Health People 2010 suggests that the current models and efforts to identify and decrease the root causes of health disparities are inadequate and new approaches are needed. Although improvements have been made, the striking continued racial disparities, particularly in birth outcomes, indicate that much remains to be done. 41 One hypothesis for the persistent racial gaps in health equality and birth outcomes is that current interventions are not addressing the underlying biological mechanisms early enough. Another hypothesis is that these health disparities reflect the trans-generational transmission of early adversity or chronic stress.42 These two hypotheses are not mutually exclusive, and in either case, determining the presence of racial differences in TL—an epigenetic biomarker of adversity and cellular aging—in newborns is a significant next scientific step toward reducing and eliminating health disparities. The rapidly expanding literature linking TL to negative health outcomes associated with both health disparities and psychosocial stress, when combined with our results demonstrating racial differences in newborn TL, suggest that TL maybe reflective of underlying biological processes contributing to the persistence of health disparities.
Our finding of longer newborn TL in Black infants, particularly females, initially may appear inconsistent with the increased negative health outcomes found in Blacks. However, as longer initial TL is associated with accelerated TL decline, longer newborn TL may increase the risk for accelerated aging, particularly with exposure to stressors, both psychosocial and environmental, known to impact telomere dynamics. Accelerated shortening of TL and an enhanced negative effect of stressors on TL within Blacks is consistent with the weathering hypothesis proposed by Geronimus et al, in which racial differences in exposure to chronic stress contribute to accelerated aging, particular in Black women.43,27 Although Blacks have significantly higher morbidity and mortality during middle age, there appears to be a mortality cross over after age 70 years, where Blacks who live past 70 years of age appear to have improved health outcomes relative to same age Whites. 44 The existence of a similar cross over may be present earlier in the life course and represents an interesting direction of future study. An alternative consideration is that racial differences in the heritability of TL have been reported.13 As such, the molecular genetic architecture underlying TL, and therefore TL associations with health outcomes, may also exhibit racial differences. The specific length at which negative health outcomes appear could therefore differ by race, with negative health outcomes appearing when telomeres are relatively longer in Blacks than in Whites.
Although methodological concerns exist in TL studies 37 and the mechanistic links between TL and negative health outcomes are as of yet undefined, future well-designed longitudinal studies of TL trajectory may provide novel mechanistic insight into persistent health disparities. Replication of our findings in larger studies, as well as studies exploring racial differences in TL trajectory across development, in conjunction with factors associated with TL are needed next steps. Enhanced understanding of the factors controlling telomere length dynamics may represent novel future research targeting the reduction in health disparities.
Acknowledgments
Funded by the National Institutes of Health (2K12HD043451-06, 1 R01 MH101533-01, 3R01MH101533-02S3 [all to S.D.]), the Tulane University Oliver Fund (to S.D.), and the Eunice Kennedy Shriver National Institute of Child Health & Human Development (K12HD043451). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Abbreviations
- TL
Telomere length
- IUGR
Intra uterine growth restriction
- CV
Coefficients of variations
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Youngren K, Jeanclos E, Aviv H, Kimura M, Stock J, Hanna M, et al. Synchrony in telomere length of the human fetus. Hum Genet. 1998;102:640–3. doi: 10.1007/s004390050755. [DOI] [PubMed] [Google Scholar]
- 2.Friedrich U, Griese E, Schwab M, Fritz P, Thon K, Klotz U. Telomere length in different tissues of elderly patients. Mechanisms of Ageing and Development. 2000;119:89–99. doi: 10.1016/s0047-6374(00)00173-1. [DOI] [PubMed] [Google Scholar]
- 3.Thomas P, O'Callaghan NJ, Fenech M, O'Callaghan NJ. Telomere length in white blood cells, buccal cells and brain tissue and its variation with ageing in Alzheimer's disease. Mechanism of ageing and development. 2008;129:183–90. doi: 10.1016/j.mad.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Gilley D, Herbert BS, Huda N, Tanaka H, Reed T. Factors impacting human telomere homeostasis and age-related disease. Mechanisms of Ageing and Development. 2008;129:27–34. doi: 10.1016/j.mad.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Chae D, Nuru-Jeter A, Adler N, Brody G, Lin J, Blackburn E, et al. Discrimination, racial bias and telomere length in African-American men. American Journal of Preventive Medicine. 2014;46:103–11. doi: 10.1016/j.amepre.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rewak M, Buka S, Prescott J, De Vivo I, Loucks E, Kawachi I, et al. Race-related health disparities and biological aging: Does rate of telomere shortening differ across blacks and whites? Biological psychology. 2014;99:92–9. doi: 10.1016/j.biopsycho.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drury S, Mabile E, Brett S, Esteves K, Jones E, Shirtcliff E, et al. The Association of Telomere Length with Family Violence and Disruption. Pediatrics. 2014;134:e128–e37. doi: 10.1542/peds.2013-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shalev I, Moffitt T, Sugden K, Williams B, Houts R, Danese A, et al. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Molecular Psychiatry. 2013;18:576–81. doi: 10.1038/mp.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buxton J, Walters R, Visvikis-Siest S, Meyre D, Froguel P, Blakemore A. Childhood obesity is associated with shorter leukocyte telomere length. Journal of Clinical Endocrinology & Metabolism. 2011;96:1500–5. doi: 10.1210/jc.2010-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz A, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. American Journal of Preventive Medicine. 1998;14:245–58. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 11.Diez Roux A, Ranjit N, Jenny N, Shea S, Cushman M, Fitzpatrick A, et al. Race/ethnicity and telomere length in the Multi-Ethinic Study of Atherosclerosis. Aging Cell. 2009;8:251–7. doi: 10.1111/j.1474-9726.2009.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu H, Wang X, Gutin B, Davis C, Keeton D, Thomas J, et al. Leukocyte telomere length in healthy Caucasian and African-American adolescents: relationships with race, sex, adiposity, adipokines, and physical activity. The Journal of Pediatrics. 2011;158:215–20. doi: 10.1016/j.jpeds.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunt S, Chen W, Gardner J, Kimura M, Srinivasan S, Eckfeldt J, et al. Leukocyte telomeres are longer in African Americans than in whites: the National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell. 2008;7:451–8. doi: 10.1111/j.1474-9726.2008.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitzpatrick A, Kronmal R, Gardner J, Psaty B, Jenny N, Tracy R, et al. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. American Journal of Epidemiology. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 15.Hertzman C, Boyce T. How experience gets under the skin to create gradients in developmental health. Annual Review of Public Health. 2010;31:329–47. doi: 10.1146/annurev.publhealth.012809.103538. [DOI] [PubMed] [Google Scholar]
- 16.Imam T, Jitratkosol M, Soudeyns H, Sattha B, Gadawski I, Maan E, et al. Leukocyte telomere length in HIV-infected pregnant women treated with antiretroviral drugs during pregnancy and their uninfected infants. Journal of Acquired Immune Deficiency Syndromes. 2012;60:495–502. doi: 10.1097/QAI.0b013e31825aa89c. [DOI] [PubMed] [Google Scholar]
- 17.Xu J, Ye J, Wu Y, Zhang H, Luo Q, Han C, et al. Reduced fetal telomere length in gestational diabetes. PLoS ONE. 2014;9:e86161. doi: 10.1371/journal.pone.0086161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Entringer S, Epel ES, Kumsta R, Lin J, Hellhammer DH, Blackburn EH, et al. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proceedings of the National Academy of Sciences of the United States. 2011;108:E513–E8. doi: 10.1073/pnas.1107759108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menon R, Yu J, Basanta-Henry P, Brou L, Berga S, Fortunato S, et al. Shorter fetal leukocyte telomere length and preterm prelabor rupture of the membranes. Plos One. 2012;7:e31136. doi: 10.1371/journal.pone.0031136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biron-Shental T, Sukenik Halevy R, Goldberg-Bittman L, Kidron D, Fejgin M, Amiel A. Telomeres are shorter in placental trophoblasts of pregnancies complicated with intrauterine growth restriction (IUGR). Early Human Development. 2010;86:451–6. doi: 10.1016/j.earlhumdev.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Okuda K, Bardeguez A, Gardner J, Rodriguez P, Ganesh V, Kimura M, et al. Telomere Length in the Newborn. Pediatric Research. 2002;52:377–81. doi: 10.1203/00006450-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Drury S, Shirtcliff E, Shachat A, Mabile E, Phan J, Brett Z, et al. Growing up or growing old? Cellular aging linked with testosterone reactivity to stress in youth. American Journal of Medical Sciences. 2014 doi: 10.1097/MAJ.0000000000000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gardner M, Bann D, Wiley L, Cooper R, Hardy R, Nitsch D, et al. Gender and telomere length: Systematic review and meta-analysis. Experimental Gerontology. 2014;51:15–27. doi: 10.1016/j.exger.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker S, Cubitt W. The use of the dried blood spot sample in epidemiological studies. Journal of Clinical Pathology. 1999;52:633–9. doi: 10.1136/jcp.52.9.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zanet D, Saberi S, Oliveira L, Sattha B, Gadawski I, Cote H. Blood and dried blood spot telomere length measurement by qPCR: Assay considerations. PLoS ONE. 2013;8:e57787. doi: 10.1371/journal.pone.0057787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cawthon R. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Research. 2009;37:e21. doi: 10.1093/nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geronimus A, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. American Journal of Public Health. 2006;96:826–33. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geronimus A, Hicken M, Pearson J, Seashols S, Brown K, Cruz T. Do US Black Women Experience Stress-Related Accelerated Biological Aging? Human Nature. 2010;21:19–38. doi: 10.1007/s12110-010-9078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drury S, Theall K, Gleason M, Smyke A, De Vivo I, Wong J, et al. Telomere length and early severe social deprivation: linking early adversity and cellular aging. Molecular Psychiatry. 2012;17:719–27. doi: 10.1038/mp.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barrett ELB, Richardson DS. Sex differences in telomeres and lifespan. Aging Cell. 2011;10:913–21. doi: 10.1111/j.1474-9726.2011.00741.x. [DOI] [PubMed] [Google Scholar]
- 31.Effros RB. Telomere/telomerase dynamics within the human immune system: Effect of chronic infection and stress. Experimental Gerontology. 2011;46:135–40. doi: 10.1016/j.exger.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demissie S, Levy D, Benjamin EJ, Cupples LA, Gardner JP, Herbert A, et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5:325–30. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 33.Akkad A, Hastings R, Konje JC, Bell SC, Thurston H, Williams B. Telomere length in small-for gestational-age babies. BJOG: An International Journal of Obstetrics & Gynaecology. 2006;113:318–23. doi: 10.1111/j.1471-0528.2005.00839.x. [DOI] [PubMed] [Google Scholar]
- 34.Friedrick U, Schwab M, Griese E, Fritz P, Klotz U. Telomeres in neonates: New insights into fetal hematopoiesis. Pediatric Research. 2001;9:252–6. doi: 10.1203/00006450-200102000-00020. [DOI] [PubMed] [Google Scholar]
- 35.Haussmann M, Longenecker A, Marchetto N, Juliano S, Bowden R. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proceedings of the Royal Society of B: Biological Sciences. 2012;279:1447–56. doi: 10.1098/rspb.2011.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsang K, Wong A, Cheung M, Tang S, Leung Y, Li C, et al. Implication of maternal-cell contamination in the clinical banking of umbilical cord blood. Cytotherapy. 2002;4:375–83. doi: 10.1080/146532402760271163. [DOI] [PubMed] [Google Scholar]
- 37.Aviv A, Hunt SC, Lin J, Cao X, Kimura M, Blackburn E. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Research. 2011;39:e134. doi: 10.1093/nar/gkr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aubert G, Hills M, Lansdorp P. Telomere length measurement—Caveats and a critical assessment of the available technologies and tools. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2012;730:59–67. doi: 10.1016/j.mrfmmm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmutz N, Henry E, Jopling J, Christensen R. Expected ranges for blood neutrophil concentrations of neonates: the Manroe and Mouzinho charts revisited. Journal of Perinatology. 2008;28:275–81. doi: 10.1038/sj.jp.7211916. [DOI] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention (CDC) Centers for Disease Control and Prevention (CDC), editor. CDC Health Disparities and Inequalities Report -- United States, 2013. Morbidity and Mortality Weekly Report (MMWR) 2013:1–186. [Google Scholar]
- 41.Christopher G, Simpson P. Improving Birth Outcomes Requires Closing the Racial Gap. American Journal of Public Health. 2014;104:S10–S2. doi: 10.2105/AJPH.2013.301817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao Y, Robinson A, Zucchi F, Robbins J, Babenko O, Kavalchuk O, et al. Ancestral exposure to stress epigenetically programs preterm birth risk and adverse maternal and newborn outcomes. BMC Medicine. 2014:12. doi: 10.1186/s12916-014-0121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geronimus A. The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethnicity & Disease. 1992;2:207–21. [PubMed] [Google Scholar]
- 44.Corti M, Guralink J, Ferruci L, Izmirlian G, Leveille S, Pahor M, et al. Evidence for black-white crossover in all-cause and coronary artery disease mortality in an older population:The North Carolina EPESE. American Journal of Public Health. 1999;89:308–14. doi: 10.2105/ajph.89.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]