Abstract
Aims
Decreased insulin sensitivity is a cardiovascular risk factor (CVRF) in youth with type 1 diabetes (T1D). Whether baseline insulin sensitivity is independently associated with changes in early arterial stiffness (pulse wave velocity (PWV)) over time in youth with T1D is not known.
Methods
298 youth with T1D in the SEARCH CVD study had PWV measured ~five years apart. Insulin sensitivity and other CVRFs were measured at baseline. The association between baseline insulin sensitivity with PWV over time was explored using linear mixed models. Models were adjusted for baseline age, sex and race, with subsequent adjustment for CVRFs.
Results
There was a significant interaction (p=0. 0326) between baseline insulin sensitivity and time on PWV, independent of CVRFs, indicating that higher insulin sensitivity levels were associated with lower rate of change in PWV over time. Other significant predictors of PWV change were baseline age [β=0.007 (p=0.03) increase in logPWV/ year increase in age] and mean arterial blood pressure (MAP) [β=0.005 (p<0.01) increase in logPWV/ mmHg increase in MAP] and smoking status (current vs. never smoker).
Conclusions
Lower insulin sensitivity at baseline appears to be an important risk factor for increased arterial stiffness over time in youth with T1D. This identifies a potentially modifiable therapeutic target.
Keywords: pulse wave velocity, pediatrics, insulin resistance, cardiovascular disease, type 1 diabetes
Introduction
Cardiovascular disease (CVD) is the leading cause of death in adults with type 1 diabetes (T1D) and is associated with a tenfold increase in CVD-related mortality compared to the general population (Soedamah-Muthu et al., 2006). This process begins early in life and there is evidence that subclinical vascular changes indicative of future CVD, such as increased arterial stiffness, are present among children and teens with T1D (Urbina et al., 2010). Vessel thickening or stiffness is due to a combination of deposition of glycosylated end products, endothelial damage and dysfunctional repair, as well as structural changes characterized by an overproduction of abnormal collagen and diminished quantities of normal elastin, in addition to deposition of lipids, calcium or other metabolic products (Rask-Madsen and King, 2013). Vessel stiffness can be quantitated by pulse wave velocity (PWV), the gold standard measure of peripheral vascular stiffness (Laurent et al., 2006). PWV is strongly correlated with cardiovascular events and all-cause mortality (Vlachopoulos et al., 2010).
Insulin resistance (decreased insulin sensitivity) is a well-accepted benchmark for type 2 diabetes (T2D), and is associated with worsening cardiovascular outcomes. Insulin resistance is less studied as risk factor in the development of macrovascular complications in T1D (Nadeau and Reusch, 2011) but recent studies show youth with T1D have increased insulin resistance compared to healthy youth (Nadeau et al., 2010). The presence of insulin resistance in T1D may explain at least some of the elevated CVD risk not associated with hyperglycemia (Snell-Bergeon and Nadeau, 2012).
In a previous cross-sectional study in youth without diabetes, insulin sensitivity assessed using the Homeostatic Model Assessment (HOMA-IR) was not independently associated with PWV (Urbina et al., 2012). However, HOMA-IR is not usable for interpretation in insulin-treated patients (Dabelea et al., 2011). The aim of this study was to characterize the association between a validated marker of insulin sensitivity (10) and changes in PWV over time in youth with T1D participating in the longitudinal component of the SEARCH CVD study. Our hypothesis was that higher insulin sensitivity (less insulin resistance) at baseline will be associated with a slower increase in PWV from baseline to follow up. In addition, we hypothesized that these associations will be independent of demographic and other CVD risk factors.
Subjects, Materials and Methods
Study Design and Participants
SEARCH CVD was an ancillary study to the SEARCH for Diabetes in Youth study (Group, 2004). The longitudinal component of SEARCH CVD included a cohort of 298 youth with provider diagnosed T1D from Colorado and Ohio who had data on arterial stiffness measured approximately five years apart, in 2004–05 and 2009–11, starting when youth were on average 14.5 (SD =2.8) years old and had a mean duration of diabetes of 4.8 (SD=3.8) years. All participants had demographics, anthropometric and metabolic factors, including insulin sensitivity measured at baseline. Characteristics of SEARCH study participants with provider diagnosed T1D have been examined previously and are consistent with the clinical diagnosis including the presence of diabetes-related auto antibodies (GAD65 or IA2) (Dabelea et al., 2011). ICA512 and ZnT8 were not tested. All participants had demographics, anthropometric and metabolic factors, including insulin sensitivity measured at baseline. Participants with Maturity-Onset-Diabetes in the Young (MODY) were excluded.
Measurements
SEARCH CVD participants had a baseline research visit conducted after an 8 hour overnight fast. Medications, including short-acting insulin, were withheld the morning of the visit until after the blood draw was completed. Race/ethnicity was self-reported and the participants were categorized as non-Hispanic white (NHW) and other racial/ethnic group (including Hispanic, African-American and Asian/Pacific Islander racial/ethnic groups). Participants completed standardized questionnaires for medical history, medications, smoking status [never, former (no cigarettes in the last 30 days) or current smoker], daily insulin dose, and family history. BMI was calculated as weight (kg) divided by height in meters2, and age and sex-specific BMI z-scores were derived (Kuczmarski et al., 2002). Waist circumference was measured to the nearest 0.1 centimeter with the National Health and Nutrition Examination Survey (NHANES) protocol. Resting systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured three times while the subjects were seated for at least 5 minutes and the average of the 3 measurements was taken. Mean arterial blood pressure (MAP) was calculated as (2DBP+SBP)/3. Laboratory samples were obtained under conditions of metabolic stability, defined as no episode of diabetic ketoacidosis during the previous month. High-performance liquid chromatography (TOSOH Bioscience, Inc., San Francisco, CA) was used to measure HbA1c. Measurements of cholesterol, triglycerides (TG) and HDL-cholesterol (HDL-C) were obtained using Roche reagent on a Roche Modular P autoanalyzer (Roche Diagnostics, Indianapolis, IN). LDL-cholesterol (LDL-C) was calculated by the Friedewald equation for individuals with triglyceride concentration < 400 mg/dl and by the Beta Quantification procedure for those with triglycerides O(≥ 400 mg/dl. Albumin and creatinine were measured in first morning urine samples and were used to compute albumin-creatinine ratio (ACR). Urinary albumin was measured using Siemens reagent on a Siemens BNII nephelometer (Siemens Healthcare Diagnostics Inc., Newark, DE). Urinary creatinine was measured by the Jaffe method using Roche Diagnostics reagent on a Roche Modular P autoanalyzer. Elevated ACR was defined as ≥ 30 μg albumin/mg creatinine.
Insulin sensitivity was estimated using the following equation:
This equation was developed and validated using direct measurements of glucose disposal rate from euglycemic-hyperinsulinemic clamps conducted among 85 SEARCH participants with T1D and T2D and 22 matched nondiabetic control subjects (Dabelea et al., 2011). The study was reviewed and approved by the local institutional review boards in Ohio and Colorado and all participants provided signed informed consent or assent.
Vascular Outcome
Pulse Wave Velocity (PWV) was measured at baseline (2004/05) and at the follow up CVD visit (2009–11) using the SphygmoCor device (AtCor Medical, Itasca, IL) after 10 minutes of supine rest (Urbina et al., 2009). Distance from the proximal (carotid artery) to the distal (femoral artery) was measured to the nearest 0.1 cm twice and averaged. A tonometer was used to collect proximal and distal arterial waveforms gated by the R-wave on the simultaneously recorded ECG. The PWV was the difference in the carotid-to-femoral path length divided by the difference in R-wave–to–waveform foot times. Results were the average of 3 measures taken sequentially. A higher PWV (meters/second or m/s) indicates increased vascular stiffness. Repeated measures showed excellent reproducibility with coefficients of variability < 7% (Urbina et al., 2011).
Statistical analyses
Baseline insulin sensitivity, demographic and CVD risk factors were examined as predictors of PWV over time using linear mixed models (Proc Mixed, SAS 9.2). Linear mixed models adjust for the correlation between repeated observations in the same individual and assume that each participant in the dataset has his/her own trajectory over time. Since time was based on the disease duration between baseline and follow-up visits, this method allowed for a random intercept and slope in disease duration to account for within-individual dependence. The outcome was log-transformed time-varying PWV. The primary determinant of interest was the interaction term between baseline insulin sensitivity and follow up time to examine the impact of insulin sensitivity on the rate of change in PWV. We first ran a model adjusted for baseline age, sex and race/ethnicity, and baseline insulin sensitivity, while the final model was additionally adjusted for BMIz, blood pressure, lipids, microalbuminuria and smoking status. All included variables were normally distributed. Waist circumference, HbA1c and TG were excluded from the models because they are incorporated in the insulin sensitivity score. Studentized and conditional residuals were analyzed for model fit.
Results
Table 1 presents the characteristics of SEARCH CVD participants at baseline. The average age of the study participants at baseline was 14.5 years (SD 2.8), their diabetes duration was 4.8 years (SD 3.8) and the majority were NHW. BMIz at baseline indicates that the group, on average, was not overweight or obese. Approximately 9% had microalbuminuria, 3.4% were current smokers, 8.0% were former smokers and 88.6% were nonsmokers. 75% were positive for either IA-2 or GAD. The average LDL-C and HDL-C were 97mg/dl (SD 23) and 55 mg/dl (SD 13), respectively.
Table 1.
Baseline Characteristics of Study Participants (N=298)
| Variable | Mean (SD) or number (%) |
|---|---|
| Age (years) | 14.5 (2.8) |
| Diabetes Duration (years) | 4.8 (3.8) |
| Sex: Females | 138 (46.3%) |
| Race/Ethnicity: Non-Hispanic White | 261 (87.6%) |
| Body mass index z-score | 0.53 (0.92) |
| Waist circumference (cm) | 75 (12) |
| LDL cholesterol (mg/dl) | 97 (23) |
| HDL cholesterol (mg/dl) | 55 (13) |
| Triglycerides (mg/dl) | 70 (38) |
| Systolic blood pressure (mmHg) | 116 (10) |
| Diastolic blood pressure (mmHg) | 67 (7) |
| Insulin resistance* | 105 (35.2%) |
| Albumin to creatinine ratio ≥ 30μg/mg | 23(9.3%) |
| HbA1c (%) | 8.2 (1.4) |
| Current Smokers | 223 (88.6) |
| Former Smokers | 21 (8.0) |
| Never Smokers | 9 (3.4) |
Insulin resistance defined as an insulin sensitivity score <8.15 (Dabelea et al., 2011)
Over 5 years PWV increased from 5.2 m/s at baseline to 5.9 m/s at follow up (p<0.0001) indicating increasing stiffness. After controlling for age, sex and race/ethnicity, an increase in insulin sensitivity from the first quartile (insulin sensitivity score ≤7.3) to the fourth quartile (insulin sensitivity score ≥11.0) was associated with a 0.051 m/s decrease (−0.072, −0.030) in log PWV.
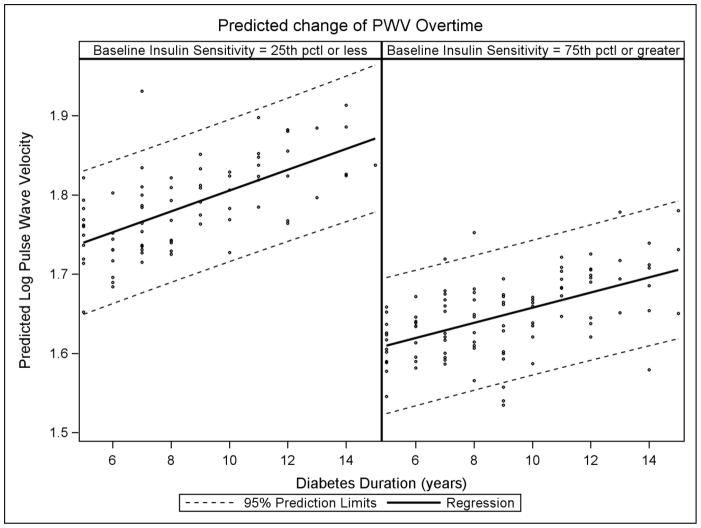
Table 2 presents the association between baseline variables of interest and PWV over time. The model included the following covariates: age, sex, race/ethnicity, BMIz, MAP, LDL-C, HDL-C and the presence of microalbuminuria. Associations between continuous variables and PWV are expressed as percent increase change in PWV over time per one unit change in baseline covariate levels. Significant predictors of increased change in PWV were older baseline age [β=0.007 (p=0.03) increase in log PWV per one year increase age], higher baseline MAP [β=0.005 (p<0.0001) increase in log PWV per 1 mmHg increase in MAP] and current smoking status (compared to non-smokers) at baseline. These relationships were linear across the range of data. There was a statistically significant interaction (p=0.033) between baseline insulin sensitivity and time (diabetes duration) on rate of change in PWV, independent of demographics, BMIz, blood pressure, lipids and microalbuminuria. This suggests that the rate of change of PWV overtime is dependent on baseline insulin sensitivity. Specifically, higher insulin sensitivity at baseline is associated with a lower rate of change in PWV over time independent of the model covariates. Figure 1 illustrates this interaction and shows that for levels of baseline insulin sensitivity at the first quartile (insulin sensitivity score ≤7.3) vs. the fourth quartile (≥ 11.0 insulin sensitivity) of observed data. For example, for a participant within the first quartile, or lowest levels of insulin sensitivity at baseline, the model predicted an increase of 0.08 m/s in log PWV over five years(95% CI: 0.06–0.09). However, for a participant within the fourth quartile, or highest levels of insulin sensitivity at baseline, the model predicted only a 0.05 m/s increase in log PWV over five years (95% CI: 0.03–0.06).
Table 2.
Independent associations between baseline demographic and cardiovascular risk factors and rate of change pulse wave velocity
| Log Time-varying PVW | |||
|---|---|---|---|
| Beta | 95%CI | p-value* | |
| Age at visit (per year) | 0.007 | 0.001, 0.012 | 0.03 |
| Sex (female vs male) | −0.014 | −0.040, 0.011 | 0.27 |
| Race/Ethnicity (NHW vs other) | 0.015 | −0.024, 0.053 | 0.466 |
| BMI z score (per 1 unit) | −0.009 | −0.025, 0.006 | 0.2431 |
| Mean arterial pressure (per mmHg) | 0.005 | 0.003, 0.007 | <0.0001 |
| LDL cholesterol (per mg/dl) | 0.000043 | −0.001, 0.001 | 0.88 |
| HDL cholesterol (per mg/dl) | −0.00011 | −0.001, 0.001 | 0.84 |
| Microalbuminuria (yes vs. no) | −0.011 | −0.057, 0.035 | 0.64 |
| Current smoker (vs. never smoker) | 0.04443 | 0.013, 0.075 | 0.005 |
| Former smoker (vs. never smoker) | 0.01764 | −0.012, 0.049 | 0.2358 |
| Insulin sensitivity score | −0.00617 | −0.014, −0.0013 | 0.11 |
| Diabetes duration (year) | 0.01476 | 0.012, 0.0176 | <.0001 |
| Insulin sensitivity score × Diabetes Duration | −0.001 | −0.002, −0.00001 | 0.0326 |
Outcome pulse wave velocity was log transformed.
p-value from mixed linear model, with age, body mass index, mean arterial pressure, LDL-C, HDL-C and baseline insulin sensitivity included as continuous variables, and race/ethnicity, sex, elevated albumin to creatinine ratio and smoking as categorical variables. Duration was included as a measure of time between visits. Data represent a percent change in PWV over time per one unit change in baseline covariate levels.
Figure 1.
Predicted log PWV over time by baseline quartile of insulin sensitivity, where time is based on diabetes duration. Data is adjusted for age, sex, race, body mass index z score, mean arterial pressure, LDL-C, HDL-C and albumin to creatinine ratio.
Discussion
This results of our study indicate that baseline insulin sensitivity, assessed using a validated surrogate marker based on routine clinical measures, influences the progression in PWV over an average follow up period of 5 years among youth with relatively short duration of T1D, such that the lower the baseline insulin sensitivity (the more insulin resistant), the greater the increase in PWV over time. This association was independent of demographics, BMIz, lipid and blood pressure levels and presence of microalbuminuria. In addition, older age, higher mean blood pressure levels and smoking at baseline were also associated with higher rate of change in PWV.
There are a number of cross sectional studies of arterial stiffness in youth with T1D (Haller et al., 2004, Heilman et al., 2009, Palombo et al., 2011, Urbina et al., 2010, Yu et al., 2012) but few prospective studies (Dabelea et al., 2013). Two prospective studies in non-diabetic adults found that the presence of metabolic syndrome (a cluster of CVD risk factors) at baseline was associated with faster progression of arterial stiffness over time (Safar et al., 2006, Tomiyama et al., 2006). Prince et al. examined risk markers of arterial stiffness among adults with youth onset T1D and found that prior presence of cardiovascular autonomic neuropathy, lower HDL-C, higher HbA1c and smoking history predicted worse measures of arterial stiffness 18 years later (Prince et al., 2010). We previously reported in SEARCH CVD that presence, clustering and worsening of CVD risk factors were associated with increased arterial stiffness (PWV) over time in youth with T1D (Dabelea et al., 2013). Our current results support and extend our previous observations. Individual CVD risk factors were significantly associated with higher baseline and follow up PWV, but not with the rate of change in PWV over time (Dabelea et al., 2013). Our current findings now document that insulin sensitivity as assessed by a surrogate marker based on routine clinical measurements (waist circumference, TG and HbA1c levels) significantly influences the rate of change (or the progression) of arterial stiffness in youth with type 1 diabetes.
Very few studies have examined insulin sensitivity as a potential risk factor for vascular complications in patients with T1D. Using a different surrogate measure of insulin sensitivity validated against hyperinsulinemic-euglycemic clamps in adults with T1D (Williams et al., 2000), Chillaron et al. found an association between decreased insulin sensitivity and presence of microvascular complications (neuropathy, nephropathy and retinopathy) (Chillaron et al., 2009). The Diabetes Control and Complications Trial (DCCT) also reported an association between decreased insulin sensitivity at baseline and the development of both microvascular and macrovascular complications among adults with T1D (Kilpatrick et al., 2007). More recently, the Coronary Artery Calcification in Type 1 Diabetes study (CACTI) showed that directly measured insulin sensitivity was associated with coronary artery calcification,(Schauer et al., 2011) a surrogate marker of coronary artery disease predictive of future CVD outcomes. Expanding on the studies in adults, our study in now in youth with T1D shows that decreased insulin sensitivity is associated with increased progression of PWV over a follow up period of approximately 5 years. This is the first study to document the influence of insulin sensitivity, estimated using a validated pediatric-specific equation, at a relatively early stage in the pathway leading to CVD in T1D. Because T1D is associated with increased and earlier CVD morbidity and mortality (Snell-Bergeon and Nadeau, 2012) and since hyperglycemia, lipids, blood pressure do not completely explain this risk differential (Balagopal et al., 2011, Mannucci et al., 2013), our data provide potentially novel insights into the pathogenesis of early CVD in T1D.
It has been well documented that insulin sensitivity is decreased in adults with T1D (DeFronzo et al., 1982). Recent hyperinsulinemic-euglycemic clamp studies have shown that significant insulin resistance also exists in adolescents with T1D compared with age-, sex-, pubertal stage-, BMI and activity-matched controls (Nadeau et al., 2010). Using the same insulin sensitivity index used here, one study showed that nearly 33% of youth with T1D had insulin resistance (Specht et al., 2013). The mechanisms responsible for the association between insulin resistance and increased arterial stiffness in T1D are incompletely understood. However, endothelial dysfunction, low grade inflammation and increased oxidative stress, common in obesity and insulin resistant states, have also been implicated in arterial stiffness (Ferreira et al., 2007) as have elevated levels of acute glycation end products (Aronson, 2003), and may represent additional pathways leading to CVD in patients with T1D and insulin resistance.
This study has some limitations. First, the lack of a non-diabetic control group limits the ability to determine if the documented influence of insulin sensitivity on the rate of change in PWV is generalizable to healthy populations. Second, we did not directly measure insulin sensitivity via a hyperinsulinemic-euglycemic clamp in this study, but used instead an estimate of insulin sensitivity validated against the gold-standard. Estimation of insulin sensitivity among patients with insulin-treated diabetes is problematic, as fasting levels of glucose and insulin reflect insulin treatment rather than underlying insulin and glucose metabolism. Moreover, surrogate estimates of insulin sensitivity, such as HOMA-insulin resistance (HOMA-IR) or the quantitative insulin sensitivity check index (QUICKI), cannot be used in insulin-treated patients and may be inaccurate in youths with T1D, as they assume preserved insulin and C-peptide secretion and normal glucose levels. Using a surrogate marker of insulin sensitivity may introduce error; however it is unlikely that such measurement error would be differential with respect to the outcome of interest and therefore, at most, this could have biased our results toward the null. Moreover, our estimated measure of insulin sensitivity was quite robust and explained 74% of the variance in glucose disposal rate (Dabelea et al., 2011). We did not collected puberty data but included age in the models to serve as a surrogate. Finally, we only had two time points for assessment of arterial stiffness and a limited follow up period (approximately 5 years), thus limiting our ability to determine the full temporal sequence of events and assess long term effects. However, we had a well characterized group of youth with T1D early in the course of their diabetes, allowing us to determine predictors of arterial stiffness at a young age and stage of disease before other micro and macrovascular complications have occurred.
In conclusion, we found that lower insulin sensitivity (increased insulin resistance) is associated with increased progression of arterial stiffness in youth with T1D, independent of other demographic and cardiovascular risk factors. Increased blood pressure levels are also associated with higher levels of arterial stiffness over time. Our findings have important implications by highlighting insulin resistance as a potentially modifiable therapeutic target at an early stage in the natural evolution of cardiovascular disease in patients with T1D.
Acknowledgments
The SEARCH CVD Study is indebted to the many youth and their families, and their health care providers, whose participation made this study possible. We also would like to thank all of the SEARCH for Diabetes in Youth investigators and study staff whose help was essential in moving this project forward.
Sources of Funding: SEARCH CVD was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK, R01DK078542, PI Dabelea).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM. All-cause mortality rates in patients with type 1 diabetes mellitus compared with a non-diabetic population from the UK general practice research database, 1992–1999. Diabetologia. 2006;49:660–666. doi: 10.1007/s00125-005-0120-4. [DOI] [PubMed] [Google Scholar]
- 2.Urbina EM, Wadwa RP, Davis C, Snively BM, Dolan LM, Daniels SR, Hamman RF, Dabelea D. Prevalence of increased arterial stiffness in children with type 1 diabetes mellitus differs by measurement site and sex: the SEARCH for Diabetes in Youth Study. J Pediatr. 2010;156:731–737. 737 e731. doi: 10.1016/j.jpeds.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Rask-Madsen C, King GL. Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metab. 2013;17:20–33. doi: 10.1016/j.cmet.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 5.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 6.Nadeau KJ, Reusch JE. Cardiovascular function/dysfunction in adolescents with type 1 diabetes. Curr Diab Rep. 2011;11:185–192. doi: 10.1007/s11892-011-0180-4. [DOI] [PubMed] [Google Scholar]
- 7.Nadeau KJ, Regensteiner JG, Bauer TA, Brown MS, Dorosz JL, Hull A, Zeitler P, Draznin B, Reusch JE. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab. 2010;95:513–521. doi: 10.1210/jc.2009-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snell-Bergeon JK, Nadeau K. Cardiovascular disease risk in young people with type 1 diabetes. J Cardiovasc Transl Res. 2012;5:446–462. doi: 10.1007/s12265-012-9363-x. [DOI] [PubMed] [Google Scholar]
- 9.Urbina EM, Gao Z, Khoury PR, Martin LJ, Dolan LM. Insulin resistance and arterial stiffness in healthy adolescents and young adults. Diabetologia. 2012;55:625–631. doi: 10.1007/s00125-011-2412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dabelea D, D’Agostino RB, Jr, Mason CC, West N, Hamman RF, Mayer-Davis EJ, Maahs D, Klingensmith G, Knowler WC, Nadeau K. Development, validation and use of an insulin sensitivity score in youths with diabetes: the SEARCH for Diabetes in Youth study. Diabetologia. 2011;54:78–86. doi: 10.1007/s00125-010-1911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.SS Group. SEARCH for Diabetes in Youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials. 2004;25:458–471. doi: 10.1016/j.cct.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Dabelea D, Pihoker C, Talton JW, D’Agostino RB, Jr, Fujimoto W, Klingensmith GJ, Lawrence JM, Linder B, Marcovina SM, Mayer-Davis EJ, Imperatore G, Dolan LM. Etiological approach to characterization of diabetes type: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2011;34:1628–1633. doi: 10.2337/dc10-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11:1–190. [PubMed] [Google Scholar]
- 14.Urbina EM, Williams RV, Alpert BS, Collins RT, Daniels SR, Hayman L, Jacobson M, Mahoney L, Mietus-Snyder M, Rocchini A, Steinberger J, McCrindle B American Heart Association Atherosclerosis, H, Obesity in Youth Committee of the Council on Cardiovascular Disease in the Y. Noninvasive assessment of subclinical atherosclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension. 2009;54:919–950. doi: 10.1161/HYPERTENSIONAHA.109.192639. [DOI] [PubMed] [Google Scholar]
- 15.Urbina EM, Khoury PR, McCoy C, Daniels SR, Kimball TR, Dolan LM. Cardiac and vascular consequences of pre-hypertension in youth. J Clin Hypertens (Greenwich) 2011;13:332–342. doi: 10.1111/j.1751-7176.2011.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haller MJ, Samyn M, Nichols WW, Brusko T, Wasserfall C, Schwartz RF, Atkinson M, Shuster JJ, Pierce GL, Silverstein JH. Radial artery tonometry demonstrates arterial stiffness in children with type 1 diabetes. Diabetes Care. 2004;27:2911–2917. doi: 10.2337/diacare.27.12.2911. [DOI] [PubMed] [Google Scholar]
- 17.Heilman K, Zilmer M, Zilmer K, Lintrop M, Kampus P, Kals J, Tillmann V. Arterial stiffness, carotid artery intima-media thickness and plasma myeloperoxidase level in children with type 1 diabetes. Diabetes Res Clin Pract. 2009;84:168–173. doi: 10.1016/j.diabres.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Palombo C, Kozakova M, Morizzo C, Gnesi L, Barsotti MC, Spontoni P, Massart F, Salvi P, Balbarini A, Saggese G, Di Stefano R, Federico G. Circulating endothelial progenitor cells and large artery structure and function in young subjects with uncomplicated type 1 diabetes. Cardiovasc Diabetol. 2011;10:88. doi: 10.1186/1475-2840-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu MC, Lo FS, Yu MK, Huang WH, Lee F. Arterial stiffness is not increased in teens with early uncomplicated type 1 diabetes mellitus. Eur J Pediatr. 2012;171:855–858. doi: 10.1007/s00431-012-1679-7. [DOI] [PubMed] [Google Scholar]
- 20.Dabelea D, Talton JW, D’Agostino R, Jr, Wadwa RP, Urbina EM, Dolan LM, Daniels SR, Marcovina SM, Hamman RF. Cardiovascular risk factors are associated with increased arterial stiffness in youth with type 1 diabetes: the SEARCH CVD study. Diabetes Care. 2013;36:3938–3943. doi: 10.2337/dc13-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Safar ME, Thomas F, Blacher J, Nzietchueng R, Bureau JM, Pannier B, Benetos A. Metabolic syndrome and age-related progression of aortic stiffness. J Am Coll Cardiol. 2006;47:72–75. doi: 10.1016/j.jacc.2005.08.052. [DOI] [PubMed] [Google Scholar]
- 22.Tomiyama H, Hirayama Y, Hashimoto H, Yambe M, Yamada J, Koji Y, Motobe K, Shiina K, Yamamoto Y, Yamashinai A. The effects of changes in the metabolic syndrome detection status on arterial stiffening: a prospective study. Hypertens Res. 2006;29:673–678. doi: 10.1291/hypres.29.673. [DOI] [PubMed] [Google Scholar]
- 23.Prince CT, Secrest AM, Mackey RH, Arena VC, Kingsley LA, Orchard TJ. Cardiovascular autonomic neuropathy, HDL cholesterol, and smoking correlate with arterial stiffness markers determined 18 years later in type 1 diabetes. Diabetes Care. 2010;33:652–657. doi: 10.2337/dc09-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams KV, Erbey JR, Becker D, Arslanian S, Orchard TJ. Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes. 2000;49:626–632. doi: 10.2337/diabetes.49.4.626. [DOI] [PubMed] [Google Scholar]
- 25.Chillaron JJ, Goday A, Flores-Le-Roux JA, Benaiges D, Carrera MJ, Puig J, Cano-Perez JF, Pedro-Botet J. Estimated glucose disposal rate in assessment of the metabolic syndrome and microvascular complications in patients with type 1 diabetes. J Clin Endocrinol Metab. 2009;94:3530–3534. doi: 10.1210/jc.2009-0960. [DOI] [PubMed] [Google Scholar]
- 26.Kilpatrick ES, Rigby AS, Atkin SL. Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes: “double diabetes” in the Diabetes Control and Complications Trial. Diabetes Care. 2007;30:707–712. doi: 10.2337/dc06-1982. [DOI] [PubMed] [Google Scholar]
- 27.Schauer IE, Snell-Bergeon JK, Bergman BC, Maahs DM, Kretowski A, Eckel RH, Rewers M. Insulin resistance, defective insulin-mediated fatty acid suppression, and coronary artery calcification in subjects with and without type 1 diabetes: The CACTI study. Diabetes. 2011;60:306–314. doi: 10.2337/db10-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balagopal PB, de Ferranti SD, Cook S, Daniels SR, Gidding SS, Hayman LL, McCrindle BW, Mietus-Snyder ML, Steinberger J. Nontraditional risk factors and biomarkers for cardiovascular disease: mechanistic, research, and clinical considerations for youth: a scientific statement from the American Heart Association. Circulation. 2011;123:2749–2769. doi: 10.1161/CIR.0b013e31821c7c64. [DOI] [PubMed] [Google Scholar]
- 29.Mannucci E, Dicembrini I, Lauria A, Pozzilli P. Is glucose control important for prevention of cardiovascular disease in diabetes? Diabetes Care. 2013;36(Suppl 2):S259–263. doi: 10.2337/dcS13-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeFronzo RA, Hendler R, Simonson D. Insulin resistance is a prominent feature of insulin-dependent diabetes. Diabetes. 1982;31:795–801. doi: 10.2337/diab.31.9.795. [DOI] [PubMed] [Google Scholar]
- 31.Specht BJ, Wadwa RP, Snell-Bergeon JK, Nadeau KJ, Bishop FK, Maahs DM. Estimated insulin sensitivity and cardiovascular disease risk factors in adolescents with and without type 1 diabetes. J Pediatr. 2013;162:297–301. doi: 10.1016/j.jpeds.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferreira I, Boreham CA, Twisk JWR, Gallagher AM, Young IS, Murray LJ, Stehouwer CDA. Clustering of metabolic syndrome risk factors and arterial stiffness in young adults: the Northern Ireland Young Hearts Project. Journal of Hypertension. 2007;25:1009–1020. doi: 10.1097/HJH.0b013e3280a94e76. [DOI] [PubMed] [Google Scholar]
- 33.Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. Journal of Hypertension. 2003;21:3–12. doi: 10.1097/00004872-200301000-00002. [DOI] [PubMed] [Google Scholar]