Abstract
This study sought to investigate the relationship between myocardial perfusion and N-terminal pro-brain natriuretic peptide (NT-proBNP) in asymptomatic individuals without overt coronary artery disease. NT-proBNP is a cardiac neurohormone secreted from the ventricles in response to ventricular volume expansion and pressure overload, and may also be elevated in the setting of reduced myocardial perfusion. We hypothesized that reduced myocardial perfusion reserve (MPR) would be associated with elevated NT-proBNP in individuals free of overt cardiovascular disease. MPR was measured by cardiac magnetic resonance, before and after adenosine infusion, in 184 MESA participants (mean age 60 ± 10.4, 58% white, 42% Hispanic, 44% female) without overt cardiovascular disease. MPR was modeled as hyperemic myocardial blood flow (MBF) adjusted for resting MBF. A linear regression analysis, adjusted for demographics, established cardiovascular risk factors, left ventricular mass, coronary calcium score, body mass index and medications, was used to determine the association between MPR and NT-proBNP. Individuals with low hyperemic MBF were more likely to be older, male, diabetic, have higher blood pressure and higher coronary artery calcium score. Mean hyperemic MBF was 3.04 ± 0.829 ml/min/g. MPR was inversely associated with NT-proBNP levels. In a fully adjusted model, every one standard deviation decrement in MPR was associated with a 21 % increment in NT-proBNP (p=0.04). In conclusion, MPR is inversely associated with NT-proBNP level in this cross sectional study of asymptomatic individuals free of overt coronary artery disease, suggesting that higher NT-proBNP levels may reflect subclinical myocardial microvascular dysfunction.
Keywords: myocardial perfusion, myocardial blood flow, NT-pro-BNP
Introduction
To our knowledge the relationship between N-terminal pro-brain natriuretic peptide (NT-proBNP) and myocardial perfusion reserve (MPR) among asymptomatic individuals without overt cardiovascular disease has not been described. These findings will be useful in understanding the effect of impaired microvascular structure on neurohormonal levels and may help explain the prognostic implications of elevated BNP in asymptomatic individuals. We hypothesized that MPR would be inversely related to serum NT-proBNP level among participants of the Multi-Ethnic Study of Atherosclerosis (MESA).
Methods
MESA is a prospective, multicenter cohort study aimed to determine characteristics related to progression of subclinical to clinical cardiovascular disease. Information regarding the design and scope of the study has been published previously1. At baseline, MESA participants were 45–84 years old and had no prior history or coronary artery disease. In a MESA ancillary study, all Minnesota participants (N=1,066) were contacted immediately following the baseline clinic exam or later by mail in a recruitment effort to obtain MR perfusion studies. A total of 234 participants consented to and underwent CMR perfusion studies at the Minnesota field center.
Participants were excluded if they had any of the following: prevalent cardiovascular disease at baseline (n=1), missing major perfusion measurements (resting or hyperemic myocardial blood flow, or perfusion ratio, n= 5), caffeine intake within 12 hours before the CMR scanning (n=7), missing NT-proBNP measurements (n=20), or missing any other covariate information (n=17). After exclusions, 184 participants were included in the final analysis.
CMR studies were performed on a 1.5T clinical MRI scanner (Sonata 1.5T, Siemens Medical Systems, Malvern, PA). The protocol used has been described in previous publications2. Briefly, IV access was obtained and the patient was positioned supine. Vital signs and an electrocardiogram were monitored throughout the examination. Scout MR images were acquired to determine the orientation of the short and long axis of the left ventricle. T1 weighted imaging with a fast gradient echo sequence was used to track the first pass on an injected contrast agent through the right and left ventricle. The first perfusion scan was performed during rest, and the second was performed approximately 15 minutes later during maximal vasodilation induced by 0.14 mg/kg/min of IV adenosine injected over three minutes before the scan.
Myocardial blood flow (MBF) was estimated before and after adenosine infusion in eight segments: anterior, anterolateral, posterolateral, posteroinferior, inferior, and posterior/mid and anterior septum. Region-of-interest signal intensity (SI) curves were generated with the MASS CMR image analysis software (Laboratory for Clinical and Experimental Image Processing, Leiden University, Leiden, the Netherlands). MBF was estimated from the initial amplitude of the myocardial impulse response by deconvolution analysis of the myocardial SI curves. The global averages of resting and hyperemic MBF over the eight myocardial segments and two to three slices were used for analysis. MPR was modeled as hyperemic MBF with adjustment for resting MBF3.
As part of the study protocol, all patients had serum samples drawn at enrollment and stored at −70°C. NT-proBNP levels were measured using a highly sensitive and specific Elecsys electrochemiluminescence immunoassay (Roche Diagnostics Corporation, Indianapolis, IN).
Demographic characteristics and established cardiovascular risk factors were examined across hyperemic MBF tertiles. Continuous variables are presented as a mean ± standard deviation and categorical variables as percentages. Logarithmic transformation was applied to variables with skewed distributions. To explore the shape of the association between NT-proBNP and MPR (hyperemic MBF with adjustment for resting MBF), we modeled MPR as a restricted cubic spline in a multivariable linear model adjusted for age, race, and sex. Linear regression was used to assess the association of MPR and NT-proBNP with adjustment for covariates listed below. The regression analyses was carried out in stages as follows: Model I is unadjusted. Model II is adjusted for age, gender, and race. Model III is adjusted for age, gender, race, body mass index, coronary artery calcium (CAC) score, education level, fasting glucose, systolic blood pressure and diastolic blood pressure, heart rate, HDL-C, LDL-C, medications (antihypertensive, statins, and diabetic medications), physical activity, smoking status, and left ventricular mass.
Similarly, the association between regional MPR among each of the eight myocardial segments and NT-proBNP was examined using linear regression with adjustment for covariates according to Model I, II, and III.
Statistical analyses were performed using SAS v9.2 (SAS Inc., Cary, NC). Statistical significance was inferred at 2-sided p-value<0.05.
Results
Overall, 184 study participants had both serum NT-proBNP measurements and cardiac MR quantification of MPR. Table 1 shows the baseline characteristics of this cohort stratified by hyperemic MBF. Patients in the lowest tertile of hyperemic MBF were older and predominantly male. They also had higher BMI, CAC score, fasting glucose, systolic and diastolic blood pressure, and were more likely to be on antihypertensive, diabetic, or statin medications (Table 1).
Table 1.
Baseline characteristics by hyperemic myocardial blood flow (MBF) tertile, Multi-Ethnic Study of Atherosclerosis, 2000 to 2002.
| Variable | Hyperemic MBF (mL/min/g)
|
P-Value | ||
|---|---|---|---|---|
| 0.98–2.64 (n=61) | 2.65–3.40 (n=62) | 3.41–5.63 (n=61) | ||
| Age, years | 65 ± 9 | 59 ± 11 | 57 ± 10 | 0.0002 |
| Women | 16 % | 45 % | 70 % | <0.0001 |
| White | 56 % | 63 % | 54 % | 0.58 |
| Hispanic | 44 % | 37 % | 46 % | 0.58 |
| Body Mass Index (kg/m2) | 30 ± 5 | 28 ± 4 | 29 ± 5 | 0.03 |
| CAC Score >0 | 80 % | 50 % | 39 % | <0.0001 |
| CAC Score* | 53 ± 383 | 7 ± 80 | 4 ± 19 | <0.0001 |
| Current Smoker | 8 % | 16 % | 20 % | 0.06 |
| Fasting Glucose (mg/dL) | 101 ± 24 | 91 ± 25 | 92 ± 24 | 0.05 |
| Heart Rate (beats/min) | 63 ± 10 | 60 ± 10 | 64 ± 8 | 0.05 |
| High School Graduate | 66 % | 60 % | 59 % | 0.81 |
| HDL Cholesterol (mg/dL) | 47 ± 15 | 50 ± 14 | 49 ± 13 | 0.37 |
| LDL Cholesterol (mg/dL) | 122 ± 26 | 116 ± 32 | 111 ± 24 | 0.12 |
| Systolic Blood Pressure (mmHg) | 125 ± 16 | 118 ± 19 | 120 ± 21 | 0.08 |
| Diastolic Blood Pressure (mmHg) | 74 ± 9 | 69 ± 10 | 69 ± 10 | 0.01 |
| Antihypertensive Medication | 33 % | 13 % | 18 % | 0.02 |
| Diabetes Medication | 15 % | 2 % | 8 % | 0.03 |
| Statins | 21 % | 5 % | 11 % | 0.02 |
| Physical Activity (MET-min/week) | 9290 ± 6440 | 6641 ± 4791 | 7081 ± 5967 | 0.03 |
| Left Ventricular Mass (g) | 183 ± 41 | 158 ± 42 | 145 ± 35 | <0.0001 |
| Left Ventricular Ejection Fraction | 64 ± 8 | 66 ± 4 | 69 ± 6 | 0.003 |
| Diabetes | 18 % | 6 % | 10 % | 0.12 |
| Calcium Channel Blocker | 10 % | 0 % | 7 % | 0.05 |
| CAC >300 | 33 % | 10 % | 8 % | 0.0002 |
| Resting Mean MBF (mL/min/g) | 0.9 ± 0.2 | 1.0 ± 0.2 | 1.1 0.2 | <0.0001 |
| Hyperemic Mean MBF (mL/min/g) | 2.1 ± 0.4 | 3.0 ± 0.2 | 4.0 ± 0.5 | <0.0001 |
| Myocardial Perfusion Reserve | 2.4 ± 0.7 | 3.2 ± 0.6 | 3.6 ± 0.7 | <0.0001 |
Values are arithmetic mean ± SD when appropriate, except *=Geometric mean (Interquartile Range).
CAC=Coronary Artery Calcium
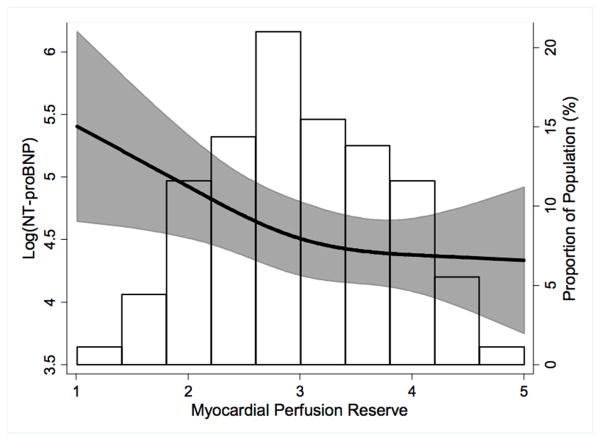
The mean and standard deviation of hyperemic MBF was 3.04 ± 0.829 ml/min/g. The median NT-proBNP was 51.6 pg/μl (IQR 26.6 – 121.2, range 4.9 – 1085). The association of MPR, modeled as a restricted cubic spline, with log(NT-proBNP) is presented in figure 1, which illustrates the predicted values and 95% CIs for a 59 year old, white woman. MPR was inversely associated with log(NT-proBNP), and the association was roughly linear. Results of the multivariable linear regression analysis are summarized in Table 2. Table 3 displays results of the multivariable linear regression analysis examining the relationship between regional MPR and NT-proBNP. NT-proBNP level was significantly associated with reduced regional MPR in the inferoseptal segment (Model I) and the inferior and inferoseptal segments (Model II and III).
Figure 1.
Independent association of plasma log (NT-proBNP) and myocardial perfusion reserve. The black line and shaded area represent predicted values and 95% CIs of myocardial perfusion reserve (hyperemic myocardial blood flow with adjustment for resting myocardial blood flow) for a 59 year old, white woman, modeling myocardial perfusion reserve with a restricted cubic spline in a multivariable linear model adjusted for age, race, and sex. The histogram represents the frequency distribution of hyperemic myocardial blood flow in the study sample.
Table 2.
Independent association of plasma log N-terminal pro-brain natriuretic peptide (NT-proBNP) level and myocardial perfusion reserve (MPR).
| Model | n | NT-proBNP
|
||
|---|---|---|---|---|
| β (95% CI)* | % Difference (95% CI)** | p-value | ||
| I | 184 | 0.18 (−0.01, 0.36) | 19 (−1, 44) | 0.06 |
| II | 184 | 0.21 (0.03, 0.39) | 23 (3, 48) | 0.02 |
| III | 184 | 0.19 (0.01, 0.37) | 21 (1, 45) | 0.04 |
|
| ||||
| Model I: Unadjusted | ||||
| Model II: Adjusted for age, gender, and race | ||||
| Model III: Model II + BMI, education level, fasting glucose, systolic and diastolic blood pressure, heart rate, HDL-C, LDL-C, medications (antihypertensive, statins, and diabetic medications), physical activity, smoking status, coronary artery calcium score, and left ventricular mass. | ||||
Increment in log(NT-proBNP) in pg/mL per 1-standard deviation (SD) decrement in MPR (1-SD=0.829 mL/min/g).
Percent increment in NT-proBNP per 1-SD decrement in MPR.
CI=Confidence Interval
Table 3.
Independent association of plasma log(NT-proBNP) level and myocardial perfusion reserve (MPR) by regional segment.
| n | Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β (95% CI)* | % Difference (95% CI)** | P-value | β (95% CI)* | % Difference (95% CI)** | P-value | β (95% CI)* | % Difference (95% CI)** | P-value | ||
| Anterior Wall | 180 | 0.10 (−0.09, 0.29) | 10 (−9, 33) | 0.30 | 0.15 (−0.02, 0.32) | 16 (−2, 38) | 0.09 | 0.13 (−0.04, 0.31) | 14 (−4, 36) | 0.13 |
| Anterior-Lateral Wall | 184 | 0.11 (−0.07, 0.29) | 11 (−7, 33) | 0.24 | 0.13 (−0.04, 0.30) | 14 (−4, 34) | 0.13 | 0.10 (−0.07, 0.28) | 11 (−7, 32) | 0.24 |
| Lateral-Posterior Wall | 182 | 0.12 (−0.08, 0.31) | 12 (−7, 36) | 0.23 | 0.17 (−0.004,0.34) | 18 (0, 40) | 0.06 | 0.14 (−0.04, 0.31) | 15 (−4, 37) | 0.12 |
| Posterior Wall | 183 | 0.06 (−0.13, 0.24) | 6 (−12, 28) | 0.55 | 0.12 (−0.06, 0.30) | 13 (−6, 36) | 0.20 | 0.10 (−0.08, 0.29) | 11 (−8, 33) | 0.26 |
| Inferior Wall | 181 | 0.14 (−0.05, 0.33) | 15 (−4, 40) | 0.13 | 0.22 (0.04, 0.40) | 24 (4, 49) | 0.02 | 0.21 (0.02, 0.39) | 23 (2, 47) | 0.03 |
| Inferior-Septal Wall | 178 | 0.27 (0.08, 0.45) | 31 (9, 57) | 0.004 | 0.27 (0.11, 0.43) | 31 (12, 54) | 0.001 | 0.24 (0.08, 0.41) | 28 (9, 50) | 0.003 |
| Mid-Septal Wall | 170 | 0.19 (0.004, 0.37) | 20 (0, 45) | 0.05 | 0.21 (0.04, 0.38) | 23 (4, 46) | 0.02 | 0.21 (0.05, 0.38) | 24 (5, 46) | 0.01 |
| Anterior- Septal Wall | 169 | 0.15 (−0.04, 0.34) | 17 (−3,41) | 0.11 | 0.13 (−0.04, 0.30) | 14 (−4, 35) | 0.13 | 0.11 (−0.06, 0.28) | 12 (−6, 32) | 0.21 |
|
| ||||||||||
| Global | 184 | 0.18 (−0.01, 0.36) | 19 (−1, 44) | 0.06 | 0.21 (0.03, 0.39) | 23 (3, 48) | 0.02 | 0.19 (0.01, 0.37) | 21 (1, 45) | 0.04 |
|
| ||||||||||
| Model I: Unadjusted | ||||||||||
| Model II: Adjusted for age, gender, and race | ||||||||||
| Model III: Model II + BMI, education level, fasting glucose, systolic and diastolic blood pressure, heart rate, HDL-C, LDL-C, medications (antihypertensive, statins, and diabetic medications), physical activity, smoking status, coronary artery calcium score, and left ventricular mass. | ||||||||||
Increment in log(NT-proBNP) in pg/mL per 1-standard deviation (SD) decrement in MPR (1-SD=0.829 mL/min/g).
Percent increment in NT-proBNP per 1-SD decrement in MPR.
CI=Confidence Interval
Discussion
In this cross sectional study of asymptomatic individuals without overt coronary artery disease, MPR was inversely associated with serum NT-proBNP level. This inverse association remained significant even after adjustment for demographics, traditional CVD risk factors, CAC, and left ventricular mass. These data suggest that elevated levels of NT-proBNP may reflect subclinical myocardial microvascular dysfunction.
BNP is primarily used as a diagnostic test for clinical heart failure, but elevated BNP is also a prognostic indicator for future cardiovascular events or death, even at levels well below current threshold used in the diagnosis of heart failure 4–7. While the underlying mechanism responsible for this association has been unclear, our results suggest that elevated NT-proBNP could be a link between microvascular dysfunction and cardiovascular risk.
MPR is a reflection of the vasodilator capacity of the coronary microcirculation, and impaired perfusion reserve implies microvascular dysfunction due to endothelial dysfunction and/or impaired smooth muscle relaxation8. Several studies have demonstrated the relationship between impaired perfusion reserve and microvascular dysfunction 9–11, and impaired MPR is associated with an increased risk of cardiovascular events 12–16.
The mechanism by which microvascular dysfunction may lead to higher levels of NT-proBNP is unclear. It could be that reduced MPR and elevated NT-proBNP identify individuals with underlying diastolic dysfunction. Alternatively, it is possible that individuals with microvascular dysfunction live in a state of chronic, relative hypoxia compared to those with normal microvascular function. In fact, others have found that myocardial ischemia alone can trigger excess production of NT-proBNP 17, 18. One small study showed that patients with impaired coronary blood flow had increased levels of NT-proBNP before and after exercise compared to controls 19. Nadir et al. recently demonstrated that BNP can identify existing silent cardiac target organ damage detected by transthoracic echocardiography, stress echocardiography, and/or myocardial perfusion imaging in a primary prevention population 20.
This study links prior observations that both impaired MPR and elevated NT-proBNP confer increased cardiac risk in asymptomatic individuals. Elevated NT-proBNP and impaired MPR are likely a reflection of endothelial or smooth muscle dysfunction of the coronary microvasculature. Elevated NT-BNP may be a compensatory mechanism for smooth muscle dysfunction, as BNP is known to have vasodilatory effects on the coronary vasculature 21. Previous studies have also demonstrated a relationship between elevated NT-proBNP and endothelial dysfunction, further supporting this assertion 22, 23.
For reasons that remain unclear, the inverse association of MPR and NT-proBNP appears to be stronger in the inferior, inferoseptal, and inferolateral segments. Interestingly, a previous MESA study on the same cohort described a significant association between decreased regional LV function evaluated as peak systolic circumferential strain and reduced regional MPR predominantly in the inferior and posterior walls of the left ventricle 24. Rosen at al. speculate that this could be explained by decreased sympathetic innervation in the LV inferior and posterior wall, leading to a reduced sympathetic response and a less vasodilation in response to reduction of local blood flow.
Strengths of the study include its community based sample population, comprehensive clinical data, and the use of sensitive, reproducible laboratory techniques. There are some limitations to this study to acknowledge. First, it is a cross-sectional study, therefore cause-and-effect relations between NT-proBNP and MPR cannot be ascertained. Second, prospective cardiovascular outcomes are too few in this small sample to be considered in the analysis. Third, diastolic function was not assessed in these individuals. Last, coronary angiography was not performed to definitively rule out atherosclerotic obstruction.
Acknowledgments
This study was funded by the National Heart, Lung, and Blood Institute (NHLBI).
Footnotes
Disclosures
The contents in this manuscript have not been previously published and it is not under consideration elsewhere. All authors have read and approved the manuscript and have nothing to disclose. We have no relationships to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 2.Wang L, Jerosch-Herold M, Jacobs DR, Jr, Shahar E, Folsom AR. Coronary risk factors and myocardial perfusion in asymptomatic adults: the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Coll Cardiol. 2006;47:565–572. doi: 10.1016/j.jacc.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman JI. Maximal coronary flow and the concept of coronary vascular reserve. Circulation. 1984;70:153–159. doi: 10.1161/01.cir.70.2.153. [DOI] [PubMed] [Google Scholar]
- 4.Doust JA, Pietrzak E, Dobson A, Glasziou P. How well does B-type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. BMJ. 2005;330:625. doi: 10.1136/bmj.330.7492.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 6.McDonagh TA, Cunningham AD, Morrison CE, McMurray JJ, Ford I, Morton JJ, Dargie HJ. Left ventricular dysfunction, natriuretic peptides, and mortality in an urban population. Heart. 2001;86:21–26. doi: 10.1136/heart.86.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kara K, Mahabadi AA, Berg MH, Lehmann N, Mohlenkamp S, Kalsch H, Bauer M, Moebus S, Dragano N, Jockel KH, Neumann T, Erbel R. Predicting risk of coronary events and all-cause mortality: role of B-type natriuretic peptide above traditional risk factors and coronary artery calcium scoring in the general population: the Heinz Nixdorf Recall Study. Eur J Prev Cardiol. 2013 doi: 10.1177/2047487313490256. [DOI] [PubMed] [Google Scholar]
- 8.Hasdai D, Gibbons RJ, Holmes DR, Jr, Higano ST, Lerman A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997;96:3390–3395. doi: 10.1161/01.cir.96.10.3390. [DOI] [PubMed] [Google Scholar]
- 9.Reis SE, Holubkov R, Lee JS, Sharaf B, Reichek N, Rogers WJ, Walsh EG, Fuisz AR, Kerensky R, Detre KM, Sopko G, Pepine CJ. Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease. Results from the pilot phase of the Women's Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol. 1999;33:1469–1475. doi: 10.1016/s0735-1097(99)00072-8. [DOI] [PubMed] [Google Scholar]
- 10.Zeiher AM, Drexler H, Wollschlager H, Just H. Endothelial dysfunction of the coronary microvasculature is associated with coronary blood flow regulation in patients with early atherosclerosis. Circulation. 1991;84:1984–1992. doi: 10.1161/01.cir.84.5.1984. [DOI] [PubMed] [Google Scholar]
- 11.Marcus ML, Chilian WM, Kanatsuka H, Dellsperger KC, Eastham CL, Lamping KG. Understanding the coronary circulation through studies at the microvascular level. Circulation. 1990;82:1–7. doi: 10.1161/01.cir.82.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–658. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 13.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 14.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 15.Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G, Bairey Merz CN. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–2832. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakanishi K, Fukuda S, Shimada K, Miyazaki C, Otsuka K, Maeda K, Miyahana R, Kawarabayashi T, Watanabe H, Yoshikawa J, Yoshiyama M. Impaired coronary flow reserve as a marker of microvascular dysfunction to predict long-term cardiovascular outcomes, acute coronary syndrome and the development of heart failure. Circ J. 2012;76:1958–1964. doi: 10.1253/circj.cj-12-0245. [DOI] [PubMed] [Google Scholar]
- 17.Mollmann H, Nef HM, Kostin S, Dragu A, Maack C, Weber M, Troidl C, Rolf A, Elsasser A, Bohm M, Brantner R, Hamm CW, Holubarsch CJ. Ischemia triggers BNP expression in the human myocardium independent from mechanical stress. Int J Cardiol. 2010;143:289–297. doi: 10.1016/j.ijcard.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Goetze JP, Christoffersen C, Perko M, Arendrup H, Rehfeld JF, Kastrup J, Nielsen LB. Increased cardiac BNP expression associated with myocardial ischemia. FASEB J. 2003;17:1105–1107. doi: 10.1096/fj.02-0796fje. [DOI] [PubMed] [Google Scholar]
- 19.Yurtdas M, Ozcan IT, Camsar A, Cicek D, Tamer L, Cin VG, Doven O, Seyis AS, Akkus MN. NT-Pro-BNP levels and their response to exercise in patients with slow coronary flow. Arq Bras Cardiol. 2012;99:1115–1122. [PubMed] [Google Scholar]
- 20.Nadir MA, Rekhraj S, Wei L, Lim TK, Davidson J, MacDonald TM, Lang CC, Dow E, Struthers AD. Improving the primary prevention of cardiovascular events by using biomarkers to identify individuals with silent heart disease. J Am Coll Cardiol. 2012;60:960–968. doi: 10.1016/j.jacc.2012.04.049. [DOI] [PubMed] [Google Scholar]
- 21.Okumura K, Yasue H, Fujii H, Kugiyama K, Matsuyama K, Yoshimura M, Jougasaki M, Kikuta K, Kato H, Tanaka H, Sumida H, Nakao K. Effects of brain (B-type) natriuretic peptide on coronary artery diameter and coronary hemodynamic variables in humans: comparison with effects on systemic hemodynamic variables. J Am Coll Cardiol. 1995;25:342–348. doi: 10.1016/0735-1097(94)00407-h. [DOI] [PubMed] [Google Scholar]
- 22.Pauriah M, Khan F, Lim TK, Elder DH, Godfrey V, Kennedy G, Belch JJ, Booth NA, Struthers AD, Lang CC. B-type natriuretic peptide is an independent predictor of endothelial function in man. Clin Sci (Lond) 2012;123:307–312. doi: 10.1042/CS20110168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ninomiya Y, Hamasaki S, Ishida S, Kataoka T, Saihara K, Okui H, Orihara K, Fukudome T, Shinsato T, Ichiki T, Mizoguchi E, Otsuji Y, Tei C. Elevated levels of brain natriuretic peptide as a predictor of impaired coronary endothelial function in patients with left ventricular remodeling. J Cardiol. 2006;48:125–132. [PubMed] [Google Scholar]
- 24.Rosen BD, Lima JA, Nasir K, Edvardsen T, Folsom AR, Lai S, Bluemke DA, Jerosch-Herold M. Lower myocardial perfusion reserve is associated with decreased regional left ventricular function in asymptomatic participants of the multi-ethnic study of atherosclerosis. Circulation. 2006;114:289–297. doi: 10.1161/CIRCULATIONAHA.105.588525. [DOI] [PubMed] [Google Scholar]