Abstract
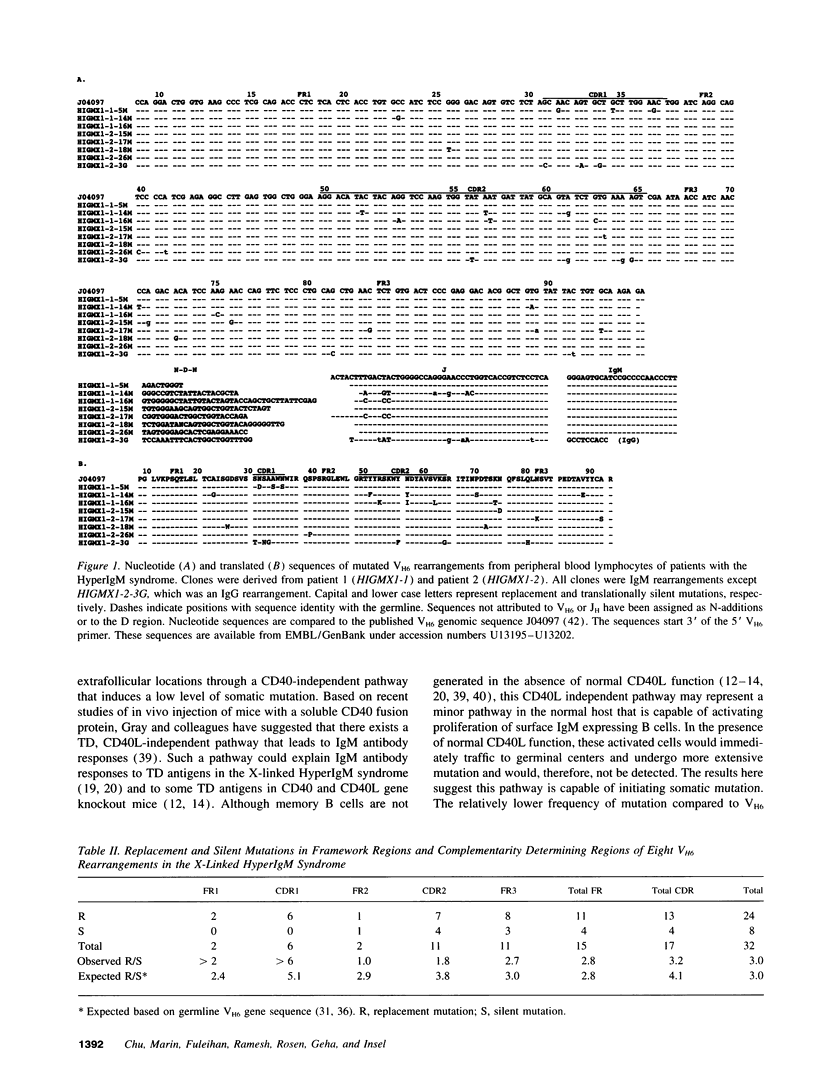
Somatic mutation of Ig variable regions occurs prominently in germinal centers, but it has been debated whether the mutation process initiates in germinal centers or is activated before germinal center entry of B cells. We have analyzed for the presence of somatic mutation in Ig gene rearrangements of the nonpolymorphic human VH6 gene in the X-linked HyperIgM syndrome, which is associated with defective CD40 ligand expression and absence of germinal centers and generation of memory B lymphocytes. IgM and rare IgG VH6 productive rearrangements were isolated from PBL of patients with X-linked HyperIgM syndrome. Although the majority of both the IgM and IgG VH6 rearrangements had a germline VH6 sequence, 7 of 102 VH6 IgM and 1 of 6 IgG rearrangements had a mutated VH6 gene. The mutation frequency (mutations/bp) was 1.4% with a range of 2-9 mutations per clone, a mutation frequency lower, however, than that observed in IgM (3.2%) and IgG (5.4%) VH6 rearrangements of normal individuals. These results suggest that somatic mutation may be initiated in a CD40 ligand-independent pathway before entry of B cells into germinal centers, but fails to achieve the high mutation frequency observed in the presence of germinal centers.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akahori Y., Kurosawa Y., Kamachi Y., Torii S., Matsuoka H. Presence of immunoglobulin (Ig) M and IgG double isotype-bearing cells and defect of switch recombination in hyper IgM immunodeficiency. J Clin Invest. 1990 Jun;85(6):1722–1727. doi: 10.1172/JCI114627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J., Bazan F., Blanchard D., Brière F., Galizzi J. P., van Kooten C., Liu Y. J., Rousset F., Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- Berek C., Berger A., Apel M. Maturation of the immune response in germinal centers. Cell. 1991 Dec 20;67(6):1121–1129. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- Berek C. The development of B cells and the B-cell repertoire in the microenvironment of the germinal center. Immunol Rev. 1992 Apr;126:5–19. doi: 10.1111/j.1600-065x.1992.tb00628.x. [DOI] [PubMed] [Google Scholar]
- Callard R. E., Armitage R. J., Fanslow W. C., Spriggs M. K. CD40 ligand and its role in X-linked hyper-IgM syndrome. Immunol Today. 1993 Nov;14(11):559–564. doi: 10.1016/0167-5699(93)90188-Q. [DOI] [PubMed] [Google Scholar]
- Castigli E., Alt F. W., Davidson L., Bottaro A., Mizoguchi E., Bhan A. K., Geha R. S. CD40-deficient mice generated by recombination-activating gene-2-deficient blastocyst complementation. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):12135–12139. doi: 10.1073/pnas.91.25.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B., Casali P. The CDR1 sequences of a major proportion of human germline Ig VH genes are inherently susceptible to amino acid replacement. Immunol Today. 1994 Aug;15(8):367–373. doi: 10.1016/0167-5699(94)90175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M., Gerrard B. Helpful hints for the detection of single-stranded conformation polymorphisms. Biotechniques. 1991 Mar;10(3):332–333. [PubMed] [Google Scholar]
- Foy T. M., Laman J. D., Ledbetter J. A., Aruffo A., Claassen E., Noelle R. J. gp39-CD40 interactions are essential for germinal center formation and the development of B cell memory. J Exp Med. 1994 Jul 1;180(1):157–163. doi: 10.1084/jem.180.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French D. L., Laskov R., Scharff M. D. The role of somatic hypermutation in the generation of antibody diversity. Science. 1989 Jun 9;244(4909):1152–1157. doi: 10.1126/science.2658060. [DOI] [PubMed] [Google Scholar]
- Fuleihan R., Ramesh N., Loh R., Jabara H., Rosen R. S., Chatila T., Fu S. M., Stamenkovic I., Geha R. S. Defective expression of the CD40 ligand in X chromosome-linked immunoglobulin deficiency with normal or elevated IgM. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2170–2173. doi: 10.1073/pnas.90.6.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D., Dullforce P., Jainandunsing S. Memory B cell development but not germinal center formation is impaired by in vivo blockade of CD40-CD40 ligand interaction. J Exp Med. 1994 Jul 1;180(1):141–155. doi: 10.1084/jem.180.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbaugh D., Ochs H. D., Noelle R. J., Ledbetter J. A., Aruffo A. The role of CD40 and its ligand in the regulation of the immune response. Immunol Rev. 1994 Apr;138:23–37. doi: 10.1111/j.1600-065x.1994.tb00845.x. [DOI] [PubMed] [Google Scholar]
- Hollenbaugh D., Wu L. H., Ochs H. D., Nonoyama S., Grosmaire L. S., Ledbetter J. A., Noelle R. J., Hill H., Aruffo A. The random inactivation of the X chromosome carrying the defective gene responsible for X-linked hyper IgM syndrome (X-HIM) in female carriers of HIGM1. J Clin Invest. 1994 Aug;94(2):616–622. doi: 10.1172/JCI117377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel R. A., Varade W. S. Bias in somatic hypermutation of human VH genes. Int Immunol. 1994 Sep;6(9):1437–1443. doi: 10.1093/intimm/6.9.1437. [DOI] [PubMed] [Google Scholar]
- Insel R. A., Varade W. S., Marin E. Human splenic IgM immunoglobulin transcripts are mutated at high frequency. Mol Immunol. 1994 Apr;31(5):383–392. doi: 10.1016/0161-5890(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Jacob J., Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. II. A common clonal origin for periarteriolar lymphoid sheath-associated foci and germinal centers. J Exp Med. 1992 Sep 1;176(3):679–687. doi: 10.1084/jem.176.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J., Kelsoe G., Rajewsky K., Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991 Dec 5;354(6352):389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- Jacob J., Przylepa J., Miller C., Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. III. The kinetics of V region mutation and selection in germinal center B cells. J Exp Med. 1993 Oct 1;178(4):1293–1307. doi: 10.1084/jem.178.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe T., Naka T., Yoshida K., Tanaka T., Fujiwara H., Suematsu S., Yoshida N., Kishimoto T., Kikutani H. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994 Jun;1(3):167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Klein U., Küppers R., Rajewsky K. Human IgM+IgD+ B cells, the major B cell subset in the peripheral blood, express V kappa genes with no or little somatic mutation throughout life. Eur J Immunol. 1993 Dec;23(12):3272–3277. doi: 10.1002/eji.1830231232. [DOI] [PubMed] [Google Scholar]
- Kocks C., Rajewsky K. Stable expression and somatic hypermutation of antibody V regions in B-cell developmental pathways. Annu Rev Immunol. 1989;7:537–559. doi: 10.1146/annurev.iy.07.040189.002541. [DOI] [PubMed] [Google Scholar]
- Kroczek R. A., Graf D., Brugnoni D., Giliani S., Korthüer U., Ugazio A., Senger G., Mages H. W., Villa A., Notarangelo L. D. Defective expression of CD40 ligand on T cells causes "X-linked immunodeficiency with hyper-IgM (HIGM1)". Immunol Rev. 1994 Apr;138:39–59. doi: 10.1111/j.1600-065x.1994.tb00846.x. [DOI] [PubMed] [Google Scholar]
- Leanderson T., Källberg E., Gray D. Expansion, selection and mutation of antigen-specific B cells in germinal centers. Immunol Rev. 1992 Apr;126:47–61. doi: 10.1111/j.1600-065x.1992.tb00630.x. [DOI] [PubMed] [Google Scholar]
- Lederman S., Yellin M. J., Inghirami G., Lee J. J., Knowles D. M., Chess L. Molecular interactions mediating T-B lymphocyte collaboration in human lymphoid follicles. Roles of T cell-B-cell-activating molecule (5c8 antigen) and CD40 in contact-dependent help. J Immunol. 1992 Dec 15;149(12):3817–3826. [PubMed] [Google Scholar]
- MacLennan I. C. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- McHeyzer-Williams M. G., McLean M. J., Lalor P. A., Nossal G. J. Antigen-driven B cell differentiation in vivo. J Exp Med. 1993 Jul 1;178(1):295–307. doi: 10.1084/jem.178.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonoyama S., Hollenbaugh D., Aruffo A., Ledbetter J. A., Ochs H. D. B cell activation via CD40 is required for specific antibody production by antigen-stimulated human B cells. J Exp Med. 1993 Sep 1;178(3):1097–1102. doi: 10.1084/jem.178.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J. Differentiation of the secondary B-lymphocyte repertoire: the germinal center reaction. Immunol Rev. 1994 Feb;137:173–183. doi: 10.1111/j.1600-065x.1994.tb00664.x. [DOI] [PubMed] [Google Scholar]
- Notarangelo L. D., Duse M., Ugazio A. G. Immunodeficiency with hyper-IgM (HIM). Immunodefic Rev. 1992;3(2):101–121. [PubMed] [Google Scholar]
- Rada C., Gupta S. K., Gherardi E., Milstein C. Mutation and selection during the secondary response to 2-phenyloxazolone. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5508–5512. doi: 10.1073/pnas.88.13.5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh N., Fuleihan R., Geha R. Molecular pathology of X-linked immunoglobulin deficiency with normal or elevated IgM (HIGMX-1). Immunol Rev. 1994 Apr;138:87–104. doi: 10.1111/j.1600-065x.1994.tb00848.x. [DOI] [PubMed] [Google Scholar]
- Ramesh N., Fuleihan R., Ramesh V., Lederman S., Yellin M. J., Sharma S., Chess L., Rosen F. S., Geha R. S. Deletions in the ligand for CD40 in X-linked immunoglobulin deficiency with normal or elevated IgM (HIGMX-1). Int Immunol. 1993 Jul;5(7):769–773. doi: 10.1093/intimm/5.7.769. [DOI] [PubMed] [Google Scholar]
- Sanz I., Kelly P., Williams C., Scholl S., Tucker P., Capra J. D. The smaller human VH gene families display remarkably little polymorphism. EMBO J. 1989 Dec 1;8(12):3741–3748. doi: 10.1002/j.1460-2075.1989.tb08550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Walter M. A., Hofker M. H., Ebens A., Willems van Dijk K., Liao L. C., Cox D. W., Milner E. C., Perlmutter R. M. Physical linkage of a human immunoglobulin heavy chain variable region gene segment to diversity and joining region elements. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8196–8200. doi: 10.1073/pnas.85.21.8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubata T., Wu J., Honjo T. B-cell apoptosis induced by antigen receptor crosslinking is blocked by a T-cell signal through CD40. Nature. 1993 Aug 12;364(6438):645–648. doi: 10.1038/364645a0. [DOI] [PubMed] [Google Scholar]
- Van den Eertwegh A. J., Noelle R. J., Roy M., Shepherd D. M., Aruffo A., Ledbetter J. A., Boersma W. J., Claassen E. In vivo CD40-gp39 interactions are essential for thymus-dependent humoral immunity. I. In vivo expression of CD40 ligand, cytokines, and antibody production delineates sites of cognate T-B cell interactions. J Exp Med. 1993 Nov 1;178(5):1555–1565. doi: 10.1084/jem.178.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varade W. S., Marin E., Kittelberger A. M., Insel R. A. Use of the most JH-proximal human Ig H chain V region gene, VH6, in the expressed immune repertoire. J Immunol. 1993 Jun 1;150(11):4985–4995. [PubMed] [Google Scholar]
- Weigert M. G., Cesari I. M., Yonkovich S. J., Cohn M. Variability in the lambda light chain sequences of mouse antibody. Nature. 1970 Dec 12;228(5276):1045–1047. doi: 10.1038/2281045a0. [DOI] [PubMed] [Google Scholar]
- Willems van Dijk K., Schroeder H. W., Jr, Perlmutter R. M., Milner E. C. Heterogeneity in the human Ig VH locus. J Immunol. 1989 Apr 1;142(7):2547–2554. [PubMed] [Google Scholar]
- Xu J., Foy T. M., Laman J. D., Elliott E. A., Dunn J. J., Waldschmidt T. J., Elsemore J., Noelle R. J., Flavell R. A. Mice deficient for the CD40 ligand. Immunity. 1994 Aug;1(5):423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- van Es J. H., Meyling F. H., Logtenberg T. High frequency of somatically mutated IgM molecules in the human adult blood B cell repertoire. Eur J Immunol. 1992 Oct;22(10):2761–2764. doi: 10.1002/eji.1830221046. [DOI] [PubMed] [Google Scholar]



