Abstract
Genetic variants in il2 and il2ra have been associated with autoimmune disease susceptibility in both genome-wide association studies (GWAS) in humans and in genetic linkage studies in experimental models of autoimmunity. Specifically, genetic variants resulting in a low IL-2 phenotype are susceptibility alleles while variants resulting in a high IL-2 phenotype are resistance alleles. The association of high IL-2 phenotypes with resistance has been attributed primarily to the T cell intrinsic promotion of regulatory T cell development, maintenance, and function; however, IL-2 can also act T cell intrinsically to dampen differentiation of pathogenic IL-17-producing Th17 cells. Here, we have uncovered a novel T cell extrinsic mechanism whereby IL-2 promotes both IFN-γ and IL-27 production from tissue resident macrophages which in turn dampen the differentiation of pathogenic Th17 cells.
Keywords: IL-17, T cells, Macrophages, Cytokines
1. Introduction
Single nucleotide polymorphisms (SNPs) in Il2 as well as Il2ra have scored highly in genome-wide association studies (GWAS) in multiple human autoimmune diseases, including type 1 diabetes (T1D) and multiple sclerosis (MS) (1). Similarly, the Idd3 genetic interval that encodes Il2 has been identified as a key determinant of susceptibility in multiple experimental autoimmune diseases, including type 1 diabetes (2), experimental autoimmune encephalomyelitis (EAE) (3), and autoimmune ovarian dysgenesis (AOD) (4). NOD mice that carry the Idd3 interval derived from diabetes-resistant C57BL/6 mice (NOD.Idd3 mice) are resistant to autoimmunity and T cells from NOD.Idd3 mice produce more IL-2 than T cells from autoimmune-susceptible NOD mice (5–9). Thus, a high IL-2 phenotype is associated with resistance to autoimmunity while a low IL-2 phenotype is associated with susceptibility to autoimmunity.
The association of high IL-2 with resistance to autoimmunity has been primarily attributed to its requirement for the proper development, function, and maintenance of Foxp3+ regulatory T cells (Treg) (10). However, IL-2 can also constrain the differentiation of pro-inflammatory IL-17-producing, Th17 cells (11,12). Thus, a high IL-2 phenotype would be expected to exhibit potent Treg function and dampened Th17 differentiation. Indeed, we (13), and others (5,8), have found that Treg from NOD.Idd3 mice are more suppressive than Treg from NOD. We further found that NOD.Idd3 T cells exhibit defective Th17 differentiation relative to NOD T cells (6). Most interestingly, we found that it was the CD11b+CD11c− antigen presenting cells that were key determinants of both the differential Treg suppressor function and Th17 differentiation observed in NOD versus NOD.Idd3 T cells (6,13). Together these observations raised the possibility that the T cell-derived cytokine IL-2 could act in a T cell extrinsic manner to modify the ability of CD11b+ APC to support Treg suppressor function and/or inhibit Th17 differentiation. Here, we identify a mechanism by which IL-2 acts T cell extrinsically on tissue resident macrophages to dampen their ability to support Th17 differentiation.
2. Materials and Methods
2.1. Animals
6–9 week old Female NOD and NOD.Idd3 mice were purchased from Taconic. NOD.FoxP3-GFP knock-in (KI) mice were bred and maintained at Taconic. All mice were housed in accordance with the guidelines established by the animal care and use committee at Harvard Medical School (Boston, MA).
2.2. Flow Cytometry
Single cell suspensions were stained with antibodies against CD4, CXCR3, CD11b, CD11c, MHC II, Ly-6C, and CD25 (BioLegend). For intracellular staining, cells were fixed in 4% paraformaldehyde, permeabilized in PBS 0.05% saponin and stained with antibodies against IL-27p28, IL-17A, IFN-γ, IL-10, or Tbet (BioLegend). All data were collected on a LSRII Flow Cytometer (BD Biosciences).
2.3. CD11b+ stimulation assays
CD11b+CD11c− cells were isolated from spleen by cell sorting. CpG was used at 0.5 μM, PGN 5 μg/ml, and IL-2 25 ng/ml. Cells were harvested at 4 h for RNA isolation and analyzed by real-time PCR. Culture supernatant was collected at 24 h for cytokine measurement by cytometric bead array (CBA) (BD Biosciences). For intracellular staining, cells were stimulated for 18 hrs, washed, and incubated for 4 h with Golgi Stop (BD Biosciences) prior to staining.
2.4. Th17 differentiation
Naïve CD4+ CD62Lhi CD44lo T cells were isolated by cell sorting and activated with soluble anti-CD3 (1 μg/ml), TGF-β (5 ng/ml), IL-6 (30 ng/ml) and irradiated T-depleted APC that had been cultured for 18 hours with CpG or CpG plus IL-2 and washed extensively prior to use. In some assays, anti-IFN-γ (XMG1.2) or anti-IL-27p28 (MM27-7B1) antibody was used at 10 μg/mL. Polarization was determined on day 3 by intracytoplasmic cytokine staining for IL-17A, IFN-γ and IL-10 after stimulation with 50ng/mL PMA and 1μg/mL ionomycin (Sigma-Aldrich) in the presence of Golgi-stop for 4 h.
2.5. Treg differentiation
Natural Treg were isolated from NOD.FoxP3-GFP KI mice by cell sorting and activated with soluble anti-CD3 (1 μg/ml) in the presence of irradiated T-depleted APC that had been cultured for 18 hours with CpG or CpG plus IL-2 and washed extensively prior to use. After 48 h, nTreg were harvested and examined for T-bet and CXCR3 expression by flow cytometry.
2.6. Real-time PCR
RNA was purified using RNeasy Plus Mini Kit (Qiagen) and cDNA synthesized using iScript (Bio-Rad). Real-time PCR was performed on either a 7900 system or ViiA 7 system (ABI) using TaqMan assays for IL-2rβ, IL-2rγc, IL-2rα, Ebi3, and IL-27 p28 (ABI). Transcript expression was normalized to β-actin.
3. Results
3.1. Tissue resident macrophages express high affinity IL-2R
Our previous work showed that the CD11b+CD11c− antigen presenting cell population (CD11b+ APC) is a major determinant of the differential Th17 differentiation in NOD vs NOD.Idd3 mice (6). However, at that time, we did not address whether T cell-derived differences in IL-2 had a role in determining the ability of CD11b+ APC to support Th17 differentiation. To address this possibility, we determined the expression of IL-2 receptor in CD11b+CD11c− cells from both NOD and NOD.Idd3. We first examined the expression of IL-2 receptor beta chain (IL2Rβ) and gamma chain (IL2Rγc) mRNA and found that both IL2Rβ and IL2Rγc are constitutively expressed on CD11b+CD11c− cells (Supplemental Fig. 1A). We examined whether this expression might change upon activation and found that although the CD11b+CD11c− cells from NOD.Idd3 showed an increase in IL2Rβ and IL2Rγc in some experiments, this was inconsistent across experiments and did not reach statistical significance. We further confirmed surface expression of IL2Rβ and IL2Rγc on CD11b+CD11c− cells by flow cytometry (Supplemental Fig 1B).
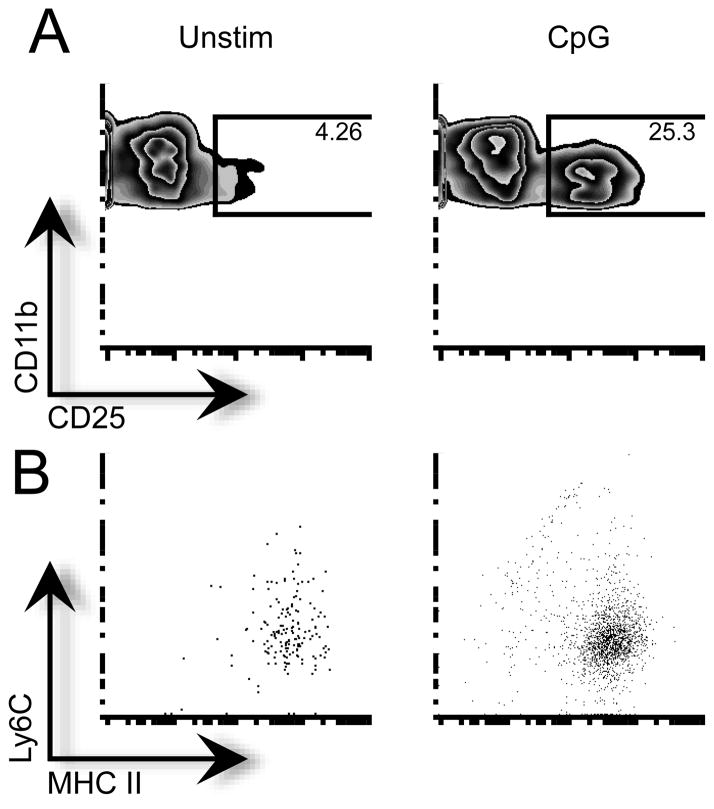
We next examined the expression of high affinity IL-2 receptor alpha chain (CD25) on CD11b+CD11c− cells. Interestingly, we found that while resting CD11b+CD11c− cells express very little IL-2Rα mRNA, it is significantly up-regulated to similar levels in both NOD and NOD.Idd3-derived cells upon activation through either Toll-like receptor (TLR) 9 (CpG) or TLR2 (peptidoglycan) (Supplemental Fig. 1C). We further characterized the population of CD11b+ CD11c− cells that up-regulated CD25 as CD11b+CD11c− MHC Class II+ Ly6Clow tissue resident macrophages (Fig. 1B). Thus, tissue-resident splenic macrophages express high affinity IL-2R upon activation and are able to respond to IL-2.
Figure 1. Tissue resident macrophages up-regulate high affinity IL-2R.
CD11b+ cells from NOD were cultured with media or CpG for 24 h. A) CD25 expression on CD11b+CD11c− cells. B) Expression of MHC Class II and Ly6C on CD11b+CD11c−CD25+ cells. Representative data are shown.
3.2. IL-2 modifies CD11b+ APC to suppress Th17 differentiation
IL-2 can suppress Th17 differentiation through a T cell intrinsic mechanism that requires STAT-5 and Aiolos (11,12). Our observation that activated tissue resident macrophages express high affinity IL-2 receptor led us to hypothesize that IL-2 could also act in a T cell extrinsic manner to suppress Th17 differentiation through modulation of tissue resident macrophages. Indeed, that NOD.Idd3 T cells are high producers of IL-2 together with our finding that NOD.Idd3-derived CD11b+ APC are defective in supporting Th17 differentiation (6) support the hypothesis that, in addition to T cell intrinsic effects, IL-2 may suppress Th17 differentiation via modulation of APC function. To test this, we examined the effect of IL-2 on the Th17-promoting ability of NOD-derived APC, which are normally effective at supporting Th17 differentiation (6). We found that while activation of NOD APC did not significantly affect Th17 differentiation, exposure to IL-2 significantly dampened the ability of activated NOD APC to drive Th17 differentiation (Fig. 2).
Figure 2. IL-2 modifies the ability of APC to support Th17 differentiation.
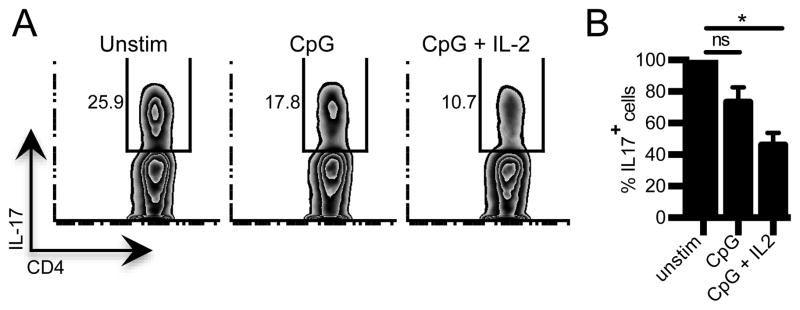
Naïve NOD CD4+ T cells were cultured under Th17 conditions with syngeneic T cell-depleted APC that were cultured for 18h with media (unstim), CpG, or CpG+ IL-2. Left panel, Representative flow cytometry data showing frequency of IL-17-producing cells in Th17 differentiation cultures. Right panel, summary data showing frequency of IL-17-producing T cells in Th17 differentiation cultures. Data are normalized to frequency of Th17 cells in cultures with unstim APC (n=3). *p=0.0034, one-way ANOVA, Tukey’s multiple comparison test. ns=not significant.
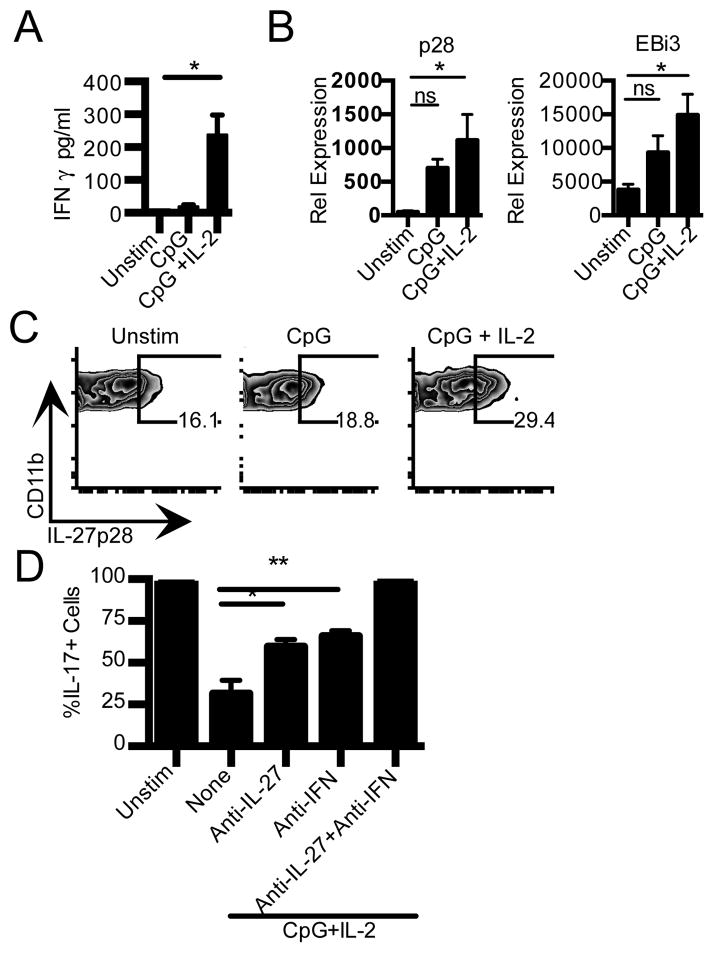
IFN-γ is known to suppress Th17 differentiation (14,15). Indeed, our previous data implicated IFN-γ as one of the mechanisms by which NOD.Idd3-derived CD11b+ APC suppress Th17 differentiation (6). The immunosuppressive cytokine IL-27 also suppresses Th17 differentiation (16,17) and has been shown to induce IFN-γ and IL-10 and be protective in both EAE and type 1 diabetes (18–20). Accordingly, we examined whether IL-2 promotes IFN-γ and/or IL-27 production in CD11b+ APC to suppress Th17 differentiation. We found that activated CD11b+ CD11c− cells produced very little IFN-γ; however, activation in the presence of IL-2 significantly augmented IFN-γ production (Fig. 3A and Supplemental Fig. 1D). We next examined whether IL-2 similarly promotes IL-27 production in CD11b+ CD11c− cells. We found that activation results in a trend towards increased p28 and EBi3 transcripts that does not reach statistical significance. However, activation in the presence of IL-2 results in a significant increase in both p28 and EBi3 (Fig. 3B). In line with these data, we found that intracellular IL-27 p28 is significantly increased only in CD11b+ CD11c− cells activated in the presence of IL-2 (Fig. 3C). We further found that IL-2-driven IL-27 production is a unique property of CD11b+ CD11c- cells as it is not observed in either B cells or dendritic cells (Supplemental Fig. 2).
Figure 3. IL-2 promotes IFN-γ and IL-27 production from CD11b+ CD11c− APC.
A) CD11b+CD11c− cells from NOD were stimulated with CpG, or CpG+ IL-2. IFN-γ production in culture supernatant at 24 h (n=5) was determined by CBA. *p=0.0074, t-test. B) CD11b+CD11c− cells were stimulated as in (A) for 4 h. Cells were then lysed for RNA isolation and use in real-time PCR. Data are an average of 2–3 independent experiments. Left panel, IL-27 p28. Right panel, EBi3. *p<0.05, One-Way ANOVA, Tukey’s multiple comparison test. C) NOD splenocytes were T-depleted and stimulated for 18h with media (unstim), CpG, or CpG+IL-2. Staining for IL27 p28 in CD11b+CD11c− cells is shown. Data shown are representative of five independent experiments. D) Th17 differentiation of naïve NOD CD4+ T cells cultured with syngeneic T cell-depleted APC that were cultured for 18h with media (unstim), CpG+ IL-2 in the presence or absence of anti-IFN-γ and anti-IL-27 p28 as indicated. Data are normalized to frequency of Th17 cells in cultures with untreated APC. *p=0.0334,**p=0.0148, One-Way ANOVA, Tukey’s multiple comparison test.
We next addressed whether IL-2 driven IFN-γ and IL-27 were responsible for the reduced ability of IL-2-treated CD11b+ APC to support Th17 differentiation. We found that neutralizing either IFN-γ or IL-27 reduced the extent by which IL-2 treated CD11b+ APC suppress Th17 differentiation. However, only neutralizing both IFN-γ and IL-27 was able to fully restore Th17 differentiation to the levels observed with untreated APC (Fig. 3D). Thus, IL-2 drives both IFN-γ and IL-27 production in CD11b+ APC and together these two cytokines dampen the generation of pathogenic Th17 cells.
3.3. IL-2 does not affect Tr1 and Th1 Treg induction by CD11b+ APC
IL-27 has also been shown to promote the differentiation of IL-10-producing T regulatory 1 (Tr1 cells) that have an important role in suppressing autoimmunity (19,21,22). Our observation of IL-2 driving IL-27 production from CD11b+ APC therefore raised the possibility that IL-2-driven increases in IL-27 in CD11b+ APC may not only dampen Th17 responses but also promote the generation of protective Tr1 cells. Accordingly, we addressed whether IL-2 can modulate the ability of CD11b+ APC to promote differentiation of Tr1 cells. We found that IL-2 treatment had no effect on the ability of APC to promote the generation of IL-10-producing cells from naïve T cells (Fig. 4A). In addition to Tr1 cells, Th17 cells can also produce IL-10 (23). Indeed, IL-10-producing Th17 cells constitute a non-pathogenic subset of Th17 cells. We therefore asked whether IL-2 treated APC can promote the generation of IL-10-producing non-pathogenic Th17 cells and found that IL-2 did not promote the generation of these cells (Fig. 4B). Lastly, both IFN-γ and IL-27 have recently been implicated in the promotion of Tbet+CXCR3+ Treg that exhibit specialized function for suppressing Th1 responses (24). Accordingly, we examined whether IL-2 stimulated APC can promote the generation of this Treg subset. We found that IL-2 did not significantly alter the differentiation of natural Treg (nTreg) to Tbet+CXCR3+ Treg (Fig. 4C). Collectively, our data show that IL-2 can act T cell extrinsically to dampen the ability of CD11b+ APC to support pathogenic Th17 differentiation, thereby contributing to resistance to autoimmunity.
Figure 4. Generation of IL-10 producing cells by IL-2 stimulated APC.
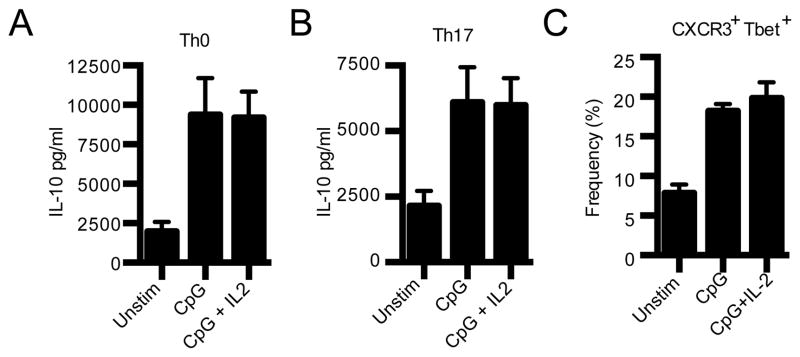
Naïve NOD CD4+ T cells were cultured under neutral Th0 (A) or Th17 (B) conditions with T cell-depleted APC that were stimulated as indicated for 18h. On day 3 IL-10 in culture supernatant was determined by CBA (n=5). C) nTreg from NOD.FoxP3 KI mice were cultured with T cell-depleted syngeneic APC that were stimulated as indicated for 18h. Frequency of nTreg expressing T-bet and CXCR3 was determined by flow cytometry after 48h (n=3).
4. Discussion
Genetic variants that result in low IL-2 phenotypes have been linked with susceptibility to autoimmunity in both mice and man. In mice, the low IL-2 NOD strain is highly susceptible to T1D, EAE, and AOD while the high IL-2 NOD.Idd3 strain is protected from disease (2–4). In humans, the SNPs that have scored in il2r in GWAS studies of MS result in increased production of soluble IL-2RA (25), which in effect lowers the amount of available IL-2. As IL-2 is critical for Treg function and maintenance, the association of low IL-2 phenotypes with autoimmunity has been largely attributed to a compromised regulatory T cell compartment. Our data uncover a novel T cell extrinsic mechanism by which IL-2 acts to promote tolerance over autoimmunity; IL-2 drives IFN-γ and IL-27 production in CD11b+ APC to dampen pathogenic Th17 differentiation.
In addition to Il2, the Idd3 interval encodes Il21 and SNPs in Il21 have also scored in GWAS in several human autoimmune diseases. Indeed, it has been observed that T cells from NOD and NOD.Idd3 differ in their production of IL-21. T cells from NOD produce high IL-21 while T cells NOD.Idd3 produce low IL-21 (7). Thus, a high IL-2/low IL-21 phenotype is associated with resistance to autoimmunity while a low IL-2/high IL-21 phenotype is associated with autoimmune susceptibility. IL-21 is known to act T cell intrinsically to suppress Treg differentiation and promote Th17 differentiation (26,27). Importantly, our previous work has shown that IL-21 can also act T cell extrinsically on CD11b+ APC to promote Th17 differentiation and suppress Treg function via promotion of IL-6 and PGE2 (6). Our data now show that the IL-2 cytokine circuit also has an important T cell extrinsic component. We hypothesize that the T cell extrinsic action of IL-2 on CD11b+ tissue resident macrophages serves as a re-enforcing mechanism to stabilize T cell phenotypes that determine susceptibility versus resistance to autoimmunity. Our findings identify a novel non-T cell role for IL-2 and increase our current understanding of the mechanisms by which genetic variants that result in low IL-2 phenotypes determine susceptibility to autoimmune disease.
Supplementary Material
Supplemental Figure 1. Expression of IL-2 receptor chains and IL-2 promotion of IFN-γ production in CD11b+ CD11c− cells.
A) CD11b+CD11c− cells were isolated from the spleens of NOD and NOD.Idd3 mice by cell sorting and stimulated with CpG for 4 h as indicated. Cells were then lysed for RNA isolation and use in real-time PCR. IL2Rβ (n= 3), IL2Rγc (n=3). ns=not significant. B) Expression of IL2Rβ and IL2Rγc on NOD CD11b+CD11c− cells before (black line) and after stimulation with CpG (gray line). Filled histogram, fluorescence minus one control. C) CD11b+CD11c− cells were prepared as in (A) and stimulated with either CpG or PGN. Left panel, CD25 (IL2Rα) (n=4),*p=0.0003, **p=0.006, t-test. ns=not significant. Middle and right panels, data from two independent experiments. C) CD11b+CD11c− cells were isolated from the spleens of NOD mice and stimulated with PGN in the presence or absence of 25ng/mL IL-2 for 24h, (n=5). *p=0.0447, t-test. Cytokine measured by CBA.
Supplemental Figure 2. IL-27 p28 is mainly produced by CD11b+ CD11c− cells.
NOD APC were T-depleted and stimulated for 18h with media, CpG, or CpG+IL-2. Cells were washed, incubated with GolgiStop for 4h and intracellular cytokine staining preformed. Data are representative of five experiments.
Highlights.
Variants in Il2 are genetically linked to autoimmune susceptibility
High IL-2 phenotypes are associated with resistance
Activated tissue resident macrophages can sense IL-2
IL-2 induces IFN-g and IL-27 in macrophages to dampen pathogenic Th17 differentiation
IL-2 can act T cell extrinsically to influence autoimmune susceptibility
Acknowledgments
The authors would like to acknowledge James Nevin for technical assistance. This work was supported by grants from the National Institutes of Health to VKK (R01DK096138;R56AI044880), from the National Multiple Sclerosis Society (NMSS RG2571 to VKK and NMSSRG4374 to ACA) and by a career development award from the Brigham and Women’s Hospital (to ACA).
Footnotes
Conflict of interest disclosure
The authors declare no conflicts of interest related to this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vandenbroeck K. Cytokine gene polymorphisms and human autoimmune disease in the era of genome-wide association studies. J Interferon Cytokine Res. 2012;32:139–151. doi: 10.1089/jir.2011.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyons PA, Armitage N, Argentina F, Denny P, Hill NJ, Lord CJ, Wilusz MB, Peterson LB, Wicker LS, Todd JA. Congenic mapping of the type 1 diabetes locus, Idd3, to a 780-kb region of mouse chromosome 3: identification of a candidate segment of ancestral DNA by haplotype mapping. Genome Res. 2000;10:446–453. doi: 10.1101/gr.10.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Encinas JA, Wicker LS, Peterson LB, Mukasa A, Teuscher C, Sobel R, Weiner HL, Seidman CE, Seidman JG, Kuchroo VK. QTL influencing autoimmune diabetes and encephalomyelitis map to a 0.15-cM region containing IL2. Nat Genet. 1999;21:158–160. doi: 10.1038/5941. [DOI] [PubMed] [Google Scholar]
- 4.Teuscher C, Wardell BB, Luncerford JK, Michael SD, Tung KSK. Aod2, the locus controlling development of atrophy in neonatal thymectomy-induced autoimmune ovarian dysgenesis, co-localizes with IL2, Fgfb and Idd3. J Exp Med. 1996;183:631–637. doi: 10.1084/jem.183.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamanouchi J, Rainbow D, Serra P, Howlett S, Hunter K, Garner VES, Gonzalez-Munoz A, Clark J, Veijola R, Cubbon R, Show-Ling C, Rosa R, Cumiskey AM, Serreze D, Gregory S, Rogers J, Lyons PA, Healy B, Smink LJ, Todd JA, Peterson LB, Wicker LS, Santamaria P. Interleukin-2 gene variation impairs regulatory T cell function and causes autoimmunity. Nat Genet. 2007;39:329–337. doi: 10.1038/ng1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu SM, Lee DH, Sullivan JM, Chung D, Jager A, Shum BO, Sarvetnick NE, Anderson AC, Kuchroo VK. Differential IL-21 signaling in APCs leads to disparate Th17 differentiation in diabetes-susceptible NOD and diabetes-resistant NOD. Idd3 mice. J Clin Invest. 2011;121:4303–4310. doi: 10.1172/JCI46187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Immunity. 2004;117:265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 8.Sgouroudis E, Albanese A, Piccirillo CA. Impact of protective IL-2 allelic variants on CD4+ FoxP3+ regulatory T cell function in situ and resistance to autoimmune diabetes in NOD mice. J Immunol. 2008;181:6283–6292. doi: 10.4049/jimmunol.181.9.6283. [DOI] [PubMed] [Google Scholar]
- 9.McGuire HM, Vogelzang A, Hill N, Flodstrom-Tullberg M, Sprent J, King C. Loss of parity between IL-2 and IL-21 in the NOD Idd3 locus. Proc Natl Acad Sci U S A. 2009;106:19438–19443. doi: 10.1073/pnas.0903561106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malek TR, Bayer AL. Tolerance, not immunity crucially depends on IL-2. Nat Rev Immunol. 2004;4:665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 11.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O’Shea JJ. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Quintana FJ, Jin H, Burns EJ, Nadeau M, Yeste A, Kumar D, Rangachari M, Zhu C, Xiao S, Seavitt J, Georgopoulos K, Kuchroo VK. Aiolos promotes TH17 differentiation by directly silencing Il2 expression. Nat Immunol. 2012;13:770–777. doi: 10.1038/ni.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson AC, Chandwaskar R, Lee DH, Kuchroo VK. Cutting Edge: the Idd3 genetic interval determines regulatory T cell function through CD11b+CD11c− APC. J Immunol. 2008;181:7449–7452. doi: 10.4049/jimmunol.181.11.7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 15.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin-17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin-27 limits autoimmune encephalomyelitis by suppressing the development of interleukin-17 producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 17.Murugaiyan G, Mittal A, Lopez-Diego R, Maier LM, Anderson DE, Weiner HL. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J Immunol. 2009;183:2435–2443. doi: 10.4049/jimmunol.0900568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujimoto H, Hirase T, Miyazaki Y, Hara H, Ide-Iwata N, Nishimoto-Hazuku A, Saris CJ, Yoshida H, Node K. IL-27 inhibits hyperglycemia and pancreatic islet inflammation induced by streptozotocin in mice. Am J Pathol. 2011;179:2327–2336. doi: 10.1016/j.ajpath.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 20.Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Das Sarma J, Gran B, Zhang GX, Rostami A. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 21.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 22.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O’Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 23.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 24.Hall AO, Beiting DP, Tato C, John B, Oldenhove G, Lombana CG, Pritchard GH, Silver JS, Bouladoux N, Stumhofer JS, Harris TH, Grainger J, Wojno ED, Wagage S, Roos DS, Scott P, Turka LA, Cherry S, Reiner SL, Cua D, Belkaid Y, Elloso MM, Hunter CA. The cytokines interleukin 27 and interferon-gamma promote distinct Treg cell populations required to limit infection-induced pathology. Immunity. 2012;37:511–523. doi: 10.1016/j.immuni.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maier LM, Anderson DE, Severson CA, Baecher-Allan C, Healy B, Liu DV, Wittrup KD, De Jager PL, Hafler DA. Soluble IL-2RA levels in multiple sclerosis subjects and the effect of soluble IL-2RA on immune responses. J Immunol. 2009;182:1541–1547. doi: 10.4049/jimmunol.182.3.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory Th17 cells. Nature. 2007;448:484–488. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Expression of IL-2 receptor chains and IL-2 promotion of IFN-γ production in CD11b+ CD11c− cells.
A) CD11b+CD11c− cells were isolated from the spleens of NOD and NOD.Idd3 mice by cell sorting and stimulated with CpG for 4 h as indicated. Cells were then lysed for RNA isolation and use in real-time PCR. IL2Rβ (n= 3), IL2Rγc (n=3). ns=not significant. B) Expression of IL2Rβ and IL2Rγc on NOD CD11b+CD11c− cells before (black line) and after stimulation with CpG (gray line). Filled histogram, fluorescence minus one control. C) CD11b+CD11c− cells were prepared as in (A) and stimulated with either CpG or PGN. Left panel, CD25 (IL2Rα) (n=4),*p=0.0003, **p=0.006, t-test. ns=not significant. Middle and right panels, data from two independent experiments. C) CD11b+CD11c− cells were isolated from the spleens of NOD mice and stimulated with PGN in the presence or absence of 25ng/mL IL-2 for 24h, (n=5). *p=0.0447, t-test. Cytokine measured by CBA.
Supplemental Figure 2. IL-27 p28 is mainly produced by CD11b+ CD11c− cells.
NOD APC were T-depleted and stimulated for 18h with media, CpG, or CpG+IL-2. Cells were washed, incubated with GolgiStop for 4h and intracellular cytokine staining preformed. Data are representative of five experiments.