Abstract
Psychosocial stress is associated with altered immunity, anxiety and depression. Previously we showed that repeated social defeat (RSD) promoted microglia activation and social avoidance behavior that persisted for 24 days after cessation of RSD. The aim of the present study was to determine if imipramine (a tricyclic antidepressant) would reverse RSD-induced social avoidance and ameliorate neuroinflammatory responses. To test this, C57BL/6 mice were divided into treatment groups. One group from RSD and controls received daily injections of imipramine for 24 days, following 6 cycles of RSD. Two other groups were treated with saline. RSD mice spent significantly less time in the interaction zone when an aggressor was present in the cage. Administration of imipramine reversed social avoidance behavior, significantly increasing the interaction time, so that it was similar to that of control mice. Moreover, 24 days of imipramine treatment in RSD mice significantly decreased stress-induced mRNA levels for IL-6 in brain microglia. Following ex vivo LPS stimulation, microglia from mice exposed to RSD, had higher mRNA expression of IL-6, TNF-α, and IL-1β, and this was reversed by imipramine treatment. In a second experiment, imipramine was added to drinking water confirming the reversal of social avoidant behavior and decrease in mRNA expression of IL-6 in microglia. These data suggest that the antidepressant imipramine may exert its effect, in part, by down-regulating microglial activation.
Keywords: Psychosocial stress, Social defeat, Imipramine, Social avoidance, Microglia
1. Introduction
Psychosocial stress stimulates the hypothalamic–pituitary– adrenal (HPA) axis and the sympathetic nervous system (SNS), triggering the release of catecholamines, glucocorticoids and pro-inflammatory cytokines such as interleukin (IL)-6, (IL)-1, and tumor necrosis factor (TNF)-α. Activation of neuroendocrine and autonomic pathways has a profound impact in physiological responses in both humans and rodents (Blanchard et al., 2001; Kiecolt-Glaser and Glaser, 2002; Kinsey et al., 2007). Converging translational evidence suggests that psychosocial stress-induced, peripheral immune dysregulation and neuroinflammation, contribute to the development of depressive-like and anxiety-like behaviors (Voorhees et al., 2013; Wohleb et al., 2011, 2013, 2014). Pro-inflammatory cytokines such as IL-6, IL-1β, and TNF-α modulate neuronal activity (Ozaktay et al., 2006). Likely, stress-induced neuroinflammatory signaling increases neuroplasticity that can lead to modification in the connectivity between neurons and neuronal circuits underlying behavioral disorders such as prolonged anxiety and depressive symptoms (Koo and Duman, 2008; Koo et al., 2010; Elliott et al., 2010; Christoffel et al., 2011).
The clinically relevant psychosocial stress model of repeated social defeat (RSD), promotes brain region-specific activation of brain CD11b+ cells (microglia/macrophages) that leads to anxiety-like behavior. In addition, microglia isolated from socially defeated mice have high levels of IL1-β mRNA expression and reduced levels of glucocorticoid responsive genes (glucocorticoid-induced leucine zipper (GILZ) and FK506 binding protein-51 (FKBP51) (Wohleb et al., 2011). Furthermore, microglia isolated from these mice and cultured ex vivo produced increased levels of IL-6, TNF-α, and monocyte chemoattractant protein-1 (MCP-1/CCL-2) following mitogen-stimulation with lipopolysaccharide (LPS) compared to microglia from home cage controls (Wohleb et al., 2011). RSD enhances reactivity of microglia and macrophages in a brain-dependent manner. In a previous study we determined reactivity of microglia and macrophages through Iba-1 staining in the medial amygdala, pre-frontal cortex (PFC), and paraventricular nucleus of the hypothalamus, after 6 days of RSD. These findings showed that social defeat enhanced the active microglia phenotype in several areas of the brain associated with fear and threat appraisal, after 6 days of RSD (Wohleb et al., 2011). Iba-1 labeling of microglia and increased Iba-1 proportional area was detected in the PFC at .5, 8, and 24 days after RSD. Additionally, immunoreactivity was also detected in the amygdala, hippocampus (HPC)-cornu ammonis 3 (CA3), and HPC-dentate gyrus at .5 and 8 days after RSD, but no longer was detected by 24 days. These data suggest that microglia return to a surveying state after RSD, in a time-dependent manner (Wohleb et al., 2014).
Clinical and experimental approaches indicate that antidepressants attenuate brain expression of pro-inflammatory cytokines and evoke neuroprotective and immunomodulatory effects (Sluzewska et al., 1995; Xia et al., 1996; Yirmiya et al., 2001; Castanon et al., 2002; Hashioka et al., 2007; Hwang et al., 2008). In clinical studies, the therapeutic effects of antidepressants seem to be related to the immune status of depressed patients when treatment is initiated. For example, when depressed patients had enhanced immune activation, antidepressants attenuated secretion of cytokines. Elevated plasma levels of IL-6 in patients suffering from acute depression were reduced when these patients were treated with fluoxetine, a selective serotonin reuptake inhibitor (Sluzewska et al., 1995). Antidepressants also normalized increased counts of monocytes, leukocytes and neutrophils in depressed patients (Seidel et al., 1996; Maes et al., 1997). Conversely, when immunity was not altered in depressed patients at the initiation of treatment, antidepressants had no effects on immune function.
In animal models, imipramine (a tricyclic antidepressant) and fluoxetine produce immune suppression and anti-inflammatory effects by suppressing the production of cytokines such as TNF-α, IL-1β, and IL-6 by glial cells (Ha et al., 2006; Lim et al., 2009; Liu et al., 2011). Imipramine inhibited interferon (IFN)-γ stimulated microglial production of IL-6 and nitric oxide (Hashioka et al., 2007), and TNF-α production in microglia and astrocyte cultures (Hwang et al., 2008). In addition to the effects on immune function, antidepressants can also modulate behavior. Specifically, imipramine treatment ameliorated LPS-induced depressive-like behavior in rats, decreased anhedonia, anorexia, weight loss, reduced social, locomotor, and exploratory behaviors (Yirmiya, 1996; Yirmiya et al., 2001). In mice subjected to social stress, 28 days of chronic administration of fluoxetine or imipramine, but not acute administration (1 day), improved social interaction in the social avoidance behavioral test (Berton et al., 2006; Tsankova et al., 2006).
Recent findings from our laboratory showed that RSD promotes long-lasting microglial activation associated with social avoidance behavior, which is maintained for at least 24 days after cessation of RSD (Wohleb et al., 2014). Thus, we aimed to determine: (1) if imipramine treatment reversed RSD-induced social avoidance behavior and (2) if the stress-induced neuroinflammatory profile, maintained at 24 days after RSD, was attenuated with imipramine treatment.
2. Materials and methods
2.1. Animals
Male C57BL/6 (6–8 weeks old) and CD-1 (12 months, retired breeders) mice were obtained from Charles River Breeding Laboratories (Wilmington, Massachusetts) and allowed to acclimate to their surroundings for 7–10 days before initiation of experimental procedures. C57BL/6 mice were housed in cohorts of three and CD-1 mice were singly housed and maintained at 21 °C under a 12:12 h light: dark cycle with ad libitum access to water and rodent chow in the animal facility at The Ohio State University. All procedures were in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the Ohio State University Institutional Laboratory Animal Care and Use Committee.
2.2. Repeated social defeat (RSD)
RSD was performed as described previously (Wohleb et al., 2011). Briefly, an intruder male CD-1 mouse was introduced into home cages of male C57BL/6 mice (three per cage) for 2 h on 6 consecutive nights. Behavior was observed to make certain that the intruder was aggressive. If the intruder did not initiate an attack within 5–10 min or was attacked by resident mice, a new intruder was introduced. At the end of the 2 h the intruder was removed and the resident mice were left undisturbed until the next day when the same paradigm was repeated. During RSD, resident mice display submissive behaviors such as upright posture, fleeing, and crouching (Avitsur, 2001; Stark et al., 2001; Hanke et al., 2012). Home cage control (HCC) cohorts were left undisturbed in a separate room.
2.3. Pharmacological treatments and administration procedures
C57BL/6 mice subjected to RSD and HCC were randomly selected for inclusion into different experimental treatment groups. The groups were: RSD/imipramine, RSD/vehicle, HCC/imipramine, and HCC/vehicle. Mice in the RSD/imipramine received daily intraperitoneal (i.p.) injections of imipramine (20 mg/kg) for 24 days after the 6 cycles of RSD. HCC/imipramine received daily i.p. imipramine at the same dose while RSD/vehicle and HCC/vehicle groups received i.p. injections of vehicle (sodium chloride, 0.9%) for 24 days at the same time point (Fig. 1A). This dose and timing was chosen since previous studies had shown that chronic (4 weeks) but not acute (1 day) imipramine treatment after social defeat, at this concentration, reversed social avoidance behavior in C57BL/6 mice (Berton et al., 2006; Tsankova et al., 2006). Additionally, we had previously shown social avoidance behavior was still present 24 days after RSD (Wohleb et al., 2014). Therefore, based on these studies, we decided to treat the animals for 24 days after the last cycle of RSD to assess if there was a reversal of social avoidance. The day after the last injection of imipramine the interaction and avoidance toward an unfamiliar CD-1 mouse was measured.
Fig. 1.
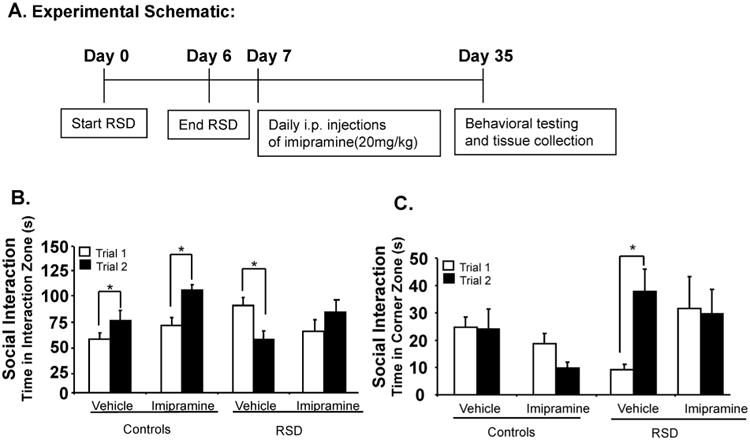
Imipramine treatment reversed RSD-induced social avoidance 24 days after stress cessation. (A) Experimental schematic. (B) Social withdrawal was exacerbated when an aggressor was introduced in the social target trial (Trial 2) in RSD mice treated with vehicle, with a decreased time spent in the interaction zone and this was reversed with imipramine i.p. treatment (20 mg/kg). (C) RSD mice treated with vehicle spent more time in the corners during the social target trial (Trial 2). Bars represent the mean ± SEM. Means with asterisk (*) are significantly different from each other (p < 0.05). Trial 1: time without intruder; Trial 2 = time with intruder.
2.4. Social avoidance test
Social avoidance behavior was determined as previously described (Wohleb et al., 2014). In brief, the social avoidance test consists of two trials. In the first trial, an experimental mouse was placed into the arena with an empty wire mesh cage and activity was recorded for 2.5 min. In the second trial, the experimental mouse was placed in the arena with an unfamiliar CD-1 mouse in the wire mesh cage and activity was recorded for the same amount of time. Time in the interaction zone and time spent in the corners was video-recorded and analyzed using Noldus EthoVision Software (Leesburg, Virginia) (n = 12–15 per group).
2.5. Splenocyte isolation and culture conditions
Spleens from experimental and control mice were aseptically removed and mechanically disrupted in 5 ml of ice-cold Hanks balanced salt solution (HBSS) using a Model 80 Biomaster Lab System Stomacher (Seward, Riverview, FL) as previously described (Avitsur, 2001 and Stark et al., 2001). The homogenized solution was centrifuged at 4 °C and 1800 rpm for 8 min. The supernatant was discarded and red bloods cells from the resulting pellet were lysed with 1 ml of room temperature red blood cell lysis buffer (4.4 g NH4Cl, 0.5 g KHCO3, 0.019 g EDTA, 500 ml distilled H2O) for two minutes followed by 5 ml HBSS + 10% heat-inactivated fetal bovine serum (FBS) to neutralize the lysis reaction. The solution was filtered through a 70 μm pore nylon filter and centrifuged at 4 °C at 1800 rpm for 8 min. The supernatant was discarded and the resulting pellet was resuspended (2.5 × 106 cells/ml) in 5 ml of supplemented RPMI medium (10% heat-inactivated FBS, 0.075% sodium bicarbonate, 10 mM HEPES buffer, 100U/ml penicillin G, 100 μg streptomycin sulfate/ml, 1.5 mM l-glutamine, and 0.00035% 2-mercaptoethanol). Cell counts were obtained using a Z2 Coulter Counter (Beckman-Coulter, Brea, CA). During processing, all solutions were kept on ice. Cells were plated at a concentration of one million cells per well. One million cells were resuspended in 1 ml of 10%/FBS/RPMI. Total splenocytes were harvested at this concentration for RNA isolation using TRIzol® reagent (Invitrogen Inc.) (n = 6 per group).
2.6. Splenocyte RNA isolation and Real time PCR
Samples were centrifuged at 4 °C and 12,000g for 10 min. Supernatant was collected and each sample received 200 μl of chloroform, followed by vortexing and centrifugation at 12,000g for 15 min. The upper aqueous phase was decanted into a fresh tube, to which isopropanol was added to precipitate the RNA. This solution was centrifuged at 12,000g for 10 min, supernatant was removed, and the resulting pellet was washed with 1 ml 75% EtOH to remove residual protein. The pellet was resuspended in 20 μl nuclease free water. RNA concentration was measured by spectrophotometry (Implen, Westlake Village, CA) and RNA was reverse transcribed to cDNA using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Quantitative PCR was performed using a Taqman Gene Expression Assay to observe for potential changes in expression of IL-6, TNF-α, and IL-1β. Expression was analyzed using an ABI Prism 7000 Sequence Detection System (Applied Biosystems; Foster City, CA) using the 2–ΔΔ Ct method with normalization glyceraldehyde-3 phosphate dehydrogenase (GAPDH) (n = 6 per group).
2.7. Isolation of microglia
Isolation of microglia was performed from whole-brain homogenates as described previously (Henry et al., 2008, 2009; Wynne et al., 2010; Wohleb et al., 2011). Briefly, brains were homogenized in HBSS, pH 7.4, by mashing the brain through a 70 μm nylon mesh cell strainer. The homogenates were centrifuged at 500×g for 6 min at 10 °C. Supernatants were decanted and the pellets were resuspended in 70% isotonic Percoll (GE Healthcare, Pittsburgh, PA) at room temperature. A Percoll density gradient was layered in this manner: 70%, 50%, 35%, and 0% isotonic Percoll. The gradient was centrifuged at 2000×g for 20 min at 10 °C and microglia was taken by aspirating the interphase between the 50% and 70% Percoll layers (Frank et al., 2006; Nair et al., 2007; Wohleb et al., 2011). The retrieved cells were washed and resuspended in sterile HBSS and centrifuged at 600×g for 6 min at 10 °C. The supernatant was decanted and viable cells were counted using 0.1% trypan blue staining in an automated cell counter (Luna-FL™ dual fluorescence cell counting, Logos Biosystems, Annandale, VA). Each brain yielded approximately 6.5 × 105 cells. Previous studies (Henry et al., 2009; Wynne et al., 2010) demonstrate that viable cells isolated from brain homogenates through this protocol yields >90% microglia.
2.8. RNA isolation and real-time PCR
RNA from Percoll-isolated microglia was extracted with the RNeasy plus mini-kit (Qiagen®). RNA concentration was assessed by a spectrophotometer (Eppendorf, Hamburg, Germany). RNA was reverse transcribed to obtain cDNA with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems)® and quantitative PCR was done using the Applied Biosystems® by Life Technologies Assay-on-Demand Gene Expression protocol as previously described (Godbout et al., 2005; Wohleb et al., 2011). Briefly, amplification of cDNA was accomplished by real-time PCR. A target cDNA (IL-6, IL1β, and TNF-α) and a reference cDNA (glyceraldehyde-3-phosphate dehydrogenase) were amplified at the same time using an oligonucleotide probe with fluorescent reporter dye (FAM). An ABI PRISM® 7300-sequence detection system (Applied Biosystems® by Life Technologies, Grand Island, New York) was used to determine fluorescence. Data were analyzed by the comparative threshold cycle and the results are given as the fold difference detected (n = 6 per group).
2.9. Flow cytometry
Staining of microglia surface antigens was performed as previously described (Henry et al., 2008, 2009 and Wohleb et al., 2011). In brief, Fc receptors were blocked with anti-CD16/CD32 antibody (eBioscience, San Diego, CA). The cells were then incubated with anti-CD11b-APC, anti-CD45-FITC, and anti-MHC-II-PE antibodies (eBioscience, San Diego, CA). Expression of these surface receptors was determined using a Becton-Dickinson FACSCalibur™ four-color cytometer. Ten thousand events were recorded and microglia were identified by CD11b+ and CD45low expression (Nair and Bonneau, 2006). For each antibody, gating was determined based on appropriate isotype-stained controls. Flow data were analyzed using FlowJo software (Tree Star, San Carlos, CA).
2.10. Ex vivo microglia and cytokine measurement by real-time PCR and ELISA
Microglia were stimulated ex vivo with LPS as described previously (Wohleb et al., 2011). In brief, microglia isolated by Percoll gradient separation were counted and plated on poly-l-lysine-coated 96-well plates. Cells were placed in complete RPMI containing 10% heat inactivated fetal bovine serum, 0.075% sodium bicarbonate, 10 mM HEPES buffer, 100U/ml penicillin G, 100 μg/ml streptomycin sulfate, 1.5 nM l-glutamine, and 0.0035% 2-mercaptoethanol. Cells were then stimulated with 400 nanograms/ml LPS (Sigma-Aldrich®, St. Louis, MO), for 18 h and incubated at 37 °C in 5% CO2 and controls were left unstimulated at same time point and temperature. After cell harvesting, the gene expression of pro-inflammatory cytokines (IL-6, IL-1β, TNF-α) was measured using real time-PCR as described above. Supernatants were collected and the concentration of IL-1 β was detected using Quantikine ELISA Mouse IL-1β/IL-1F2 (R&D Systems™, Minneapolis, MN) (n = 4–6 per group).
2.11. Pharmacological treatment in drinking water
The experiment was repeated for social avoidance behavior and detection of pro-inflammatory cytokines in microglia, and modified by giving experimental mice imipramine (15 mg/kg) in their drinking water. The dose of imipramine was based on a previous study with C57BL/6 mice, in which chronic administration at 15 mg/kg in drinking water effectively increased sucrose and water intake, as well as enhanced home-cage and novelty exploration activities in naïve animals (Strekalova et al., 2013). C57BL/6 mice cohorts subjected to RSD and HCC were randomly selected for inclusion into the four different experimental treatment groups as described in Section 2.3. Mice in the RSD/imipramine group were treated with imipramine in the drinking water for 24 days after the 6 cycles of RSD. HCC/imipramine were also given the same treatment at the same dose at this time point. Animals in the RSD/vehicle and HCC/vehicle groups drank untreated water throughout the experiment (Fig. 3A) (n = 8–9 per group).
Fig. 3.
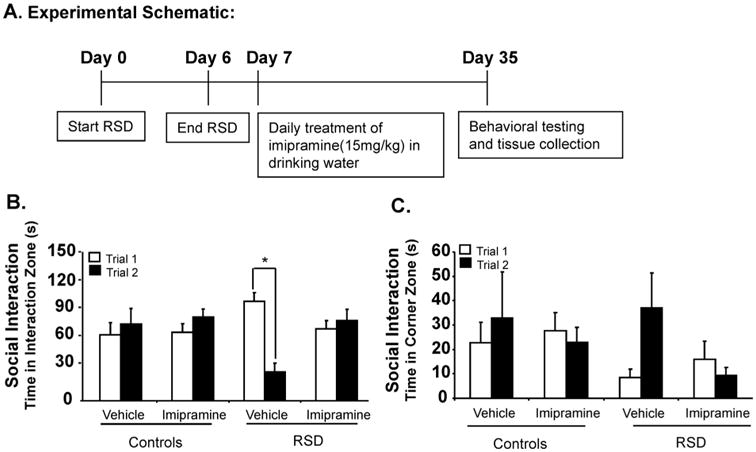
Imipramine diluted in the animal's drinking water reversed RSD-induced social avoidance behavior 24 days after stress cessation. Social Avoidance test was repeated, but this time imipramine was given orally in the animal's drinking water (15 mg/kg). (A) Experimental schematic is shown. (B) Time spent in the interaction zone was significantly decreased in RSD mice treated with vehicle, when an aggressor was introduced in the social target trial (Trial 2), and was reversed with Imipramine treatment. (C) No differences were detected in the time spent in the corners between the groups. Bars represent the mean ± SEM. Means with asterisk (*) are significantly different from each other (p < 0.05). Trial 1: time without intruder; Trial 2 = time with intruder.
The amount of water consumed for each cage was registered daily throughout the experiment (from Day 0 to Day 35). The calculation of the concentration of imipramine in drinking water was based on the evaluated mean volume of daily water consumption, assessed by weighing the bottles daily (from Day 0 to Day 6). An average of 9.0 ml per day/per cage intake was calculated. Water consumption in the four groups of mice was in fact similar. Based on this average, the desirable dosage of treatment (15 mg/kg/day) was established.
3. Statistical analysis
The data were subjected to Shapiro–Wilk tests to ensure a normal distribution using SPSS Statistics version 21. Data are expressed as means ± SEM. In order to determine significant main effects and interactions between variables, data were subjected to one-way (trial, stress, imipramine), two-way (trial × stress); (trial × imipramine); (stress × imipramine) or three-way (trial × stress × imipramine); (stress × imipramine × LPS) analysis of variances (ANOVAs). When appropriate, differences between treatment group means were analyzed by an F-protected t test using the least-significant difference method. In all cases, the level of significance was set at p ≤ .05.
4. Results
4.1. Imipramine reversed RSD-induced social avoidance behavior
Since RSD promotes long-lasting social avoidance behavior that is maintained for at least 24 days after RSD cessation (Wohleb et al., 2014), our aim was to determine in this experiment if stress-induced social avoidance behavior could be reversed in mice exposed to RSD with imipramine treatment. Mice were subjected to 6 cycles of RSD or left undisturbed (HCC) and subsequently treated chronically with daily i.p. injections of imipramine or vehicle for 24 days. At 24 days after RSD, social avoidance was determined using a two-trial interaction paradigm with an empty social target trial (Trial 1) followed by a social target trial (Trial 2). Fig. 1B shows that mice in the control group treated with either vehicle or imipramine increased time spent in the interaction zone (p < 0.05 for both) when a social target was introduced in the second trial (main effect trial; F(1,28) = 14.53, p < 0.01; main effect imipramine; F(1,28) = 0.7, p > 0.05; trial × imipramine interaction; F(1,28) = 0.0006, p = 0.98). Time spent in the interaction zone was significantly decreased in RSD mice treated with vehicle (p < 0.01), when an aggressor was introduced in the social trial (main effect stress; F(1,55) = 9.62, p < 0.01; main effect imipramine; F(1,55) = 4.41, p < 0.05; stress × imipramine; F(1,55) = 5.22, p < 0.05) and this was reversed with imipramine treatment (trial × stress × imipramine interaction; F(1,55) = 4.90, p = 0.03). Fig. 1C shows that there were no differences between the control treatment groups in the time spent in corners (p = 0.39), however, during the social trial RSD mice treated with vehicle spent more time in the corners and less time in the interaction zone (p < 0.01) (main effect stress; F(1,55) = 9.62, p < 0.01; main effect imipramine; F(1,55) = 4.41, p < 0.05; stress × imipramine; F(1,55) = 5.22, p < 0.05). Together, these data indicate that imipramine reversed stress-induced social avoidance behavior.
4.2. RSD enhanced TNF-α, IL-6 and IL1-β expression in total splenocytes 24 days after stress cessation
In this experiment we sought to determine if RSD increased pro-inflammatory cytokine production by total spleen cells 24 days after cessation of stress (Table 1). TNF-α relative gene expression was significantly enhanced in total splenocytes of RSD mice treated with vehicle in comparison to HCC and RSD mice treated with imipramine (p < 0.05); (main effect stress; F(1,23) = 11.81, p < 0.01; main effect imipramine; F(1,23) = 8.53, p < 0.01). Stress induced the relative gene expression of IL-6 (main effect stress; F(1,23) = 14.08, p < 0.01) and a trending decrease of the expression of this cytokine was observed in RSD mice treated with imipramine (main effect imipramine; F(1,23) = 3.74, p = 0.065). Likely, RSD mice had increased levels of IL1-β in total spleen cells (effect of stress; F(1,23) = 15.08, p < 0.01), and a trending decrease of expression was detected with imipramine treatment in RSD mice (effect of imipramine; F(1,23) = 3.16, p = 0.088). In sum, RSD promoted the mRNA expression of pro-inflammatory cytokines 24 days after stress cessation in total splenocytes, and imipramine partially attenuated the production of IL-6 and IL-1β in these cells.
Table 1.
Total splenocytes of RSD animals treated with vehicle had increased mRNA expression of TNF-α at 24 days after cessation of stress, and this was attenuated with imipramine i.p. treatment (20 mg/kg). Enhanced levels of IL-6 and IL-1β mRNA were detected in both stressed groups at this time point. Imipramine did not fully block the expression of either IL-6 or IL1-β.
| Gene | Total splenocytes mean relative gene expression (24 days after RSD) | |||
|---|---|---|---|---|
|
| ||||
| Vehicle | Imipramine | |||
|
|
|
|||
| VCON | VRSD | ICON | IRSD | |
| TNF-α | 1.01 ± 0.15a,c | 1.91 ± 0.38b | 0.58 ± 0.18a | 1.11 ± 0.06c |
| IL-6 | 1.11 ± 0.08a | 2.92 ± 0.79b | 0.67 ± 0.20a | 1.72 ± 0.23c |
| IL-1β | 1.10 ± 0.23a | 2.43 ± 0.58b | 0.39 ± 0.19a | 1.76 ± 0.32b |
Means with different letters (a,b,c) are significantly different from each other (p < 0.05). CON = home caged controls; RSD = repeated social defeat; VCON = vehicle controls; ICON = imipramine controls; VRSD = vehicle repeated social defeat; IRSD = imipramine repeated social defeat.
4.3. Imipramine ameliorates RSD-induced over expression of IL-6 in microglia
To elucidate if the RSD-induced neuroinflammatory profile at 24 days after RSD was attenuated by imipramine treatment, gene expression of three major pro-inflammatory cytokines, IL-6, IL1-β, and TNF-α was examined in microglia (Table 2). Increased relative gene expression of IL-6 was maintained at 24 days after cessation of RSD in mice treated with vehicle (main effect stress; F(1,23) = 25.20, p < 0.01 and main effect imipramine; F(1,23) = 18.34, p < 0.01). Imipramine attenuated relative gene expression of IL-6 (stress × imipramine interaction; F(1,23) = 4.91, p = 0.04). There were no differences in TNF-α and IL-1β relative gene expression at 24 days between the RSD and control groups (p > 0.05 for both). These data suggest that imipramine may reduce long-lasting RSD-induced neuroinflammation by decreasing gene expression of IL-6.
Table 2.
Increased neuroinflammatory signaling was still observed 24 days after stress cessation in mice treated with vehicle and ameliorated in RSD mice treated with imipramine. Following behavioral testing, microglia were collected by Percoll gradient separation. RSD markedly increased relative gene expression of IL-6 even at 24 days after cessation of stress, and this was decreased with imipramine i.p. treatment. No differences in IL1-β and TNF-α mRNA expression at 24 days between the RSD and control groups were seen.
| Gene | Microglia mean relative gene expression (24 days after RSD) | |||
|---|---|---|---|---|
|
| ||||
| Vehicle | Imipramine | |||
|
|
|
|||
| VCON | VRSD | ICON | IRSD | |
| IL-6 | 1.01 ± 0.24a | 2.35 ± 0.21b | 0.61 ± 0.06a | 1.14 ±0.18a |
| TNF-α | 1.10 ± 0.52a | 1.89 ± 0.92b | 0.14 ± 0.36a | 0.90 ± 0.38a |
| IL-1β | 1.01 ± 0.31a | 2.64 ± 0.88a,c | 0.29 ± 0.15a,b | 1.06 ±0.36a,b,c |
Means with different letters (a,b,c) are significantly different from each other (p < 0.05). CON = home caged controls; RSD = repeated social defeat; VCON = vehicle controls; ICON = imipramine controls; VRSD = vehicle repeated social defeat; IRSD = imipramine repeated social defeat.
4.4. Imipramine attenuates RSD-induced reactivity of microglia to LPS stimulation
We had previously reported that microglia isolated from RSD mice and cultured ex vivo produced markedly higher levels of pro-inflammatory cytokines (IL-6 and TNF-α) compared to HCC (Wohleb et al., 2011), after 6 cycles of RSD. Therefore, the objective of the next experiment was to determine if microglia from RSD mice have increased reactivity after LPS stimulation 24 days after RSD cessation, and determine if imipramine treatment reduced microglia response to LPS stimulation. To address this objective, brains were collected at 24 days after RSD. Microglia were collected by Percoll gradient separation and cultured ex vivo. Microglia were plated in growth media, in a 96-well tissue culture plate at 1 × 105 cells per well. Cells were incubated with LPS and supernatants and cells were collected 18 h later. Relative gene expression of IL-1β, IL-6, and TNF-α was determined from these cells. Stimulation with LPS induced higher gene expression of IL-1β, IL-6, and TNF-α (Table 3) in microglia from RSD mice treated with vehicle, 24 days after RSD cessation (main effect stress; F(1,41) = 9.2, p < 0.01, F(1,42) = 6.2, p = 0.02,F(1,40) = 5.6, p = 0.02, respectively), and this over expression was reduced with imipramine treatment (stress × imipramine × LPS interaction; F(1,41) = 4.4, p = 0.04 for IL-1β, F(1,42) = 5.58, p = 0.02 for IL-6, and F(1,40) = 5.25, p = 0.03 for TNF-α). Fig. 2 shows increased protein levels of IL1-b in supernatants of RSD mice treated with vehicle after LPS stimulation compared to all the other mitogen-stimulated groups (p < 0.01 between each group), meaning that imipramine reversed the exaggerated response to mitogen-stimulation (stress × imipramine × LPS interaction; F(1,41) = 17.00, p < 0.01). Collectively, these data suggests that RSD microglia are more reactive and have an increased response to a subsequent inflammatory stimuli, even 24 days after the RSD.
Table 3.
RSD increased reactivity of microglia 24 days after stress cessation. Imipramine treatment blocked microglia activation. Male C57BL/6 mice were subjected to six cycles of RSD and treated with imipramine i.p. injections as described in Fig. 1A. Brains were collected at 24 days after RSD. Enriched microglia were collected and cultured ex vivo. Cells were incubated with LPS (0.1 μM) and IL-1β, IL-6, and TNF-α relative gene expression was determined from these cells collected 18 h later. Means represent the relative fold change increase over vehicle controls in media ± SEM.
| Gene | Microglia mean relative gene expression after LPS stimulation (normalized to VCON media) 24 days after RSD | |||
|---|---|---|---|---|
|
| ||||
| Vehicle | Imipramine | |||
|
|
|
|||
| VCON | VRSD | ICON | IRSD | |
| IL-1β | 18.05 ± 3.49a | 67.32 ± 14.86b | 7.60±4.27a | 17.06 ±6.69a |
| IL-6 | 6.84 ± 1.56a | 30.53 ±9.87b | 4.73 ± 1.61a | 6.04 ± 0.95a |
| TNF-α | 12.72 ± 0.38a | 106.21 ±39.04b | 20.11 ± 8.34a | 21.87 ± 7.93a |
Means with different letters (a, b) are significantly different from each other (p < 0.05). CON = home caged controls; RSD = repeated social defeat; VCON = vehicle controls; ICON = imipramine controls; VRSD = vehicle repeated social defeat; IRSD = imipramine repeated social defeat.
Fig. 2.
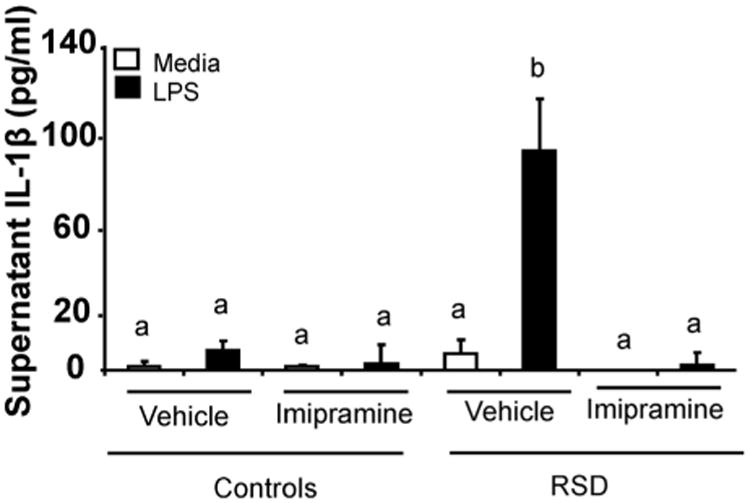
IL-1β protein levels were determined in the supernatant collected 18 h after LPS stimulation. Bars represent the relative fold change increase over vehicle controls in media ± SEM determined by RT-PCR, and bars represent protein levels (pg/ml) determined by ELISA. Means with different letters (a,b,c,d) are significantly different from each other (p < 0.05). CON = home caged controls; RSD = repeated social defeat; VCON = vehicle controls; ICON = imipramine controls; VRSD = vehicle repeated social defeat; IRSD = imipramine repeated social defeat.
4.5. Treatment with imipramine in the drinking water reversed stress-induced social avoidance behavior
In order to confirm the reversal of RSD-induced social avoidance behavior with imipramine treatment, the drug was diluted in the animal's drinking water. Animals from the RSD/imipramine group were given imipramine (15 mg/kg) daily via drinking water, for 24 days after 6 cycles of RSD. HCC/imipramine group started the same treatment at this time point and dose, while RSD/vehicle and HCC/vehicle groups drank plain water throughout the whole experiment. Social avoidance was determined using the two-trial interaction paradigm with an empty social target trial followed by a social trial. Mice in the control group treated with either vehicle or imipramine showed no differences between the first and second trial in the interaction zone when a social target was introduced in the second trial (main effect trial, F(1,28) = 0.25, p = 0.62; trial × imipramine interaction, F(1,28) = 0.13, p = 0.72). Time spent in the interaction zone was significantly decreased in RSD mice treated with vehicle (p < 0.01), when an unfamiliar CD-1 mouse was introduced in the social trial (main effect stress; F(1,28) = 5.76, p < 0.05; stress × imipramine interaction; F(1,28) = 4.69, p < 0.05; trial × stress interaction; F(1,28) = 15.7, p < 0.01) and was reversed with imipramine treatment in the drinking water (trial × stress × imipramine interaction; F(1,28) = 10.97, p < 0.01) (Fig. 3B). There were no differences between the groups in time spent in the corners between trial 1 and trial 2 (main effect stress; F(1,28) = 0.02, p > 0.05; main effect imipramine; F(1,28) = 0.27, p > 0.05; stress × imipramine interaction; F(1,28) = 0.28, p > 0.05 (Fig. 3C).
4.6. Imipramine in the drinking water reduced RSD-induced IL-6 relative gene expression in microglia
Relative gene expression was determined in animals treated with imipramine (15 mg/kg) in the drinking water (Table 4). RSD markedly increased relative gene expression of IL-6 in microglia 24 days after stress cessation (main effect stress; F(1,32) = 10.11, p < 0.01), and this was decreased with imipramine treatment diluted in the drinking water (stress × imipramine interaction; F(1,32) = 12.44, p < 0.01). TNF-α relative gene expression was increased in the RSD/vehicle group compared to HCC of either groups (main effect stress; F(1,32) = 10.06, p < 0.01), nonetheless there was no difference when compared to RSD/imipramine group (main effect imipramine; F(1,32) = 2.34, p = 0.14). An increased relative gene expression level of IL-1β was present in the RSD groups treated with vehicle and imipramine (main effect stress; F(1,32) = 11.46, p < 0.01, main effect imipramine; F(1,32) = 0.83, p = 0.37) compared to HCC.
Table 4.
Increased neuroinflammatory signaling was observed 24 days after stress cessation in mice treated with vehicle. Imipramine given orally ameliorated neuroinflammatory responses in RSD mice. RSD markedly increased relative gene expression of IL-6 in microglia after 24 days of stress cessation, and this was decreased with imipramine treatment diluted in the drinking water (15 mg/kg). TNF-α relative gene expression and IL1-β relative gene expression are shown. Means represent the relative fold change increase over vehicle controls in media ± SEM.
| Gene | Microglia mean relative gene expression 24 days after RSD | |||
|---|---|---|---|---|
|
| ||||
| Vehicle | Imipramine | |||
|
|
|
|||
| VCON | VRSD | ICON | IRSD | |
| IL-6 | 0.95 ±0.21a | 5.30 ± 1.09b | 1.09 ±0.39a | 0.87 ± 0.24a |
| TNF-α | 0.99 ± 0.09a | 1.73 ±0.24b | 1.09 ±0.05a | 1.24 ±0.15a,b |
| IL-1β | 0.91 ± 0.07a | 1.69 ±0.22b | 1.04 ±0.02a | 1.28 ±0.05b |
Means with different letters (a, b) are significantly different from each other (p < 0.05). CON = home caged controls; RSD = repeated social defeat; VCON = vehicle controls; ICON = imipramine controls; VRSD = vehicle repeated social defeat; IRSD = imipramine repeated social defeat.
5. Discussion
The present study provides evidence that chronic treatment with imipramine by i.p. injection (20 mg/kg) or by oral administration (in drinking water) (15 mg/kg), caused reversal of stress-induced social avoidance behavior. Imipramine inhibits the reuptake of serotonin, a neurotransmitter that plays a pivotal role in aggressive and socioaffective appraisal cues (Challis et al., 2014). An increase in serotonin, by pharmacological manipulation in several species, promotes socioaffective stimuli, whereas depletion of serotonin negatively shifts social responses to avoidance and aggressive behaviors (Canli and Lesch, 2007; Passamonti et al., 2012).
We had previously shown that 24 days after RSD cessation markers of immune alterations associated with RSD, such as splenomegaly, plasma IL-6, and the number of circulating CD11b+ cells returned to control levels. Moreover, brain macrophage population returned to control levels by 24 days after RSD. IL-1β and TNF-α mRNA levels returned to baseline by 24 days, nonetheless IL-6 mRNA level was still elevated at 24 days (Wohleb et al., 2014). In the present follow-up series of experiments, in parallel to the behavior, RT-qPCR analysis showed that imipramine either administered by i.p. injection or orally, significantly reduced stress-induced expression of IL-6. One of the ways imipramine may be attenuating stress-induced neuroinflammatory signaling is the fact that microglia have serotonin receptors. It has been suggested, that physiological concentrations of serotonin are required to have a balance, low intracellular levels of serotonin are required for a reduced production of pro-inflammmatory cytokines (Kenis and Maes, 2002). Furthermore, one of imipramine's mechanism of medicinal action is antagonizing adreno-receptors, hence its anti-anxiety properties. Previous work has demonstrated that RSD triggered anxiety-like behavior and enhanced the inflammatory state in the periphery and in the central nervous system in a β-adrenergic dependent manner (Hanke et al., 2012; Wohleb et al., 2011). Pretreatment of RSD mice with propranolol, a nonselective adrenergic receptor antagonist, blocked RSD-induced microglia/macrophage activation and social defeat-induced anxiety, as well as expression of c-Fos in brain regions associated with fear and threat appraisal (Wohleb et al., 2011). It is plausible that imipramine may be antagonizing directly the activation of these adrenergic receptors and decreasing pro-inflammatory cytokine production. Moreover, a number of in vivo studies have suggested that antidepressants increase intracellular levels of cAMP in immune cells, through activation of monoamine receptors (Xia et al., 1996; Edgar et al., 1999). As a result, pharmacological enhancement of cAMP dampens the expression of pro-inflammatory cytokines (Benbernou et al., 1997; Brideau et al., 1999; Eigler et al., 1998; Platzer et al., 1999) by inhibiting the protein kinase A (PKA) pathway (Xia et al., 1996; Hashioka et al., 2007).
In agreement with another study (Yirmiya et al., 2001), imipramine blunts inflammatory activation of microglia but may not fully suppress pro-inflammatory cytokine production in total splenocytes. Microglia from RSD mice treated with imipramine failed to produce exaggerated levels of pro-inflammatory cytokines IL-6, IL-1β, TNF-α, ex vivo, after LPS stimulation, compared to RSD mice treated with vehicle. As a matter of interest, there was still a pro-inflammatory phenotype in total splenocytes, 24 days after stress cessation. Imipramine failed to fully attenuate mRNA expression of pro-inflammatory cytokines in total splenocytes. It is plausible, that microglia are more sensitive to the anti-inflammatory effect of imipramine than splenocytes. There is evidence that shows a different regulation and responsiveness to LPS administration in central and peripheral cytokine production (Yirmiya et al., 2001).
Microglia from RSD mice, have long-lasting enhanced sensitivity to inflammatory challenges and an amplified response to external stimuli. Several lines of evidence indicate that antidepressants can negatively regulate LPS-induced nuclear translocation of NF-κβ p65 subunit (Obuchowicz et al., 2014) in microglia. It has been reported that imipramine can shift the balance in the production of pro-inflammatory cytokines such as TNF-α and IL1-β toward IL-10, an anti-inflammatory cytokine, and can suppress LPS-stimulated cytokine release even at very low concentrations in rat glial cultures (Obuchowicz et al., 2014). It has also been found that imipramine reduced the production of IL1-β, TNF-α in LPS-activated murine BV2 microglia cells (Hwang et al., 2008). Imipramine added to culture medium of LPS-stimulated mixed glial cells prevented morphological alterations induced by LPS and transformed microglia cells into cells with a neuron-like morphology (Obuchowicz et al., 2014).
Microglia and brain macrophages directly influence neurophysiology and behavior via cytokines and other secondary mediators. Pro-inflammatory cytokines enhance anorexia, weight loss, and sleep alterations in animal models (Kelly et al., 1997), as well as decrease interest in social exploration (Castanon et al., 2002). Hence, the present results confirm the beneficial anti-inflammatory properties of antidepressants and the effect on preventing glial activation and decreasing expression of pro-inflammatory molecules. In concert, the anti-inflammatory effect of antidepressants is associated with neuroprotection against cell death in microglia/neuron co-cultures (Hwang et al., 2008).
Nowadays it has been postulated that neuroinflammatory signaling plays a crucial role in the development of anxiety and depression. Pro-inflammatory cytokines can modify neurogenesis in the hippocampus (Koo and Duman, 2009) and affect mood. In contrast, treatment with antidepressants stimulates hippocampal neurogenesis and could potentially block atrophy and damage caused by repeated stress (Van Bokhoven et al., 2011). Evidence indicates that circulating levels of IL-6 are associated with behavioral deficits and depressive-like behavior (Voorhees et al., 2013). Likewise, NF-κβ signaling, is essential to show deficits in social aversion (Christoffel et al., 2011). Taken together, our data suggest that the long-lasting stress-induced microglial reactivity and pro-inflammatory phenotype at 24 days after RSD cessation, may be important factors in prolonged social avoidance behavior. Imipramine treatment of RSD mice conferred protection against the production of pro-inflammatory molecules in microglia, impeding the social interaction deficit.
This study demonstrates that imipramine may exert its effects, in part, by down regulating neuroinflammation. It is not well known whether immunomodulation is a side effect or part of the clinical activity of imipramine. Since, hyper activation of the neuroimmune system is believed to be involved in pathogenesis/development of mood disorders and anxiety, the present study suggests that the anti-inflammatory effect of antidepressants may have protective effects by silencing RSD-induced priming and activation of microglia, thus down regulating the biosynthesis of high levels of pro-inflammatory cytokines. By elucidating the underlying mechanism of antidepressant action and neuroimmune responses clinicians may be able to optimize therapeutic interventions treatment of mood disorders and anxiety.
Acknowledgments
NIH/NIMH Grants to JFS R01 MH097243-02, R01 MH093473-03 and NIH/NIDCR training Grant T32 DE014320-13 supported this research.
Contributor Information
Karol Ramirez, Email: ramirezchan.1@buckeyemail.osu.edu.
Daniel T. Shea, Email: Shea@osumc.edu.
Daniel B. McKim, Email: Daniel.McKim@osumc.edu.
Reader B.F., Email: Reader@osumc.edu.
John F. Sheridan, Email: sheridan.1@osu.edu.
References
- Avitsur R. Social stress induces glucocorticoid resistance in subordinate animals. Horm Behav. 2001;39:247–257. doi: 10.1006/hbeh.2001.1653. [DOI] [PubMed] [Google Scholar]
- Benbernou N, Esnault S, Shin HC, Fekkar H, Guenounou M. Differential regulation of IFN-gamma, IL-10 and inducible nitric oxide synthase in human T cells by cyclic AMP-dependent signal transduction pathway. Immunology. 1997;91(3):361–368. doi: 10.1046/j.1365-2567.1997.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science (New York, NY) 2006;311(5762):864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, McKittrick CR, Blanchard DC. Animal models of social stress: effects on behavior and brain neurochemical systems. Physiol Behav. 2001;73:261–271. doi: 10.1016/s0031-9384(01)00449-8. [DOI] [PubMed] [Google Scholar]
- Brideau C, Van Staden C, Styhler A, Rodger IW, Chan CC. The effects of phosphodiesterase type 4 inhibitors on tumour necrosis factor-alpha and leukotriene B4 in a novel human whole blood assay. Br J Pharmacol. 1999;126:979–988. doi: 10.1038/sj.bjp.0702387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nat Neurosci. 2007;10:1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Castanon N, Leonard B, Neveu P, Yirmiya R. Effects of antidepressants on cytokine production and actions. Brain Behav Immun. 2002;16:569–574. doi: 10.1016/s0889-1591(02)00008-9. [DOI] [PubMed] [Google Scholar]
- Challis C, Beck SG, Berton O. Optogenetic modulation of descending prefrontocortical inputs to the dorsal raphe bidirectionally bias socioaffective choices after social defeat. Front Behav Neurosci. 2014;8:43. doi: 10.3389/fnbeh.2014.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, Krishnan V, Reyes CM, Han MH, Ables JL, Eisch AJ, Dietz DM, Ferguson D, Neve RL, Greengard P, Kim Y, Morrison JH, Russo SJ. IκB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci. 2011;31:314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar VA, Sterin-Borda L, Cremaschi GA, Genaro AM. Role of protein kinase C and cAMP in fluoxetine effects on human T-cell proliferation. Eur J Pharmacol. 1999;372:65–73. doi: 10.1016/s0014-2999(99)00142-9. [DOI] [PubMed] [Google Scholar]
- Eigler A, Siegmund B, Emmerich U, Baumann KH, Hartmann G, Endres S. Anti-inflammatory activities of cAMP-elevating agents: enhancement of IL-10 synthesis and concurrent suppression of TNF production. J Leukoc Biol. 1998;63:101–107. doi: 10.1002/jlb.63.1.101. [DOI] [PubMed] [Google Scholar]
- Elliott E, Ezra-Nevo G, Regev L, Neufeld-Cohen A, Chen A. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat Neurosci. 2010;13:1351–1353. doi: 10.1038/nn.2642. [DOI] [PubMed] [Google Scholar]
- Frank MG, Wieseler-Frank JL, Watkins LR, Maier SF. Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus: immunophenotypic and functional characteristics. J Neurosci Methods. 2006;151:121–130. doi: 10.1016/j.jneumeth.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Ha E, Jung KH, Choe BK, Bae JH, Shin DH, Yim SV, Baik HH. Fluoxetine increases the nitric oxide production via nuclear factor kappa B-mediated pathway in BV2 murine microglial cells. Neurosci Lett. 2006;397:185–189. doi: 10.1016/j.neulet.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Hanke ML, Powell ND, Stiner LM, Bailey MT, Sheridan JF. Beta adrenergic blockade decreases the immunomodulatory effects of social disruption stress. Brain Behav Immun. 2012;26:1150–1159. doi: 10.1016/j.bbi.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashioka S, Klegeris A, Monji A, Kato T, Sawada M, McGeer PL, Kanba S. Antidepressants inhibit interferon-gamma-induced microglial production of IL-6 and nitric oxide. Exp Neurol. 2007;206:33–42. doi: 10.1016/j.expneurol.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huan Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1β and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J, Zheng LT, Ock J, Lee MG, Kim SH, Lee HW, Lee WH, Park HC, Suk K. Inhibition of glial inflammatory activation and neurotoxicity by tricyclic antidepressants. Neuropharmacology. 2008;55(5):826–834. doi: 10.1016/j.neuropharm.2008.06.045. [DOI] [PubMed] [Google Scholar]
- Kelly JP, Wrynn AS, Leonard BE. The olfactory bulbectomized rat as a model of depression: an update. Pharmacol Ther. 1997;74:299–316. doi: 10.1016/s0163-7258(97)00004-1. [DOI] [PubMed] [Google Scholar]
- Kenis G, Maes M. Effects of antidepressants on the production of cytokines. Int J Neuropsychopharmacol. 2002 Dec;5:401–412. doi: 10.1017/S1461145702003164. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R. Depression and immune function: central pathways to morbidity and mortality. J Psychosom Res. 2002;53:873–876. doi: 10.1016/s0022-3999(02)00309-4. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, Bailey MT, Sheridan JF, Padgett DA, Avitsur R. Repeated social defeat causes increased anxiety-like behavior and alters splenocyte function in C57BL/6 and CD-1 mice. Behav Immun. 2007;21:458–466. doi: 10.1016/j.bbi.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci USA. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappa B is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci USA. 2010;107:2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Duman RS. Interleukin-1 receptor null mutant mice show decreased anxiety-like behavior and enhanced fear memory. Neurosci Lett. 2009;456:39–43. doi: 10.1016/j.neulet.2009.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CM, Kim SW, Park JY, Kim C, Yoon SH, Lee JK. Fluoxetine affords robust neuroprotection in the postischemic brain via its anti-inflammatory effect. J Neurosci Res. 2009;87:1037–1045. doi: 10.1002/jnr.21899. [DOI] [PubMed] [Google Scholar]
- Liu D, Wang Z, Liu S, Wang F, Zhao S, Hao A. Anti-inflammatory effects of fluoxetine in lipopolysaccharide (LPS)-stimulated microglial cells. Neuropharmacology. 2011;61(4):592–599. doi: 10.1016/j.neuropharm.2011.04.033. [DOI] [PubMed] [Google Scholar]
- Maes M, Vandoolaeghe E, Van Hunsel F, Bril T, Demedts P, Wauters A, Neels H. Immune disturbances in treatment-resistant depression: modulation by antidepressive treatments. Hum Psychopharm Clin Exp. 1997;12 [Google Scholar]
- Nair A, Bonneau RH. Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. J Neuroimmunol. 2006;171:1–2. 72–85. doi: 10.1016/j.jneuroim.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Nair A, Hunzeker J, Bonneau RH. Modulation of microglia and CD8+ T cell activation during the development of stress-induced herpes simplex virus type-1 encephalitis. Brain Behav Immun. 2007;21:791–806. doi: 10.1016/j.bbi.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Obuchowicz E, Bielecka AM, Paul-Samojedny M, Pudełko A, Kowalski J. Imipramine and fluoxetine inhibit LPS-induced activation and affect morphology of microglial cells in the rat glial culture. Pharmacol Rep. 2014;66:34–43. doi: 10.1016/j.pharep.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Ozaktay AC, Kallakuri S, Takebayashi T, Cavanaugh JM, Asik I, DeLeo JA, Weinstein JN. Effects of interleukin-1 beta, interleukin-6, and tumor necrosis factor on sensitivity of dorsal root ganglion and peripheral receptive fields in rats. Eur Spine J. 2006;15 doi: 10.1007/s00586-005-0058-8. Author abstract. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Crockett MJ, Apergis-Schoute AM, Clark L, Rowe JB, Calder AJ, Robbins TW. Effects of acute tryptophan depletion on prefrontal-amygdala connectivity while viewing facial signals of aggression. Biol Psychiatry. 2012;71(1):36–43. doi: 10.1016/j.biopsych.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platzer C, Fritsch E, Elsner T, Lehmann MH, Volk HD, Prösch S. Cyclic adenosine monophosphate-responsive elements are involved in the transcriptional activation of the human IL-10 gene in monocytic cells. Eur J Immunol. 1999;29:3098–3104. doi: 10.1002/(SICI)1521-4141(199910)29:10<3098::AID-IMMU3098>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Seidel A, Arolt V, Hunstiger M, Rink L, Behnisch A, Kirchner H. Major depressive disorder is associated with elevated monocyte counts. Acta Psychiatr Scand. 1996;94:198–204. doi: 10.1111/j.1600-0447.1996.tb09849.x. [DOI] [PubMed] [Google Scholar]
- Sluzewska A, Rybakowski J, Laciak M, Mackiewicz A, Sobieska M, Wiktorowicv K. Interleukin-6 serum levels in depressed patients before and after treatment with fluoxetine. Ann N Y Acad Sci. 1995;762:474–476. doi: 10.1111/j.1749-6632.1995.tb32372.x. [DOI] [PubMed] [Google Scholar]
- Stark JL, Avitsur R, Padgett DA, Campbell KA, Beck FM, Sheridan JF. Social stress induces glucocorticoid resistance in macrophages. Am J Physiol Regul Integr Comp Physiol. 2001;49 doi: 10.1152/ajpregu.2001.280.6.R1799. [DOI] [PubMed] [Google Scholar]
- Strekalova T, Anthony DC, Dolgov O, Anokhin K, Kubatiev A, Steinbusch HM, Schroeter C. The differential effects of chronic imipramine or citalopram administration on physiological and behavioral outcomes in naïve mice. Behav Brain Res. 2013 May;15:101–106. doi: 10.1016/j.bbr.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Van Bokhoven P, Oomen CA, Hoogendijk WJ, Smit AB, Lucassen PJ, Spijker S. Reduction in hippocampal neurogenesis after social defeat is long-lasting and responsive to late antidepressant treatment. Eur J Neurosci. 2011;33:1833–1840. doi: 10.1111/j.1460-9568.2011.07668.x. [DOI] [PubMed] [Google Scholar]
- Voorhees JL, Tarr AJ, Wohleb ES, Godbout JP, Mo X, Sheridan JF, Eubank TD, Marsh CB. Prolonged restraint stress increases IL-6, reduces IL-10, and causes persistent depressive-like behavior that is reversed by recombinant IL-10. PloS one. 2013;8(3) doi: 10.1371/journal.pone.0058488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF. β-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31:6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, McKim DB, Shea DT, Powell ND, Tarr AJ, Sheridan JF, Godbout JP. Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol Psychiatry. 2014;75(12):970–981. doi: 10.1016/j.biopsych.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci. 2013;33:13820–13833. doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne AM, Henry CJ, Huang Y, Cleland A, Godbout JP. Protracted downregulation of CX 3CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain Behav Immun. 2010;24:1190–1201. doi: 10.1016/j.bbi.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, DePierre JW, Nassberger L. Tricyclic antidepressants inhibit IL-6, IL-1b and TNF-a release in human blood monocytes and IL-2 and interferon-g in T cells. Immunopharmacology. 1996;34(2–3):27. doi: 10.1016/0162-3109(96)00111-7. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Pollak Y, Barak O, Avitsur R, Ovadia H, Bette M, Weihe E, Weidenfeld J. Effects of antidepressant drugs on the behavioral and physiological responses to lipopolysaccharide (LPS) in rodents. Neuropsychopharmacology. 2001;24(5):531–544. doi: 10.1016/S0893-133X(00)00226-8. [DOI] [PubMed] [Google Scholar]
- Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996;711:163–174. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]


