Abstract
Receptor-interacting protein 140 (RIP140) is highly expressed in the brain, and acts in neurons and microglia to affect emotional responses. The present study reveals an additional function of RIP140 in the brain, which is to regulate brain lipid homeostasis via its action in astrocytes. We found forced swim stress (FSS) significantly reduces the expression level of RIP140 and elevates cholesterol content in the brain. Mechanistically, FSS elevates endoplasmic reticulum stress, which suppresses RIP140 expression by increasing microRNA 33 (miR33) that targets RIP140 mRNA’s 3′-untranslated region. Consequentially, cholesterol biosynthesis and export are dramatically increased in astrocyte, the major source of brain cholesterol. These results demonstrate that RIP140 plays an important role in maintaining brain cholesterol homeostasis through, partially, regulating cholesterol metabolism in, and mobilization from, astrocyte. Altering RIP140 levels can disrupt brain cholesterol homeostasis, which may contribute to behavioral stress-induced neurological disorders.
Keywords: RIP140, astrocyte, cholesterol, stress
1. Introduction
Stressful experiences can contribute to mood changes and psychological and neurological disorders. For instance, chronic stress leads to hippocampal structure remodeling and cognitive impairment in rodents (McEwen et al., 2012). While alteration in adrenal steroid and neurotransmitter release can cause brain structural changes and behavioral disorders (McEwen, 2012), the molecular mechanism underlying stress-induced neurological disorder is poorly understood. Recent studies indicated that, chronic stress such as social defeats can disrupt lipid metabolism by increasing transcriptional activity of genes involved in cholesterol synthesis (Chuang et al., 2010); as a result, the level of circulating cholesterol is significantly elevated minutes after exposure to psychological stressor (Muldoon et al., 1992). Brain contains the highest level of cholesterol, and almost all the brain cholesterol is formed by de novo synthesis due to efficient blockade in the uptake of circulating cholesterol by the blood-brain barrier (Wang and Eckel, 2014). A disruption in brain cholesterol level has also been related to cognitive impairment (Martin et al., 2010). However, whether and how brain cholesterol homeostasis may be directly affected by stress is unclear.
Receptor-interacting protein 140 (RIP140), a transcriptional co-regulator for numerous transcription factors and a signal transduction regulator, is recently implicated in the regulation of cognitive function, emotional response and neuron health via its activities in microglia and neurons (Feng et al., 2014, Flaisher-Grinberg et al., 2014). RIP140 is first known to co-regulate the activities of many nuclear receptors/transcription factors that regulate lipid metabolism. For instance, in adipose tissues, RIP140 regulates the storage of lipids by inhibiting the expression of genes involved in fatty acid oxidation (Leonardsson et al., 2004, Ho et al., 2011). In hepatocytes and macrophages, RIP140 is involved in both positive and negative actions of liver X receptor (LXR)-regulated lipid and glucose metabolism (Herzog et al., 2007). In microphages, cholesterol overload activates microRNA 33 (miR33), a regulator of hepatocyte cholesterol homeostasis which decreases RIP140 mRNA level (Ping-Chih Ho, 2011). RIP140 is highly expressed in the brain (Lee et al., 1998) and has been detected in various cell types including neurons, astrocytes and microglia. Whole body RIP140 knockout mice are lean and exhibit memory deficits and increased stress responses, in addition to numerous defects particularly in reproduction (Duclot et al., 2012). Macrophage specific RIP140 knockdown mice have decreased microglia RIP140 level in ventromedial hypothalamus and the cingulate cortex, and exhibit increased anxiety- and depressive-like behaviors (Flaisher-Grinberg et al., 2014). More recently, we found that loss of RIP140 in hippocampal neurons can result in increased vulnerability to ER stress-induced death (Feng et al., 2014). Whether RIP140 plays a role in another prominent cell type of the brain, astrocyte, is still unclear.
In the current study, we aim to determine if RIP140 expression in the brain responds to stress, and whether this response is causally related to brain cholesterol metabolism. Forced swim stress (FSS) was used to generate the behaviorally stressed animal model. RIP140 level and cholesterol metabolic genes expression, as well as cholesterol content, in the brain and primary cultured astrocyte were examined. The experiments results show that a stressor like FSS can decrease RIP140 level in the brain, and simultaneously increase brain cholesterol level. Further, RIP140 negatively regulates cholesterol biosynthesis in, and cholesterol exportation from, astrocyte. Thus, a reduction in RIP140 expression such as in astrocytes, which can be caused by FSS, can result in brain cholesterol accumulation. This may contribute to certain stress-induced pathological outcomes in the brain including cognitive impairment.
2. Materials and Methods
2.1. Porsolt forced swim stress (FSS) and behavioral test
Male C57BL/6 mice (8–9 weeks old), from Charles River Laboratories, were maintained and experimental procedures were conducted according to NIH guidelines and approved by the University of Minnesota Institutional Animal Care and Use Committee (Protocol No. 1306A30679). All behavioral assessments were performed as previously described (Flaisher-Grinberg et al., 2012, Flaisher-Grinberg et al., 2014). The general activity was assessed by the automated open field environments. Motor coordination and balance were determined by the grid walk test and the rotarod apparatus to indicate the physical abnormalities (Crawley, 2008).
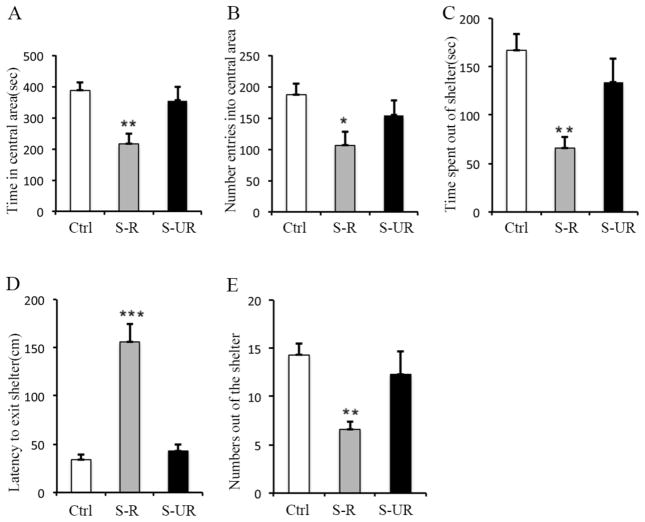
Repeated exposure to a modified Porsolt forced swim test (FST) was used to induce stress (FSS). Mice were placed in a transparent cylindrical container (30 cm tall, 20 cm in diameter), filled to a depth of 20 cm with tap water at 30°C, to swim for 15 min each trial. Between each trial, the water was replaced and the mice were towel dried and returned to their home cage placed on heat pad to keep the mice warm. The trial was repeated for 14 days (1 trial per day). At the day 15, all mice were subjected to the stress-like behavioral tested with automated open field test and emergence test. In the automated open field test, the time spent in the central area of open field, number of enters into the central area of open field, and as well as the distance travelled in the central were recorded. In the emergence test, the time spent out of the shelter, latency to exit shelter and number of out of the shelter were recorded.
The stress-like behaviors of the normal control (n=9) and FSS (n=12) groups were assessed according to the five recordings (Rec 1: time in the central area; Rec 2: number enters into the central area; Rec 3: time spent out of the shelter; Rec 4: latency to exit shelter; Rec 5: number of out of the shelter). For each recording, the average of the control group was defined as “standard”, indicating normal behaviors with regard to this particular recording. Each recording of a stressed mouse was converted into a percentage change, compared to the standard, for that particular recording. An overall percentage change (indicated as “Average”) was determined by averaging the five recordings for each animal. The stressed animals were subdivided into stress responsive (S-R) and non-responsive (S-UR) groups based on the average percentage change: stressed animal displaying > 30% difference in its average percentage change was regarded as stress responsive; stressed animal displaying < 10% difference in its average percentage change was regarded as stress non-responsive. Stressed animals with an average percentage change of 10%–30% were excluded from further experiments.
2.2 Cell culture
Primary astrocyte from day 1 mice pups was performed as previously described (Feng et al., 2011). Briefly, whole cortex were isolated from neonatal mice and digested in 0.25% trypsin at 37°C. The dissociated cells were cultured in poly-D-lysine (5 μg/mL) coated flasks in DMEM/F12 (10% FBS) at 37°C under 5% CO2. Neurons and microglia were removed by shaking the flasks in the culture box for 15 h at 250 rpm, and the 3-week old cells were used for the experiments. Cultures consisting of more than 95% astrocytes were determined by glial fibrillary acidic protein (GFAP) immunocytochemical staining.
Mouse hippocampal neuron cells (HT22 cells, from Salk Institute) were maintained with DMEM supplemented with 10% FBS and differentiated in neurobasal medium with N2 supplement for 3 days before treatment.
2.3. siRNAs and transfection
Scrambled RNA and siRNAs for RIP140 (5′-CGGCGTTGACATCAAAGAA-3′ and 5′-GCTTCTTTCTTTAATCTAA-3′, SI02698759, Qiagen) were transfected into astrocytes or HT22 cells to knockdown RIP140 using HiPeFect transfect reagent (301707, QIAGEN) according to the manufactural instruction. siRNA for miR33 (SI02655450, Qiagen) was transfected into astrocytes to knockdown miR33 by HiPeFect transfect reagent.
2.4. Gene expression assay by quantitative real-time PCR (qPCR)
Total RNA was isolated from astrocytes and HT22 cells or the different brain region by TRIzol reagent (Invitrogen, Carlsbad, CA, USA). RNA samples’ cDNA was synthesized using Omniscript RT kit (QIAGEN), and qPCR was performed with SYBR-Green QPCR reagent (Agilent, Santa Clara, CA, USA) and detected by the Mx3005P QPCR system (Agilent). QPCR primer sequences were presented in supplemental table1.
The miR33 expression level was detected using real-time PCR with miR33 specific primer (MS00032697, Qiagen).
2.5. Immunofluorescence (IF) assay
Animals were anesthetized and transcardially perfused with 0.9% saline followed by 4% paraformaldehyde. Brain samples were post fixed with 4% paraformaldehyde overnight and equilibrated in 20% and 30% sucrose. Coronal sections of 15 μm were prepared with a sliding microtome. IF was performed using as primary antibody rabbit anti-RIP140 and Bip or goat anti-GFAP IgG (Abcam, ab42126, ab21685, ab7260) and as secondary antibody FITC, Cy3 or Cy5 conjugated monkey anti-rabbit IgG (Santa Cruz). Nuclei were stained by DAPI (Sigma-Aldrich). Images were acquired with an Olympus FluoView 1000 IX2 upright confocal microscope. The fluorescence intensity presenting RIP140 or Bip level was calculated with Zen 2011(ZEISS) software.
2.6. Cholesterol assay
Cellular and brain tissue cholesterol content were detected with Amplex® Red Cholesterol Assay Kit (Invitrogen, A12216) according to the manufactural instruction. The free cholesterol in hippocampal neurons was labeled by filipin staining and images were acquired with an Olympus FluoView 1000 IX2 upright confocal microscope. The fluorescence intensity presenting cholesterol level was calculated with Zen 2011software.
2.7. Mouse stereotaxic apparatus injection
The 2′O-Methyl RNA based antisense miR33 (miR33 inhibitor: mC/ZEN/mA mAmUmG mCmAmA mCmUmA mCmAmA mUmGmC mA/3ZEN/) and control RNA were purchase from Integrated DNA Technology. The miR33 inhibitor (25 μM in 2 μl saline) or scrambled RNA was delivered to hippocampus using stereotaxic apparatus at anteroposterior (AP) 2.0 mm, medial-lateral (ML) 1.2 mm and dorsoventral (DV)1.6 mm according to previous describe (Feng et al., 2014). The level of RIP140 mRNA and miR33 was detected 4 days later using qPCR.
2.8. Statistical Analyses
Statistical significance for multiple comparisons was determined by Student’s t-test and one-way analysis of variance (ANOVA) using SPSS17.0 software and summarized as the mean ± SEM of repeated measures; p< 0.05 was considered statistically significant at the 95% level.
3. Results
3.1. Forced swim stress elevates brain cholesterol biosynthesis
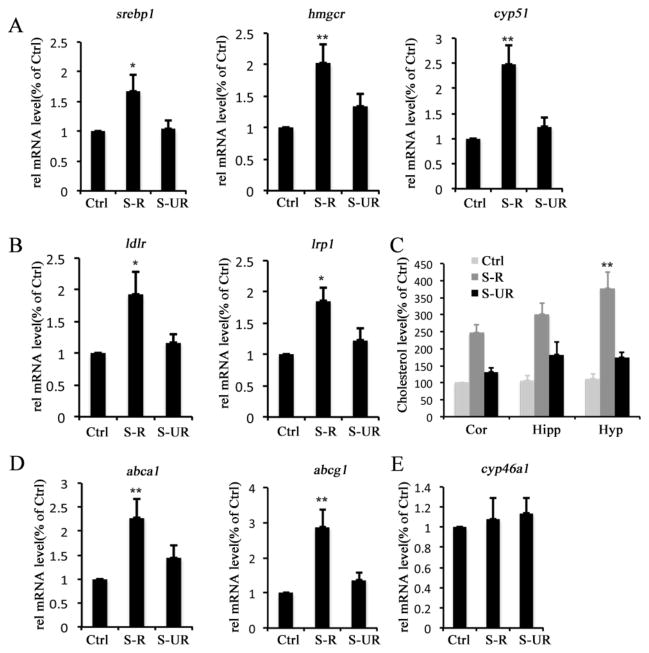
To determine the effect of behavioral stress on brain cholesterol metabolism, we performed forced swim stress (FSS) experiments using mice. Following FSS, the stress-like behavior was evaluated by automated open field and emergence tests. Animals exhibited significant (>30% of the difference of behavioral score between stressed animal and normal controlled animal) increase in the latency to exit shelter and decrease in time spent out of shelter, as well as decreased entries into the central areas of open field or out of shelter were regarded as stress-responsive group (S-R) (Fig. 1). The animals exhibited similar behavior (<10% of the difference of behavioral score between stressed animals and normal controlled animals) as the normal controlled animals (non-stressed animals) were regarded as stress unresponsive group (S-UR) (Fig. 1). Original behavioral scores are provided in supplemental table 2. Gene expression in hippocampi involved in cholesterol metabolism was examined using quantitative PCR. The results show that the levels of srebp1, hmgcr and cyp51 gene are all significantly increased in stress-responsive animals after FSS (Fig. 2A), and the expression levels of genes mediating neuronal uptake of cholesterol, such as ldlr and lrp1, or astroglial exportation of cholesterol, such as abca1 and abcg1, are also increased (Fig. 2B and D). Additionally, total cholesterol level is elevated in the cortex (Cor), hippocampus (Hipp) and hypothalamus (Hyp) of stress-responsive animals (Fig. 2C). However, there is no significant difference in the expression level of cyp46a1 that mediates the turnover of cholesterol to 24S-hydrocholesterol in neurons between normal and stressed animals (Fig. 2E). These results demonstrate that behavioral stress, such as FSS, can alter the homeostasis of brain cholesterol.
Figure 1. Forced swim stress (FSS) induces behavioral stress.
There 12 mice were subjected to forced swim stress (FSS) daily for 14 days, at day 14, following FSS, the stress like behavioral was assessed using automated open field and emergence tests. A, Reduction of time in the central area of open field from stress-responsive group (5 mice) compared with normal controlled group (9 mice, without FSS) and stress-unresponsive group (5 mice). B, Reduced number of enters into the central area of open field from stress-responsive group (5 mice) compared with normal controlled group (9 mice, without FSS) and stress-unresponsive group (5 mice). C, Reduction of time spent out of the shelter from stress-responsive group (5 mice) compared with normal controlled group (9 mice, without FSS) and stress-unresponsive group (5 mice). D, Trend towards an increase in latency to exit shelter from stress-responsive group (5 mice) compared with normal controlled group (9 mice, without FSS) and stress-unresponsive group (5 mice). E, Numbers out of the shelter. The statistic results are presented as means ± SEM., *p<0.05, **p<0.01 and ***p<0.001 compare to control group determined by one-way analysis of variance.
Figure 2. Behavioral stress disrupts brain cholesterol homeostasis.
The animals were subjected to forced-swim stress (FSS) to induce behavioral stress. The stress-like behavior was evaluated using automated open field test and emergence test. According to the different behavioral response after FSS, the animals were divided to two groups, stress responsive, which displayed stress-like behavior and stress unresponsive, which exhibited no stress-like behavior. A, Expression levels of cholesterogenic genes of hippocampus from normal control group (Ctrl), stress-responsive group (S-R) and stress unresponsive group (S-UR). B, Expression levels of lipoprotein receptor genes in hippocampus from Ctrl, S-R and S-UR groups. C, Cholesterol levels of cortex (Cor), hippocampi (Hipp) and hypothalamus (Hyp) from Ctrl, S-R and S-UR groups. D, Expression levels of genes mediates cholesterol exportation from astrocytes in hippocampus from Ctrl, S-R and S-UR groups. E, Expression level of cyp46a1, which mediates cholesterol removal from neurons, in hippocampi of Ctrl, S-R and S-UR groups. The statistic results are presented as means ± SEM., (n=5–9), *p<0.05 and **p<0.01 compare to control group determined by one-way analysis of variance.
3.2. FSS decreases RIP140 expression level
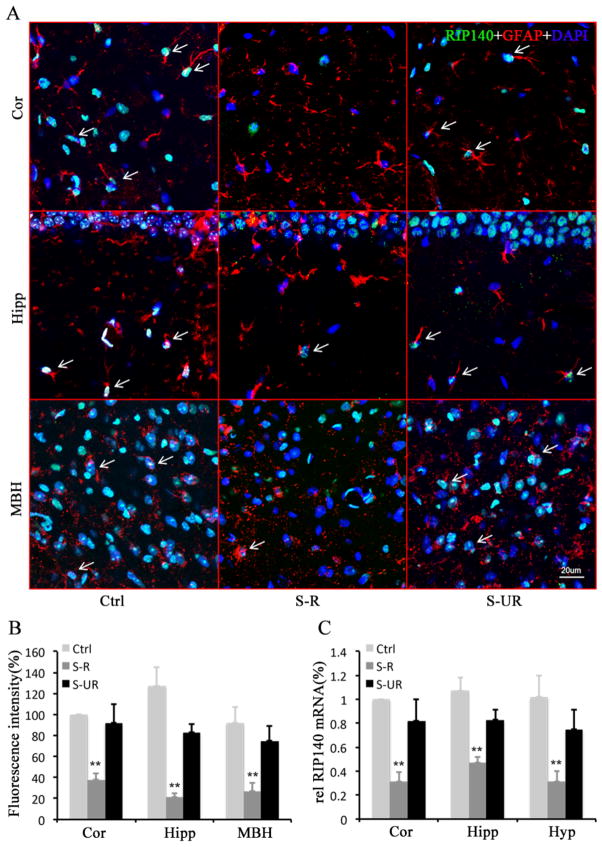
Since RIP140 is known to play important role in regulating lipid metabolism, brain function and animal behavior, we then determined whether FSS affected RIP140, such as its expression, in the brain. We monitored RIP140 protein in cortex, hippocampus and hypothalamus of normal control, stress-responsive and stress-unresponsive animals by immunofluorescence staining. The results show that immunoreactive RIP140 signal in astrocyte is significantly reduced in stress-responsive animals, but it remains largely unaltered in stress-unresponsive animals (Fig. 3A and B). Consistently, RIP140 mRNA level is down-regulated in different brain areas of stress-responsive animals (Fig. 3C). These results indicate that this type of behavioral stress down-regulates the expression of RIP140 in specific brain regions at both mRNA and protein levels.
Figure 3. Behavioral stress decreases brain RIP140 expression level.
A, Confocal microscopy images showing decreased RIP140 (green) expression in astrocyte (red) of the cortex (Cor), hippocampus (Hipp) and medial basal hypothalamus (MBH) from stress-responsive group (S-R) compared with normal control group (Ctrl) and stress unresponsive group (S-UR). B, Quantified results of fluorescence intensity representing RIP140 levels from 7 different fields of hippocampus (anteroposterior 2.0 mm, medial-lateral 1.2 mm and dorsoventral 1.6 mm). C, Expression level of RIP140 mRNA in the cortex (Cor), hippocampi (Hipp) and hypothalamus (Hyp) from Ctrl, S-R and S-UR groups. Scale bar, 20 μm. The statistic results are presented as means ± SEM., (n=7), **p<0.01 compare to control group determined by one-way analysis of variance.
3.3. Behavioral stress-induced reduction in RIP140 involves ER stress and miR33
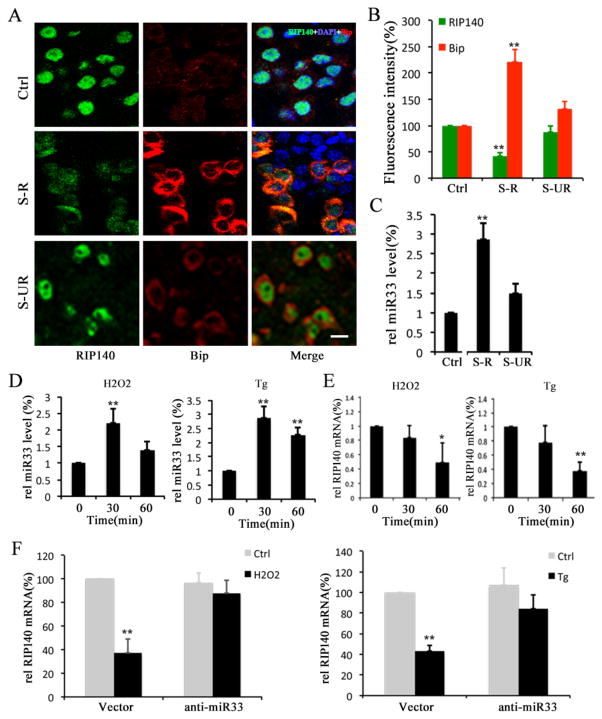
Recent studies have shown that elevated ER stress and oxidative stress are involved in certain behavioral stress-induced neurological disorders (Madrigal et al., 2001, Lindholm et al., 2006, Lee et al., 2009). We speculated whether ER stress was involved in the FSS-induced behavioral stress, therefore we monitored ER stress marker Bip. As shown in Figure 4 A and B, Bip protein level is increased in the brain of stress-responsive animals, but not in stress-unresponsive animals, suggesting that elevated ER stress is associated with the manifestation of this type of behavioral stress.
Figure 4. Activated microRNA33 (miR33) following behavioral stress contributes to a decrease in RIP140 level.
A, Confocal microscopy images showing RIP140 (Green) expression and ER stress marker Bip (Red) in hippocampi from normal control group (Ctrl), stress-responsive group (S-R) and stress unresponsive group (S-UR). Scale bar, 20 μm. B, Quantified results of fluorescence intensity showing RIP140 and Bip expression from 7 different fields of hippocampus. C, Expression level of miR33 in hippocampi of Ctrl, S-R and S-UR groups. D, Expression level of miR33 in astrocytes treated with oxidative stress inducer H2O2 and ER stress inducer thapsigargin (Tg). E, Expression level of RIP140 mRNA in astrocytes treated with H2O2 and Tg. F, Expression level of RIP140 mRNA in astrocytes, with or without miR33 knockdown, treated with H2O2 and Tg. The statistic results are presented as means ± SEM., (n=5–7), *p<0.05 and **p<0.01 compare to control group determined by one-way analysis of variance.
We next determined whether microRNAs involved in regulating RIP140 and/or cholesterol metabolism might have been affected by behavioral stress. We monitored the expression levels of miR33 (released by SREBP activation and down-regulating RIP140 mRNA), miR106b and miR758 (both highly expressed in brain and regulating cholesterol level) (Ramirez et al., 2011, Kim et al., 2012). As shown in Figure 4 C, miR33 level indeed is robustly elevated in stress-responsive animals, but not in stress-unresponsive animals. We also examined three other microRNAs that have been implicated in either cholesterol metabolism (miR106b and miR758) or stress response (miR34c). We found no apparent changes in either miR106b or miR758 level between the control and the stress groups, but an increase in miR34c in stressed mice (Supplemental Figure1). These results support the success of our stress induction, and rule out potential contribution by other microRNAs in our experimental system.
We further examined whether RIP140 mRNA level can be elevated by directly decreasing miR33 level in vivo. The 2′O-Methyl RNA-based antisense miR33 or control RNA was delivered into hippocampus CA1. The miR33 and RIP140 levels were detected 4 days later. The results show that the miR33 inhibitor indeed significantly suppresses the miR33 level in the injected area (80% decrease) (Supplemental Figure 2 A). Consequentially, the level of RIP140 mRNA is increased (Supplemental Figure 2 B). These results validate that RIP140 mRNA can be stabilized by inhibiting miR33 in the brain. Alteration in miR33 in the brain, such as that triggered by the behavioral stress, can affect RIP140 mRNA levels in the brain.
We followed up with this observation to determine whether miR33 elevation in the brain of stressed animals was caused by ER stress and/or oxidative stress. Since cholesterol metabolism in the brain involves, mainly, astrocytes, we thus examined this stress pathway using primary cultures of astrocytes. The astrocyte cultures were treated with oxidative stress inducer H2O2 or ER stress inducer thapsigargin (Tg), and the expression levels of miR33 and RIP140 were both determined. As shown in Figure 4D and E, both H2O2 and Tg elevated the miR33 level, with a corresponding decrease in the RIP140 mRNA level. Further, knockdown of miR33 in these cells before their exposure to H2O2 or Tg down-regulated the RIP140 mRNA level (Fig. 4F). These results demonstrate that behavioral stress such as FSS can be manifested as oxidative and/or ER stress. In astrocytes, this then down-regulates RIP140 level in a miR33-dependent manner.
3.4. RIP140 inhibits cholesterol biosynthesis in astrocytes
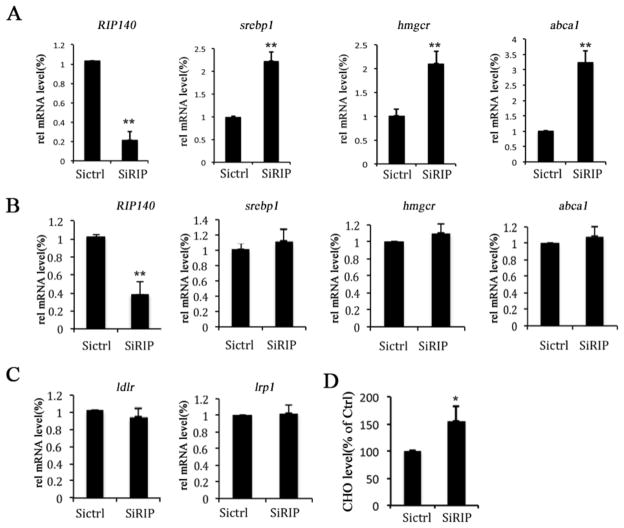
In adult brains, astrocyte provides the main source of cholesterol, whereas neurons uptake astrocyte-derived cholesterol, mediated mainly through the lipoprotein receptor pathway. We speculated that a reduction in astrocyte’s RIP140 level, as shown above, might regulate astrocyte cholesterol metabolism, which will affect cholesterol homeostasis in the brain. It appears that RIP140 knockdown in astrocytes indeed significantly increases the expression of genes related to cholesterol metabolism such as srebp1, hmgcr and abca1 (Fig. 5A), as well as an elevation in the cholesterol content (Fig. 5D). Interestingly, reducing RIP140 in neurons does not change the expression of cholesterol-metabolizing genes (Fig. 5B and C). These results demonstrate that RIP140 plays a role in regulating cholesterol metabolism in the brain, specifically in the astrocyte.
Figure 5. RIP140 negatively regulates brain cholesterol biosynthesis.
Cholesterol metabolic genes expression in primary cortex astrocytes and hippocampal neurons with (SiRIP) or without (SiCtrl) RIP140 knockdown was examined. A, Expression levels of RIP140 and cholesterogenic genes in astrocytes with or without RIP140 knockdown. B, Expression levels of RIP140 and cholesterogenic genes in hippocampal neurons (HT22 cells) with or without RIP140 knockdown. C, Expression levels of lipoprotein receptor genes in hippocampal neurons with or without RIP140 knockdown. D, Cholesterol level in astrocytes with or without RIP140 knockdown. The statistic results are presented as means ± SEM., (n=3), *p<0.05 and **p<0.01 compare to control group determined by student’s t-test.
3.5. RIP140 inhibits cholesterol transport from astrocyte to neurons through an Apo protein-dependent pathway
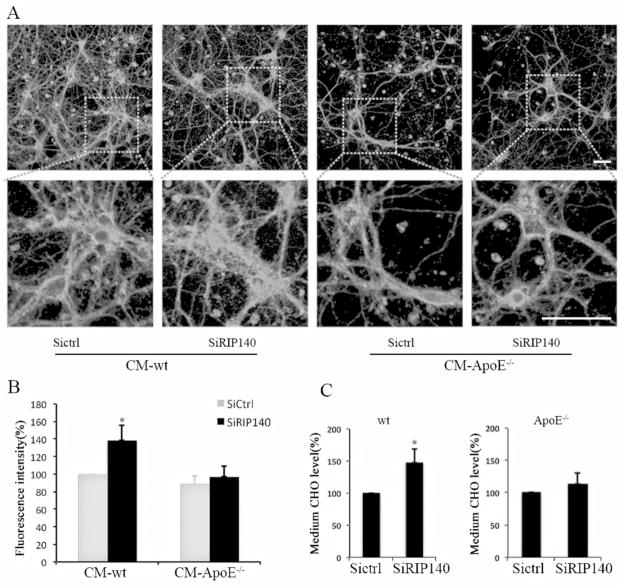
Astrocyte-derived cholesterol can be exported in an apoprotein-dependent pathway via the activation of ABCA1 and ABCG1, which is then taken by neurons (Pfrieger and Ungerer, 2011). To assess the potential activity of RIP140 in regulating cholesterol transportation from astrocyte to neurons, we monitored the intracellular free cholesterol pool of primary hippocampal neurons using filipin staining after incubating these cells with different astrocyte-derived conditioned media (CM). It appears that the cholesterol pool in neurons cultured with the CM of wild type astrocytes where RIP140 is knocked down is significantly increased (indicating increased cholesterol export from astrocyte); however, the intracellular cholesterol pool remains largely unchanged in neurons cultured with CM obtained from ApoE−/− astrocyte, with or without RIP140 knockdown (Fig. 6 A and B). We further confirmed that the cholesterol content in the CM from wild type astrocytes with RIP140 knockdown is increased, but this increase is abolished in those experiments using ApoE−/− astrocyte (Fig. 6C). These results demonstrate that RIP140 negatively regulates cholesterol exportation from astrocyte to neurons.
Figure 6. Reducing astrocyte’s RIP140 accelerates cholesterol exportation from astrocyte and uptake by neurons.
A, Confocal microscopy images showing cholesterol labeled by filipin staining in hippocampal neurons cultured with conditioned medium (CM) from wild-type (wt) or apolipoprotein E (apoE−/−) null astrocyte with (SiRIP) or without (SiCtrl) RIP140 knockdown. Scale bar, 20 μm. B, Quantified results of fluorescence intensity showing free cholesterol levels from 7 different experiments. The statistic results are presented as means ± SEM., *p<0.05 compare to wt-CM of siRNA control group, determined by one-way analysis of variance. C, Cholesterol content in the medium of wt or apoE−/− astrocyte with or without RIP140 knockdown. The statistic results are presented as means ± SEM., (n=3), *p<0.05 compare to siRNA control group determined by student’s t-test.
4. Discussion
RIP140 is highly expressed in the brain and plays multiple roles in maintaining normal brain function. In macrophage/microglia, it functions as a coactivator for NF-κB to activate inflammation (Ho et al., 2012). In neurons exposed to ER stress, RIP140 can be exported to cytoplasm to interact with inositol 1, 4, 5-trisphosphate receptor on ER membrane, thereby modulating calcium mobilization (Feng et al., 2014). The present study uncovers an important response of RIP140 in the brain upon behavioral stress, which is its down regulation through a miR33-dependent pathway. This study also reveals an additional function of RIP140 in the manifestation of behavioral stress, which is to regulate brain cholesterol biosynthesis particularly in the astrocyte.
Disruption in the homeostasis of brain cholesterol, which provides major membrane components especially for the neuron, can be related to neuronal degeneration and cognitive decline (Kotti et al., 2006, Di Paolo and Kim, 2011). Studies have also found that the level of cholesterol in circulation is significantly elevated after exposure to psychological stressor (Muldoon et al., 1992). Since the blood-brain barrier (BBB) can effectively prevent the uptake of cholesterol from circulation into the brain, it is believed that all brain cholesterol is formed by de novo synthesis (Chang et al., 2006). However, the stress-induced disruption in BBB can increase the penetration of circulated cholesterol through BBB (Friedman et al., 1996). Nevertheless, our current study presents the evidence for behavioral stress-induced increase in brain cholesterol biosynthesis and an elevation in the brain cholesterol level, which occurs only in stress-responsive animals. This would suggest that increased brain cholesterol biosynthesis by behavioral stress may elevate the brain cholesterol content. Given that brain cholesterol serves as the principal precursor for steroid hormones and myelin, the increase in brain cholesterol biosynthesis may also facilitate the release of stress hormones and myelination. On this note, behavioral stress has also been implicated in the aberrant activity of synaptic plasticity (Kim et al., 1996).
Behavioral stress has profound effects, including altering brain structure and function. For instance, stress induces spatial memory impairments through modifying synaptic plasticity (Kim et al., 2007). Recent studies indicated that stress-induced alteration of brain function could be related to the epigenetic changes such as through histone modifications (Sailaja et al., 2012). In the current study, after repeated FSS, some mice displayed resistance to FSS stressor. This may be caused by the difference in animals’ resilience which could be due to different epigenetic changes in those mice. Studies have shown that early-life stress experience can induce epigenetic modification of genes related to stress and thus altered response to stress (Meaney and Szyf, 2005). However, effects caused by variations in the epigenetic profiles of these mice could not be excluded (Franklin et al., 2012). Interestingly, we found that the expression levels of RIP140 in the brains of stress-unresponsive animals stay high but decrease in the brains of stress-responsible animals. The stability of RIP140 mRNA is regulated manly by miR33 (Ping-Chih Ho, 2011). We detected significantly increased miR33 only in the brain of stress-responsible animals. Thus, a fluctuation in miR33 may mediate behavioral responses to stress by regulating RIP140 which modulates brain cholesterol metabolism.
Several other microRNAs, such as miR34, miR132 and miR19b, are known to regulate stress response (Haramati et al., 2011, Sailaja et al., 2012, Rodgers et al., 2013). However, none of these has been reported to regulate cholesterol metabolism (Najafi-Shoushtari et al., 2010). The present study shows that in behavioral stress sensitive animals, the expression level of miR33, along with activation of SREPB1, the major isoform of SREBP family in the brain, is dramatically increased following exposure to stress, which contributes to the reduced RIP140 level in multiple brain areas including hippocampus. Study has shown that SREBP can also be activated by oxidative stress and ER stress (Wang et al., 2005). It is tempting to speculate that the activation of SREBP1 in behaviorally stressed animals’ brains may be due to an elevation in ER stress. Indeed, our results show that behavioral stress results in increased expression in ER stress marker Bip. Further, in vitro study using primary astrocyte culture treated with ER stressor thapsigargin (Tg) and oxidative stressor H2O2 show that both stressors can elevate miR33 levels, and correspondingly, decrease RIP140 mRNA level. Further, miR33 knockdown abolishes Tg and H2O2-triggered decrease in RIP140 mRNA level, supporting that the decrease in RIP140 level is dependent on the activation of miR33 in astrocyte.
The major source of cholesterol in adult brain is provided by astrocyte; neurons uptake cholesterol from astrocytes (Dietschy and Turley, 2004, Pfrieger and Ungerer, 2011). Regulation in the exportation of astrocyte-derived cholesterol involves liver X receptor (LXR), which can be co-regulated by RIP140(Herzog et al., 2007). As a target gene of LXR, abca1 expression is robustly increased in the brain upon stress (Fig. 2) and cultured astrocyte upon RIP140 knockdown (Fig. 5). Thus, RIP140 may also modulate neuronal cholesterol uptake through regulating astrocyte cholesterol export in LXR-apolipoprotein E (apoE)-dependent pathway. To dissect brain cholesterol mobilization at the cellular level, we employed conditioned medium (CM) in the experiments. Indeed, depleting RIP140 in astrocyte can increase its expression of genes related to cholesterol metabolism, as well as the cholesterol content in neurons cultured with CM obtained from this astrocyte culture, supporting that RIP140 negatively regulates astrocyte cholesterol biosynthesis and exportation. Importantly, it is not the case in experiments using apoE-null astrocyte culture, supporting that the function of RIP140 in the regulation of astrocyte cholesterol exportation is related to the LXR-dependent pathway. In the adult brain, ApoE is mostly expressed in astrocyte to mediate LXR-regulated cholesterol export from astrocyte, whereas CYP46 is expressed mostly in neurons to remove cholesterol from brain (Pfrieger and Ungerer, 2011). Interestingly, RIP140 knockdown in neurons did not affect cholesterol metabolism in neurons, supporting that adult neurons do not actively synthesize cholesterol in neurons.
In conclusion, the current study reports that behavioral stress significantly reduces RIP140 expression in the mouse brain, which is associated with altered brain cholesterol homeostasis. Mechanistically, behavioral stress suppresses RIP140 expression by increasing miR33 that targets RIP140 mRNA’s 3′-untranslated region. Cholesterol biosynthesis and exportation in astrocyte, the major source of adult brain cholesterol, is dramatically increased as a result of reduction in RIP140 level. This study demonstrates that RIP140 plays an important role in maintaining brain cholesterol homeostasis through regulating cholesterol metabolism in astrocyte, and that miR33 is an important mediator of behavioral stress to regulate RIP140 level. Altering RIP140 levels can disrupt brain cholesterol homeostasis, which may underline behavioral stress-induced neurological disorders.
Supplementary Material
Highlights.
RIP140 expression is reduced in behaviorally stressed mouse brain.
RIP140 expression is associated with cholesterol homeostasis in the brain
RIP140 represses cholesterol biosynthesis in, and exportation from, astrocyte.
Reduction in brain RIP140 expression and corresponding disruption in brain cholesterol homeostasis may underline behavioral stress-induced neurological disorders.
Acknowledgments
This work was supported by DK54733, DK60521, DK54733-11S, Dean’s Commitment, and the Distinguished McKnight Professorship of University of Minnesota to LNW.
Abbreviations
- ABCA
ATP-binding cassette transporter
- ABCG1
ATP-binding cassette sub-family G
- ApoE
Apolipoprotein E
- CYP46
cholesterol 24S-hydroxylase
- CYP51
Sterol 14 alpha-demethylase
- ER
endoplasmic reticulum
- FSS
forced swim stress
- H2O2
Hydrogen peroxide
- HMGCR
3-hydroxy-3-methyl-glutaryl-CoA reductase
- LDLR
low density lipoprotein receptor
- LPR
lipoprotein receptor
- LXR
liver X receptor
- miR33
microRNA 33
- RIP140
Receptor-interacting protein 140
- SREBP
Sterol regulatory element-binding proteins
Footnotes
Statement of Interest
The authors declare that there is no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chang TY, Chang CC, Ohgami N, Yamauchi Y. Cholesterol sensing, trafficking, and esterification. Annual review of cell and developmental biology. 2006;22:129–157. doi: 10.1146/annurev.cellbio.22.010305.104656. [DOI] [PubMed] [Google Scholar]
- Chuang JC, Cui H, Mason BL, Mahgoub M, Bookout AL, Yu HG, Perello M, Elmquist JK, Repa JJ, Zigman JM, Lutter M. Chronic social defeat stress disrupts regulation of lipid synthesis. Journal of lipid research. 2010;51:1344–1353. doi: 10.1194/jlr.M002196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping strategies for mutant mice. Neuron. 2008;57:809–818. doi: 10.1016/j.neuron.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Di Paolo G, Kim TW. Linking lipids to Alzheimer’s disease: cholesterol and beyond. Nature reviews Neuroscience. 2011;12:284–296. doi: 10.1038/nrn3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Thematic review series: brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. Journal of lipid research. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- Duclot F, Lapierre M, Fritsch S, White R, Parker MG, Maurice T, Cavailles V. Cognitive impairments in adult mice with constitutive inactivation of RIP140 gene expression. Genes, brain, and behavior. 2012;11:69–78. doi: 10.1111/j.1601-183X.2011.00731.x. [DOI] [PubMed] [Google Scholar]
- Feng X, Krogh KA, Wu CY, Lin YW, Tsai HC, Thayer SA, Wei LN. Receptor-interacting protein 140 attenuates endoplasmic reticulum stress in neurons and protects against cell death. Nature communications. 2014;5:4487. doi: 10.1038/ncomms5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng XD, Xia Q, Yuan L, Huang HF, Yang XD, Wang K. Gadolinium triggers unfolded protein responses (UPRs) in primary cultured rat cortical astrocytes via promotion of an influx of extracellular Ca2+ Cell biology and toxicology. 2011;27:1–12. doi: 10.1007/s10565-010-9166-2. [DOI] [PubMed] [Google Scholar]
- Flaisher-Grinberg S, Persaud SD, Loh HH, Wei LN. Stress-induced epigenetic regulation of kappa-opioid receptor gene involves transcription factor c-Myc. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:9167–9172. doi: 10.1073/pnas.1205565109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaisher-Grinberg S, Tsai HC, Feng X, Wei LN. Emotional regulatory function of receptor interacting protein 140 revealed in the ventromedial hypothalamus. Brain, behavior, and immunity. 2014;40:226–234. doi: 10.1016/j.bbi.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TB, Saab BJ, Mansuy IM. Neural mechanisms of stress resilience and vulnerability. Neuron. 2012;75:747–761. doi: 10.1016/j.neuron.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Friedman A, Kaufer D, Shemer J, Hendler I, Soreq H, Tur-Kaspa I. Pyridostigmine brain penetration under stress enhances neuronal excitability and induces early immediate transcriptional response. Nature medicine. 1996;2:1382–1385. doi: 10.1038/nm1296-1382. [DOI] [PubMed] [Google Scholar]
- Haramati S, Navon I, Issler O, Ezra-Nevo G, Gil S, Zwang R, Hornstein E, Chen A. MicroRNA as repressors of stress-induced anxiety: the case of amygdalar miR-34. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:14191–14203. doi: 10.1523/JNEUROSCI.1673-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog B, Hallberg M, Seth A, Woods A, White R, Parker MG. The nuclear receptor cofactor, receptor-interacting protein 140, is required for the regulation of hepatic lipid and glucose metabolism by liver X receptor. Mol Endocrinol. 2007;21:2687–2697. doi: 10.1210/me.2007-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho PC, Chuang YS, Hung CH, Wei LN. Cytoplasmic receptor-interacting protein 140 (RIP140) interacts with perilipin to regulate lipolysis. Cellular signalling. 2011;23:1396–1403. doi: 10.1016/j.cellsig.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho PC, Tsui YC, Feng X, Greaves DR, Wei LN. NF-kappaB-mediated degradation of the coactivator RIP140 regulates inflammatory responses and contributes to endotoxin tolerance. Nature immunology. 2012;13:379–386. doi: 10.1038/ni.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Yoon H, Ramirez CM, Lee SM, Hoe HS, Fernandez-Hernando C. MiR-106b impairs cholesterol efflux and increases Abeta levels by repressing ABCA1 expression. Experimental neurology. 2012;235:476–483. doi: 10.1016/j.expneurol.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Foy MR, Thompson RF. Behavioral stress modifies hippocampal plasticity through N-methyl-D-aspartate receptor activation. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:4750–4753. doi: 10.1073/pnas.93.10.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Lee HJ, Welday AC, Song E, Cho J, Sharp PE, Jung MW, Blair HT. Stress-induced alterations in hippocampal plasticity, place cells, and spatial memory. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18297–18302. doi: 10.1073/pnas.0708644104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotti TJ, Ramirez DM, Pfeiffer BE, Huber KM, Russell DW. Brain cholesterol turnover required for geranylgeraniol production and learning in mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3869–3874. doi: 10.1073/pnas.0600316103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Chinpaisal C, Wei LN. Cloning and characterization of mouse RIP140, a corepressor for nuclear orphan receptor TR2. Molecular and cellular biology. 1998;18:6745–6755. doi: 10.1128/mcb.18.11.6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KW, Kim JB, Seo JS, Kim TK, Im JY, Baek IS, Kim KS, Lee JK, Han PL. Behavioral stress accelerates plaque pathogenesis in the brain of Tg2576 mice via generation of metabolic oxidative stress. Journal of neurochemistry. 2009;108:165–175. doi: 10.1111/j.1471-4159.2008.05769.x. [DOI] [PubMed] [Google Scholar]
- Leonardsson G, Steel JH, Christian M, Pocock V, Milligan S, Bell J, So PW, Medina-Gomez G, Vidal-Puig A, White R, Parker MG. Nuclear receptor corepressor RIP140 regulates fat accumulation. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8437–8442. doi: 10.1073/pnas.0401013101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell death and differentiation. 2006;13:385–392. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]
- Madrigal JL, Olivenza R, Moro MA, Lizasoain I, Lorenzo P, Rodrigo J, Leza JC. Glutathione depletion, lipid peroxidation and mitochondrial dysfunction are induced by chronic stress in rat brain. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2001;24:420–429. doi: 10.1016/S0893-133X(00)00208-6. [DOI] [PubMed] [Google Scholar]
- Martin M, Dotti CG, Ledesma MD. Brain cholesterol in normal and pathological aging. Biochimica et biophysica acta. 2010;1801:934–944. doi: 10.1016/j.bbalip.2010.03.011. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The ever-changing brain: cellular and molecular mechanisms for the effects of stressful experiences. Developmental neurobiology. 2012;72:878–890. doi: 10.1002/dneu.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012;62:3–12. doi: 10.1016/j.neuropharm.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues in clinical neuroscience. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldoon MF, Bachen EA, Manuck SB, Waldstein SR, Bricker PL, Bennett JA. Acute cholesterol responses to mental stress and change in posture. Archives of internal medicine. 1992;152:775–780. [PubMed] [Google Scholar]
- Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, Gerszten RE, Naar AM. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science. 2010;328:1566–1569. doi: 10.1126/science.1189123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfrieger FW, Ungerer N. Cholesterol metabolism in neurons and astrocytes. Progress in lipid research. 2011;50:357–371. doi: 10.1016/j.plipres.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Ping-Chih Ho K-CC, Chuang Ya-Shan, Wei Li-Na. Cholesterol regulation of receptor-interacting protein 140 via microRNA-33 in inflammatory cytokine production. The FASEB Journal • Research Communication. 2011:25. doi: 10.1096/fj.10-179267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez CM, Davalos A, Goedeke L, Salerno AG, Warrier N, Cirera-Salinas D, Suarez Y, Fernandez-Hernando C. MicroRNA-758 regulates cholesterol efflux through posttranscriptional repression of ATP-binding cassette transporter A1. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:2707–2714. doi: 10.1161/ATVBAHA.111.232066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:9003–9012. doi: 10.1523/JNEUROSCI.0914-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailaja BS, Cohen-Carmon D, Zimmerman G, Soreq H, Meshorer E. Stress-induced epigenetic transcriptional memory of acetylcholinesterase by HDAC4. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E3687–3695. doi: 10.1073/pnas.1209990110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Eckel RH. What are lipoproteins doing in the brain? Trends in endocrinology and metabolism: TEM. 2014;25:8–14. doi: 10.1016/j.tem.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Kouri G, Wollheim CB. ER stress and SREBP-1 activation are implicated in beta-cell glucolipotoxicity. Journal of cell science. 2005;118:3905–3915. doi: 10.1242/jcs.02513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.