Abstract
Objective
Takayasu’s arteritis is a rare large vessel vasculitis with incompletely understood etiology. We performed the first unbiased genome-wide association study (GWAS) in Takayasu’s arteritis.
Methods
Two independent Takayasu’s arteritis cohorts from Turkey and North America were included in our study. The Turkish cohort consisted of 559 patients and 489 controls, and the North American cohort consisted of 134 European-derived patients and 1,047 controls. Genotyping was performed using the Omni1-Quad and Omni2.5 genotyping arrays. Genotyping data were subjected to rigorous quality control measures and subsequently analyzed to discover genetic susceptibility loci for Takayasu’s arteritis.
Results
We identified genetic susceptibility loci for Takayasu’s arteritis with a genome-wide level of significance in IL6 (rs2069837, OR= 2.07, P= 6.70×10−9), RPS9/LILRB3 (rs11666543, OR= 1.65, P= 2.34×10−8), and an intergenic locus on chromosome 21q22 (rs2836878, OR= 1.79, P= 3.62×10−10). The genetic susceptibility locus in RPS9/LILRB3 is located within the leukocyte receptor complex (LRC) gene cluster on chromosome 19q13.4, and the disease risk variant in this locus correlates with reduced expression of multiple genes including the inhibitory leukocyte immunoglobulin-like receptor gene LILRB3 (P= 2.29×10−8). In addition, we identified candidate susceptibility genes with suggestive levels of association (P <1×10−5) including PCSK5, LILRA3, PPM1G/NRBP1, and PTK2B in Takayasu’s arteritis.
Conclusion
This study identified novel genetic susceptibility loci for Takayasu’s arteritis and uncovered potentially important aspects in the pathophysiology of this form of vasculitis.
INTRODUCTION
Takayasu’s arteritis is a rare inflammatory disease that typically involves the aorta and its major branches (1-3). The disease causes arterial stenosis, blood-vessel wall thickening, dilation, and progressive occlusion, leading to potentially life-threatening ischemia, aortic regurgitation, and absent or reduced pulses (1-3). Takayasu’s arteritis can manifest with a broad range of non-specific symptoms including fever, fatigue, arthralgia, myalgia, and weight-loss, and has a typical age of onset between 20 and 40 years of age (4, 5). The disease occurs worldwide and in all ethnicities, but the highest prevalence has been reported in East Asia, India, and Mexico. It is much more common in women, although the extent of this sex bias seems to be ethnicity-dependent (4, 6).
The etiology of Takayasu’s arteritis remains elusive. However, there is strong evidence for genetic contribution to the disease pathogenesis supported by the repeatedly confirmed genetic association with HLA-B*52 across multiple ethnicities (7-10). Recently, the genetic association between Takayasu’s arteritis and the HLA extended region was investigated using dense genotyping and imputation analysis (11). These data, derived by examining two sets of patients and controls from two different ethnicities, established the presence of two independent genetic associations within the HLA region in Takayasu’s arteritis (11). The strongest such association is in the HLA-B/MICA region and the second genetic association is in the HLA-DQB1/HLA-DRB1 locus in HLA class II. Outside the HLA, we have previously established the genetic association between Takayasu’s arteritis and genetic variants in IL12B (encoding the P40 regulatory subunit of IL-12 and IL-23 cytokines), and in the genetic region encoding Fc-γ receptors IIA and IIIA with a genome-wide level of significance (11). The genetic association with the same genetic variants in IL12B was simultaneously described and confirmed in a Japanese cohort of Takayasu’s arteritis (12).
In this study, we performed the first unbiased genome-wide association study in Takayasu’s arteritis in two ethnically divergent cohorts of patients and controls.
METHODS
Patients and controls
We studied two ethnically divergent cohorts of patients with Takayasu’s arteritis and controls from Turkey and North America. The Turkish cohort included 559 patients enrolled by the Turkish Takayasu’s Study Group and 489 healthy controls, and the North American cohort included 134 European-American (EA) patients enrolled in the Vasculitis Clinical Research Consortium Longitudinal Study of Takayasu’s Arteritis and 1,047 EA controls. All patients fulfilled the 1990 American College of Rheumatology classification criteria for Takayasu’s arteritis (13). Our sample size has ~90% power to detect a genetic effect with an odds ratio of 1.55 and with a genome-wide significant P value of 5×10−8, for variants with a minor allele frequency (MAF) of 0.35, with an estimated disease prevalence of 2 per million for Takayasu’s arteritis, and using an additive genetic model. Genotyping data from the 1,047 EA controls were derived from the database of Genotypes and Phenotypes (dbGaP, study accession: phs000187.v1.p1). The study was approved by the Institutional Review Boards and the Ethics Committees at all participating institutions, and all study participants signed an informed written consent.
Genotyping and data analysis
Genotyping of patients and controls was performed using the Omni1-Quad and Omni2.5 genotyping platforms (Illumina). Genotyping data from SNPs included on both platforms were available for evaluation in both cohorts. Following genotyping, we followed rigorous quality control measures as previously described (11, 14). In brief, samples were excluded from the analysis based on population stratification by principal components analysis (>4 standard deviations), identity by descent (IBD>0.4), and autosomal heterozygosity (>2 standard deviation around the mean). A 10-component principal components analysis was performed using Eigenstrat version 4.2 (Supplementary Figure 1) (15), and IBD and heterozygosity analysis were performed with PLINK (16). Genotyped markers were filtered for minor allele frequency (MAF>0.01), genotype success rate (GSR>0.9), and Hardy-Weinberg equilibrium P value (HWPControls>0.01, HWPCases>0.0001). Markers with differential missingness between patients and controls (P<0.05) were also excluded from the analysis. After applying the quality control measures detailed above, a total of 474,442 variants were evaluated in the Turkish cohort and 547,389 in the EA cohort. A total of 516 patients and 462 controls in the Turkish cohort, and 119 patients and 993 controls in the EA cohort were included in the final analysis. Genomic control (GC) was performed using filtered non-HLA variants with minor allele frequencies > 0.02, and showed no to minimum evidence of population stratification in our cohorts (λGCTurkish= 1.05, λGCEA= 1.00). Genetic association analyses were performed using a basic allelic chi-square test with 1 degree of freedom, and the results were given as asymptotic P values. Meta-analysis was then performed using a fixed-effects model, and the results were filtered to exclude SNPs with a Cochrane's Q-statistic P value <0.05. Meta-analysis was performed using PLINK and haplotype structure analysis was performed using Haploview 4.2 (17).
Additional genetic variants up to the 1000 Genomes Project density were imputed in the three non-HLA genetic loci that were detected with a GWAS level of association with Takayasu’s arteritis. Imputation was performed using Impute 2 (18) and a combined reference panel consisting of 1,092 individuals (19). We applied a posterior probability imputation threshold of 0.9, and filtered imputed variants based on MAF (>1%), imputation success rate (> 90% of individuals), and HWP (> 0.0001) in controls prior to analysis, as previously described (11). Adjusted associations between SNPs were performed using conditional logistic regression in PLINK. Regional linkage disequilibrium (LD) plots were generated using the programing language R version 3.1.1.
Expression quantitative trait loci (eQTL) analysis
Expression quantitative trait loci analysis was performed to detect correlation between the presence or absence of the risk alleles in the identified Takayasu’s arteritis susceptibility loci and transcript expression levels in whole blood and lymphoblastoid cell lines. This was performed using the Genotype-Tissue Expression (GTEx) Project (20) and GENe Expression VARiation (Genevar) expression quantitative trait loci databases (21).
RESULTS
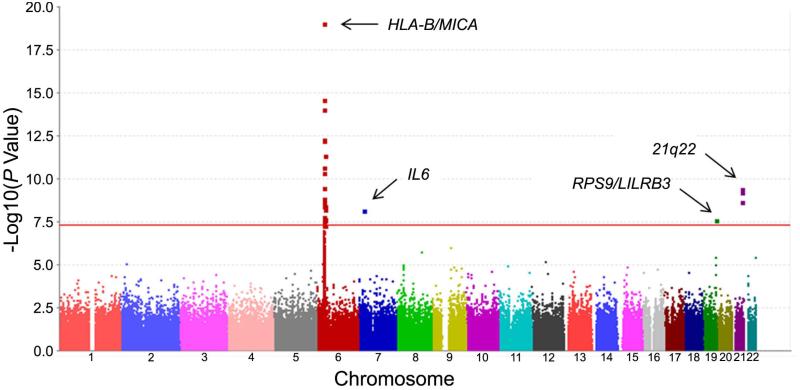
We identified four association peaks that passed the level of genome-wide significance. In addition to the association with the HLA regions (rs12524487, P= 8.17×10−20), three genetic associations in non-HLA loci were identified (Figure 1). We identified the genetic association between Takayasu’s arteritis and IL6 (rs2069837, P= 6.70×10−9), RPS9/LILRB3 (rs11666543, P= 2.34×10−8), and an intergenic locus on chromosome 21q22 that is closest to PSMG1 (rs2836878, P=3.62×10−10) (Table 1).
Figure 1.
Manhattan plot showing the meta-analysis results for genotyped variants in the Turkish and European-American cohorts. The red line represents the threshold for genome-wide level of significance (P = 5×10−8).
Table 1.
Genome-wide association analysis results showing genetic variants outside of the HLA region that are significantly associated (P <5×10−8) with Takayasu’s arteritis in the Turkish and European-American cohorts.
| Turkish Cohort |
European-American Cohort |
Meta-Analysis |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Locus/ Variant |
Minor Alleles |
Case MAF |
Control MAF |
OR | 95% CI | P Value | Case MAF |
Control MAF |
OR | 95% CI | P Value | OR | P Value |
Q-statistic P Value |
| IL6 | ||||||||||||||
| rs2069837 | G | 0.10 | 0.19 | 0.51 | 0.39-0.66 | 1.92E-07 | 0.03 | 0.09 | 0.32 | 0.15-0.69 | 2.32E-03 | 0.48 | 6.70E-09 | 0.274 |
| RPS9/LILRB3 | ||||||||||||||
| rs11666543 | A | 0.19 | 0.30 | 0.56 | 0.45-0.69 | 3.55E-08 | 0.24 | 0.29 | 0.74 | 0.54-1.02 | 6.27E-02 | 0.61 | 2.34E-08 | 0.134 |
| 21q22 | ||||||||||||||
| rs2242944 | A | 0.30 | 0.40 | 0.65 | 0.54-0.78 | 4.98E-06 | 0.22 | 0.36 | 0.51 | 0.37-0.70 | 3.07E-05 | 0.61 | 1.93E-09 | 0.211 |
| rs2836878 | A | 0.19 | 0.29 | 0.56 | 0.46-0.70 | 9.24E-08 | 0.17 | 0.27 | 0.55 | 0.39-0.78 | 7.23E-04 | 0.56 | 3.62E-10 | 0.912 |
| rs2836881 | T | 0.19 | 0.29 | 0.57 | 0.46-0.70 | 1.40E-07 | 0.17 | 0.27 | 0.55 | 0.39-0.78 | 6.85E-04 | 0.56 | 5.16E-10 | 0.879 |
MAF=minor allele frequency, OR=odds ratio, CI=confidence interval
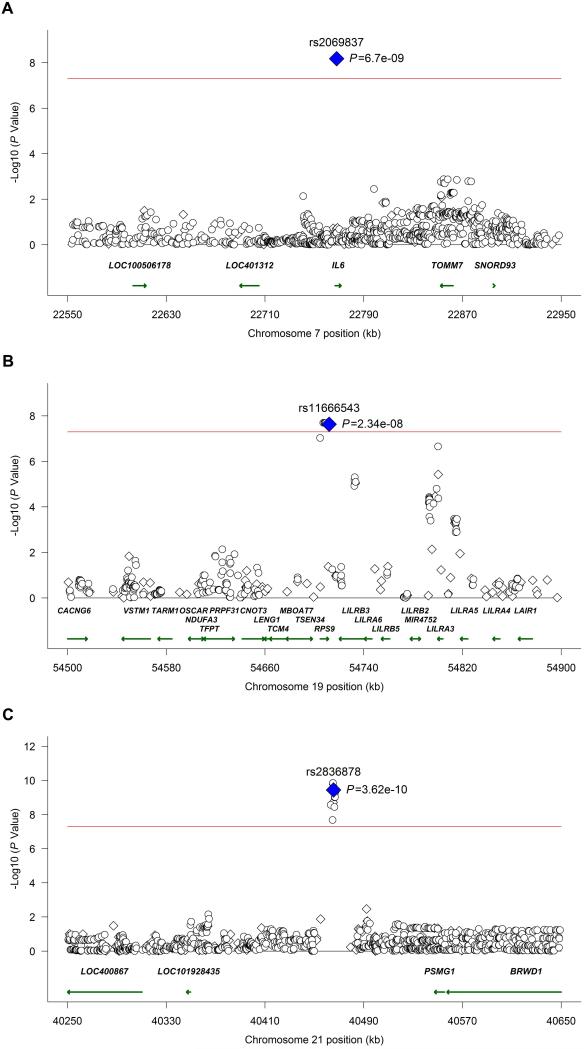
Using the imputation approach described above, we identified additional genetic variants within these loci that are associated with the risk for Takayasu’s arteritis (Figure 2, Supplementary Figures 2, 3, and 4, and Supplementary Tables 1 and 2). There are a total of 10 and 11 genotyped or imputed SNPs with evidence for at least modest genetic association (P<0.05) that are in LD (r2>0.7) with the index SNP rs2836878 in the chromosome 21q22 genetic region, in the Turkish and European-American cohorts, respectively. The high LD in this locus precluded localization of this genetic effect to a single genetic variant using conditional regression analysis. However, the LD structure in this locus, informed by a trans-ancestral data from the Turkish and the European-American cohorts, indicates that this association in chromosome 21q22 is explained by a relatively small genetic region extending from 40,463,283-40,466,744 (HG19) located in the intergenic region between PSMG1 and LOC101928435 (Supplementary Figure 2).
Figure 2.
Regional meta-analysis results for genotyped and imputed variants in both the Turkish and European-American cohorts. Association results are shown in the IL6, RPS9/LILRB3, and chromosome 21q22 loci, in panels A, B, and C, respectively. Genotyped variants are represented as diamonds and imputed variants as circles. The red line shows the threshold for a genome-wide level of significance (P = 5×10−8).
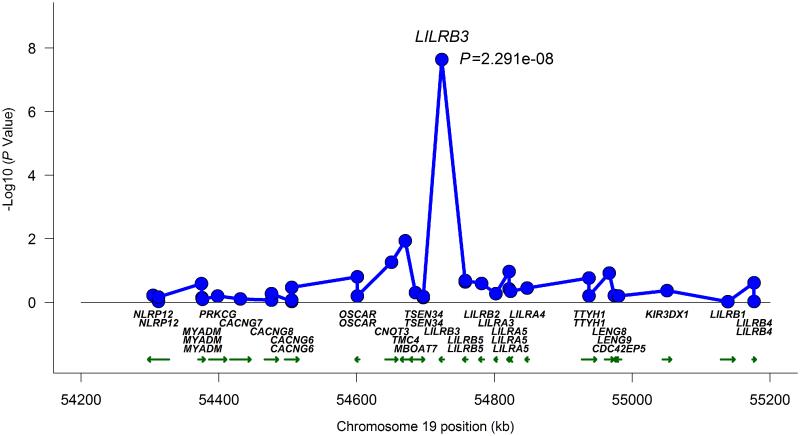
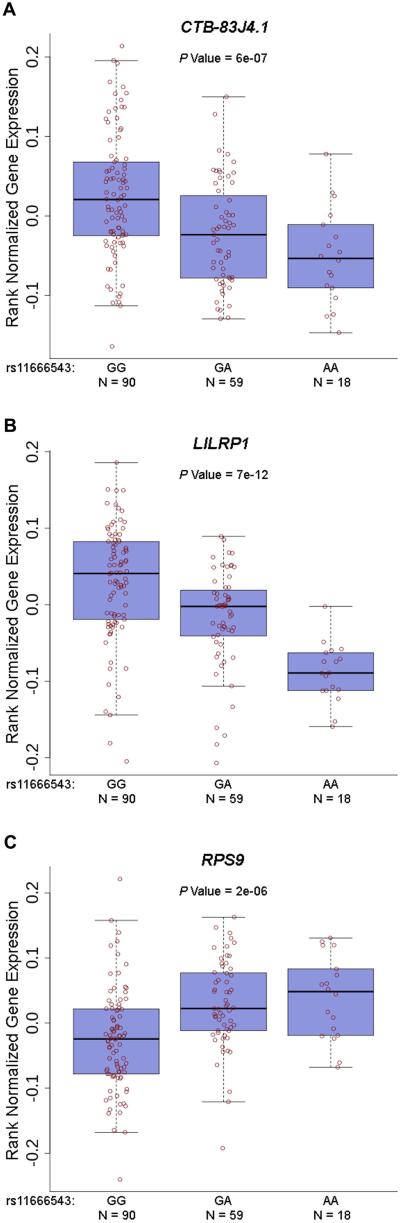
A similar approach was attempted to further localize the novel genetic association we identified in Takayasu’s arteritis in the RPS9/LILRB3 locus located on chromosome 19q13.4. This gene-rich locus includes multiple genes in the leukocyte immunoglobulin-like receptor family that are known to be expressed on antigen presenting and other immunocompetent cells and interact with HLA class I. The LD structure and genetic association results, using genotyped and densely imputed genetic variants in this region, localized the genetic effect tagged by the index SNP in this locus (rs11666543) to a region that includes RPS9 and LILRB3 (Supplementary Figure 3). Similar to the genetic effect in chromosome 21q22, very high to complete LD precluded further localization to a single genetic variant. As this genetic effect is in a gene rich region, it is possible that the functional effect of the identified genetic variants might extend to other genes on this same locus. Therefore, we performed eQTL analysis to determine if the index SNP in this locus (rs11666543) affects expression levels of any of the genes or transcripts located within 1 million base pairs upstream and downstream from this SNP. We detected significant reduction in the expression of LILRB3 in lymphoblastoid cell lines in the presence of the risk allele (G) in rs11666543 (P =2.29×10−8) (Figure 3). The genetic variant rs11666543 is also associated with significant down regulation of RPS9, and upregulation of a long non-coding (lnc) RNA (CTB-83J4.1), and the pseudogene LILRP1 in a whole blood expression eQTL database (Figure 4). CTB-83J4.1 and LILRP1 are located ~16kb and 500kb from rs11666543, respectively. Together, these data suggest that the genetic risk variant tagged by the SNP rs11666543 is a putative functional variant that alters the expression of multiple transcripts within this gene-rich region on chromosome 19q13.
Figure 3.
Expression quantitative trait loci (eQTL) association between rs11666543 and chromosome 19q13.4 genes in lymphoblastoid cell lines. The risk allele (G) was associated with significant reduction in mRNA expression of leukocyte immunoglobulin-like receptor gene LILRB3 (P= 2.29×10−8).
Figure 4.
Expression quantitative trait loci (eQTL) associations between rs11666543 and several transcripts in 19q13.4 in whole blood. The risk allele (G) in rs11666543 correlates with increased expression of the lncRNA CTB-83J4.1 and LILRP1, and decreased expression of RPS9 in whole blood (A, B, and C, respectively).
We also identified a novel genetic association between IL6 and Takayasu’s arteritis (rs2069837, PTurkish= 1.92×10−7, PEA= 2.32×10−3, Pmeta= 6.70×10−9). This genetic variant located within the second intron of IL6 is not in LD with any other variant that we genotyped or imputed in this locus. This is also consistent with the LD data in HapMap, and explains why only a single variant in this genetic locus was identified as a risk variant for Takayasu’s arteritis. We used ENCODE data to determine if this genetic variants in IL6 localizes to a regulatory genetic region. We found that rs2069837 in IL6 overlaps with an H3K27 acetylated region indicating that this genetic variant is located within an active enhancer.
In addition to identifying genetic associations in IL6, RPS9/LILRB3, and chromosome 21q22 with a genome level of significance (P <5×10−8), we identified several novel genetic susceptibility loci for Takayasu’s arteritis with a suggestive evidence of association with the disease (P< 1×10−5). These include PCSK5, ZFPM2, LOC100289420/FAM19A5, LILRA3, SLC16A7/LOC100289417, PPM1G/NRBP1, and PTK2B (Table 2).
Table 2.
Genetic variants with a suggestive evidence for association in Takayasu’s arteritis (meta-analysis P value <1×10−5).
| SNP | Minor Allele | Position | Gene Symbol | Gene Location |
OR | P Value |
Q-statistic P
Value |
|---|---|---|---|---|---|---|---|
| rs6560480 | C | Chr9: 78599133 | PCSK5 | INTRON | 1.49 | 9.34E-07 | 0.676 |
| rs1113601 | G | Chr8: 106338217 | ZFPM2 | INTRON | 0.56 | 1.69E-06 | 0.834 |
| rs9615754 | T | Chr22: 48479166 | LOC100289420/FAM19A5 | INTERGENIC | 0.58 | 3.70E-06 | 0.195 |
| rs410852 | G | Chr19: 54800371 | LILRA3 | INTRON | 1.47 | 3.74E-06 | 0.966 |
| rs7956657 | A | Chr12: 60228857 | SLC16A7/LOC100289417 | INTERGENIC | 1.67 | 6.13E-06 | 0.188 |
| rs11675428 | C | Chr2: 27642734 | PPM1G/NRBP1 | INTERGENIC | 0.54 | 8.06E-06 | 0.316 |
| rs13260543 | G | Chr8: 27251325 | PTK2B | INTRON | 0.70 | 8.97E-06 | 0.156 |
| rs7005183 | G | Chr8: 27260484 | PTK2B | INTRON | 0.70 | 9.01E-06 | 0.141 |
MAF=minor allele frequency, OR=odds ratio, CI=confidence interval.
Genetic association results (P< 1×10−5) in the two independent cohorts are presented in Supplementary Tables 3 and 4.
DISCUSSION
We performed the first unbiased genome-wide association study in Takayasu’s arteritis and discovered and characterized novel genetic susceptibility loci that predispose to Takayasu’s arteritis in independent cohorts from Turkey and North America. We established three risk loci for the disease, outside of the HLA region, with a genome-wide level of significance (P< 5×10−8). Two of these loci, IL6 and RPS9/LILRB3, point to important immuno-regulatory pathways that could further explain the underlying immunopathology of this large-vessel vasculitis. The third genetic locus we established with a GWAS level of significance in Takayasu’s arteritis is located in a region on chromosome 21q22. This same genetic susceptibility locus confers risk for ulcerative colitis and ankylosing spondylitis (22, 23), and the risk variant in this locus has been recently shown increase the expression of two novel lncRNA transcripts in this intergenic region (24).
A role for interleukin (IL)-6 in the pathogenesis of Takayasu’s arteritis has been suspected from previous studies reporting increased serum IL-6 levels in patients compared to healthy controls (25, 26). IL-6 plays an important role in regulating multiple aspects of the immune response, including the differentiation of T cells into T helper 17 cells and regulatory T cells (27). Previous candidate gene association studies have suggested a modest effect for genetic variants within the promoter region of IL6 in Takayasu’s arteritis (28). While our data do not show evidence for associations with these two promoter region variants (P >0.05), we report a novel genetic association in Takayasu’s arteritis with a genetic variant located in a regulatory region within the second intron of IL6. This genetic variant is located within an experimentally-identified active enhancer region, as suggested by the presence of a histone H3K27 acetylation mark within this locus and across multiple cell types. Multiple case reports have suggested successful treatment of refractory Takayasu’s arteritis with monoclonal anti-IL-6 receptor antibody (tocilizumab) (29).
Our discovery of a genetic risk locus for Takayasu’s arteritis on the leukocyte receptor complex (LRC) immune-regulatory gene rich region of chromosome 19q13.4 uncovers a potentially novel aspect of this disease. This genomic region includes genes encoding for killer immunoglobulin-like receptors (KIR), leucocyte immunoglobulin-like receptors (LILR), and leucocyte-associated immunoglobulin-like receptors (LAIR) (30). Using dense imputation and trans-ancestral mapping, we localized the genetic susceptibility locus for Takayasu’s arteritis in this region to RPS9/LILRB3. The LILR gene family encodes inhibitory receptor proteins consisting of two or four extracellular immunoglobulin domains, a transmembrane domain, and one to four cytoplasmic immunoreceptor tyrosine-based inhibitory motifs (ITIMs) (30). LILRB3 binds to HLA class I antigens and generally provide a negative inhibitory signal to limit an immune response and prevent autoreactivity. Our data indicate that the index SNP in the RPS9/LILRB3 locus tags a functional genetic variant that regulates multiple genes within this extended region. Specifically, the Takayasu’s risk allele in rs11666543 correlates with reduced mRNA expression of RPS9, LILRB3, and increased expression of the pseudogene LILRP1 located over 500kb from this SNP. In addition, the risk allele in this locus correlates with increased expression of a lncRNA (CTB-83J4.1) that is over 16kb away. These data suggest a long-range interaction within this genomic region, and a possible chromatin looping configuration that brings multiple genes spread across this complex region into close proximity to this functional regulatory locus that includes rs11666543 and that confers risk to Takayasu’s arteritis.
Our expression quantitative trait loci analysis in the chromosome 19q13.4 locus that indicates significant reduction in LILRB3 expression with the Takayasu’s risk allele suggests loss of inhibitory signaling that could result in enhanced uncontrolled immune activation upon MHC class I antigen presentation. It is intriguing that HLA class I is strongly associated with the risk for Takayasu’s arteritis. Our study was underpowered to establish epistatic interaction between the HLA class I risk locus in Takayasu’s (tagged by rs12524487 in HLA-B/MICA) and RPS9/LILRB3 (data not shown). The variant tagging the RPS9/LILRB3 genetic effect in Takayasu’s arteritis also alters the mRNA expression of RPS9 which encodes for ribosomal protein S9 and is a component of the 40S ribosomal subunit.
We have previously used the Immunochip custom-designed genotyping platform and reported significant genetic associations with IL12B and FCGR2A/FCGR3A in Takayasu’s arteritis (14). The Immunochip platform included 196,524 genetic variants and allowed for very dense coverage and genotyping in ~200 genetic loci with a previous reported association in immune-mediated diseases. These same variants in IL12B and FCGR2A/FCGR3A were not included in the GWAS platform used in this study, and could not be imputed and analyzed. The genetic association results with the genotyped variants in these two loci in this study are presented in Supplementary Figure 5. Indeed, only one genetic variant analyzed in this study was in LD with the previously reported risk variant in IL12B, and no variant was in in LD with the previously reported risk variant in FCGR2A/FCGR3A (Supplementary Tables 5 and 6). Therefore, we predict that additional genetic susceptibility loci for Takayasu’s arteritis would be discovered in future studies when more comprehensive genotyping platforms or sequencing experiments are performed.
In summary, this multi-ethnic first GWAS study in Takayasu’s arteritis established three additional genetic susceptibility loci with a genome-wide level of significance for this disease. Out study revealed important novel aspects in the pathogenesis of Takayasu’s arteritis, and brings the total number of established genetic risk loci with a genome-wide level of significance in this disease to seven. These are the two independent MHC loci in HLA class I and class II, FCGR2A/FCGR3A, IL12B, IL6, RPS9/LILRB3, and the intergenic locus on chromosome 21q22 near PSMG1. Uncovering the genetic basis for Takayasu’s arteritis has the great potential to lead to a better understanding of the disease pathogenesis and the discovery of novel therapeutic targets.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by funding from the University of Michigan and the Vasculitis Foundation. The Vasculitis Clinical Research Consortium has received support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (U54AR057319 and U01 AR51874 04), the National Center for Research Resources (U54 RR019497), and the Office of Rare Diseases Research of the National Center for Advancing Translational Sciences. Genotyping data from European-American controls were obtained from the High Density SNP Association Analysis of Melanoma: Case-Control and Outcomes Investigation dataset (dbGaP study accession: phs000187.v1.p1). Research support for this dataset was provided by 3P50CA093459, 5P50CA097007, 5R01ES011740, and 5R01CA133996.
Footnotes
Conflict of interest: The authors have no relevant conflicts of interest to disclose.
REFERENCES
- 1.Kobayashi Y, Numano F. 3. Takayasu arteritis. Internal medicine. 2002;41(1):44–6. doi: 10.2169/internalmedicine.41.44. [DOI] [PubMed] [Google Scholar]
- 2.Bicakcigil M, Aksu K, Kamali S, Ozbalkan Z, Ates A, Karadag O, et al. Takayasu's arteritis in Turkey - clinical and angiographic features of 248 patients. Clinical and experimental rheumatology. 2009;27(1 Suppl 52):S59–64. [PubMed] [Google Scholar]
- 3.Kerr GS, Hallahan CW, Giordano J, Leavitt RY, Fauci AS, Rottem M, et al. Takayasu arteritis. Annals of internal medicine. 1994;120(11):919–29. doi: 10.7326/0003-4819-120-11-199406010-00004. [DOI] [PubMed] [Google Scholar]
- 4.Johnston SL, Lock RJ, Gompels MM. Takayasu arteritis: a review. Journal of clinical pathology. 2002;55(7):481–6. doi: 10.1136/jcp.55.7.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillip R, Luqmani R. Mortality in systemic vasculitis: a systematic review. Clinical and experimental rheumatology. 2008;26(5 Suppl 51):S94–104. [PubMed] [Google Scholar]
- 6.Moriwaki R, Noda M, Yajima M, Sharma BK, Numano F. Clinical manifestations of Takayasu arteritis in India and Japan--new classification of angiographic findings. Angiology. 1997;48(5):369–79. doi: 10.1177/000331979704800501. [DOI] [PubMed] [Google Scholar]
- 7.Charoenwongse P, Kangwanshiratada O, Boonnam R, Hoomsindhu U. The association between the HLA antigens and Takayasu's arteritis in Thai patients. International journal of cardiology. 1998;66(Suppl 1):S117–20. doi: 10.1016/s0167-5273(98)00158-2. [DOI] [PubMed] [Google Scholar]
- 8.Lee SW, Kwon OJ, Park MC, Oh HB, Park YB, Lee SK. HLA alleles in Korean patients with Takayasu arteritis. Clinical and experimental rheumatology. 2007;25(1 Suppl 44):S18–22. [PubMed] [Google Scholar]
- 9.Mehra NK, Jaini R, Balamurugan A, Kanga U, Prabhakaran D, Jain S, et al. Immunogenetic analysis of Takayasu arteritis in Indian patients. International journal of cardiology. 1998;66(Suppl 1):S127–32. doi: 10.1016/s0167-5273(98)00160-0. discussion S33. [DOI] [PubMed] [Google Scholar]
- 10.Sahin Z, Bicakcigil M, Aksu K, Kamali S, Akar S, Onen F, et al. Takayasu's arteritis is associated with HLA-B*52, but not with HLA-B*51, in Turkey. Arthritis research & therapy. 2012;14(1):R27. doi: 10.1186/ar3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saruhan-Direskeneli G, Hughes T, Aksu K, Keser G, Coit P, Aydin SZ, et al. Identification of multiple genetic susceptibility loci in Takayasu arteritis. American journal of human genetics. 2013;93(2):298–305. doi: 10.1016/j.ajhg.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terao C, Yoshifuji H, Kimura A, Matsumura T, Ohmura K, Takahashi M, et al. Two susceptibility loci to Takayasu arteritis reveal a synergistic role of the IL12B and HLA-B regions in a Japanese population. American journal of human genetics. 2013;93(2):289–97. doi: 10.1016/j.ajhg.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33(8):1129–34. doi: 10.1002/art.1780330811. [DOI] [PubMed] [Google Scholar]
- 14.Hughes T, Coit P, Adler A, Yilmaz V, Aksu K, Duzgun N, et al. Identification of multiple independent susceptibility loci in the HLA region in Behcet's disease. Nature genetics. 2013;45(3):319–24. doi: 10.1038/ng.2551. [DOI] [PubMed] [Google Scholar]
- 15.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904–9. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 16.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 18.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5(6):e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.A map of human genome variation from population-scale sequencing. Nature. 2010;467(7319):1061–73. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Consortium GT. The Genotype-Tissue Expression (GTEx) project. Nature genetics. 2013;45(6):580–5. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang TP, Beazley C, Montgomery SB, Dimas AS, Gutierrez-Arcelus M, Stranger BE, et al. Genevar: a database and Java application for the analysis and visualization of SNP-gene associations in eQTL studies. Bioinformatics. 2010;26(19):2474–6. doi: 10.1093/bioinformatics/btq452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson CA, Boucher G, Lees CW, Franke A, D'Amato M, Taylor KD, et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nature genetics. 2011;43(3):246–52. doi: 10.1038/ng.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Australo-Anglo-American Spondyloarthritis C. Reveille JD, Sims AM, Danoy P, Evans DM, Leo P, et al. Genome-wide association study of ankylosing spondylitis identifies non-MHC susceptibility loci. Nature genetics. 2010;42(2):123–7. doi: 10.1038/ng.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haynes K, Kenna T, Glazov E, Brown M, Thomas G. Arthritis and Rheumatology. 2014;66(11 Supplement):S830. [Google Scholar]
- 25.Park MC, Lee SW, Park YB, Lee SK. Serum cytokine profiles and their correlations with disease activity in Takayasu's arteritis. Rheumatology. 2006;45(5):545–8. doi: 10.1093/rheumatology/kei266. [DOI] [PubMed] [Google Scholar]
- 26.Alibaz-Oner F, Yentür S, Saruhan-Direskeneli G, Direskeneli H. Serum cytokine profiles in Takayasu’s arteritis: Search for biomarkers. Clinical and Experimental Rheumatology. In Press. [PubMed] [Google Scholar]
- 27.Tanaka T, Narazaki M, Kishimoto T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harbor perspectives in biology. 2014;6(10) doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saruhan-Direskeneli G, Bicakcigil M, Yilmaz V, Kamali S, Aksu K, Fresko I, et al. Interleukin (IL)-12, IL-2, and IL-6 gene polymorphisms in Takayasu's arteritis from Turkey. Human immunology. 2006;67(9):735–40. doi: 10.1016/j.humimm.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Loricera J, Blanco R, Castaneda S, Humbria A, Ortego-Centeno N, Narvaez J, et al. Tocilizumab in refractory aortitis: study on 16 patients and literature review. Clin Exp Rheumatol. 2014;32(3 Suppl 82):S79–89. [PubMed] [Google Scholar]
- 30.Barrow AD, Trowsdale J. The extended human leukocyte receptor complex: diverse ways of modulating immune responses. Immunological reviews. 2008;224:98–123. doi: 10.1111/j.1600-065X.2008.00653.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.