Abstract
Background and purpose
Prolonged ischemia causes blood-brain barrier (BBB) damage and increases the incidence of neurovasculature complications secondary to reperfusion. Therefore, targeting ischemic BBB damage pathogenesis is critical to reducing neurovasculature complications and expanding the therapeutic time window of tissue-type plasminogen activator (tPA) thrombolysis. This study investigates whether increasing cerebral tissue pO2 through normobaric hyperoxia (NBO) treatment will slow the progression of BBB damage and thus improve the outcome of delayed tPA treatment after cerebral ischemia.
Methods
Rats were exposed to NBO (100%O2) or normoxia (21%O2) during 3, 5, or 7-h middle cerebral artery occlusion. Fifteen min before reperfusion, tPA was continuously infused to rats over 30min. Neurological score, mortality rate, and BBB permeability were determined. MMP-9 was measured by gelatin zymography, and tight junction proteins (occludin and cluadin-5) by Western blot in the isolated cerebral microvessels.
Results
NBO slowed the progression of ischemic BBB damage pathogenesis, evidenced by reduced Evan’s blue leakage, smaller edema and hemorrhagic volume in NBO-treated rats. NBO treatment reduced MMP-9 induction and the loss of tight junction proteins in ischemic cerebral microvessels. NBO-afforded BBB protection was maintained during tPA reperfusion, resulting in improved neurological functions, significant reductions in brain edema, hemorrhagic volume and mortality rate, even when tPA was given after prolonged ischemia (7-h).
Conclusions
Early NBO treatment slows ischemic BBB damage pathogenesis and significantly improves the outcome of delayed tPA treatment, providing new evidence supporting NBO as an effective adjunctive therapy to extend the time window of tPA thrombolysis for ischemic stroke.
Keywords: blood-brain barrier, ischemia, matrix metalloproteinase-9, normobaric hyperoxia, tissue-type plasminogen activator (tPA)
Introduction
Thrombolysis with tissue-type plasminogen activator (tPA) is the only FDA-approved therapy for acute ischemic stroke, but it has a narrow therapeutic time window of 3-4.5 h after stroke onset.1 Delayed tPA treatment leads to severe complications, such as intracerebral hemorrhage (ICH) and edema, due to reperfusion into weakened or necrotic brain microvasculature.2, 3 Blood-brain barrier (BBB) damage after ischemic stroke is a dynamic process with an initial damage during ischemia4 and a secondary injury during reperfusion.2, 5, 6 Prolonged ischemia leads to severe BBB damage which dramatically increases the risk of complications following tPA thrombolysis. Therefore, preserving BBB integrity during ischemia is of critical importance in ensuring that the microvasculature will safely withstand the return of blood flow.
Oxygen therapy aimed at increasing tissue partial pressure of oxygen (pO2) has long been considered a logic treatment for ischemic stroke.7 Several animal studies have shown that short duration of NBO treatment is highly neuroprotective if started early after ischemia onset.8-12 A pilot clinical study also demonstrated that NBO therapy was associated with a transient improvement of clinical deficits and magnetic resonance imaging abnormalities in patients with acute ischemic stroke.13 These studies have demonstrated that early NBO treatment can slow the progression of ischemic brain tissue to necrosis, thus improving the therapeutic time window for reperfusion therapies. We have shown that besides neuroprotection, NBO can also protect the BBB against ischemic damage through inhibiting reactive oxygen species production14,15 and matrix metalloproteinase-9 (MMP-9)-mediated degradation of tight junction proteins (TJPs)16-18 However, the time window for vasoprotection of NBO remains unknown.
In this study, we investigated how, and to what extent, early NBO treatment could slow the progression of ischemic BBB damage using a rat model of focal cerebral ischemia. Additionally, we investigated whether the vasoprotection afforded by NBO during cerebral ischemia was effective in reducing the neurovascular complications associated with delayed tPA treatment.
Materials and Methods
Animal model of focal cerebral ischemia
The Institutional Animal Care and Use Committee of Capital Medical University approved all animal experiments. Male Sprague-Dawley rats (n=144) weighing 290-320g were anesthetized with 2% isoflurane and subjected to middle cerebral artery occlusion (MCAO) followed by reperfusion using the suture occlusion model, as we previously described.18 Successful MCAO was confirmed by 2,3,5-triphenyltetrazolium chloride (TTC) staining.18
Although suture model is not directly relevant to thromboembolic ischemia in patients, this stable model would allow us to compare the different effects of NBO/normoxia on tPA’s complications under identical and controlled ischemia and reperfusion conditions. In contrast, the more clinically relevant model of injecting a preformed clot into MCA is not most suitable for this investigative study because we cannot predict how long and where the clot will occlude MCA.
Experimental design
To investigate NBO’s ability to slow the progression of ischemic BBB damage and its impact on the neurovascular complications of delayed tPA treatment, we chose three ischemia durations: 3-h (within the established 3-4.5 h thrombolytic time window), 5-h and 7-h (outside the window). We chose two reperfusion time points, 2-h after reperfusion onset and 24-h after MCAO onset. tPA was IV infused for 30min at 15min prior to reperfusion, mimicking tPA thrombolysis in clinical settings.
Rats with successful MCAO were randomly assigned to four groups: normoxia+MCAO (n=30); NBO+MCAO (n=30); normoxia+tPA+MCAO (n=27); and NBO+tPA+MCAO (n=27). Each group was further divided into 3 subgroups with 3, 5 or 7-h MCAO followed by 2-h reperfusion. Another 30 rats were randomly assigned into four groups to assess mortality rate at 24h after MCAO onset: normoxia+3h-tPA (n=6); NBO+3h-tPA (n=5); normoxia+7h-tPA (n=10); and NBO+7h-tPA (n=9). The experimental design is schematically illustrated in Figure 1A.
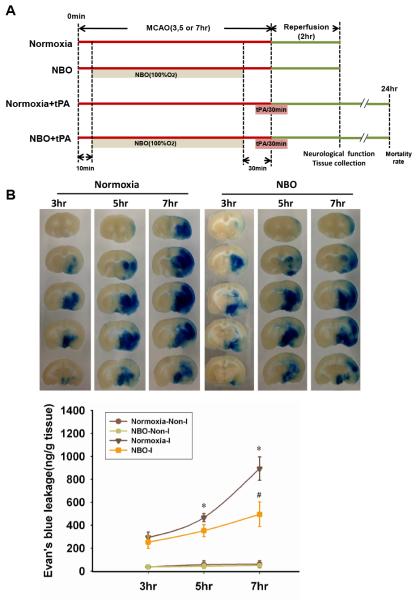
Figure 1. NBO treatment reduces Evan’s blue leakage in the ischemic brain after 3-h, 5-h or 7-h of MCAO with 2-h reperfusion.
A. Schematic diagram of the overall experimental design. B. Representative brain slices showing EB leakage in the ischemic tissue. Quantification of EB extravasation is expressed as nanogram per gram of brain tissue (ng/g). *P<0.05 versus 3-h MCAO Normoxia-I; #P<0.05 versus 7-h MCAO Normoxia-I. Data are expressed as mean ± SEM (n = 5).
We chose to assess NBO’s BBB protection at 2-h after reperfusion for two reasons :i) to minimize the impact of reperfusion on BBB damage while allowing Evan’s blue dye to circulate around the damaged BBB; ii) to minimize the number of animals needed as prolonged reperfusion causes high mortality rate.18 The reason for assessing tPA’s neurovascular complications at 24-h after ischemia was because tPA-associated ICH and mortality occurs within 36-h after tPA administration.19, 20
NBO treatment
All animals exhibited rapid recovery from anesthesia within 5-min post MCAO surgery. When rats could freely move at 10-min post MCAO onset, we put them into an anesthesia box that was ventilated (5L/min) with air (21%O2, normoxia) or 100%O2 (NBO) until 30-min before the end of MCAO. 100%O2 was chosen as the NBO treatment because our previous study demonstrated that rats breathing this O2 concentration were able to maintain the ischemic penumbral pO2 close to the preischemic level.10 For convenience and precise control, the rats were taken out of NBO exposure box for tPA infusion 30 min before reperfusion, thus terminating NBO treatment.
tPA administration
tPA (10mg/kg; Biberach, Germany) was infused into rat through the right femoral vein at 15-min before reperfusion onset and the infusion continued for another 15-min after withdrawal of the suture. This regimen achieved a fibrinolytic effect in rats and made the reperfused tissue fully exposed to tPA, mimicking clinical tPA treatment.18
Measurement of neurological deficits
At the end of 2-h reperfusion, neurological deficits were assessed double blinded with two methods: Zea-Longa score 21 and Ludmila Belayev.22
Tissue collection and measurement of infarction and edema
At the end of 2-h reperfusion, the rats were transcardially perfused with 250mL cold PBS. Brains were sectioned into six 2-mm coronal slices from a 12mm thick region 3mm away from the tip of the frontal lobe. The third slice was stained using 1% TTC to measure the infarct size. The 2-mm thick brain slices were digitally photographed to assess brain edema by measuring hemispheric enlargement. After photographing, the tissue was collected for quantitatively measuring hemorrhagic volume or for cerebromicrovessel isolation.
An indirect method for calculating infarct volume was used to minimize error introduced by edema.23 The non-infarcted region in the ipsilateral hemisphere was subtracted from that in the contralateral hemisphere. The infarct size was presented as a percentage of the contralateral hemisphere.
Evaluation of Evan’s blue leakage
Evan’s blue dye (EB, 2% wt/vol in PBS, 3mL/kg) (Sigma, USA) was administered via IV in the tail vein at the onset of reperfusion. At the end of 2-h reperfusion, the rats were transcardially perfused with PBS. The brain was then removed, sectioned, and photographed to visualize EB extravasation. BBB disruption was quantitatively assessed by measuring EB contents in ischemic hemispheric tissue as previously reported.16
Spectrophotometric assay of cerebral hemorrhage
Cerebral hemorrhage was quantified using QuantiChrom Hemoglobin Assay Kit (BioAssay Systems, Hayward, USA) as previously described. 24
Measurement of brain edema
Brain edema was assessed by measuring the hemispheric areas of each 2-mm-thick brain slice on the digital photographs obtained using Image J software (National Institutes of Health) as described previously.14 Edema was expressed as a ratio of ischemic hemispheric area versus non-ischemic hemispheric area.
Isolation of cerebral microvessels
Isolation of cerebral microvessels was performed as described in our previous study.14
Measurement of MMP-9, occludin and claudin-5 in isolated microvessels
MMP-9 levels were measured by gelatin zymography and occludin and claudin-5 protein levels were measured by Western blot in the isolated microvessels, as described in our previous studies.14,17
Statistical analysis
Data were presented as means±SEM. Statistical analysis was carried out using X2 test and one-way analysis of variance (ANOVA). A value of P<0.05 was considered statistically significant.
Results
NBO slows the evolution of ischemic BBB damage
To determine whether NBO could slow down the evolution of ischemic BBB damage, we compared the severity of BBB damage with or without NBO treatment after 3-h, 5-h or 7-h of ischemia followed by 2-h reperfusion. Fig. 1B shows the EB extravasation after 3, 5, or 7-h of cerebral ischemia. As expected, EB contents in non-ischemic hemispheric tissue were low in all tested stroke conditions (Fig. 1B). Cerebral ischemia drastically increased EB content in the ischemic hemispheric tissue of the normoxic group, and greater EB leakage was seen for longer ischemic duration. NBO treatment decreased EB extravasation for all three ischemic durations with the largest reduction occurring for 7-h ischemia.
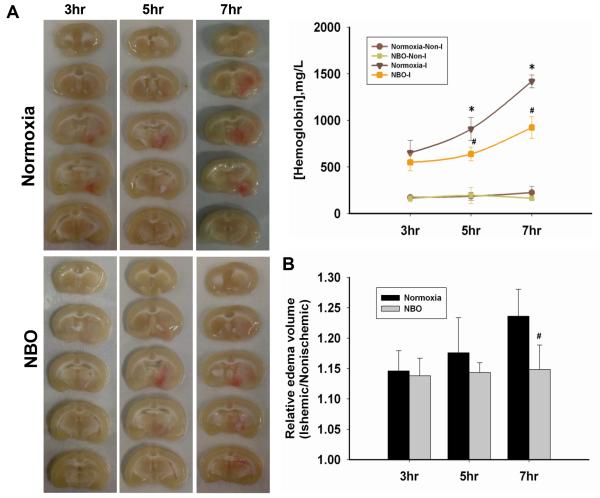
ICH was seen in the ischemic hemisphere of normoxic rats after ischemia, with longer ischemia durations inducing greater bleeding volumes (Fig. 2A). Similar to EB extravasation, NBO treatment reduced the severity of ICH for all three ischemic durations. A small amount of hemoglobin was detected in the non-ischemic hemisphere of all rats, likely due to residual blood left in the cerebral vasculature after transcardial perfusion.
Figure 2. NBO reduces hemoglobin extravasation and brain edema in the ischemic brain after 3-h, 5-h or 7-h of MCAO with 2-h reperfusion.
A. Representative brain slices showing hemoglobin extravasation and hemispheric enlargement and the quantitative analysis of hemoglobin leakage. *P<0.05 versus 3-h MCAO Normoxia-I; #P<0.05 versus the Normoxia-I with the same ischemia duration. (n=5) B. The ratio of ischemia-induced brain edema. #P<0.05 versus the Normoxia-treated with the same ischemia duration. Data are expressed as mean ± SEM (n=7).
We also assessed brain edema and observed that longer ischemia durations induced more severe brain edema in normoxic rats (Fig. 2B). When the rats were treated by NBO during cerebral ischemia, the hemispheric enlarging process appeared to be “frozen” at the level of 3-h ischemia because the edematic volume remained unchanged even if the ischemic duration was prolonged to 7-h.
These results clearly indicate that NBO treatment can slow the evolution of ischemic BBB damage, potentially expanding the therapeutic time window.
NBO slows the process of MMP-9 induction and the loss of TJPs in ischemic microvessels
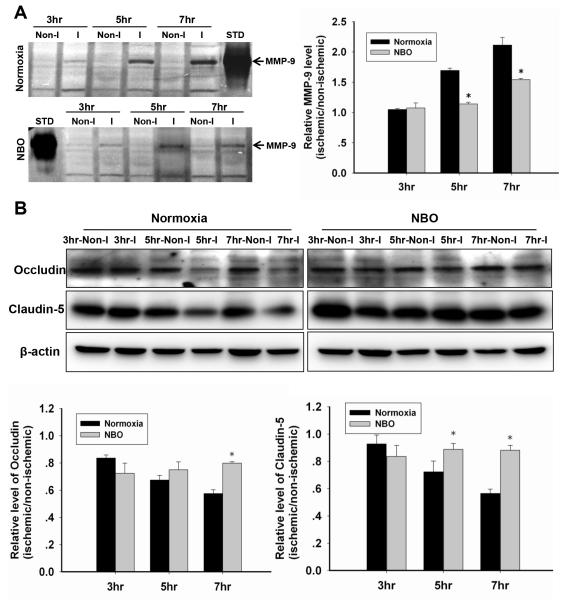
We next studied the mechanisms by which NBO treatment slowed the evolution of ischemic BBB damage. MMP-9 induction and increased TJPs degradation are two well-studied events critically contributing to BBB damage in ischemic stroke.25 MMP-9 band was faint in non-ischemic microvascular extracts of all groups (Fig. 3A). MMP-9 induction increased with increasing ischemic duration in the ischemic microvessels. Concurrently, both occludin and claudin-5 protein levels in the microvessels decreased with increasing ischemic duration (Fig. 3B). Importantly, NBO treatment attenuated MMP-9 induction and restored the loss of occludin and claudin-5 protein in ischemic microvessels. These findings suggest that NBO treatment can slow the process of MMP-9 induction and the loss of occludin and claudin-5 proteins in ischemic microvessels.
Figure 3. NBO slows the process of MMP-9 induction and the loss of TJPs in ischemic microvessels after 3-h, 5-h or 7-h MCAO with 2-h reperfusion.
A. Representative gelatin zymography showing MMP-9 activity in isolated microvessels from non-ischemic (Non-I) and ischemic (I) hemisphere, and the quantitative analysis results. STD: the standard of MMP-9. *P<0.05 versus the Normoxia-treated with the same ischemic duration. B. Representative western blots of occludin and claudin-5 and the quantitative analysis results. *P<0.05 versus the Normoxia-treated with the same ischemic duration. Data are expressed as mean±SEM (n=5).
NBO slows MMP-9 induction and tight junction protein loss in ischemic microvessels following delayed tPA administration
The results above clearly showed that NBO treatment could slow the evolution of BBB damage and inhibit two key BBB-damaging events, i.e., MMP-9 induction and TJPs degradation, over a prolonged time window. We wondered whether NBO-afforded BBB protection would remain following delayed tPA treatment and thus expanding tPA’s therapeutic time window. To test this, we assessed MMP-9 induction, TJPs protein loss, EB extravasation, hemorrhagic volume and brain edema under the same experimental regimen as described above except that tPA was administered at the end of each indicated ischemic duration.
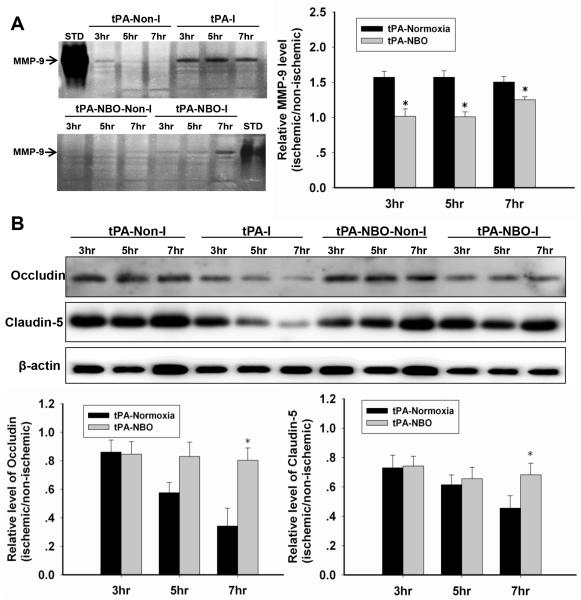
Administration of tPA did not affect MMP-9 levels in the non-ischemic microvessles of the normoxic rats (Fig. 4A). However, the induction of MMP-9 was elevated and saturated in normoxic rats following tPA administration because all three ischemic durations induced the same level of MMP-9 induction. Importantly, NBO treatment abolished MMP-9 induction in the ischemic microvessels of tPA-treated rats for all three ischemic durations.
Figure 4. NBO slows tPA-augmented MMP-9 gelatinolytic activity and tight junction proteins (occludin and claudin-5) loss.
A. Gelatin zymography showing MMP-9 activity in the Non-I and I microvessels in tPA-treated rats and the quantitative analysis result. STD: the standard of MMP-9. *P<0.05 versus the tPA+normoxia with the same ischemic duration (n=4). B. Occludin and Claudin-5 protein levels in microvessel extracts. *P<0.05 versus the tPA+normoxia with the same ischemic duration. Data are expressed as mean±SEM (n=4).
As expected, ischemia caused an ischemia duration-dependent loss of occludin and claudin-5 proteins in ischemic microvessels following tPA administration (Fig. 4B). Interestingly, NBO treatment did not reduce occludin and claudin-5 loss induced by 3-h MCAO plus tPA administration, however, it prevented the further loss of these proteins in rats with 5 and 7-h ischemic durations.
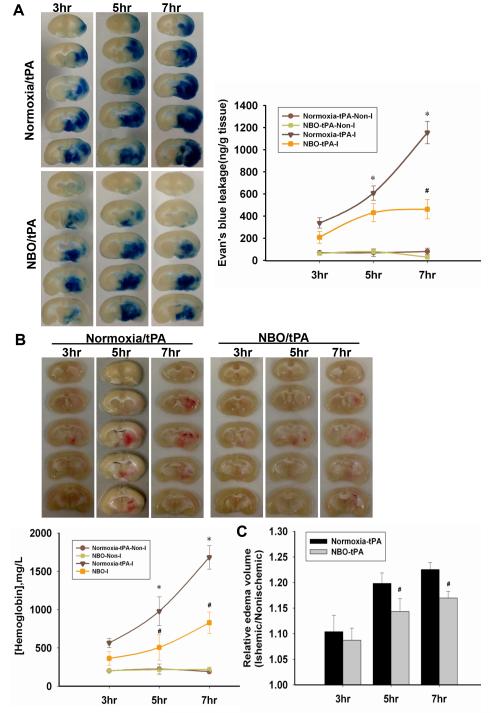
NBO slows down the deterioration of BBB in delayed tPA treatment
Cerebral ischemia with tPA administration led to an ischemia duration-dependently increased in EB extravasation in ischemic tissue (Fig. 5A). NBO treatment reduced EB extravasation in tPA-treated rats with 3, 5, or 7-h MCAO. Similarly, hemoglobin contents in the ischemic tissue of normoxia+tPA-treated rats increased in an ischemia duration-dependent manner, while NBO treatment reduced cerebral hemorrhage in tPA-treated rats (Fig. 5B). Brain edema was progressively worsened with prolongation of MCAO durations and NBO treatment slowed this deteriorating process (Fig. 5C). These results indicated that NBO-afforded BBB protection during cerebral ischemia was sustained following tPA administration.
Figure 5. NBO slows the Evan’s blue extravasation, hemoglobin extravasation and reduces brain edema in delayed tPA-treatment.
A. Representative brain slices showing EB leakage and the quantitative analysis result in rats with 3, 5, or 7-h ischemia followed 2h reperfusion. *P<0.05 versus Normoxia/tPA+3h MCAO; #P<0.05 versus Normoxia-tPA-I+7h MCAO. Data are expressed as mean ± SEM (n=5). B. Representative brain slices showing hemoglobin extravasation and edema in tPA-treated rats, and the quantitative analysis results. *P<0.05 versus Normoxia/tPA+3h MCAO; #P<0.05 versus Normoxia+tPA-treated with the same ischemic duration (n=4). C. The ratio of edema. #P<0.05 versus the tPA-treated with the same ischemia duration (n=7).
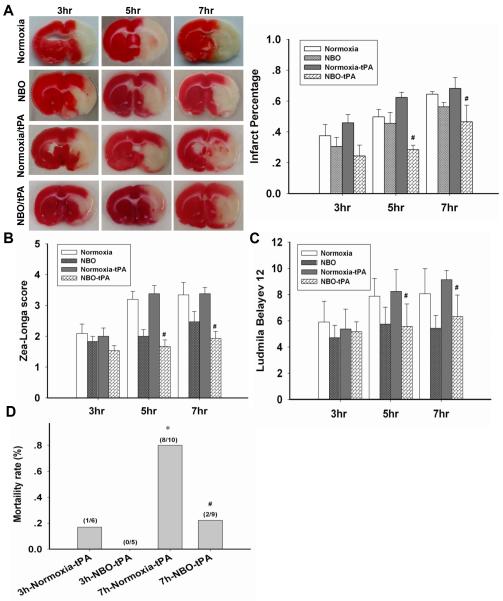
NBO reduces the neurovascular complications associated with delayed tPA treatment
Lastly, we sought to determine whether NBO-afforded BBB protection could result in reductions in the neurovascular complications associated with delayed tPA treatment. The infarction volume increased with the prolongation of MCAO duration in normoxic rats (Fig. 6A). Combined treatment of NBO with tPA reduced the infarction volume for all groups with 3, 5, and 7-h ischemic durations. Similar protective effects of NBO were observed for neurological function assessment using Zea-Longa (Fig. 6B) and Ludmila Belayev scores (Fig. 6C).
Figure 6. NBO improves the outcome of stroke rats with delayed tPA treatment.
A. Representative brain slices illustrating infarction in rats with 3-, 5- or 7-h MCAO plus 2-h reperfusion, and the quantitative analysis result. #P<0.05 versus normoxia+tPA with the same ischemia duration (n=5). B. Zea-Longa neurological scores. C. Ludmila Belayev neurological scores. #P<0.05 versus the normoxia+tPA with the same ischemia duration) (n=10 for normxia and NBO group; n=9 for Normoxia+tPA and tPA+NBO group). D. The mortality of rats at 24 h post ischemia onset. *P<0.05 versus 3h-Normoxia/tPA; #P<0.05 versus 7h-Normoxia/tPA.
Mortality rate in tPA-treated rats was assessed at the end of 24-h of reperfusion (Fig. 6D). tPA administration after 7-h MCAO under normoxia condition resulted in a high mortality rate, which was dramatically reduced by NBO treatment. These results indicate that tPA alone is safe to use within the currently prescribed 3-h window, but NBO could potentially expand tPA’s therapeutic time window for ischemic stroke beyond this time limit, at least to 7-h.
Discussion
The clinical use of tPA has been profoundly constrained due to the narrow therapeutic time window and the potentially devastating hemorrhagic complications. Previous studies showed that NBO given during cerebral ischemia is neuro- and vaso-protective in a rat model of transient cerebral ischemia.10, 14-16, 18, 26 Here, we demonstrated that NBO treatment effectively slowed the progression of ischemia-induced BBB disruption, thus expanding the window of opportunity for other drug treatment. Using tPA as an example, we showed that comparing with tPA alone, combined NBO/tPA treatment led to significant improvement in neurological outcome and reductions in brain edema, ICH severity and mortality rate following delayed tPA treatment, despite prolonged ischemia duration (up to 7-h). Our results suggest that NBO could be used as an adjunct therapy to expand the therapeutic time window of tPA thrombolysis for ischemic stroke.
To maximize tPA’s benefit in treating acute stroke patients, two conditions have to be met at the time of tPA administration: a salvageable penumbra and low risk of severe neurovascular complications. Any treatment that can slow the evolution of ischemic penumbra to irreversible injury and/or reduce tPA’s neurovascular complications would serve as an effective adjuvant therapy to improve the efficacy of tPA thrombolysis. We and others have shown that NBO given during cerebral ischemia can preserve the ischemic penumbra.10, 26-28 We have also demonstrated that NBO could protect the BBB against ischemic damage.14-18 In this context, NBO has been suggested as an ideal adjunct therapy to improve tPA’s efficacy and safety.7, 8, 29 Here, our findings show that NBO treatment can effectively reduce EB extravasation, cerebral hemorrhage and brain edema despite prolonged ischemia of up to 7-h, indicating that NBO-afforded BBB protection is sustained following tPA administration. These results indicate that NBO may have the potential to serve as an adjuvant therapy to safely widen the narrow time window of tPA thrombolysis for ischemic stroke.
MMP-9 is induced in ischemic brain tissue and critically contributes to BBB disruption, brain edema formation and ICH via proteolytic degradation of BBB structural components including tight junction proteins.30-32 Here, our data shows that MMP-9 is induced in ischemic cerebral microvessels in an ischemic duration dependent manner, and this change is accompanied by the loss of the TJPs occludin and claudin-5. NBO treatment ameliorated MMP-9 induction and TJPs loss. Interestingly, in NBO-treated rats, the changes of MMP-9 and TJPs appeared to be “frozen” to a degree observed for 3-h MCAO because no significant further changes were observed for longer ischemic durations, i.e., 5-h and 7-h MCAO. Interestingly, although NBO significantly decreased the infarct volume when compared with normoxia group, it did not “freeze” it at 3-h MCAO level, suggesting that a) “vasoproective” and “neuroprotective” effects of NBO are likely due to two separate processes, and b) NBO treatment affords greater vasoprotection than neuroprotection. Future work is warranted to understand this unexpected finding.
In conclusion, our findings demonstrate that NBO treatment can slow the progression of BBB damage even during prolonged periods of cerebral ischemia, and inhibiting MMP-9 induction and TJPs degradation may account for this protection. More importantly, NBO-afforded BBB protection sustains following tPA administration, which results in significant reductions in the neurovascular complications associated with delayed tPA treatment and improved neurological function. However, rationally-designed clinical studies with well-defined patient population are needed to validate whether NBO is a viable, safe, and efficacious adjuvant therapy for ischemic stroke.
Acknowledgments
Funding sources: This work was supported by grants from National Natural Science Foundation of China (81171242 and 81200928); Beijing Nova Program (Z141107001814045); and NIH (P30GM103400).
Footnotes
Disclosure/conflict of interest: None.
References
- 1.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 2.del Zoppo GJ, von KR, Hamann GF. Ischaemic damage of brain microvessels: inherent risks for thrombolytic treatment in stroke. J Neurol Neurosurg Psychiatry. 1998;65:1–9. doi: 10.1136/jnnp.65.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alberts MJ. tPA in acute ischemic stroke: United States experience and issues for the future. Neurology. 1998;51:S53–S55. doi: 10.1212/wnl.51.3_suppl_3.s53. [DOI] [PubMed] [Google Scholar]
- 4.Jin X, Liu J, Yang Y, Liu KJ, Yang Y, W Liu. Spatiotemporal evolution of blood brain barrier damage and tissue infarction within the first 3h after ischemia onset. Neurobiol Dis. 2012;48:309–316. doi: 10.1016/j.nbd.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Henninger N, Sicard KM, Fisher M. Spectacular shrinking deficit: insights from multimodal magnetic resonance imaging after embolic middle cerebral artery occlusion in Sprague-Dawley rats. J Cereb Blood Flow Metab. 2007;27:1756–1763. doi: 10.1038/sj.jcbfm.9600477. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Tsuji K, Lee SR, Ning M, Furie KL, Buchan AM, et al. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004;35:2726–2730. doi: 10.1161/01.STR.0000143219.16695.af. [DOI] [PubMed] [Google Scholar]
- 7.Poli S, Veltkamp R. Oxygen therapy in acute ischemic stroke - experimental efficacy and molecular mechanisms. Curr Mol Med. 2009;9:227–241. doi: 10.2174/156652409787581619. [DOI] [PubMed] [Google Scholar]
- 8.Henninger N, Fisher M. Normobaric hyperoxia - a promising approach to expand the time window for acute stroke treatment. Cerebrovasc Dis. 2006;21:134–136. doi: 10.1159/000090446. [DOI] [PubMed] [Google Scholar]
- 9.Henninger N, Bouley J, Nelligan JM, Sicard KM, Fisher M. Normobaric hyperoxia delays perfusion/diffusion mismatch evolution, reduces infarct volume, and differentially affects neuronal cell death pathways after suture middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 2007;27:1632–1642. doi: 10.1038/sj.jcbfm.9600463. [DOI] [PubMed] [Google Scholar]
- 10.Liu S, Shi H, Liu W, Furuichi T, Timmins GS, Liu KJ. Interstitial pO2 in ischemic penumbra and core are differentially affected following transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2004;24:343–349. doi: 10.1097/01.WCB.0000110047.43905.01. [DOI] [PubMed] [Google Scholar]
- 11.Singhal AB. A review of oxygen therapy in ischemic stroke. Neurol Res. 2007;29:173–183. doi: 10.1179/016164107X181815. [DOI] [PubMed] [Google Scholar]
- 12.Wu O, Lu J, Mandeville JB, Murata Y, Egi Y, Dai G, et al. Dynamic functional cerebral blood volume responses to normobaric hyperoxia in acute ischemic stroke. J Cereb Blood Flow Metab. 2012;32:1800–1809. doi: 10.1038/jcbfm.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singhal AB, Benner T, Roccatagliata L, Koroshetz WJ, Schaefer PW, Lo EH, et al. A pilot study of normobaric oxygen therapy in acute ischemic stroke. Stroke. 2005;36:797–802. doi: 10.1161/01.STR.0000158914.66827.2e. [DOI] [PubMed] [Google Scholar]
- 14.Liu W, Sood R, Chen Q, Sakoglu U, Hendren J, Cetin O, et al. Normobaric hyperoxia inhibits NADPH oxidase-mediated matrix metalloproteinase-9 induction in cerebral microvessels in experimental stroke. J Neurochem. 2008;107:1196–1205. doi: 10.1111/j.1471-4159.2008.05664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu W, Chen Q, Liu J, Liu KJ. Normobaric hyperoxia protects the blood brain barrier through inhibiting Nox2 containing NADPH oxidase in ischemic stroke. Med Gas Res. 2011;1:22. doi: 10.1186/2045-9912-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu W, Hendren J, Qin XJ, Shen J, Liu KJ. Normobaric hyperoxia attenuates early blood-brain barrier disruption by inhibiting MMP-9-mediated occludin degradation in focal cerebral ischemia. J Neurochem. 2009;108:811–820. doi: 10.1111/j.1471-4159.2008.05821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Jin X, Liu KJ, Liu W. Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. J Neurosci. 2012;32:3044–3057. doi: 10.1523/JNEUROSCI.6409-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu W, Hendren J, Qin XJ, Liu KJ. Normobaric hyperoxia reduces the neurovascular complications associated with delayed tissue plasminogen activator treatment in a rat model of focal cerebral ischemia. Stroke. 2009;40:2526–2531. doi: 10.1161/STROKEAHA.108.545483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfefferkorn T, Rosenberg GA. Closure of the blood-brain barrier by matrix metalloproteinase inhibition reduces rtPA-mediated mortality in cerebral ischemia with delayed reperfusion. Stroke. 2003;34:2025–2030. doi: 10.1161/01.STR.0000083051.93319.28. [DOI] [PubMed] [Google Scholar]
- 20.Murata Y, Rosell A, Scannevin RH, Rhodes KJ, Wang X, Lo EH. Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke. 2008;39:3372–3377. doi: 10.1161/STROKEAHA.108.514026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 22.Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27:1616–1622. doi: 10.1161/01.str.27.9.1616. [DOI] [PubMed] [Google Scholar]
- 23.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 24.Qin Z, Karabiyikoglu M, Hua Y, Silbergleit R, He Y, Keep RF, et al. Hyperbaric oxygen-induced attenuation of hemorrhagic transformation after experimental focal transient cerebral ischemia. Stroke. 2007;38:1362–1367. doi: 10.1161/01.STR.0000259660.62865.eb. [DOI] [PubMed] [Google Scholar]
- 25.Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- 26.Liu S, Liu W, Ding W, Miyake M, Rosenberg GA, Liu KJ. Electron paramagnetic resonance-guided normobaric hyperoxia treatment protects the brain by maintaining penumbral oxygenation in a rat model of transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:1274–1284. doi: 10.1038/sj.jcbfm.9600277. [DOI] [PubMed] [Google Scholar]
- 27.Kim HY, Singhal AB, Lo EH. Normobaric hyperoxia extends the reperfusion window in focal cerebral ischemia. Ann Neurol. 2005;57:571–575. doi: 10.1002/ana.20430. [DOI] [PubMed] [Google Scholar]
- 28.Singhal AB, Wang X, Sumii T, Mori T, Lo EH. Effects of normobaric hyperoxia in a rat model of focal cerebral ischemia-reperfusion. J Cereb Blood Flow Metab. 2002;22:861–868. doi: 10.1097/00004647-200207000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Qi Z, Liu W, Luo Y, Ji X, Liu KJ. Normobaric hyperoxia-based neuroprotective therapies in ischemic stroke. Med Gas Res. 2013;3:2. doi: 10.1186/2045-9912-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenberg GA, Yang Y. Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg Focus. 2007;22:E4. doi: 10.3171/foc.2007.22.5.5. [DOI] [PubMed] [Google Scholar]
- 31.Simard JM, Kent TA, Chen M, Tarasov KV, Gerzanich V. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6:258–268. doi: 10.1016/S1474-4422(07)70055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke. 2002;33:831–836. doi: 10.1161/hs0302.104542. [DOI] [PubMed] [Google Scholar]