Abstract
Both sleep disturbance and social isolation increase the risk for morbidity and mortality. Systemic inflammation is suspected as a potential mechanism of these associations. However, the complex relationships between sleep disturbance, social isolation, and inflammation have not been examined in a population-based longitudinal study. This study examined the longitudinal association between sleep disturbance and systemic inflammation, and the moderating effects of social isolation on this association. The CARDIA study is a population-based longitudinal study conducted in four US cities. Sleep disturbance – i.e., insomnia complaints and short sleep duration – was assessed in 2962 African-American and white adults at baseline (2000-2001, ages 33-45 years). Circulating C-reactive protein (CRP) was measured at baseline and follow-up (2005-2006). Interleukin-6 (IL-6) and subjective and objective social isolation (i.e., feelings of social isolation and social network size) were measured at follow-up. Sleep disturbance was a significant predictor of inflammation five years later after full adjustment for covariates (adjusted betas: 0.048, P=0.012 for CRP; 0.047, P=0.017 for IL-6). Further adjustment for baseline CRP revealed that sleep disturbance also impacted the longitudinal change in CRP levels over five years (adjusted beta: 0.044, P=0.013). Subjective social isolation was a significant moderator of this association between sleep disturbance and CRP (adjusted beta 0.131, P=0.002). Sleep disturbance was associated with heightened systemic inflammation in a general population over a five-year follow-up, and this association was significantly stronger in those who reported feelings of social isolation. Clinical interventions targeting sleep disturbances may be a potential avenue for reducing inflammation, particularly in individuals who feel socially isolated.
Keywords: sleep disturbance, systemic inflammation, C-reactive protein, interleukin-6, social isolation, population-based longitudinal study, moderation
1. INTRODUCTION
Disturbances of sleep including insomnia complaints and extremes of sleep duration have an influence on the risk of several major medical and mental illnesses including infectious disease, cardiovascular disease, cancer, and depression, ultimately contributing to all-cause mortality (Cho et al., 2008; Dew et al., 2003; Irwin, 2015; Kripke et al., 2002; Mallon et al., 2002; Vgontzas et al., 2013). One of the most intensely investigated biological mechanisms underlying these effects is systemic inflammation (Irwin, 2015; Motivala, 2011). Plasma levels of inflammatory markers have been shown to be elevated in clinical populations with sleep disturbance – e.g., end stage renal disease, alcoholism, and major depression (Chiu et al., 2009; Irwin et al., 2004; Motivala et al., 2005) – and also following experimental sleep deprivation (Irwin et al., 2006; Irwin et al., 2008). However, less is known about the relationships between sleep disturbance and systemic inflammation in community-dwelling adults. Although many community-based cross-sectional studies have examined such relationships (Christian et al., 2011; Friedman, 2011; Liukkonen et al., 2007; Okun et al., 2009; Zhang et al., 2013), few community-based studies have examined sleep variables and systemic inflammation in a prospective design. Ferrie and colleagues recently reported that decreasing sleep duration over two assessments 5 years apart was longitudinally associated with higher levels of C-reactive protein (CRP) and interleukin-6 (IL-6), but did not include an assessment of insomnia complaints or sleep quality (Ferrie et al., 2013). One other study found prospective associations between sleep and inflammation, but asked whether inflammation predicted sleep duration, as opposed to the effects of sleep on inflammation (Dowd et al., 2011). Thus, to our knowledge, community-based prospective studies have not yet examined the role of a more comprehensive sleep disturbance variable that includes both insomnia complaints and sleep duration in the more clinically meaningful direction of causality, i.e., from sleep disturbance to systemic inflammation. This approach may be particularly important because insomnia with short sleep duration is likely the most biologically severe phenotype of sleep disturbance, leading to cognitive-emotional and cortical arousal, activation of both hypothalamic-pituitary-adrenal and sympatho-adrenal-medullary axes, and a higher risk for hypertension, impaired heart rate variability, diabetes, neurocognitive impairment, and mortality (Vgontzas et al., 2013).
A large body of epidemiologic research has linked characteristics of the social environment to human physical health (Cacioppo and Hawkley, 2003; Seeman, 1996). People who are socially isolated have increased risk of all-cause mortality (Cacioppo and Hawkley, 2003; Seeman, 1996), and several infectious, neoplastic, and cardiovascular diseases (Caspi et al., 2006; Cohen et al., 1997; Cole et al., 2003; Kroenke et al., 2006). A possible biological mechanism of these effects is systemic inflammation, as social isolation has been shown to predict heightened systemic inflammation as assessed by circulating levels of inflammatory markers (Heffner et al., 2011). Furthermore, it is thought that social isolation is associated with sleep disturbance through a disruption of social zeitgebers (i.e., social cues that maintain the sleep-wake activity schedule), which are increasingly viewed as being critical in the homeostatic regulation of sleep-wake activity (Mistlberger and Skene, 2004). In fact, socially isolated individuals – either subjectively or objectively – reported poorer sleep quality and had poorer sleep efficiency compared to those who were not socially isolated (Cacioppo et al., 2002a; Cacioppo et al., 2002b; Eshkoor et al., 2014). Despite these apparently overlapping interrelationships among sleep disturbance, social isolation, and systemic inflammation, these three variables have rarely been examined simultaneously. An interesting analysis of the data from a population-based cross sectional study has revealed that social engagement – i.e., social well-being based on positive relations with others – moderated the association of sleep disturbance with circulating inflammatory markers, suggesting that social integration as opposed to social isolation may attenuate the effects of sleep disturbance on systemic inflammation (Friedman, 2011).
The current work aims to extend the prior cross-sectional observations in a longitudinal setting and to examine: 1) the longitudinal association of sleep disturbance – i.e., insomnia complaints and short sleep duration – with systemic inflammation; and 2) the moderating effect of subjective and objective social isolation – i.e., feelings of social isolation and social network size – on this association. Of note, both subjective and objective measures of social isolation have been associated with morbidity and mortality (Newall et al., 2013; Seeman, 2000; Steptoe et al., 2013), and in particular, a large body of psychological research has demonstrated a robust association between subjective social isolation (e.g., perceived emotional support, negative support and/or feelings of loneliness) and poor health, including cardiovascular disease, inflammation, and depression (Berkman et al., 1992; Cacioppo et al., 2006; Hawkley et al., 2006; Steptoe et al., 2004).
Using data from the Coronary Artery Risk Development in Young Adults (CARDIA) study, an ongoing community-based prospective cohort study, we examined whether sleep disturbance – i.e., insomnia complaints and short sleep duration – is associated with systemic inflammation as indexed by plasma CRP and IL-6 five years later. Furthermore, we examined whether subjective and objective social isolation moderates the longitudinal association between sleep disturbance and systemic inflammation. We hypothesized that: 1) sleep disturbance would be associated with elevated levels of plasma CRP and IL-6 five years later; and 2) this longitudinal association would be stronger among socially isolated individuals than among socially integrated individuals (i.e., those who report feelings of social isolation vs. those who report feelings of social connection; and those with small social networks vs. those with large social networks). Given the role of inflammation in onset and progression of major physical and mental illnesses, an investigation into the complex interrelationships among sleep disturbance, social isolation, and systemic inflammation would provide insight about the mechanisms by which sleep disturbance contributes to adverse clinical outcomes, especially among those who are socially isolated. Such understanding would be instrumental to developing treatments that might target sleep disturbance to prevent inflammatory illnesses, with attention focused on socially isolated individuals.
2. MATERIALS AND METHODS
2.1. Subjects
CARDIA is a bi-racial longitudinal study of cardiovascular risk factors in white and African-American men and women aged 18 to 30 years at study inception (1985-1986). From the inception, study participation was limited to members of the two largest racial groups in the US, whites and African-Americans (Friedman et al., 1988). Full details of the study design and methods have been published previously (Friedman et al., 1988). Briefly, 5115 individuals were recruited from four US cities (Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA) to take part in the baseline clinical examination in 1985-1986. Of the 5115 individuals originally enrolled at Year 0, 3178 participated in both Years 15 and 20, the two CARDIA examinations considered for the current analysis because Year 15 included the assessment of sleep quality, and Year 20 included measurement of CRP, IL-6, and subjective social isolation. For the purpose of the current paper, Year 15 (2000-2001) was considered as the baseline and Year 20 (2005-2006) as the follow-up. We excluded 216 participants with missing values of either Year 15 measures of sleep quality or covariates or Year 20 measures of CRP, IL-6, or subjective social isolation. After this exclusion, 2962 participants remained.
2.2. Predictor: Sleep Disturbance
Sleep disturbance was assessed at baseline (2000-2001) using a sleep questionnaire specifically devised for the CARDIA study (Cho et al., 2012; Cho et al., 2009; St-Onge et al., 2010). A summary score was produced summing up 6 items of the sleep questionnaire (score range 0-9), with higher summary scores reflecting sleep disturbance as indexed by 6 components: daytime sleepiness (individual score 0 or 1); sleep onset problem (0 or 1); sleep maintenance problem (0 or 1); early awakening (0 or 1); subjective sleep quality (0 to 4); and short sleep duration (0 or 1). For the sleep duration item, the participants were asked the following question: “During the past month, how many hours of actual sleep did you get at night? (This may be different than the number of hours you spend in bed).” The answer to this question was used to determine self-reported sleep duration. For the summary score, sleep duration was categorized into usual (6 hours or more) and short (less than 6 hours). This classification is consistent with the classifications used in the majority of previous studies on the health effects of habitual sleep duration (Patel et al., 2004; Stranges et al., 2008). Since less than 1% of the participants reported sleep duration of more than 9 hours and the evidence demonstrates the combination of insomnia complaints and short sleep duration to be a robust predictor of poor health (Vgontzas et al., 2013), a separate category of long sleep duration was not generated.
2.3. Outcome: CRP and IL-6
Plasma CRP was measured at follow-up (2005-2006) using a BNII nephelometer (Dade Behring, Deerfield, Ill) utilizing a particle enhanced immunonepholometric assay. The assay range is 0.175-1100 mg/L, intra-assay coefficients of variation range from 2.3-4.4% and inter-assay coefficients of variation range from 2.1-5.7%. Plasma IL-6 was measured by ultra-sensitive ELISA (R&D Systems, Minneapolis, MN). The lower detection limit is <0.10 pg/mL, with a detection range of 0.156-10.0 pg/mL and a routine inter-assay coefficients of variation in the lab of 10.0. IL-6 was measured by ultra-sensitive ELISA (R&D Systems, Minneapolis, MN). The lower detection limit is <0.10 pg/mL, with a detection range of 0.156-10.0 pg/mL and a routine inter-assay coefficient of variation in the lab of 10.0%.
2.4. Moderator: Subjective and Objective Social Isolation
Subjective social isolation was assessed at follow-up (2005-2006) using a composite questionnaire containing 12 items: emotional support (4 items); negative support (4 items); and loneliness (4 items). Emotional support items were reverse-coded so that higher scores indicate more subjective social isolation. Each item is on a four-point scale (0-3) and hence the total score ranges from 0 to 36, with higher total scores reflecting higher subjective social isolation. Internal consistency was measured by Cronbach’s alpha (Bland and Altman, 1997), and the data showed a high degree of internal consistency with a value of 0.82. Subjective social isolation was not assessed at baseline (2000-2001). Subjective social isolation was used as a continuous variable in its original format for moderation testing. Subsequently, for the purpose of graphic representation and subgroup analysis, the median split of the total score was used generating a binary variable (feeling socially isolated vs. feeling socially integrated). Social network size was assessed at follow-up as an objective measure of social isolation. Social network size reflects the mean of reported number of close friends and of close relatives (available answer categories for each item were: 0, 1-2, 3-5, 6-9, or 10+), hence higher scores meaning larger social networks (Seeman et al., 2014). Social network size was used as a continuous variable in its original format for moderation testing. Subsequently, for the purpose of graphic representation and subgroup analysis, the median split of the score was used generating a binary variable (small vs. large social network).
2.5. Potential Biobehavioral Confounders
Covariates were chosen based on the published recommendations on the assessment of control variables for the studies investigating linkages between behavioral factors and inflammation (O’Connor et al., 2009), and all of them were assessed at baseline. Variables known to exert confounding effects for the association between biobehavioral factors and inflammation include age, sex, ethnicity, socioeconomic status (often represented by education), smoking, alcohol consumption, body-mass index (BMI), physical activity level, and medications such as aspirin, statins, and antidepressants. Depressive symptoms were also used as a covariate. For the current analysis, smoking status, originally classified as never, former or current smoker, was re-categorized as a binary variable (current smoker or not). Medication use was defined as the use of any prescription medications that may affect systemic inflammatory state such as antihypertensives, antihyperlipidemics, antiasthmatics, aspirin, and antidepressants (yes or no). All the other variables were used in the original format without further reduction. They were binary (sex and ethnicity [White and African-American]) or continuous (age, years of education, BMI, self-reported average daily alcohol consumption, physical activity level, and depressive symptoms). Physical activity level was assessed with the CARDIA Physical Activity History Questionnaire (Sidney et al., 1991), eliciting frequency of participation in a range of specific heavy and moderate intensity activities during the previous 12 months. Physical activity score was computed and expressed in ‘exercise units’ (EU). For reference, 300 EU roughly approximates the American College of Sports Medicine recommendations for the amount of exercise needed to support weight loss (5 sessions of 300 kcal of energy expenditure weekly) (Parker et al., 2007). Depressive symptoms were assessed using the Center for Epidemiological Studies Depression Scale (CES-D) (Radloff, 1977), a valid and reliable measure of depressive symptoms in the general population, with a score range 0-60 and higher scores reflecting more severe depression.
2.6. Analysis
Baseline characteristics of 2962 participants are presented as mean (standard deviation; SD) or n (%) and their associations with plasma levels of CRP and IL-6 at follow-up were described using correlation (continuous variables) or t-tests (dichotomous variables). Then the study hypotheses were tested by performing linear regression analyses with standardized regression coefficients (beta), which facilitate comparison across models. The first hypothesis tested was whether sleep disturbance at baseline was associated with inflammation at follow-up, in which the independent variable was sleep disturbance based on insomnia complaints and short sleep duration and the dependent variables were plasma CRP or IL-6. Selection of covariates for multivariable analysis relied on empirical evidence rather than predetermined P-value criteria; the latter approach, which selects factors for inclusion in a multivariable model only if the factors are ‘statistically significant’ in bivariate screening, is considered less optimal (Steyerberg et al., 2000). All covariates were assessed at baseline. Model 1 included sociodemographic variables (age, sex, ethnicity, and education). Model 2 further included biomedical factors (BMI and medication use). Model 3 further included health-related behaviors (smoking, alcohol consumption, and physical activity level). Model 4 further included depressive symptoms. An additional multivariable model (Model 5) also including baseline CRP as a covariate was tested in order to examine how sleep disturbance impacted the prospective change in plasma levels of CRP over five years. Such an analysis could not be conducted for IL-6 as plasma IL-6 was measured only in a small subset of participants at baseline. To avoid any artifact due to different sample sizes between the nested models, participants missing the covariates were excluded. We also hypothesized that the longitudinal association between sleep disturbance and circulating inflammatory markers would be moderated by social isolation assessed at follow-up. It was expected that sleep disturbance would be more strongly associated with increases of plasma CRP or IL-6 among those who were socially isolated than among those who were socially integrated, i.e., social integration would act as a buffer against the effect of sleep disturbance on systemic inflammation. To test this hypothesis, formal moderation tests were performed using the identical multivariable approaches as shown above (Models 1 to 5). The main effects of the predictor (i.e., sleep disturbance) and the moderator (i.e., subjective social isolation or social network size) were included in the models with the interaction term (i.e., ‘sleep disturbance × subjective social isolation’ or ‘sleep disturbance × social network size’). Subsequently, we conducted subgroup analyses of longitudinal associations between sleep disturbance and circulating inflammatory markers stratified by subjective social isolation (feeling socially isolated vs. feeling socially integrated) and also by objective social isolation (small vs. large social networks). The identical multivariable analyses were conducted for subgroup analyses. All the analyses were performed using STATA version 12.1 (StataCorp, College Station, TX, USA).
3. RESULTS
3.1. Sample Characteristics
Table 1 and Table 2 describe baseline characteristics of the participants and their associations with plasma levels of CRP and IL-6 at follow-up using correlation or t-test. Two potential moderators assessed at follow-up – subjective social isolation and social network size – and their associations with plasma levels of CRP and IL-6 at follow-up are also described. Plasma CRP levels at follow-up were significantly positively correlated with sample characteristics such as sleep disturbance, BMI, depressive symptoms, and subjective social isolation; and significantly negatively correlated with education years, daily alcohol consumption, physical activity level, and social network size. Mean CRP levels at follow-up were significantly higher for female participants, African-Americans, and those using medications. Plasma IL-6 levels at follow-up were significantly positively correlated with baseline characteristics such as sleep disturbance, BMI, depressive symptoms, and subjective social isolation; and significantly negatively correlated with education years, physical activity level, and social network size. Mean IL-6 levels at follow-up were significantly higher for female participants, African-Americans, those using medications, and current smokers. Plasma levels of CRP and IL-6 were significantly correlated with each other (r=0.41, P<0.0001). Additionally, according to paired t-tests between baseline and follow-up, BMI significantly increased (from 28.6 [SD 0.1] to 29.4 [SD 0.1], P<0.0001) and sleep disturbance somewhat worsened (from 2.4 [SD 1.9] to 2.5 [SD 2.0], P=0.0006) over 5 years, while depressive symptoms barely changed (from 8.9[7.7] to 9.1 [7.8], P=0.05).
Table 1. Characteristics of 2962 participants at baseline (2000-2001) and their associations with plasma CRP levels at follow-up (2005-2006).
| Baseline characteristic (range) | N (%) | Mean CRP level (ug/mL)* |
Mean (SD) | Correlation with CRP* |
P* |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 1301 (43.9) | 1.97 | <0.0001 | ||
| Female | 1661 (56.1) | 3.33 | |||
|
| |||||
| Ethnicity | |||||
| White | 1669 (56.3) | 2.08 | <0.0001 | ||
| African-American | 1293 (43.7) | 3.58 | |||
|
| |||||
| Medication use | |||||
| No | 2306(77.9) | 2.55 | 0.0001 | ||
| Yes | 656 (22.1) | 3.39 | |||
|
| |||||
| Current smoker | |||||
| No | 2374 (80.1) | 2.66 | 0.074 | ||
| Yes | 588 (19.9) | 3.05 | |||
|
| |||||
| Age (33-45 years) | 40.3 (3.6) | −0.025 | 0.174 | ||
|
| |||||
| Education (4-20 years) | 15.1 (2.5) | −0.099 | <0.0001 | ||
|
| |||||
| BMI (15.7-65.8 kg/m2) | 28.6 (6.7) | 0.351 | <0.0001 | ||
|
| |||||
| Daily alcohol consumption (0- 564.9 mL) |
10.8 (25.0) | −0.055 | 0.003 | ||
|
| |||||
| Physical activity score (0-1818) | 351 (281) | −0.112 | <0.0001 | ||
|
| |||||
| Depressive symptoms (CES-D score 0-54) |
8.9 (7.7) | 0.066 | 0.0004 | ||
|
| |||||
| Sleep disturbance score (0-9) | 2.4 (1.9) | 0.105 | <0.0001 | ||
| Subjective social isolation§ (0-36) | 10.2 (5.5) | 0.063 | 0.0006 | ||
| Social network size§ (0-24) | 9.3 (5.7) | −0.040 | 0.028 | ||
CRP = C-reactive protein; BMI = body mass index; CES-D = Center for Epidemiological Studies Depression Scale; SD = standard deviation
Correlation coefficient between the baseline variable and plasma CRP level at follow-up when the baseline variable is continuous (P-value from correlation); and mean CRP level at follow-up by each category of the baseline variable when the baseline variable is categorical (P-value from t-test).
These 2 variables were assessed at follow-up (2005-2006).
Table 2. Characteristics of 2962 participants at baseline (2000-2001) and their associations with plasma IL-6 levels at follow-up (2005-2006).
| Baseline characteristic (range) | N (%) | Mean IL-6 level (pg/mL)* |
Mean (SD) | Correlation with IL-6* |
P* |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 1301 (43.9) | 2.37 | 0.002 | ||
| Female | 1661 (56.1) | 2.70 | |||
|
| |||||
| Ethnicity | |||||
| White | 1669 (56.3) | 2.16 | <0.0001 | ||
| African-American | 1293 (43.7) | 3.07 | |||
|
| |||||
| Medication use | |||||
| No | 2306(77.9) | 2.43 | <0.0001 | ||
| Yes | 656 (22.1) | 2.99 | |||
|
| |||||
| Current smoker | |||||
| No | 2374 (80.1) | 2.41 | <0.0001 | ||
| Yes | 588 (19.9) | 3.14 | |||
|
| |||||
| Age (33-45 years) | 40.3 (3.6) | −0.016 | 0.396 | ||
|
| |||||
| Education (4-20 years) | 15.1 (2.5) | −0.124 | <0.0001 | ||
|
| |||||
| BMI (15.7-65.8 kg/m2) | 28.6 (6.7) | 0.243 | <0.0001 | ||
|
| |||||
| Daily alcohol consumption (0- 564.9 mL) |
10.8 (25.0) | 0.004 | 0.833 | ||
|
| |||||
| Physical activity score (0-1818) | 351 (281) | −0.087 | <0.0001 | ||
|
| |||||
| Depressive symptoms (CES-D score 0-54) |
8.9 (7.7) | 0.082 | <0.0001 | ||
|
| |||||
| Sleep disturbance score (0-9) | 2.4 (1.9) | 0.104 | <0.0001 | ||
| Subjective social isolation§ (0-36) | 10.2 (5.5) | 0.075 | <0.0001 | ||
| Social network size§ (0-24) | 9.3 (5.7) | −0.046 | 0.012 | ||
IL-6 = interleukin-6; BMI = body mass index; CES-D = Center for Epidemiological Studies Depression Scale; SD = standard deviation
Correlation coefficient between the baseline variable and plasma IL-6 level at follow-up when the baseline variable is continuous (P-value from correlation); and mean IL-6 level at follow-up by each category of the baseline variable when the baseline variable is categorical (P-value from t-test).
These 2 variables were assessed at follow-up (2005-2006).
3.2. Longitudinal Associations between Sleep Disturbance and Circulating Inflammatory Markers
As shown in Table 3, sleep disturbance assessed at baseline was respectively associated with plasma levels of CRP and IL-6 at follow-up (unadjusted standardized beta coefficients: 0.105, P<0.001 for CRP; and 0.104, P<0.001 for IL-6). Table 3 also presents the adjusted standardized beta coefficients after step-wise inclusion of four sets of covariates for the associations between sleep disturbance and circulating inflammatory markers. These associations remained statistically significant after adjusting for all assessed covariates including sociodemographic characteristics, BMI, medication use, smoking, alcohol consumption, physical activity level, and depressive symptoms (adjusted betas: 0.048, P=0.012 for CRP; and 0.047, P=0.017 for IL-6). Finally, the association between baseline sleep disturbance and plasma CRP level at follow-up remained significant even after the additional adjustment for baseline CRP (adjusted beta: 0.044, P=0.013). Of note, in this fully adjusted multivariable model (Model 5), in addition to sleep disturbance, the following predictors remained significant (i.e., the following variables were independent risk factors for CRP at follow-up): female sex (adjusted beta: 0.083, P<0.001), BMI (0.191, P<0.001), and baseline CRP (0.390, P<0.001) (see Supplementary Data). Furthermore, the association between baseline CRP level and sleep disturbance at follow-up, in an opposite direction, was also significant after adjustment for the same covariates and baseline sleep disturbance (adjusted beta: 0.046, P=0.015).
Table 3. Standardized regression coefficients for longitudinal association between sleep disturbance assessed at baseline (2000-2001) and inflammatory markers (CRP and IL-6) measured at follow-up (2005-2006) (N=2962).
| Adjustment | Beta for CRP |
P | Beta for IL-6 |
P |
|---|---|---|---|---|
| Unadjusted model | 0.105 | <0.001 | 0.104 | <0.001 |
| Model 1: Sociodemographic variables (age, sex, ethnicity, and education) | 0.071 | <0.001 | 0.072 | <0.001 |
| Model 2: Model 1 + Biomedical factors (BMI and medication use) | 0.043 | 0.014 | 0.052 | 0.004 |
| Model 3: Model 2 + Health-related behaviors (smoking, alcohol consumption, and physical activity) | 0.041 | 0.020 | 0.045 | 0.014 |
| Model 4: Model 3 + Depressive symptoms | 0.048 | 0.012 | 0.047 | 0.017 |
| Model 5: Model 4 + Baseline CRP | 0.044 | 0.013 | NA | NA |
CRP = C-reactive protein; IL-6 = Interleukin-6; BMI = body mass index; Beta = standardized regression coefficient expressing the change in standardized CRP level per one standard deviation in sleep disturbance score; NA = not applicable
Short sleep duration as a separate variable assessed at baseline was respectively associated with plasma levels of CRP and IL-6 at follow-up (unadjusted standardized beta coefficients: 0.086, P<0.001 for CRP; and 0.101, P<0.001 for IL-6). After adjusting for the above-mentioned covariates, the association between short sleep duration and CRP was attenuated to non-significance, whereas the association between short sleep duration and IL-6 remained statistically significant (adjusted betas: 0.024, P=0.177 for CRP; and 0.043, P=0.019 for IL-6).
3.3. Moderation of Longitudinal Associations between Sleep Disturbance and Circulating Inflammatory Markers by Subjective Social Isolation
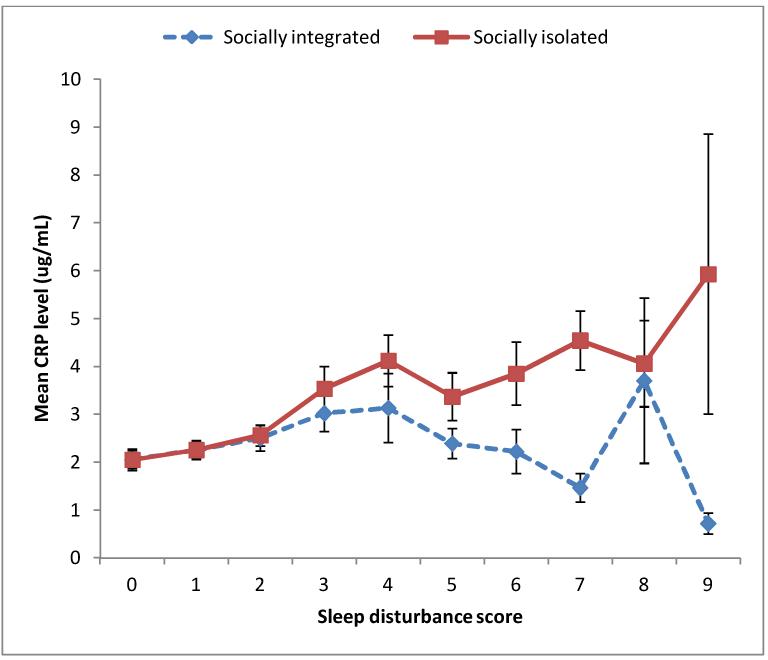
The association between sleep disturbance and plasma CRP was moderated by subjective social isolation, as formal moderation test was significant in both unadjusted and adjusted analyses (unadjusted beta 0.150, P=0.003; adjusted beta 0.134, P=0.004). Formal moderation test was also significant in the adjusted analysis that included baseline CRP in addition to all covariates (adjusted beta 0.131, P=0.002). Interestingly, although subjective social isolation was a strong moderator, it was not a predictor of systemic inflammation by itself. An exploratory analysis included subjective social isolation as an additional covariate to the fully adjusted model (i.e., Model 5 in Table 3) and revealed that subjective social isolation did not have any independent effect on plasma CRP (adjusted beta: −0.001, P= 0.949). In line with the results of formal moderation tests, the subgroup analysis revealed that the association between sleep disturbance and CRP was much stronger among those who felt socially isolated than among those who felt socially integrated. As shown in Figure 1, higher sleep disturbance score at baseline was associated with higher mean CRP level at follow-up for the subgroup of those who felt socially isolated (n=1460; unadjusted beta 0.135, P<0.001). This association remained significant even after adjusting for age, sex, ethnicity, education, BMI, medication use, smoking, alcohol consumption, physical activity level, and depressive symptoms (adjusted beta 0.073, P=0.007). However, the longitudinal association between sleep disturbance and CRP was much weaker and non-significant in both unadjusted and adjusted analyses for the subgroup of those who felt socially integrated (n=1502; unadjusted beta 0.037, P=0.149; adjusted beta 0.003, P=0.910).
Figure 1. Mean CRP level at follow-up according to basleine sleep disturbance score in socially integrated (n=1502) and socially isolated (n=1460) subgroups.
CRP = C-reactive protein; Error bars represent standard errors of mean.
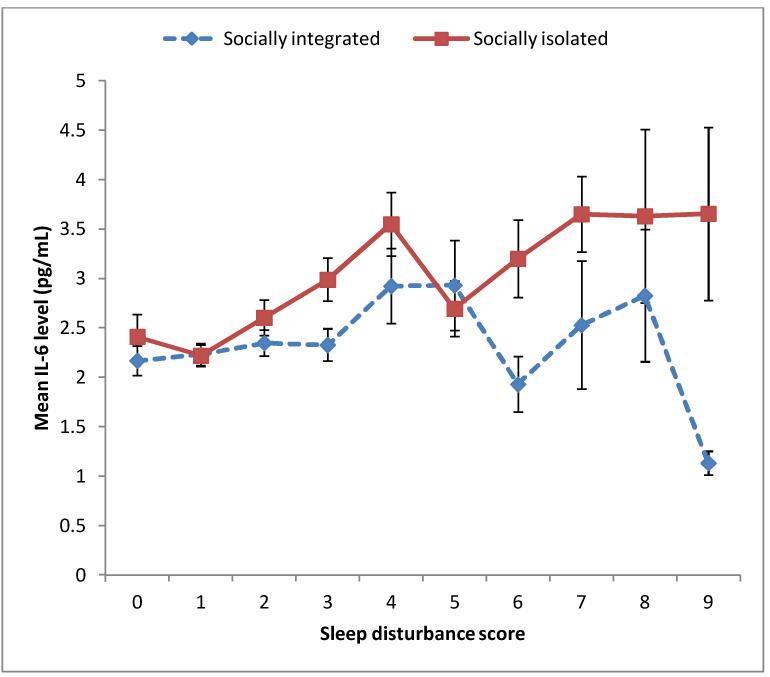
Regarding the moderation of the association between sleep disturbance and plasma IL-6 by subjective social isolation, formal moderation test was not significant in either unadjusted or adjusted analyses (unadjusted beta 0.005, P=0.923; adjusted beta −0.008, P=0.865). However, the subgroup analysis revealed that the association between sleep disturbance and IL-6 was appreciably stronger among those who felt socially isolated than among those who felt socially integrated. As shown in Figure 2, higher sleep disturbance score at baseline was associated with higher mean IL-6 level at follow-up for the subgroup of those who felt socially isolated (n=1460; unadjusted beta 0.119, P<0.001). This association remained significant even after adjusting for the above-mentioned covariates (adjusted beta 0.064, P=0.024). Comparatively, the longitudinal association between sleep disturbance and IL-6 for the subgroup of those who felt socially integrated was substantially weaker (n=1502; unadjusted beta 0.052, P=0.045; adjusted beta 0.019, P=0.478).
Figure 2. Mean IL-6 level at follow-up according to basleine sleep disturbance score in socially integrated (n=1502) and socially isolated (n=1460) subgroups.
IL-6 = Interleukin-6; Error bars represent standard errors of mean.
3.4. Moderation of Longitudinal Associations between Sleep Disturbance and Circulating Inflammatory Markers by Objective Social Isolation
In addition to testing the moderating influence of subjective social isolation, we also examined the influence of social network size on the association between sleep disturbance and systemic inflammation. Regarding the moderation of the association between sleep disturbance and plasma CRP by social network size, formal moderation test was not significant in either unadjusted or adjusted analyses (unadjusted beta −0.068, P=0.075; adjusted beta −0.064, P=0.074). However, the subgroup analysis revealed that the association between sleep disturbance and CRP was appreciably stronger among those who had a small social network than among those who had a large social network. The longitudinal association between sleep disturbance and CRP was significant for the subgroup with a small social network (n=1709; unadjusted beta 0.141, P<0.001). This association remained significant even after adjusting for age, sex, ethnicity, education, BMI, medication use, smoking, alcohol consumption, physical activity level, and depressive symptoms (adjusted beta 0.083, P=0.001). The longitudinal association between sleep disturbance and CRP was much weaker and non-significant for the subgroup with a large social network in both unadjusted and adjusted analyses (n=1253; unadjusted beta 0.052, P=0.067; adjusted beta 0.007, P=0.826).
Regarding the moderation of the association between sleep disturbance and plasma IL-6 by social network size, formal moderation test was not significant in either unadjusted or adjusted analyses (unadjusted beta −0.012, P=0.745; adjusted beta −0.003, P=0.931). According to the subgroup analysis, the association between sleep disturbance and IL-6 did not substantially differ between those who had a small social network and those who had a large social network. The longitudinal association between sleep disturbance and IL-6 was highly significant for the subgroup with a small social network (n=1709; unadjusted beta 0.090, P<0.001). This association was attenuated to non-significance after adjusting for the above-mentioned covariates (adjusted beta 0.028, P=0.286). The longitudinal association between sleep disturbance and IL-6 was significant for the subgroup with a large social network in both unadjusted and adjusted analyses (n=1253; unadjusted beta 0.117, P<0.001; adjusted beta 0.071, P=0.019).
4. DISCUSSION
In a large community-based adult sample, sleep disturbance – i.e., insomnia complaints and short sleep duration – was associated with heightened systemic inflammation five years later as represented by plasma CRP and IL-6, and this association was independent of several covariates including sociodemographic characteristics, BMI, medication use, smoking, alcohol consumption, and depressive symptoms. Furthermore, the longitudinal association between sleep disturbance and systemic inflammation was significantly moderated by subjective social isolation, as this association was considerably stronger among those who felt socially isolated compared to those who felt socially integrated. Of note, this association was not significantly moderated by objective social isolation assessed as social network sizes. Subjective sense of social integration appears to buffer the inflammatogenic effects of sleep disturbance. Interestingly, subjective social isolation by itself did not have any effects on systemic inflammation, supporting the notion that subjective social isolation enhances the inflammatogenic effect of sleep disturbance through a moderation (i.e., effect modification) rather than through a simple addition of its own inflammatogenic effect.
To our knowledge, this is the first study to demonstrate a longitudinal prediction of systemic inflammation by sleep disturbance in a general population, as the previous community studies of prospective design assessing sleep variables and systemic inflammation have either focused on sleep duration rather than sleep disturbance or examined a reversed direction of causality, i.e., from systemic inflammation to sleep duration. The current data extend the findings of prior cross-sectional studies on the association between sleep disturbance and systemic inflammation (Christian et al., 2011; Friedman, 2011; Liukkonen et al., 2007; Okun et al., 2009; Zhang et al., 2013) and on the moderating effect of social isolation on this cross-sectional association (Friedman, 2011). Our findings on the prediction of heightened systemic inflammation by sleep disturbance are unique. Given the role of inflammation in onset and progression of major physical and mental illnesses, these data also provide insight about the mechanisms by which sleep disturbance contributes to adverse clinical outcomes. Such understanding is a key to developing and implementing treatments that might target sleep disturbance to prevent inflammatory illnesses, with attention focused on those who are socially isolated and most vulnerable to the inflammatogenic effects of sleep disturbance.
Derived from a community-based prospective study, the current data overcome the limitations of prior studies, given the following strengths. First, the prospective design provided information on the directionality of association between sleep disturbance and systemic inflammation. Second, the association between sleep disturbance and systemic inflammation was independent of a series of confounding variables such as sociodemographic characteristics, obesity, medication use, health-related behaviors, and depressive symptoms. Third, the current study employed two systemic inflammatory markers involved in different steps of the inflammation process, a proinflammatory cytokine and an acute phase reactant, respectively corresponding to a proximal and a distal step of the inflammatory cascade.
The following limitations should be considered. First, the assessment of sleep disturbance relied on self-report, whereas polysomnography is arguably the objective standard to assess sleep disturbance. However, assessment of sleep problems in clinical practice primarily relies on patients’ subjective evaluation. Second, subjective social isolation was assessed only at the follow-up, although a moderator should be ideally ascertained concurrently with the predictor variable. Third, while plasma CRP was available for the entire sample at baseline, IL-6 was available only for a small subset at baseline. Hence, while we were able to examine how sleep disturbance impacted the prospective change in plasma levels of CRP over 5 years by controlling for baseline CRP, such an analysis could not be conducted for IL-6. Fourth, although we carefully performed multivariable analyses considering a series of potential risk factors, it is still possible that there is some residual confounding since this was not a randomized controlled trial, the only approach that can eliminate the confounding effect entirely. There could be unmeasured confounding variables accounting for some of the association between sleep disturbance and inflammatory markers. Thus, a causal effect for sleep disturbance on inflammation is consistent with our findings but cannot be definitively inferred from them.
In conclusion, the current study provides evidence on the longitudinal association between sleep disturbance and heightened systemic inflammation and also on the moderating influences of subjective and objective social isolation on this association, extending the findings of prior cross-sectional studies. The current data suggest that addressing sleep disturbances, which may be amenable to clinical interventions, might be a potential avenue for reducing inflammation; and this may be particularly important in those who perceive themselves as being socially isolated.
Supplementary Material
HIGHLIGHTS.
Sleep disturbance was longitudinally associated with high plasma C-reactive protein.
Sleep disturbance was longitudinally associated with high plasma interleukin-6.
These associations were stronger in those who reported feelings of social isolation.
ACKNOWLEDGMENT
The Coronary Artery Risk Development in Young Adults study was supported (or partially supported) by the following contracts from the National Heart, Lung and Blood Institute: University of Alabama at Birmingham, Coordinating Center, N01-HC-95095; University of Alabama at Birmingham, Field Center, N01-HC-48047; University of Minnesota, Field Center and Diet Reading Center (Year 20 Exam), N01-HC-48048; Northwestern University, Field Center, N01-HC-48049; Kaiser Foundation Research Institute, N01-HC-48050; University of California, Irvine, Echocardiography Reading Center (Years 5 and 10), N01-HC-45134; Harbor-University of California, Los Angeles (UCLA) Research Education Institute, Computed Tomography Reading Center (Year 15 Exam), N01-HC-05187; Wake Forest University (Year 20 Exam), N01-HC-45205; and New England Medical Center (Year 20 Exam), N01-HC-45204. The work on this manuscript was supported by 5T32MH019925-15, the APA/Lilly Research Fellowship Award, the National Center for Advancing Translational Sciences UCLA CTSI Grant UL1TR000124, and the Cousins Center for Psychoneuroimmunology to HJC, and in part by R01-AG034588; R01-AG026364; R01-119159; R01-HL079955; P30-AG028748; R01-MH091352 to MI. We gratefully acknowledge Dr. Denise Janicki Deverts for her valuable contribution to the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: All authors declare that there are no conflicts of interest.
The authors reported no biomedical financial interests or potential conflicts of interest.
REFERENCES
- Berkman LF, Leo-Summers L, Horwitz RI. Emotional Support and Survival after Myocardial InfarctionA Prospective, Population-based Study of the Elderly. Ann Intern Med. 1992;117:1003–1009. doi: 10.7326/0003-4819-117-12-1003. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Cronbach’s alpha. BMJ. 1997;314:572. doi: 10.1136/bmj.314.7080.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC. Social isolation and health, with an emphasis on underlying mechanisms. Perspect Biol Med. 2003;46:S39–52. [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Berntson GG, Ernst JM, Gibbs AC, Stickgold R, Hobson JA. Do lonely days invade the nights? Potential social modulation of sleep efficiency. Psychol Sci. 2002a;13:384–387. doi: 10.1111/1467-9280.00469. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Crawford LE, Ernst JM, Burleson MH, Kowalewski RB, Malarkey WB, Van Cauter E, Berntson GG. Loneliness and health: potential mechanisms. Psychosom Med. 2002b;64:407–417. doi: 10.1097/00006842-200205000-00005. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hughes ME, Waite LJ, Hawkley LC, Thisted RA. Loneliness as a specific risk factor for depressive symptoms: cross-sectional and longitudinal analyses. Psychol Aging. 2006;21:140–151. doi: 10.1037/0882-7974.21.1.140. [DOI] [PubMed] [Google Scholar]
- Caspi A, Harrington H, Moffitt TE, Milne BJ, Poulton R. Socially isolated children 20 years later: risk of cardiovascular disease. Arch Pediatr Adolesc Med. 2006;160:805–811. doi: 10.1001/archpedi.160.8.805. [DOI] [PubMed] [Google Scholar]
- Chiu YL, Chuang YF, Fang KC, Liu SK, Chen HY, Yang JY, Pai MF, Peng YS, Wu KD, Tsai TJ. Higher systemic inflammation is associated with poorer sleep quality in stable haemodialysis patients. Nephrol Dial Transplant. 2009;24:247–251. doi: 10.1093/ndt/gfn439. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Bower JE, Kiefe CI, Seeman TE, Irwin MR. Early life stress and inflammatory mechanisms of fatigue in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Brain Behav Immun. 2012;26:859–865. doi: 10.1016/j.bbi.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HJ, Lavretsky H, Olmstead R, Levin MJ, Oxman MN, Irwin MR. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry. 2008;165:1543–1550. doi: 10.1176/appi.ajp.2008.07121882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HJ, Seeman TE, Bower JE, Kiefe CI, Irwin MR. Prospective association between C-reactive protein and fatigue in the coronary artery risk development in young adults study. Biol Psychiatry. 2009;66:871–878. doi: 10.1016/j.biopsych.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Glaser R, Porter K, Malarkey WB, Beversdorf D, Kiecolt-Glaser JK. Poorer self-rated health is associated with elevated inflammatory markers among older adults. Psychoneuroendocrinology. 2011;36:1495–1504. doi: 10.1016/j.psyneuen.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM., Jr. Social ties and susceptibility to the common cold. JAMA. 1997;277:1940–1944. [PubMed] [Google Scholar]
- Cole SW, Kemeny ME, Fahey JL, Zack JA, Naliboff BD. Psychological risk factors for HIV pathogenesis: mediation by the autonomic nervous system. Biol Psychiatry. 2003;54:1444–1456. doi: 10.1016/s0006-3223(02)01888-7. [DOI] [PubMed] [Google Scholar]
- Dew MA, Hoch CC, Buysse DJ, Monk TH, Begley AE, Houck PR, Hall M, Kupfer DJ, Reynolds CF., 3rd Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- Dowd JB, Goldman N, Weinstein M. Sleep duration, sleep quality, and biomarkers of inflammation in a Taiwanese population. Ann Epidemiol. 2011;21:799–806. doi: 10.1016/j.annepidem.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshkoor SA, Hamid TA, Nudin S.S.a.H., Mun CY. Importance of Hypertension and Social Isolation in Causing Sleep Disruption in Dementia. Am J Alzheimers Dis Other Demen. 2014;29:61–66. doi: 10.1177/1533317513505136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrie JE, Kivimaki M, Akbaraly TN, Singh-Manoux A, Miller MA, Gimeno D, Kumari M, Davey Smith G, Shipley MJ. Associations between change in sleep duration and inflammation: findings on C-reactive protein and interleukin 6 in the Whitehall II Study. Am J Epidemiol. 2013;178:956–961. doi: 10.1093/aje/kwt072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EM. Sleep quality, social well-being, gender, and inflammation: an integrative analysis in a national sample. Ann N Y Acad Sci. 2011;1231:23–34. doi: 10.1111/j.1749-6632.2011.06040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Masi CM, Berry JD, Cacioppo JT. Loneliness is a unique predictor of age-related differences in systolic blood pressure. Psychol Aging. 2006;21:152–164. doi: 10.1037/0882-7974.21.1.152. [DOI] [PubMed] [Google Scholar]
- Heffner KL, Waring ME, Roberts MB, Eaton CB, Gramling R. Social isolation, C-reactive protein, and coronary heart disease mortality among community-dwelling adults. Soc Sci Med. 2011;72:1482–1488. doi: 10.1016/j.socscimed.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M, Rinetti G, Redwine L, Motivala S, Dang J, Ehlers C. Nocturnal proinflammatory cytokine-associated sleep disturbances in abstinent African American alcoholics. Brain Behav Immun. 2004;18:349–360. doi: 10.1016/j.bbi.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Irwin MR. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol. 2015;66:143–172. doi: 10.1146/annurev-psych-010213-115205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Wang M, Ribeiro D, Cho HJ, Olmstead R, Breen EC, Martinez-Maza O, Cole S. Sleep Loss Activates Cellular Inflammatory Signaling. Biol Psychiatry. 2008;64:538–540. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- Kroenke CH, Kubzansky LD, Schernhammer ES, Holmes MD, Kawachi I. Social networks, social support, and survival after breast cancer diagnosis. J Clin Oncol. 2006;24:1105–1111. doi: 10.1200/JCO.2005.04.2846. [DOI] [PubMed] [Google Scholar]
- Liukkonen T, Rasanen P, Ruokonen A, Laitinen J, Jokelainen J, Leinonen M, Meyer-Rochow VB, Timonen M. C-reactive protein levels and sleep disturbances: observations based on the Northern Finland 1966 Birth Cohort study. Psychosom Med. 2007;69:756–761. doi: 10.1097/PSY.0b013e318157cb96. [DOI] [PubMed] [Google Scholar]
- Mallon L, Broman JE, Hetta J. Sleep complaints predict coronary artery disease mortality in males: a 12-year follow-up study of a middle-aged Swedish population. J Intern Med. 2002;251:207–216. doi: 10.1046/j.1365-2796.2002.00941.x. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Skene DJ. Social influences on mammalian circadian rhythms: animal and human studies. Biol Rev Camb Philos Soc. 2004;79:533–556. doi: 10.1017/s1464793103006353. [DOI] [PubMed] [Google Scholar]
- Motivala SJ. Sleep and inflammation: psychoneuroimmunology in the context of cardiovascular disease. Ann Behav Med. 2011;42:141–152. doi: 10.1007/s12160-011-9280-2. [DOI] [PubMed] [Google Scholar]
- Motivala SJ, Sarfatti A, Olmos L, Irwin MR. Inflammatory markers and sleep disturbance in major depression. Psychosom Med. 2005;67:187–194. doi: 10.1097/01.psy.0000149259.72488.09. [DOI] [PubMed] [Google Scholar]
- Newall NE, Chipperfield JG, Bailis DS, Stewart TL. Consequences of loneliness on physical activity and mortality in older adults and the power of positive emotions. Health Psychol. 2013;32:921–924. doi: 10.1037/a0029413. [DOI] [PubMed] [Google Scholar]
- O’Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, Thomas KS, Irwin MR. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009;23:887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Coussons-Read M, Hall M. Disturbed sleep is associated with increased C-reactive protein in young women. Brain Behav Immun. 2009;23:351–354. doi: 10.1016/j.bbi.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker ED, Schmitz KH, Jacobs DR, Jr, Dengel DR, Schreiner PJ. Physical Activity in Young Adults and Incident Hypertension Over 15 Years of Follow-Up: The CARDIA Study. American Journal of Public Health. 2007;97:703–709. doi: 10.2105/AJPH.2004.055889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SR, Ayas NT, Malhotra MR, White DP, Schernhammer ES, Speizer FE, Stampfer MJ, Hu FB. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27:440–444. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas. 1977;1:385. [Google Scholar]
- Seeman TE. Social ties and health: the benefits of social integration. Ann Epidemiol. 1996;6:442–451. doi: 10.1016/s1047-2797(96)00095-6. [DOI] [PubMed] [Google Scholar]
- Seeman TE. Health promoting effects of friends and family on health outcomes in older adults. Am J Health Promot. 2000;14:362–370. doi: 10.4278/0890-1171-14.6.362. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Gruenewald TL, Cohen S, Williams DR, Matthews KA. Social relationships and their biological correlates: Coronary Artery Risk Development in Young Adults (CARDIA) study. Psychoneuroendocrinology. 2014;43:126–138. doi: 10.1016/j.psyneuen.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Sidney S, Jacobs DR, Haskell WL, Armstrong MA, Dimicco A, Oberman A, Savage PJ, Slattery ML, Sternfeld B, Van Hom L. Comparison of Two Methods of Assessing Physical Activity in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 1991;133:1231–1245. doi: 10.1093/oxfordjournals.aje.a115835. [DOI] [PubMed] [Google Scholar]
- St-Onge MP, Perumean-Chaney S, Desmond R, Lewis CE, Yan LL, Person SD, Allison DB. Gender Differences in the Association between Sleep Duration and Body Composition: The Cardia Study. Int J Endocrinol. 2010 doi: 10.1155/2010/726071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Owen N, Kunz-Ebrecht SR, Brydon L. Loneliness and neuroendocrine, cardiovascular, and inflammatory stress responses in middle-aged men and women. Psychoneuroendocrinology. 2004;29:593–611. doi: 10.1016/S0306-4530(03)00086-6. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Shankar A, Demakakos P, Wardle J. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci U S A. 2013;110:5797–5801. doi: 10.1073/pnas.1219686110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyerberg EW, Eijkemans MJ, Harrell FE, Jr., Habbema JD. Prognostic modelling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Stat Med. 2000;19:1059–1079. doi: 10.1002/(sici)1097-0258(20000430)19:8<1059::aid-sim412>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Stranges S, Dorn JM, Shipley MJ, Kandala NB, Trevisan M, Miller MA, Donahue RP, Hovey KM, Ferrie JE, Marmot MG, Cappuccio FP. Correlates of short and long sleep duration: a cross-cultural comparison between the United Kingdom and the United States: the Whitehall II Study and the Western New York Health Study. Am J Epidemiol. 2008;168:1353–1364. doi: 10.1093/aje/kwn337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17:241–254. doi: 10.1016/j.smrv.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Lamers F, Hickie IB, He JP, Feig E, Merikangas KR. Differentiating nonrestorative sleep from nocturnal insomnia symptoms: demographic, clinical, inflammatory, and functional correlates. Sleep. 2013;36:671–679. doi: 10.5665/sleep.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.