Abstract
Objective
Fibromyalgia (FM) is a chronic functional pain syndrome characterized by widespread pain, significant pain catastrophizing, sympathovagal dysfunction, and amplified temporal summation for evoked pain. While several studies have found altered resting brain connectivity in FM, studies have not specifically probed the somatosensory system, and its role in both somatic and non-somatic FM symptomatology. Our objective was to evaluate resting primary somatosensory cortex (S1) connectivity, and explore how sustained, evoked deep-tissue pain modulates this connectivity.
Methods
We acquired fMRI and electrocardiography data from FM patients and healthy controls (HC) during rest (REST) and sustained mechanical pressure pain (PAIN) over the lower leg. Functional connectivity associated with different S1 subregions was calculated, while S1leg (leg representation) connectivity was contrast between REST and PAIN, and correlated with clinically-relevant measures in FM.
Results
At REST, FM showed decreased connectivity between multiple ipsilateral and cross-hemispheric S1 subregions, which was correlated with clinical pain severity. PAIN, compared to REST, produced increased S1legconnectivity to bilateral anterior insula in FM, but not in HC. Moreover, in FM, sustained pain-altered S1legconnectivity to anterior insula was correlated with clinical/behavioral pain measures and autonomic responses.
Conclusion
Our study demonstrates that both somatic and non-somatic dysfunction in FM, including clinical pain, pain catastrophizing, autonomic dysfunction, and amplified temporal summation, are all closely linked with the degree to which evoked deep-tissue pain alters S1 connectivity to salience/affective pain processing regions. Additionally, diminished connectivity between S1 subregions at REST in FM may result from ongoing widespread clinical pain.
Chronic pain patients feel pain as a primarily somatosensory sensation, although it is well understood that clinical pain is much more than somatic and encompasses multiple affective and cognitive domains. Fibromyalgia (FM) is a prototypical functional pain syndrome characterized by multi-dimensional symptomatology. Symptoms include widespread pain, mood disturbance with significant pain catastrophizing, cognitive and physical fatigue, dysfunction of autonomic activity, and amplified sensitivity and temporal summation to experimental pain stimuli (1).Multiple neuroimaging studies have supported the theory that FM is primarily a multi-system disorder of central nervous system (e.g. brain) processing. However, the precise linkage between the circuitries processing somatic sensation with those underlying broader affective and cognitive domains remains unknown.
Functional connectivity magnetic resonance imaging (fcMRI) is an adaptation of fMRI that may help assess brain circuitry supporting spontaneous clinical pain. While spontaneous clinical pain(2), and negative affect (3) components of FM have been linked to altered resting (or intrinsic)functional brain connectivity, previous studies have not systematically probed the primary somatosensory cortex (S1) – a potentially integral brain area for somatic symptomatology such as pain. In FM, decreased secondary somatosensory (S2) connectivity to primary motor cortex (3), and reduced connectivity between S2 and S1 (4) were also recently reported. Interestingly, S1 connectivity is also sensitive to sustained experimental pain stimulation in healthy adults (5), suggesting malleable state-like properties for S1 connectivity networks. This view is consistent with generalized reports that functional brain connectivity can reflect both state and trait processes (6). Such state processes may even underlie the hyperalgesia, allodynia, and temporal summation commonly noted in chronic pain patients, as region-specific changes in S1 connectivity may support maladaptive changes in central processing of somatosensory afference.
Our current study investigated evoked-pain state induced alterations in S1 connectivity in chronic pain patients suffering from FM. We also explored how altered S1 connectivity is associated with clinically-relevant variables such as pain intensity and pain-related catastrophizing, key determinants of FM morbidity. Furthermore, we linked evoked deep-tissue pain modulated S1 connectivity with temporal summation of pain and core non-somatic aspects of FM pathophysiology including altered autonomic modulation. The latter investigation follows past studies that have noted autonomic dysfunction in FM patients (7), linking such dysfunction with clinically-relevant parameters (7, 8). We hypothesized that multi-system pathology, common to FM, is supported by altered functional S1 connectivity at rest and/or in response to evoked nociceptive stimuli highly relevant to FM patients – i.e. deep-tissue pain.
Materials and Methods
Participants
All participants in the study gave written informed consent in accordance with the Human Research Committee of the Massachusetts General Hospital. Inclusion criteria for FM were the following:1) between the ages of 18-70 years old 2) diagnosed with fibromyalgia as confirmed by physician and medical records, and 3) met the recently-proposed Wolfe et al criteria(9). Exclusion criteria for FM were the following: 1) history of significant neurologic disorders 2) history of anxiety disorders or significant anxiety symptoms interfering with MRI procedures 3) history of significant cardiac events 4) history of significant head injury 5) current treatment with opioids, 6) current use of recreational drugs, self reported, and 7) typical contraindications for MRI. Healthy controls (HC) were included in the same age range as above, while exclusion criteria were as for FM above in addition to chronic or acute pain. Data from 35 FM patients (32F;age=44.94±12.02) and 14 HC (10F;age=44.21±14.26) were included for data analyses. Neither sex (Fisher's exact test, p=0.091) nor age (two-sample t-test, p=0.86) distribution differed between FM and HC groups. Special statistical considerations were used when fMRI analyses included different sample size groups (see below).
During a behavioral training session (on a separate date from fMRI), subjects were familiarized with pressure pain and rating procedures and requested to complete pain catastrophizing, depression, and chronic pain specific questionnaires using the Pain Catastrophizing Scale (PCS, (10)), Beck Depression Inventory (BDI) , and the Brief Pain Inventory (BPI), respectively. FMRI included a 6-minuteresting state run (REST), 5-minute block-design pain stimuli runs (used as functional localizer), and a 6-minute continuous pain state run (PAIN), in that order. REST always preceded PAIN in order to negate any potential carry-over effects of sustained pain provocation.
Pressure Pain Stimuli
Painful pressure stimuli using cuff pain algometry were applied on the left lower leg (over the gastrocnemius muscle belly) with a velcro-adjusted pressure cuff connected to a rapid cuff inflator (HokansonInc, Bellevenue, WA, USA). Such cuff pressures timuli have been shown to preferentially target deep-tissue nociceptors and can be applied for extended periods of time without damaging tissue (11). Our group has successfully used cuff pressure algometry with neuroimaging in both healthy adults and chronic pain patients (5, 12).
MRI session
For the REST, PAIN, and block design pain runs, fMRI data were acquired using a 3T TIM Trio MRI System (Siemens) equipped for echo planar imaging with a 32-channel head coil. A whole brain T2*-weighted gradient echo BOLD EPI pulse sequence was used (TR/TE=2sec/30ms, flip angle=90°, 37 AC-PC aligned axial slices, voxel size=3.1×3.1×3.6mm). In addition to fMRI data, we also collected anatomical data using a T1-weighted multi-echo MPRAGE pulse sequence (TR/TE1/TE2/TE3/TE4=2,530/1.64/3.5/5.36/7.22ms, flip angle=7°, voxel size=1mm isotropic).
For both REST and PAIN runs, subjects were instructed to relax and lie still with their eyes open, which has been shown to improve resting connectivity estimation(13). Subjects were asked to verbally rate their clinical pain intensity after REST. A 0-100 numeric pain rating scale was used, where 0 was labeled “no pain” and 100 was labeled “the most intense pain tolerable.”
Block design fMRI cuff pain runs were used to localize the contralateral S1 sub-region associated with the cortical representation of the left lower leg for seed correlation analysis of PAIN data (i.e. functional localizer).Subjects received two cuff pain stimuli per run, which elicited a pain intensity rating of ~40/100. While robust S1 activation was noted (Supplementary Figure 1), relatively long (duration=75–105 seconds, inter-stimulus interval=52–72 seconds) pressure pain blocks were used for a separate study hypothesis. Thus, within-subject general linear model (GLM) analysis was performed with a regressor of interest modeling pressure/pain onset. Regressors of no-interest modeled the variance explained by pressure/pain offset and entire-duration cuff pressure block. Following the scan, subjects rated how well they were able to keep their attention focused on such lengthy pain stimuli on a scale of 0-100 where 0 was ‘not at all’ and 100 was ‘extremely well’. This value served as an inter-individual measure of attentiveness to sustained cuff pain.
For the PAIN run, the cuff pressure level was set to target ~40/100 pain intensity. Following the PAIN run, subjects were asked to rate cuff pain intensity, using a 0-100 numerical rating scale. Subjects rated overall pain intensity for the entire 6-minute PAIN run, as well as separate pain intensity for each of the 2-minute periods at the beginning, middle, and end of this 6-minute run. A variety of methodologies have noted that individuals are generally proficient at remembering pain intensity levels over spans of time ranging from minutes(14) to days(15, 16), though the latter may be more controversial. Moreover, previous cuff algometry studies using continuous ratings have noted relative stability of sensation over a 2-minute period (17).
Physiological data were collected simultaneously to all fMRI runs. Electrocardiogram (ECG) data were collected with an MRI-compatible Patient Monitoring system (Invivo Research Inc., Orlando, FL). Respiration data were collected using a custom built pneumatic MRI-compatible belt placed around the subject's ribcage.
Temporal summation
Using the ratings of the 2-minute periods from the PAIN run described above, we also evaluated temporal summation (potential sensitization or habituation) to the sustained cuff pain, by calculating a temporal summation index (see equation 1). This index was defined as the ratio of the “end” period pain intensity divided by the “beginning” period. In order to control for individual differences in subjects’ sensitivity to cuff pain, this ratio was divided by the pressure level (mmHg) used to elicit target pain.
| (1) |
Physiological data analyses
The ECG beat annotation and respiration data time series were used for cardiorespiratory artifact correction using RETROICOR, while nuisance regressors were formed by convolving these time series with cardiac and respiratory response functions (18). Additionally, autonomic response to cuff-pain in both FM and HC groups was estimated using heart rate variability (HRV) analyses. HRV estimation was performed using the previously validated Kubios-HRV software (19). Normalized high-frequency (0.15-0.40Hz) spectral power was computed to estimate cardiovagal modulation (20). Spectral power was calculated for the entire 6-minute REST and PAIN runs, as well as for the 2-minute beginning, middle, and end periods within these runs.
Functional connectivity analyses
Functional MRI data were pre-processed using FSL (FMRIB's Software Library, http://www.fmrib.ox.ac.uk/fsl/), AFNI (http://afni.nimh.nih.gov/afni), and FreeSurfer (http://surfer.nmr.mgh.harvard.edu/) software packages. Data were corrected for physiological artifacts, slice timing, and affine head motion, and brain extraction was performed. As recent studies suggest that head motion can significantly influence functional connectivity estimation (21, 22), root-mean-square relative motion estimates were calculated. We found no significant differences in mean relative motion between REST and PAIN, nor between FM and HC for either condition. In addition, motion during REST and PAIN was further reduced by independent component analysis, where components whose time series demonstrated significant motion-relevant spikes (comparing to estimated motion parameters) and spatial distribution consistent with motion artifacts were filtered out. Cortical surface reconstruction was completed to improve structural-functional co-registration using Free Surfer'sbb register tool (23). Functional data were then registered to standard Montreal Neurological Institute (MNI) space using FMRIB's nonlinear co-registration tool. Data were then resampled to 2-mm isotropic voxels and spatially smoothed (6-mm FWHM), followed by high-pass temporal filtering (f=0.006Hz). We chose to retain fMRI signal at high frequency (i.e. no low-pass filtering), as our recent study highlighted the importance of fMRI signal at higher frequencies (21), while other groups have reported altered cortical dynamics at higher frequencies in chronic pain patients (24, 25).
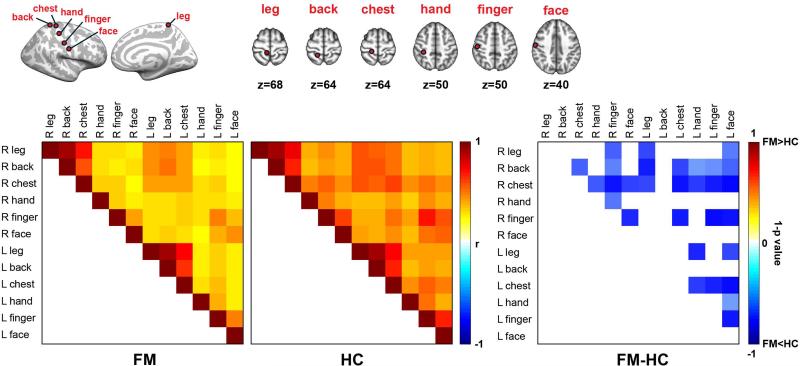
Functional connectivity was computed using seed-based correlation analysis(26). For REST data, seed locations within S1 were defined based on the block-design pain fMRI results (leg representation, S1leg, see below), and from other evoked-stimulation fMRI studies that reported S1 activation. These latter studies included somatosensory stimuli applied to back (±18, −44, 64mm in MNI coordinates, (27)), chest (±18,-36, 64, (28)), hand (±28, −30, 50, (29)), finger (±50, −16,50, (30)) and face (±60, −14,40, (31)). Seeds were mirrored across the mid-sagittal plane for analysis. For REST data analyses, we averaged fMRI signal from a 4mm radius sphere centered on each coordinate above. These time series were used to calculate a correlation matrix covering S1 subregions across both brain hemispheres. Correlation matrices were transformed by a Fisher's r-to-z transformation to impose a normal distribution, followed by an omnibus t-test contrasting FM and HC matrices.
Whole-brain voxel-wise correlation analyses were focused on the S1leg seed contralateral to the leg experiencing cuff pain. In order to use an unbiased seed location, the S1leg seed was defined by a 4mm sphere centered on the peak activation voxel (8,−38,68mmin MNI coordinates) from the group map of the block-design pain runs, combined over both FM and HC. The average fMRI timeseries from this seed was used as a GLM regress or for both REST and PAIN data. Nuisance regressors included 1) fMRI signals from deep cerebral white matter 2) fMRI signals from cerebral ventricles using previously validated masks (13), and 3) cardiorespiratory artifacts defined by convolving the heart rate and respiratory signal with appropriate transfer functions(18).Notably, we did not include the global fMRI signal in this GLM. Resultant connectivity maps, and their variance, from each individual were passed up to group level analyses to explore differences between REST and PAIN, for both FM and HC subjects, using Local Analysis of Mixed Effects (FLAME1+2 using Metropolis-Hastings Markov Chain Monte Carlo sampling for improved mixed-effects variance estimation, which is recommended in group comparisons that involve unequal sample sizes). We also performed whole-brain voxel-wise linear regression analysis to investigate the link between pain-altered S1leg connectivity and clinical and behavioral/autonomic measures. PCS scores were controlled for depression (BDI), similar to previous studies (e.g. (32)), to estimate the specific influence of catastrophizing above and beyond generalized depression. All brain maps were thresholded using cluster correction for multiple comparisons (Z>2.3 and a cluster-size threshold of p<0.05).
All clinical and behavioral data were compared between groups using independent samples two-tailed t-tests in SPSS v.22 (IBM, Armonk, NY). ANOVA models were computed for functional connectivity values taken from significant clusters’ peak voxels, in order to test for interactions between GROUP (levels FM and HC) and SCAN (levels REST and PAIN) factors, significant at p<0.05.
Results
Clinical, behavioral, and autonomic response to sustained pain
Patients with FM demonstrated significantly higher PCS (p<0.01), BDI (p<0.05), and BPI (p<0.01) scores compared to HC (Table 1). FM patients reported, on average, mild to moderate (~30/100 NRS) clinical pain at the MRI session (Table 1).
Table 1.
Clinical and behavioral data on the study subjects
| Healthy Controls (n=14) | FM Patients (n=35) | FM vs. HC p-value | |
|---|---|---|---|
| Age (years) | 44.2±14.3 | 44.9±12.0 | n.s. |
| Sex (number of female) | 10 | 32 | n.s. |
| Symptom duration (years, based on date of diagnosis) | – | 9.76±8.56 | n/a |
| Pain catastrophizing (PCS) | 5.4±5.8 | 22.2±12.9 | ** |
| Depression (BDI) | 2.8±3.8 | 13.5±8.2 | * |
| Clinical pain (BPI) | |||
| Pain severity | 0.3±0.6 | 5.1±2.0 | ** |
| Pain interference | 0.0±0.0 | 5.2±2.1 | ** |
| Clinical pain at MRI scan (0-100) | 0.0±0.0 | 29.9±22.6 | ** |
| Cuff-pressure for percept-matched PAIN run (mmHg) | 180.4±91.4 | 105.4±64.4 | ** |
| Attention to cuff pain (0-100) | 84.7±14.1 | 77.9±17.0 | n.s. |
| Pain intensity from cuff pain (0-100) overall(6-minutes) | |||
| 45.2±17.6 | 55.7±17.8 | n.s. | |
| 2-minute at the beginning | 34.4±15.0 | 46.7±13.8 | ** |
| 2-minute at the middle | 43.1±14.5 | 50.0±16.1 | n.s. |
| 2-minute at the end | 42.9±22.3 | 57.1±19.4° | * |
| Temporal summation (1/mmHg) | 0.9±0.6 | 1.5±0.8 | * |
| Change in nHFHRV (PAIN-REST) overall(6-minutes) | |||
| −6.5±3.8 | −7.8±2.5# | n.s. | |
| 2-minute at the beginning | −7.5±4.7 | −1.8±3.5 | n.s. |
| 2-minute at the middle | −8.4±5.6 | −6.5±3.4 | n.s. |
| 2-minute at the end | 0.5±4.6 | −9.7±3.4# | n.s. |
FM=fibromyalgia, HC=healthy control. PCS=Pain Catastrophizing Scores, BDI=Beck Depression Inventory, BPI=Brief Pain Inventory, nHFHRv=normalized High Frequency component of Heart Rate Variability.
p<0.01
p<0.05 two group t-test contrasting FM versus HC
p<0.05Dunnett's test contrasting 2-minute ending period with 2-minute baseline period
p<0.01 paired t-test contrasting nHFHRV of PAIN with nHFHRV of REST.
Values reported as mean ± SD except that nHFHRV reported as mean+SEM.
For the PAIN run, cuff pressures were calibrated individually to ~40/100 NRS just prior to the PAIN run. Pain intensity ratings at this calibration did not differ between FM and HC subjects (FM:43.13±7.97, HC:43.63±8.09, p=0.86).Cuff pressure over the lower leg during the PAIN run produced, on average, moderate to strong pain intensity in both FM and HC subjects (see Table 1). The overall pain intensity for 6 minutes of cuff stimulation was not statistically different between FM and HC, though there was a trend for greater cuff pain in FM (HC:45.21±17.58; FM:55.67±17.83; p=0.068) due to temporal summation (see below). All subjects also rated cuff pain intensity for three sequential 2-minute periods from this 6-minute PAIN run. Dunnett's test was performed to evaluate sensitization or habituation to the cuff pain, using the beginning 2-minute period as reference. For FM, the ending 2-minute period showed significantly greater pain intensity compared to the beginning (Beginning:46.69±13.80; End:57.11±19.42, p<0.05), while the middle 2-minute period (50.0±16.1,p=0.61) did not differ from the beginning 2-minute period. For HC, there were no significant differences between the middle (43.1±14.5; p=0.33), or ending (42.9±22.3,p=0.34) 2-minute periods compared to the beginning period (34.4±15.0). Temporal summation was greater in FM compared to HC (HC:0.9±0.6; FM:1.5±0.8, p<0.05).
We evaluated cardiovagal activity using HRV analysis and found that, compared to REST, sustained cuff-pain reduced the normalized high frequency component of HRV (nHFHRV) in FM (mean±SEM:-7.78±2.48,p<0.01), while the reduction for HC was not significant (−6.50±3.80, p=0.15, Table 1). For FM, reduction in nHFHRVwas also more robust over time (PAIN-REST, beginning 2-minute period:-1.85±3.52, p=0.60; middle:−6.47±3.42,p=0.07; end:−9.69±3.39, p<0.01). In contrast, for HC, changes in nHFHRVwere sporadic over time and not significant (PAIN-REST, beginning:−7.49±4.72, p=0.31; middle:−8.40±5.65,p=0.11; end:0.51±4.56,p=0.21).
We also found a significant association between clinical/behavioral and autonomic measures in FM patients. Temporal summation in FM showed a positive correlation with PCS (r=0.53, p<0.05). Thus, FM subjects with higher PCS were more sensitized to the cuff pain over the 6 minutes stimulation period. Individually-tailored cuff pressure was negatively correlated with PCS (r=−0.43, p<0.05). In addition, for FM, temporal summation also showed a negative correlation with pain-induced decreases in nHFHRV(calculated over the whole 6-minute run: r=−0.50, p<0.01), suggesting that FM subjects with greater temporal summation to sustained deep-tissue pain also showed more reduced cardiovagal modulation.
Altered S1 functional connectivity in FM at REST
REST correlation matrices for different bilateral somatotopic S1 subregions (leg, back, chest, hand, finger, face) were significantly different between FM and HC (omnibus test, t(65)=−17.29, p<0.01), with FM showing reduced resting connectivity between multiple different S1 subregions (Figure 1).Moreover, a negative correlation between inter-regional S1 connectivity and BPI scores was found (omnibus test following Fisher's r-to-z transform: t(65)=−12.30, p<0.001). Thus, patients reporting greater clinical pain also showed greater reduction in resting connectivity within S1.
Figure 1.
Diminished resting state S1 functional connectivity with in S1 regions in patients with fibromyalgia (FM). Correlation analysis using different S1 ROIs demonstrated disrupted interregional functional correlation at rest in FM as compared to HC.
Altered S1 functional connectivity during sustained pain stimuli (PAIN)
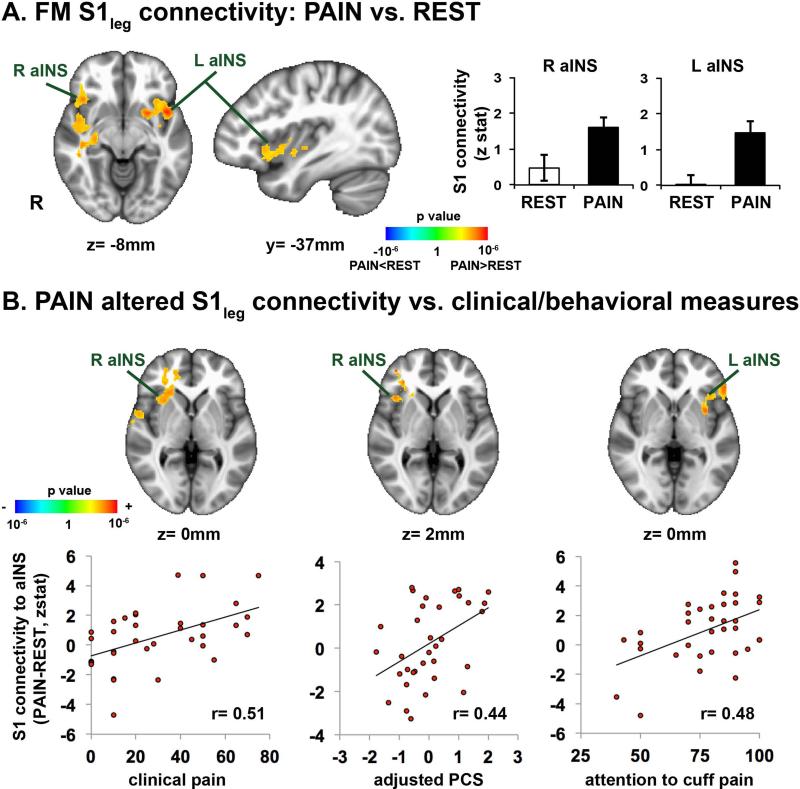
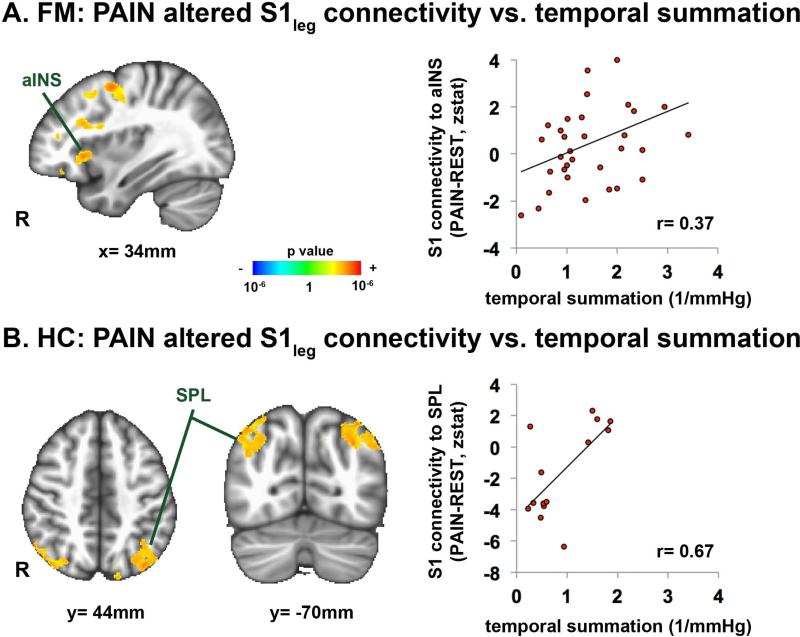
In HC, sustained cuff-pain over the lower leg produced decreased (compared to REST) S1leg connectivity to S1 subregions outside of the seed's cortical representation, similar to our previous results(5) (Supplementary Figure 2). In contrast to HC, in FM, sustained cuff-pain elicited increased S1legconnectivity to bilateral anterior insula (aINS)(Figure 2A, Supplementary Table 1). In fact, we found a significant GROUP (FM vs. HC) by SCAN (REST vs. PAIN) interaction for S1leg connectivity to right aINS (peak voxel:42, 22, −12 in MNI coordinates; F=6.98, p<0.01). A whole-brain linear regression analysis in FM found that connectivity changes (PAIN-REST)for S1leg to a INS was significantly correlated with clinical pain intensity at the MRI scan (r=0.51), PCS (r=0.44) and attention to cuff-pain scores (Figure 2B, Table 2). A whole-brain analysis also showed positive correlation between changes in S1leg connectivity to the right anterior/middle insula and temporal pain summation (Figure 3A, Table 2). For HC, individual variability in temporal summation was instead positively correlated with changes in S1leg connectivity to superior parietal lobule (SPL) (Figure 3B, Table 2).
Figure 2.
A) Sustained pain modulates S1leg seed connectivity. For FM,PAIN increased connectivity between S1leg and bilateral anterior insula (aINS). B) Association between clinical/behavioral measures in FM and sustained pain induced changes in S1leg functional connectivity to anterior insula (aINS). Increases in S1leg connectivity (PAIN-REST) to anterior insula were positively correlated with clinical pain intensity at the time of the scan, and PCS, and attention score to cuff pain.
Table 2.
Brain regions showing significant correlation between clinical/behavioral measures and sustained cuff-pain induced S1leg connectivity (PAIN-REST).
| MNI coordinates |
||||||
|---|---|---|---|---|---|---|
| side | size (mm3) | X (mm) | Y (mm) | Z (mm) | peak z-stat | |
| FM patients | ||||||
| Clinical pain intensity | ||||||
| anterior insula | R | 8,376 | 32 | 18 | 0 | 3.46 |
| dorsal anterior cingulate cortex | - | 8,376 | 0 | 22 | 12 | 4.12 |
| middle insula | R | 688 | 40 | 2 | −10 | 2.94 |
| posterior insula | R | 2,576 | 34 | −14 | 24 | 3.89 |
| superior temporal gyrus | R | 1,888 | 54 | 4 | 6 | 4.23 |
| inferior frontal gyrus | R | 1,608 | 56 | 16 | −14 | 4.48 |
| Pain catastrophizing scores | ||||||
| anterior insula | R | 6,552 | 42 | 20 | 2 | 3.99 |
| middle frontal gyrus | R | 6,552 | 46 | 40 | −4 | 3.80 |
| Attention to cuff pressure pain scores | ||||||
| anterior insula | L | 9,680 | −36 | 20 | 0 | 3.11 |
| caustrum/middle insula | L | 9,680 | −34 | 4 | 0 | 3.95 |
| inferior frontal gyrus | L | 9,680 | −54 | 26 | 0 | 3.87 |
| Cardiovagal response (nHFHRV) | ||||||
| anterior/middle insula | R | 9,864 | 34 | 12 | 0 | −3.19 |
| middle/posteriorinsula | R | 9,864 | 42 | 0 | −12 | −3.94 |
| superior temporal gyrus | R | 9,864 | 54 | 0 | −10 | −4.70 |
| inferior parietal lobule | L | 67,312 | −66 | −42 | 30 | −5.02 |
| cerebellum | L | 1,552 | −4 | −66 | −24 | −3.81 |
| Temporal summation | ||||||
| anterior insula | R | 3,472 | 34 | 16 | −2 | 2.91 |
| caudate nucleus | R | 3,472 | 14 | 4 | 2 | 4.39 |
| putamen | R | 3,472 | 24 | 10 | 6 | 3.36 |
| premotor | R | 4,904 | 34 | 0 | 52 | 4.16 |
| middle frontal gyrus | R | 3,888 | 42 | 40 | −14 | 4.61 |
| Healthy controls | ||||||
| Temporal summation | ||||||
| superior parietal lobule | L | 7,496 | −36 | −76 | 44 | 3.71 |
| superior parietal lobule | R | 5,920 | 38 | −72 | 48 | 3.78 |
Figure 3.
Temporal summation is associated with pain-induced changes in S1leg functional connectivity (PAIN-REST). A) FM subjects who were more sensitized to sustained pain showed greater increases in S1leg connectivity to anterior insula (aINS). B) In contrast, HC subjects reporting greater temporal summation to sustained pain showed greater pain-induced increases in S1leg connectivity to superior parietal lobule (SPL).
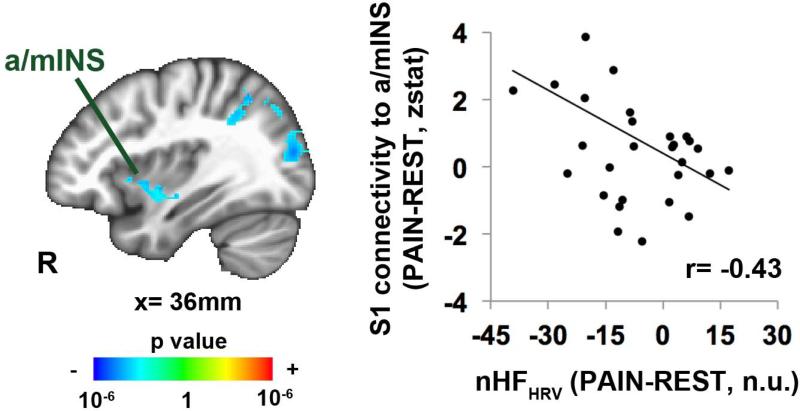
For FM, whole-brain linear regression analysis showed a negative correlation between cuff pain-induced changes in nHFHRV (PAIN-REST, entire 6-minute estimate) and changes in S1leg connectivity to the right anterior/middle insula (Figure 4, Table 2), suggesting that increased S1leg connectivity to right aINS was also associated with more reduced cardiovagal modulation.
Figure 4.
Pain-induced changes in cardiovagal (nHFHRV) response were negatively correlated with changes in S1leg connectivity to right anterior / middle insula (a/mINS). Thus, more decrease in nHFHRVin response to leg cuff pain was associated with greater connectivity between S1 (cortical representation of the leg) and right a/mIns. n.u.=normalized unit.
Discussion
FM is characterized by multi-dimensional symptomatology that varies between individuals, while somatic pain remains a consistent core feature of this chronic pain disorder. Our results demonstrated that, compared to HC,FM was characterized by diminished resting S1 connectivity, both within and between hemispheres. Lower leg cuff-pain, compared to REST, produced increased contralateral S1legconnectivity to bilateral anterior insula in FM. Moreover, in FM, pain-altered S1leg connectivity to right anterior insula was correlated with clinical pain intensity, pain catastrophizing, temporal summation, and autonomic response to evoked cuff-pain, while increased S1leg connectivity to left anterior insula was correlated with attention ratings to cuff-pain. These results highlight the clinically meaningful role of altered S1 physiology, further elaborate on the dynamic role of the anterior insula in chronic pain pathophysiology, and suggest that both somatic and non-somatic aspects of FM pathology are linked by S1 connectivity to non-somatosensory specific, salience-processing brain regions.
Previous studies have reported altered S1 connectivity in response to noxious afference in healthy adults. Riedle et al. found that exposure to repeated noxious stimulation for 10 days produced habituation in terms of pain intensity ratings, but increased functional connectivity within the somatosensory-motor network(33), suggesting that reduced pain is associated with greater intrinsic sensorimotor network connectivity. The inverse may be true for chronic pain, as we found that greater clinical pain was associated with more reduced resting connectivity within S1. Interestingly, our previous study in healthy adults showed that sustained leg cuff-pain decreased connectivity between contralateral S1legand S1 sub-regions outside the leg representation (5), while in the current study, for FM patients, PAIN did not reduce S1leg connectivity to these sub-regions. Thus, we propose that for FM, (1) reduced resting state connectivity between somatotopically different S1 sub-regions and (2) lack of PAIN-induced reduction for S1legconnectivity to other S1 sub-regions, both resulted from ongoing, widely distributed clinical pain in FM patients that leads to a tonic level of elevated somatosensory processing. As to the former, our hypothesis is supported by the negative correlation found between resting inter-subregion S1 connectivity and clinical pain (BPI) scores, demonstrating that patients reporting greater clinical pain also showed greater reduction in connectivity within S1.
We also found that evoked pain increased connectivity between the contralateral S1 sub-region activated by this stimulus (i.e. S1leg) and anterior insula in FM. Notably, while target pain levels were the same between groups, HC subjects experienced far greater cuff pressures to reach these perceptual levels, due to the well-known phenomenon of hyperalgesia in FM.The anterior insula is known as a salience processing region(34), and is also a key region for affective and attentional pain processing (35). Thus, our results showing PAIN-induced increase in S1/insula connectivity in FM suggest a neurobiological substrate for evoked pain hypersensitivity in FM. Specifically, PAIN-induced increase in S1/insula connectivity may reflect increased salience and affective processing attributed to the somatosensory dimension of evoked somatic pain. In fact, we found that changes in S1leg connectivity to the anterior insula during PAIN were associated with 1) higher clinical pain at the scan2) pain catastrophizing, and 3) reported attention to the cuff-pain, thus highlighting the clinical relevance of this brain-based response to our experimental pain stimulus. As our previous connectivity studies have demonstrated that resting anterior/mid insula connectivity to default mode network regions is associated with clinical pain intensity(36-38), there is now accumulating evidence supporting the dynamic role of the insula in both chronic pain perception and hyperalgesic response to experimental mechanical stimuli.
Temporal summation for repeated or long-lasting evoked pain stimuliis also commonly noted in chronic pain patients, including FM(39), and is likely a consequence of central sensitization. While FM patients experienced lower cuff pressures to elicit target (40/100) pain ratings, temporal summation was actually greater than in HC. Previous fMRI neuroimaging studies have implicated several brain regions that support temporal summation, including posterior (not anterior) insula, and S1 in both healthy adults (40), and a combined healthy/FM cohort (41). Our study used a much longer duration of mechanical pain stimulation and a within-subjects group level analysis to show that functional S1 connectivity to right anterior insula supports greater temporal summation in FM. In HC, temporal summation was instead associated with greater S1 connectivity to SPL, an important somatic attention processing brain region(42). Hence, our results suggest that in FM, enhanced temporal summation(compared to HC) may reflect greater linkage between somatosensory and affective/salience processing brain regions, leading to enhanced emotional attribution to evoked pain stimuli of extended duration. In contrast, temporal summation in HC may instead reflect enhanced attentional resources attributed to sustained nociceptive afference.
We observed significantly decreased nHFHRV in response to sustained pain stimuli for FM. Interestingly, autonomic dysfunction has been demonstrated in FM(43), and is thought to result from patients’ chronic pain experience (i.e. reduced cardiovagal activity due to ongoing stress). In our study, reduced cardiovagal modulation was especially pronounced in the final 2-minute period and may contribute to (or result from) the noted temporal summation, as greater reductions in nHFHRV were correlated with greater temporal summation. We further demonstrated that subjects with greatern HFHRVreductions also showed greater S1 connectivity to right anterior insula.The anterior insula is also known to control autonomic response for both internally driven processes and external sensory stimuli (44, 45), and is a core component of the central autonomic network for both sympathetic and parasympathetic modulation(46). Thus, anterior insula connectivity to S1 appears to play a crucial modulatory role in not only hyperalgesia and temporal summation, but also autonomic responsivity to evoked pain, which may reflect elevated levels of clinical pain severity and pain catastrophizing.
Interestingly, while sustained PAIN increased S1leg connectivity to bilateral anterior insula, the association between S1leg connectivity and clinical pain, catastrophizing (affective, emotional dimension), and cardiovagal response was localized to right aINS, and the association between S1leg connectivity and attention to pain (cognitive dimension) was localized to left aINS. Previous studies have suggested that laterality of aINS processing may relate to differential autonomic inputs (47), valence of emotional stimuli, and/or subject's sex (48). Furthermore, the association between S1-insula connectivity and clinical variables such as catastrophizing was not seen during rest, suggesting that a strong affective/somatic input that modulates autonomic outflow is necessary to produce this association between catastrophizing and S1-insula connectivity.
Limitations to our study should also be noted. For instance, while some analyses (e.g. nHFHRVresponse to PAIN) found significant effects in FM and only trending significance in HC, the latter group was composed of fewer subjects. However, we should note that increasing nHFHRVresponse to PAIN over time was only seen for FM (and was not trending for HC), and was correlated with a temporal summation effect specific to the chronic pain population. Additionally, recent studies have noted altered small diameter fiber density and hyperexcitable c-nociceptors in fibromyalgia patients (49, 50).Thus, FM subjects may have experienced differential peripheral signaling from cuff stimulation, in addition to a more acknowledged central amplification. Future studies should explicitly resolve the influence of peripheral factors on our results. Nearly half (49%) of the patients were on antidepressant therapy, most commonly SNRIs (e.g., duloxetine) or tricyclic antidepressants. A much smaller number were taking muscle relaxants (16%) or benzodiazepines (9%). Future studies with greater power should explicitly explore the role of different medications on brain connectivity. Lastly, we did not collect clinical pain ratings from FM subjects after the PAIN run to understand how the evoked experimental pain interacts with clinical pain. However, the association between PAIN-induced S1-aINS connectivity and clinical measures highlights the clinical relevance of the reported brain responses to cuff pain.
In summary, our results suggest that FM pain, which is somatic in origin and accompanied by symptomatology covering multiple affective and cognitive domains, may be supported by neural links between somatosensory and affective/cognitive processing brain regions. Our results highlight the clinically meaningful role of altered S1 physiology in FM, particularly in response to nociceptive afference, and the clear importance of anterior insula connectivity for hyperalgesia, temporal summation, and even autonomic dysfunction in FM.
Supplementary Material
S-Figure 1. Brain group map, combined over FM and HC, of block-design pain runswith a regressor of interest modeling pressure/pain onset.
S-Figure 2. Reduced resting connectivity between S1leg and other S1 subregions outside of the leg cortical representation in FM, compared to HC. Sustained cuff pain reduced this intra-S1 connectivity in HC (consistent with Kim et al., 2013), but had no appreciable effect in FM.
Acknowledgments
Grants:This project was supported by the NCCAM, NIH (P01-AT006663, R01-AT007550, R01-AR064367, and R21-AR057920), the Korea Institute of Oriental Medicine (K14290), German Research Foundation Grant BE4677/1-1, and funded in part by grants to RRE from the Arthritis Foundation and American College of Rheumatology.
References
- 1.Clauw DJ. Fibromyalgia: a clinical review. JAMA : the journal of the American Medical Association. 2014;311(15):1547–55. doi: 10.1001/jama.2014.3266. [DOI] [PubMed] [Google Scholar]
- 2.Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62(8):2545–55. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cifre I, Sitges C, Fraiman D, Munoz MA, Balenzuela P, Gonzalez-Roldan A, et al. Disrupted functional connectivity of the pain network in fibromyalgia. Psychosom Med. 2012;74(1):55–62. doi: 10.1097/PSY.0b013e3182408f04. [DOI] [PubMed] [Google Scholar]
- 4.Pujol J, Macia D, Garcia-Fontanals A, Blanco-Hinojo L, Lopez-Sola M, Garcia-Blanco S, et al. The contribution of sensory system functional connectivity reduction to clinical pain in fibromyalgia. Pain. 2014 doi: 10.1016/j.pain.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 5.Kim J, Loggia ML, Edwards RR, Wasan AD, Gollub RL, Napadow V. Sustained deep-tissue pain alters functional brain connectivity. Pain. 2013;154(8):1343–51. doi: 10.1016/j.pain.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckner RL, Krienen FM, Yeo BT. Opportunities and limitations of intrinsic functional connectivity MRI. Nature neuroscience. 2013;16(7):832–7. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- 7.Cohen H, Neumann L, Shore M, Amir M, Cassuto Y, Buskila D. Autonomic dysfunction in patients with fibromyalgia: application of power spectral analysis of heart rate variability. Seminars in arthritis and rheumatism. 2000;29(4):217–27. doi: 10.1016/s0049-0172(00)80010-4. [DOI] [PubMed] [Google Scholar]
- 8.Reyes Del Paso GA, Garrido S, Pulgar A, Martin-Vazquez M, Duschek S. Aberrances in autonomic cardiovascular regulation in fibromyalgia syndrome and their relevance for clinical pain reports. Psychosom Med. 2010;72(5):462–70. doi: 10.1097/PSY.0b013e3181da91f1. [DOI] [PubMed] [Google Scholar]
- 9.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis care & research. 2010;62(5):600–10. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and Validation. Psychol Assess. 1995;7:524–32. [Google Scholar]
- 11.Polianskis R, Graven-Nielsen T, Arendt-Nielsen L. Pressure-pain function in desensitized and hypersensitized muscle and skin assessed by cuff algometry. The journal of pain : official journal of the American Pain Society. 2002;3(1):28–37. doi: 10.1054/jpai.2002.27140. [DOI] [PubMed] [Google Scholar]
- 12.Loggia ML, Berna C, Kim J, Cahalan CM, Gollub RL, Wasan AD, et al. Disrupted brain circuitry for pain-related reward/punishment in fibromyalgia. Arthritis Rheum. 2013 doi: 10.1002/art.38191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. Journal of neurophysiology. 2010;103(1):297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verhoeven K, Van Damme S, Eccleston C, Van Ryckeghem DM, Legrain V, Crombez G. Distraction from pain and executive functioning: an experimental investigation of the role of inhibition, task switching and working memory. Eur J Pain. 2011;15(8):866–73. doi: 10.1016/j.ejpain.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Schneider S, Stone AA, Schwartz JE, Broderick JE. Peak and end effects in patients' daily recall of pain and fatigue: a within-subjects analysis. The journal of pain : official journal of the American Pain Society. 2011;12(2):228–35. doi: 10.1016/j.jpain.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terry R, Brodie EE, Niven CA. Exploring the phenomenology of memory for pain: is previously experienced acute pain consciously remembered or simply known? The journal of pain : official journal of the American Pain Society. 2007;8(6):467–75. doi: 10.1016/j.jpain.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Polianskis R, Graven-Nielsen T, Arendt-Nielsen L. Spatial and temporal aspects of deep tissue pain assessed by cuff algometry. Pain. 2002;100(1-2):19–26. doi: 10.1016/s0304-3959(02)00162-8. [DOI] [PubMed] [Google Scholar]
- 18.Chang C, Cunningham JP, Glover GH. Influence of heart rate on the BOLD signal: the cardiac response function. NeuroImage. 2009;44(3):857–69. doi: 10.1016/j.neuroimage.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-Aho PO, Karjalainen PA. Kubios HRV--heart rate variability analysis software. Computer methods and programs in biomedicine. 2014;113(1):210–20. doi: 10.1016/j.cmpb.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 20.Malik M, Bigger JT, Jr., Camm AJ, Kleiger RE, Malliani A, Moss AJ, et al. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. European heart journal. 1996;17(3):354–81. [PubMed] [Google Scholar]
- 21.Kim J, Van Dijk KR, Libby A, Napadow V. Frequency-dependent relationship between resting-state functional magnetic resonance imaging signal power and head motion is localized within distributed association networks. Brain connectivity. 2014;4(1):30–9. doi: 10.1089/brain.2013.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2012;59(1):431–8. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48(1):63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baliki MN, Baria AT, Apkarian AV. The cortical rhythms of chronic back pain. J Neurosci. 2011;31(39):13981–90. doi: 10.1523/JNEUROSCI.1984-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malinen S, Vartiainen N, Hlushchuk Y, Koskinen M, Ramkumar P, Forss N, et al. Aberrant temporal and spatial brain activity during rest in patients with chronic pain. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(14):6493–7. doi: 10.1073/pnas.1001504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lloyd D, Findlay G, Roberts N, Nurmikko T. Differences in low back pain behavior are reflected in the cerebral response to tactile stimulation of the lower back. Spine. 2008;33(12):1372–7. doi: 10.1097/BRS.0b013e3181734a8a. [DOI] [PubMed] [Google Scholar]
- 28.Strigo IA, Duncan GH, Boivin M, Bushnell MC. Differentiation of visceral and cutaneous pain in the human brain. Journal of neurophysiology. 2003;89(6):3294–303. doi: 10.1152/jn.01048.2002. [DOI] [PubMed] [Google Scholar]
- 29.Bingel U, Lorenz J, Glauche V, Knab R, Glascher J, Weiller C, et al. Somatotopic organization of human somatosensory cortices for pain: a single trial fMRI study. NeuroImage. 2004;23(1):224–32. doi: 10.1016/j.neuroimage.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 30.Napadow V, Liu J, Li M, Kettner N, Ryan A, Kwong KK, et al. Somatosensory cortical plasticity in carpal tunnel syndrome treated by acupuncture. Human brain mapping. 2007;28(3):159–71. doi: 10.1002/hbm.20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moulton EA, Pendse G, Morris S, Aiello-Lammens M, Becerra L, Borsook D. Segmentally arranged somatotopy within the face representation of human primary somatosensory cortex. Human brain mapping. 2009;30(3):757–65. doi: 10.1002/hbm.20541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gracely RH, Geisser ME, Giesecke T, Grant MA, Petzke F, Williams DA, et al. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain : a journal of neurology. 2004;127(Pt 4):835–43. doi: 10.1093/brain/awh098. [DOI] [PubMed] [Google Scholar]
- 33.Riedl V, Valet M, Woller A, Sorg C, Vogel D, Sprenger T, et al. Repeated pain induces adaptations of intrinsic brain activity to reflect past and predict future pain. NeuroImage. 2011;57(1):206–13. doi: 10.1016/j.neuroimage.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiech K, Lin CS, Brodersen KH, Bingel U, Ploner M, Tracey I. Anterior insula integrates information about salience into perceptual decisions about pain. J Neurosci. 2010;30(48):16324–31. doi: 10.1523/JNEUROSCI.2087-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loggia ML, Kim J, Gollub RL, Vangel MG, Kirsch I, Kong J, et al. Default mode network connectivity encodes clinical pain: an arterial spin labeling study. Pain. 2013;154(1):24–33. doi: 10.1016/j.pain.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Napadow V, Kim J, Clauw DJ, Harris RE. Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis Rheum. 2012;64(7):2398–403. doi: 10.1002/art.34412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris RE, Napadow V, Huggins JP, Pauer L, Kim J, Hampson J, et al. Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology. 2013;119(6):1453–64. doi: 10.1097/ALN.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 39.Staud R, Robinson ME, Price DD. Temporal summation of second pain and its maintenance are useful for characterizing widespread central sensitization of fibromyalgia patients. The journal of pain : official journal of the American Pain Society. 2007;8(11):893–901. doi: 10.1016/j.jpain.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tran TD, Wang H, Tandon A, Hernandez-Garcia L, Casey KL. Temporal summation of heat pain in humans: Evidence supporting thalamocortical modulation. Pain. 2010;150(1):93–102. doi: 10.1016/j.pain.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Staud R, Craggs JG, Perlstein WM, Robinson ME, Price DD. Brain activity associated with slow temporal summation of C-fiber evoked pain in fibromyalgia patients and healthy controls. Eur J Pain. 2008;12(8):1078–89. doi: 10.1016/j.ejpain.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lobanov OV, Quevedo AS, Hadsel MS, Kraft RA, Coghill RC. Frontoparietal mechanisms supporting attention to location and intensity of painful stimuli. Pain. 2013;154(9):1758–68. doi: 10.1016/j.pain.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meeus M, Goubert D, De Backer F, Struyf F, Hermans L, Coppieters I, et al. Heart rate variability in patients with fibromyalgia and patients with chronic fatigue syndrome: a systematic review. Seminars in arthritis and rheumatism. 2013;43(2):279–87. doi: 10.1016/j.semarthrit.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Craig AD. How do you feel--now? The anterior insula and human awareness. Nature reviews Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 45.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature neuroscience. 2004;7(2):189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 46.Beissner F, Meissner K, Bar KJ, Napadow V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci. 2013;33(25):10503–11. doi: 10.1523/JNEUROSCI.1103-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Craig AD. Forebrain emotional asymmetry: a neuroanatomical basis? Trends in cognitive sciences. 2005;9(12):566–71. doi: 10.1016/j.tics.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 48.Duerden EG, Arsalidou M, Lee M, Taylor MJ. Lateralization of affective processing in the insula. NeuroImage. 2013;78:159–75. doi: 10.1016/j.neuroimage.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 49.Caro XJ, Winter EF. Evidence of abnormal epidermal nerve fiber density in fibromyalgia: clinical and immunologic implications. Arthritis & rheumatology. 2014;66(7):1945–54. doi: 10.1002/art.38662. [DOI] [PubMed] [Google Scholar]
- 50.Serra J, Collado A, Sola R, Antonelli F, Torres X, Salgueiro M, et al. Hyperexcitable C nociceptors in fibromyalgia. Annals of neurology. 2014;75(2):196–208. doi: 10.1002/ana.24065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S-Figure 1. Brain group map, combined over FM and HC, of block-design pain runswith a regressor of interest modeling pressure/pain onset.
S-Figure 2. Reduced resting connectivity between S1leg and other S1 subregions outside of the leg cortical representation in FM, compared to HC. Sustained cuff pain reduced this intra-S1 connectivity in HC (consistent with Kim et al., 2013), but had no appreciable effect in FM.