Abstract
Wnt-1 inducible signalling pathway protein 1 (WISP-1/CCN4) is an extracellular matrix protein that belongs to the Cyr61 (cysteine-rich protein 61), CTGF (connective tissue growth factor) and NOV (CCN) family and plays a role in multiple cellular processes. No specific WISP-1 receptors have been identified but emerging evidence suggests WISP-1 mediates its downstream effects by binding to integrins. Here we describe a functional analysis of integrin receptor usage by WISP-1. Truncated WISP-1 proteins were produced using a baculovirus expression system. Full length WISP-1 and truncated proteins were evaluated for their ability to induce adhesion in A549 epithelial cells and β-catenin activation and CXCL3 secretion in fibroblasts (NRK49-F cells). Subsequent inhibition of these responses by neutralising integrin antibodies was evaluated. A549 cells demonstrated adhesion to full-length WISP-1 whilst truncated proteins containing VWC, TSP or CT domains also induced adhesion, with highest activity observed with proteins containing the C-terminal TSP and CT domains. Likewise the ability to induce β-catenin activation and CXCL3 secretion was retained in truncations containing C-terminal domains. Pre-treatment of A549s with either integrin αVβ5, αVβ3 or β1 neutralising antibodies partially inhibited full length WISP-1 induced adhesion whilst combining integrin αVβ5 and β1 antibodies increased the potency of this effect. Incubation of NRK49-F cells with integrin neutralising antibodies failed to effect β-catenin translocation or CXCL3 secretion. Analysis of natural WISP-1 derived from human lung tissue showed the native protein is a high order oligomer. Our data suggest that WISP-1 mediated adhesion of A549 cells is an integrin-driven event regulated by the C-terminal domains of the protein. Activation of β-catenin signalling and CXCL3 secretion also resides within the C-terminal domains of WISP-1 but are not regulated by integrins. The oligomeric nature of native WISP-1 may drive a high avidity interaction with these receptors in vivo.
Keywords: WISP-1, Integrin receptors, CCN proteins
Introduction
The CCN proteins are a family of highly conserved secreted matricellular proteins which are linked to roles in embryogenesis, wound repair, fibrosis and tumour genesis. All family members share a modular structure comprised of a signal peptide followed by three domains with homology to insulin-like growth factor (IGF) binding proteins, von Willebrand type C (vWC) factor and thrombospondin type 1(TSP1) repeat plus a fourth domain which contains a cysteine-knot (CT) motif. A variable hinge region between domains two and three is susceptible to proteolytic cleavage and results in truncated CCN proteins which have both overlapping and distinct biological activities to the full length proteins (reviewed by (Perbal 2009)).
The CCN proteins described to date have no known unique cellular receptors but instead interact with multiple partners including integrins, heparin sulphate proteoglycans, bone morphogenetic proteins (BMPs) and low density lipoprotein receptor-related proteins (LRPs) (reviewed by (Holbourn et al. 2008)). This promiscuity has been cited as the reason behind their pleiotropic functions and cell-specific behaviour and may underlie the complexity of the literature concerning this protein family.
Wnt-inducible signaling protein-1 (WISP-1/CCN4) is a downstream mediator of Wnt signalling which is upregulated in a number of chronic fibrotic disorders effecting the lung, liver and kidney (Jiang et al. 2006; Wang et al. 2011; Zulato et al. 2011). Functionally, WISP-1 has been shown to induce proliferation and drive epithelial to mesenchymal transition in alveolar epithelial cells whilst increasing the synthesis of extracellular matrix components (ECM) in fibroblasts. Furthermore, antibody-mediated neutralisation of WISP-1 conferred a survival benefit and improved lung function when administered therapeutically in the bleomycin model of pulmonary fibrosis (Konigshoff et al. 2009). Despite the emerging evidence for a role for WISP-1 in fibrosis, the biology of the protein remains poorly described. Structural variants of WISP-1 generated by differential splicing or proteolysis have been detected in a number of pathological settings but their function remains to be elucidated (Cervello et al. 2004; Yanagita et al. 2007). WISP-1 has been speculated to perform pleiotropic, cell-specific functions with potential distinct paracrine and autocrine functions which may be attributed to the use of differing cell-surface receptors in different cell types. Furthermore, whilst WISP-1 has been shown to interact with the small leucine rich proteoglycans biglycan and decorin (Desnoyers et al. 2001) the mechanism by which it activates the Akt signalling pathway is not understood (Su et al. 2002). The WISP-1 proteins contains putative integrin recognition sites in the vWC, TSP1 and CT domains and recently two groups reported an interaction between full length WISP-1 and the αVβ5 integrin (Hou et al. 2013; Liu et al. 2013; Ono et al. 2011) although the domains responsible for these interactions were not identified.
Here we describe a functional role for WISP-1 as a mediator of cell to matrix adhesion in epithelial cells and secretion of pro-angiogenic chemokines in fibroblasts via activation of the β-catenin signalling pathway. Furthermore we demonstrate that these functions reside within the C-terminal domains of the protein and whilst adhesion is an integrin-mediated event, β-catenin activation and chemokine secretion are integrin independent.
Materials and methods
Cell culture and reagents
The rat fibroblast cell line NRK-49F and human A549 alveolar epithelial cells were grown in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10 % foetal bovine serum (FBS) and 0.1 mM NEAA and Pen/Strep. Recombinant full-length human WISP-1 was purchased from Peprotech and used in functional assays. Neutralising polyclonal antibodies against WISP-1 and integrin neutralising antibodies were purchased from R&D Systems.
Generation of truncated and full-length WISP-1 proteins
Truncated and full length WISP-1 proteins were generated in the baculovirus expression system. DNA sequences were synthesised by GenScript in the pDEST8 baculovirus expression vector (Invitrogen) using the honey bee melittin signal sequence and an amino terminus APP tag. Max Efficiency DH10Bac chemically competent E.coli (Invitrogen) were used to amplify Bacmid DNA which was extracted using a Qiagen kit according to manufacturer’s instructions. For P1 infections, Sp21 cells were transfected with Bacmid DNA and Cellfectin (Invitrogen) diluted in Grace’s media (Gibco) and virus-containing supernates harvested after 96 h. Two further rounds of infection were performed using supernates from the subsequent round to infect Sp21 cells. Proteins were purified from supernates of P3 infections using sepharose columns conjugated to anti-APP antibodies.
Adhesion assays
WISP-1 was added to black-walled, 96-well plates and incubated at 37 °C for 4 h. Experiments were performed in triplicate and PBS only used as a negative control. Plates were washed with PBS and blocked for 1 h at RT in PBS + 1 % BSA. For inhibition assays, anti-WISP-1 antibodies were diluted in PBS and added to plates after the blocking step for 1 h at RT. Sub-confluent A549 cells were detached using cell dissociation buffer and added to plates at a concentration of 1.25 × 104cells/well in DMEM (without phenol red) plus 0.5 % BSA. After incubating for 2 h at 37 °C, media was removed and plates washed twice in PBS prior to freezing at −80 °C overnight. Plates were thawed and 10 % formalin was added for 10 min at RT and replaced with CyQUANT detection solution (Life Technologies) for 5 min at RT. Fluorescence was then read using setting of excitation 480 nm and emission 520 nm. For integrin neutralisation experiments, antibodies were diluted to 50 μg/mL in DMEM plus 0.5 % BSA and pre-incubated with an equal volume of cell suspension for 30 min at 37 °C prior to seeding.
Microarrays analysis
NRK49-F cells were stimulated with 10 or 100 nM WISP-1 or control for 24 h. Total RNA was extracted from cells using TRIZOL (Life Technologies) as described by the manufacturer and further purified using the RNeasy mini-columns from Qiagen (Valencia, CA, USA). And RNA samples were then stored at −80 ° C. The Rat230_2 Array (Affymetrix, Santa Clara, CA, USA) containing 31,099 probe set was used for expression profiling.
Data analysis was performed using the GeneSpring GX11.0.2 software and the MAS5 algorithm. Data were normalized for each microarray using the logarithmic mean of total signal intensity. A 2-fold difference was used as cut-off to identify differentially expressed genes. In our experience >90 % of microarray hits can be verified by an independent method (e.g. TaqMan reverse transcription real-time polymerase chain reaction). For the annotation of hits the NCBI Unigene database (http://www.ncbi.nlm.nih.gov/unigene) was used.
CXCL3 ELISA
NRK-49F cells were maintained in DMEM supplemented with 10 % FBS, 0.1 mM NEAA and Pen/Strep. For assays, cells were seeded in 96-well plates at 20,000 cells per well and left to adhere overnight before starving for 24 h in DMEM + 0.1 % FBS. WISP-1 was incubated with 100 ug/mL polymixin B for 30 min at RT before adding to plates for 24 h. CXCL3 levels in conditioned medium were then analysed by ELISA (R&D Systems) according to manufacturer’s instructions. For integrin inhibition assays, cells were incubated with antibodies in serum-free DMEM for 1 h prior to seeding and WISP-1 used at a concentration of 100 nM.
Reporter gene assay
STF-lentivirus in the pLenti vector (Invitrogen) was generated using the Viral Power Lentiviral Expression System kit (Invitrogen) according to manufacturer’s instructions. Briefly HEK-FT cells were transfected with viral packaging mix (Invitrogen) and the pLenti-STF vector using Lipofectamine 2000 (Invitrogen). Virus was harvested at 96 h and used to infect NRK49-F cells in the presence of 5 μg/mL polybrene. Media was removed after 24 h and cells cultured in the presence of 4 μg/mL blasticidin. For assay, 20,000 cells per well were seeded in 96-well plates and left to adhere overnight before starving for 24 h in DMEM + 0.1 % BSA. Cells were stimulated with WISP-1 for 48 h and luminescence measured using Steady Glo (Promega) according to manufacturer’s instructions. For integrin inhibition assays, cells were incubated with antibodies in serum-free DMEM for 1 h prior to seeding and WISP-1 used at a concentration of 250 nM.
Cut human lung slices
Human lung tissue was obtained from Tissue Solutions (www.tissue-solutions.com). All human tissues provided by Tissue Solutions are obtained according to the legal and ethical requirements of the country of collection, with the approval of an ethics committee (or similar) and with anonymous consent from the donor or nearest relative. Tissue was stored in Aquix medium and used no later than 48 h following resection. Material was sliced into 300–400 μM slices using a tissue chopper and one slice per well placed in a 48-well plate in DMEM supplemented with 10 % human serum. Slices were left overnight and then starved in DMEM supplemented with 0.5 % BSA. Supernates were then harvested and stored at −70 °C prior to analysis.
Polyacrylamide gel electrophoresis and western blotting
For Western blotting, cells were lysed in buffer containing 1 % Nonident P-40, 150 mM NaCl, 50 mM Tris, 1 mM sodium orthovanadate and 5 mM sodium fluoride. Samples were then diluted in NuPAGE 4x LDS Sample Buffer (Life Technologies) and DTT added to a final concentration of 50 mM before boiling at 70 °C for 10 min. Samples were then loaded onto NuPAGE 4–12 % Bis-Tris gels (Invitrogen) for gel electrophoresis. For non-reducing gels, DTT and boiling were omitted from sample preparation.
For coomassie staining, gels were rinsed in deionised water and SimplyBlue SafeStain (Life Technologies) added for 1 h at RT. Gels were washed in water for 1 h before imaging. For Western blotting, gels were transferred onto nitrocellulose membranes and then blocked in 5 % milk in TBST. Membranes were incubated with biotinylated anti-WISP-1 pAb (R&D Systems) for 2 h at RT and washed in TBST. Streptavidin-HRP was then added for 1 h at RT and membranes washed again in TBST prior to visualisation using ECL detection.
Results
Generation of truncated WISP-1 proteins
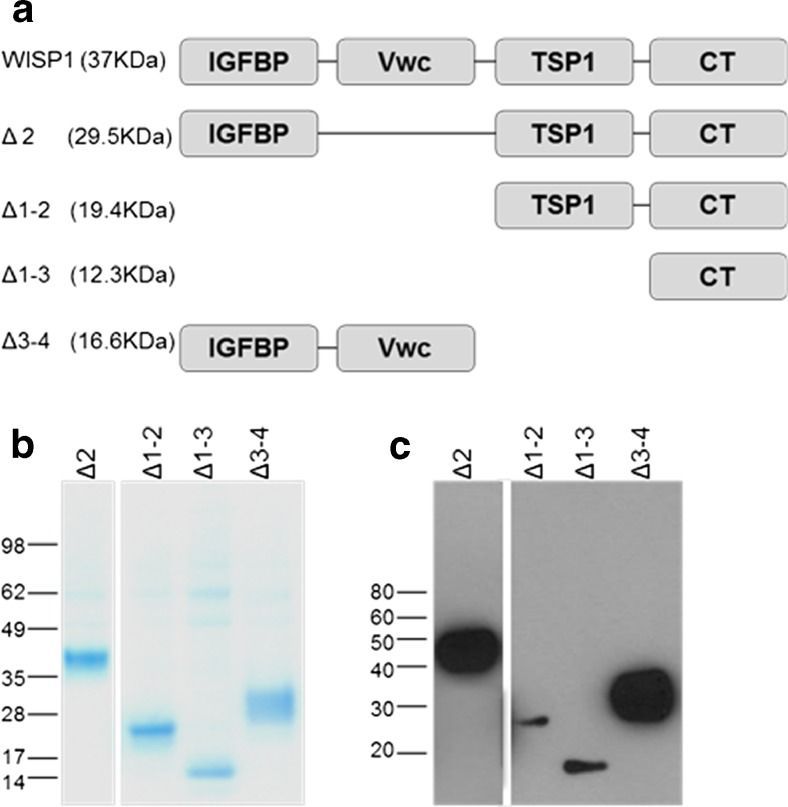
Truncated WISP-1 proteins were designed based on the splice variants and cleaved proteins reported to exist for WISP-1 (Δ2) (Cervello et al. 2004; Yanagita et al. 2007) or for other CCN proteins (Δ1-2, Δ3-4, Δ1-3) (Perbal 2009) (Fig 1a). Sequences were preceded by the honey bee melittin signal sequence and an amino terminus APP tag prior to having been codon-optimised for the insect expression system. Proteins were generated using the baculovirus expression system and isolated by sepharose column coupled anti-APP antibodies. Coomassie gels and Western blotting using a polyclonal anti-WISP-1 antibody showed that the resulting proteins were of the expected size (Fig. 1b and c). Proteins were analysed by mass spectroscopy after N-deglycosylation and in all cases the measured mass was found to match expected mass. N-terminal sequence analysis confirmed expected sequences for all proteins (data not shown).
Fig. 1.
Generation of truncated WISP-1 proteins. Truncated WISP-1 proteins comprising different combinations of the four domains observed in the full length protein were designed as shown schematically (a). APP-tagged proteins generated in the baculovirus expression system showed the expected sizes by coomassie staining (b) and were recognized by α-WISP-1 pAb in Western blotting (c)
WISP-1 mediates cell to matrix adhesion through the C-terminal domains
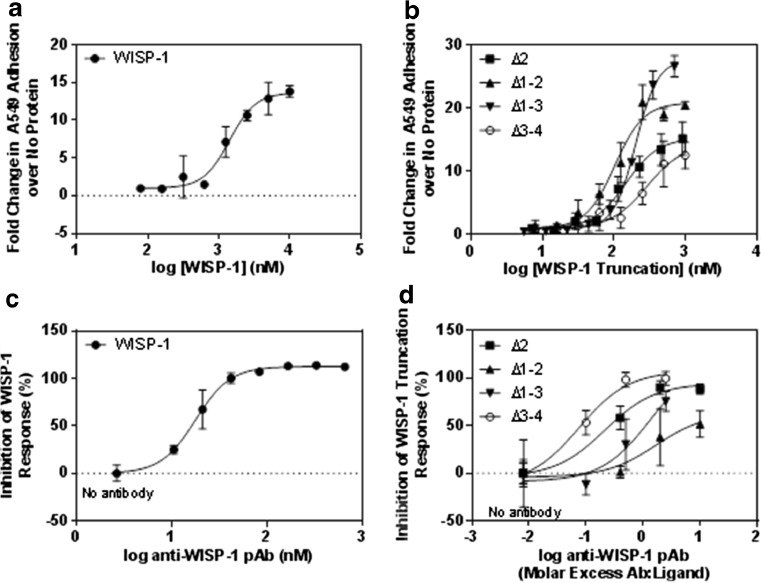
As matrix components, multiple members of the CCN family have been reported to induce cell to matrix adhesion. In order to determine if this was the case for WISP-1, the ability of recombinant full-length protein to mediate adhesion of A549 cells was determined (Fig. 2a). A dose dependent increase in adhesion was observed with an EC50 value of 1.4 μM and a maximal luminescent signal 15-fold higher than that detected in the absence of protein. To determine which domains of WISP-1 are responsible for binding of A549 cells, truncated WISP-1 proteins were used in adhesion assays (Fig. 2b). When domains 3 and 4 were removed from WISP-1 (Δ3-4) the ability to mediate adhesion was significantly reduced compared to the other truncations with a maximal signal 10-fold higher than background and an EC50 value of 291 nM. In the presence of the TSP1 and CT domains however, maximal signals ranged from 15 to 30 fold higher than background and EC50 values were lower at 103 nM (Δ1-2), 147 nM (Δ2) and 202 nM (Δ1-3). This suggests that the C-terminal domains are key drivers of adhesion but also that the interaction is not attributable to any one single domain since all truncations induced some degree of adhesion. The ability of multiple domains to act in concert is a feature of CCN proteins and integrin recognition sites are present in 3 separate domains of WISP-1 (vWC, TSP1 and CT) (Holbourn et al. 2009) making such behaviour plausible.
Fig. 2.
The C terminal domains of WISP-1 mediate cell adhesion. Wells were coated with full length (a) or truncated WISP-1 (b) at various concentrations and blocked with BSA. A549 cells were allowed to adhere to the coated wells for 2 h and non-adherent cells were washed off. Fold change in adherence is relative to the level of fluorescence observed in the absence of WISP-1. For inhibition studies, neutralising antibodies were incubated with WISP-1 prior to the addition of A549 cells. Full length WISP-1 was used at 400 nM (c) and truncated WISP-1 proteins used at 250 nM (Δ2 and Δ1-2) or 500 nM (Δ1-3 and Δ3-4) (d). Results are expressed as the mean ± SD (n = 3)
We next demonstrated the specificity of WISP-1 mediated adhesion to A549 cells by means of antibody inhibition. For this we used a polyclonal antibody which recognised multiple epitopes in the full-length protein as demonstrated by its ability to detect all WISP-1 truncations by Western blot (data not shown). This was necessary to ensure that the antibody could inhibit not only full-length WISP-1 but also all the truncated proteins. Adhesion induced by full-length WISP-1 was inhibited in a dose dependent manner by pre-incubating 400 nM WISP-1 with neutralising polyclonal antibodies prior to the addition of A549 cells (2C). Due to the differing levels of adhesion observed with truncated WISP-1 proteins, WISP-1 Δ2 and Δ1-2 were used at 250 nM and WISP-1 Δ1-3 and Δ3-4 at 500 nM (2D). The activity of the Δ3-4 protein was inhibited by a lower antibody:ligand ratio than any other truncation, as may be expected given this construct also induced the weakest adhesion. Conversely, the Δ1-3 protein induced the greatest fold change in luminescent signal and a maximal inhibition of just 50 % was achieved by the antibody:ligand ratios tested. This further supports the hypothesis that the C-terminal domains are key mediators of epithelial adhesion.
WISP-1 induces secretion of pro-angiogenic chemokines in fibroblasts
In order to identify novel signalling pathways modulated by WISP-1, we used gene microarrays to investigate downstream effects of WISP-1 stimulation. WISP-1 has been speculated to perform pleiotropic, cell-specific functions which may be attributed to its ability to bind and activate multiple distinct receptors present on different cells. For this reason, we wanted to investigate the effect of WISP-1 stimulation in different cells types. Since the cellular receptors of WISP-1 are not known, we selected cell lines previously described in the literature as being WISP-1 responsive. Specifically we used NRK-49F fibroblasts and A549 epithelial cells.
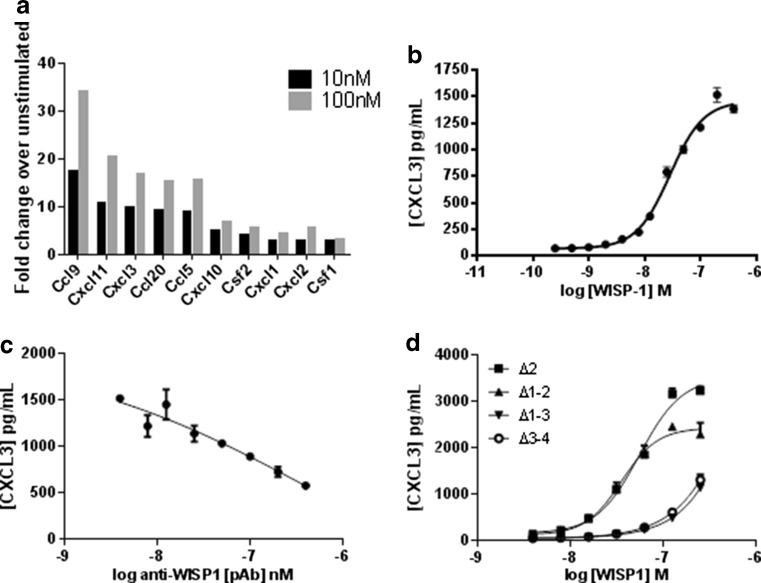
Among the 1222 differentially regulated genes in NRK-49F cells (data not shown) we detected the induction of several cytokines and chemokines with roles in angiogenesis (Fig. 3a) which has not previously been described as a WISP-1 mediated effect. Interestingly the same gene signature was not observed on WISP-1 stimulation in A549 cells. We selected CXCL3 for further analysis due to the high level of induction on WISP-1 stimulation and the availability of tools. We analysed the CXCL3 inducing effect by quantifying CXCL3 secretion by ELISA. NRK-49F fibroblasts were stimulated with full length WISP-1 for 24 h and conditioned medium harvested for analysis. A dose dependent induction of CXCL3 secretion was observed in response to WISP-1 stimulation (3B) with an EC50 value of 28 nM. This effect could be inhibited by pre-incubation of the ligand with WISP-1 neutralising polyclonal antibodies (Fig. 3c). When truncated WISP-1 proteins were investigated in this assay, the TSP-1 domain was found to be key in driving the functional effect (3D). Both Δ2 and Δ1-2 proteins performed similarly to full-length WISP-1 with EC50 values of 55 and 35 nM respectively. However, the Δ3-4 and Δ1-3 proteins lacking the TSP-1 domain both had comparably weak effects and calculation of an EC50 value was not possible. This indicates that the domains which regulate CXCL3 secretion are distinct from those which mediate adhesion since the activity pattern shown by the truncated proteins is different in each case. Interestingly full-length WISP-1 induced maximal of around 1500 pg/mL which is comparable to the maximal levels seen in the weaker performing truncated proteins. As with adhesion, this difference may be due to the differing ways in which the proteins were generated.
Fig. 3.
WISP-1 mediates the secretion of pro-angiogenic chemokines in fibroblasts. NRK-49F cells were stimulated with full-length WISP-1 prior to RNA extraction for gene microarray analysis. Data analysis using the GeneSpring GX11.0.2 software identified the enrichment of a subset of cytokines and chemokines (a). NRK-49F cells were stimulated with full-length (b) or truncated (d) WISP-1 and conditioned media assayed for CXCL3 content by ELISA. For inhibition studies, full-length WISP-1 was pre-incubated with anti-WISP-1 pAbs prior to addition to NRK-49F cells (c). Results for B-D are expressed as the mean ± SD (n = 3)
The C-terminal domains of WISP-1 stimulate β-catenin activation in fibroblasts
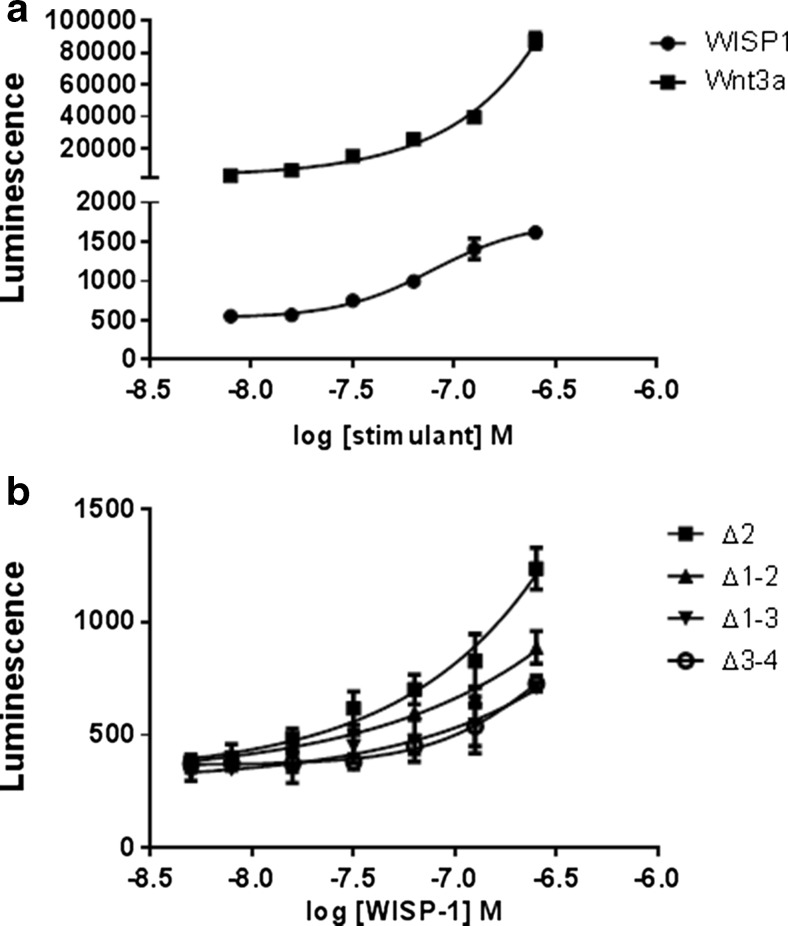
Several groups have reported Akt phosphorylation and subsequent β-catenin activation in WISP-1 stimulated cells, suggesting that WISP-1 may participate in autocrine feedback and hence play a role in regulating its own expression (Colston et al. 2007; Shimo et al. 1999; Su et al. 2002). Using the Super Topflash reporter vector which contains TCF/LEF (T-Cell-Specific Transcription Factor/ Lymphoid Enhancer-binding Factor) binding sites we determined that β-catenin activation occurred in NRK-49F cells following WISP-1 stimulation however, this effect appeared to be weak in comparison to activation with the Wnt ligand Wnt3a, that is known to stimulated robust canonical Wnt pathway activation (Fig. 4a). We also investigated which domains of the protein were responsible for this function by testing the ability of truncated WISP-1 proteins to induce β-catenin activation (Fig. 4b). All truncations induced dose dependent activation of β-catenin transcriptional activity and this effect was marginally stronger in the Δ2 truncation and not individually attributable to either the C-terminal or N-terminal domains. These data indicate that multiple domains potentially acting in concert are required for activation of the β-catenin signalling pathway and the autocrine regulation of WISP-1 expression.
Fig. 4.
WISP-1 activates the β-catenin signalling pathway in fibroblasts. NRK-49F fibroblasts stably transfected with the β-catenin responsive Super Topflash reporter construct were stimulated with Wnt3a for 6 h or full-length WISP-1 for 48 h (a) or truncated WISP-1 proteins for 48 h (b). Cells were then lysed and luminescence quantified. Results are expressed as the mean ± SD (n = 3)
WISP-1-induced adhesion but not CXCL3 secretion is mediated by integrins
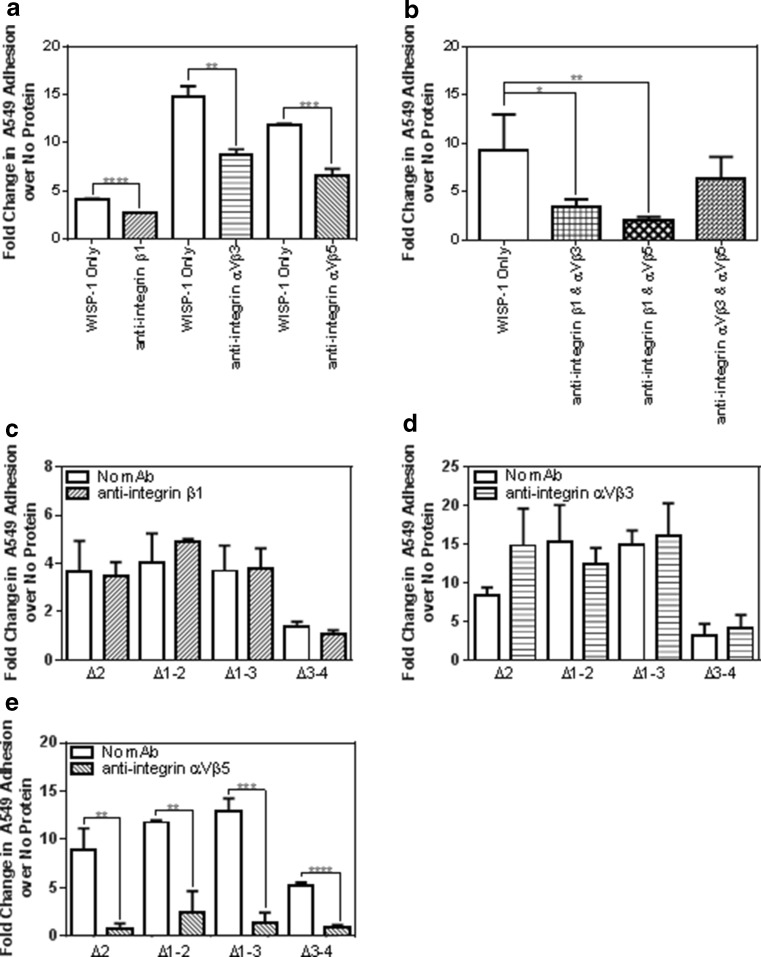
The ability of CCN proteins to induce adhesion of different cell types including fibroblasts, endothelial and epithelial cells has been reported to be an integrin-driven interaction (reviewed by (Chen et al. 2014) but this has not previously been investigated for WISP-1. Putative integrin recognition sites are found in vWC, TSP1 and CT domains of WISP-1 (Holbourn et al. 2009) and other groups have reported functional effects of WISP-1 mediated by αVβ5, αVβ3 and β1 integrins (Chuang et al. 2013; Hou et al. 2013; Liu et al. 2013; Ono et al. 2011). We found that pre-treatment of A549 cells with integrin αVβ5, αVβ3 or β1 neutralising antibodies individually partially inhibited full length WISP-1 induced cell adhesion (Fig. 5a) but inhibition of one integrin alone was insufficient to completely block adhesion. Combining integrin αVβ5 and β1 antibodies or αVβ3 and β1 resulted in further reductions in WISP-1 induced A549 adhesion, inhibiting the response by up to 80 % but combining the αVβ5 and αVβ3 antibodies had no greater effect than neutralising each integrin individually (Fig. 5b). This indicates that the β1 integrin subunit is crucial to the adhesion of full-length WISP-1. We next sought to identify the domains responsible for this interaction by utilising truncated WISP-1 proteins. No reduction in adhesion was seen with any truncated protein when cells were incubated with β1 and αVβ3 antibodies (Fig. 5c and d) whereas the αVβ5 neutralising antibody caused robust statistically significant reductions in adhesion mediated by all truncated proteins (Fig. 5e). This suggests that recognition sites for the αVβ5 integrin may be present in both C-terminal and N-terminal domains of WISP-1 and that the differing levels of adhesion mediated by the truncated proteins may be due to the combined interactions of multiple integrin binding sites.
Fig. 5.
WISP-1 mediated epithelial cell adhesion is regulated by integrins. A549 cells were pre-incubated with integrin-neutralising antibodies before use in adhesion assays. For full-length WISP-1, plates were coated with 2.5 μM ligand and the effect of antibodies evaluated either individually (a) or in combination (b). For truncated WISP-1 proteins, plates were coated with 250 nM ligand (Δ2 and Δ1-2) or 500 nM ligand (Δ1-3 and Δ3-4) and the effect of neutralisation of integrins β1 (c), ɑVβ3 (d) and ɑVβ5 (e) evaluated individually. Results are expressed as the mean ± SD (n = 3), one-way ANOVA performed for B and T-tests for A, and C-E
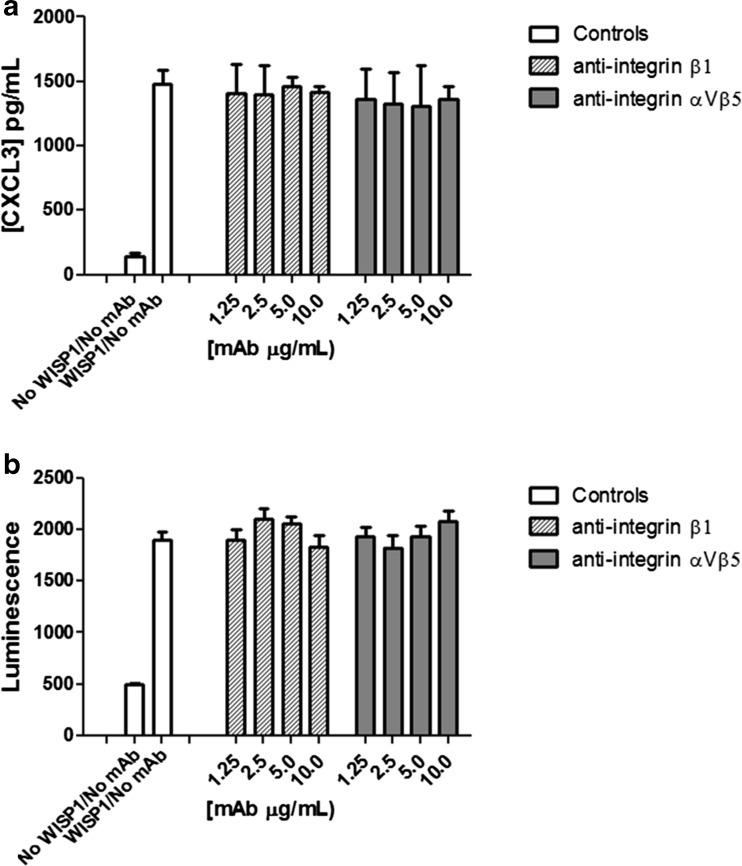
We next aimed to determine if CXCL3 secretion and activation of the β-catenin signalling pathway are also integrin-dependent events. For both experiments, NRK-49F cells were incubated for 1 h with integrin-neutralising antibodies prior to seeding. Neither the anti-β1 nor αVβ5 neutralising antibodies inhibited CXCL3 secretion or activation of the β-catenin signalling pathway in NRK-49F cells (Fig. 6a and b) at concentrations of up to 10 μg/mL. This suggests that these functions of WISP-1 are distinct from adhesion both in terms of domains responsible for activity and of cellular interacting partners.
Fig. 6.
WISP-1 mediated CXCL3 secretion and β-catenin activation in fibroblasts is not regulated by integrins. Wild-type or STF reporter NRK-49F cells were pre-incubated with integrin neutralising antibodies prior to stimulation with full-length WISP-1. Conditioned media was harvested after 24 h and analysed for CXCL3 content by ELISA for wild-type cells (a) or cells lysed and luminescent signal detected after 48 h for STF reporter cells (b). Results are expressed as the mean ± SD (n = 3)
Natural WISP-1 is a high molecular weight oligomer
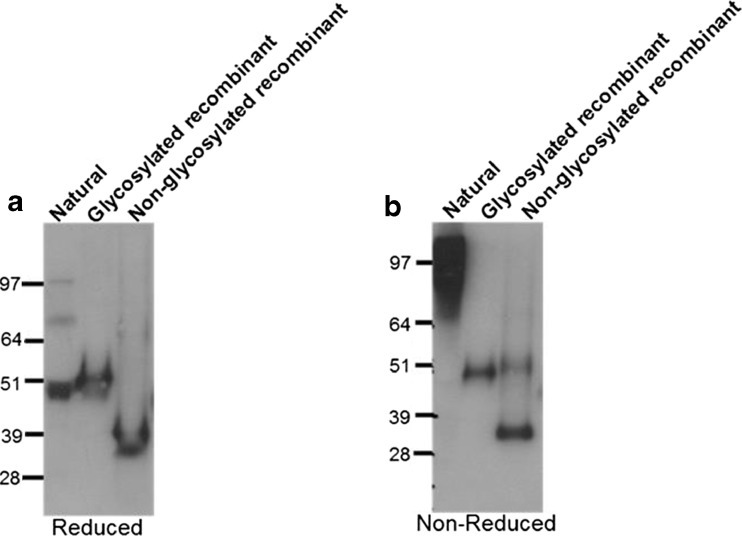
The interaction between individual integrins and their binding partners is known to be weak and depends on avidity in many settings (Carman and Springer 2003). To further dissect the role of integrins in the WISP-1/A549 interaction we investigated the protein chemistry of natural WISP-1. Human lung tissue was sliced into 300–400 μM slices using a tissue chopper and incubated in media for 24 h prior to harvest of supernatants. Western blotting for WISP-1 was then performed under both reducing and non-reducing conditions (Fig. 7). WISP-1 is not normally expressed in healthy adults but was present in supernatants generated in this system, possibly because of the tissue injury caused by mechanical processing since WISP-1 has been implicated in the wound healing process (Wang et al. 2009). Importantly, under non-reducing conditions WISP-1 appears as a high molecular weight oligomer although the exact size of the multimer cannot be determined through this analysis alone. Multimerisation motifs are found in both the TSP1 and vWC domains of the protein and the ability to form dimers and higher order oligomers has been described for other CCN family members (Holbourn et al. 2008). This is the first time however that WISP-1 has been shown to be a multimer and is in keeping with data already presented in this paper showing WISP-1 driven effects can be integrin mediated. Interestingly recombinant WISP-1 protein does not show the same pattern. Under reducing conditions glycosylated WISP-1 is comparable to natural protein whilst non-glycosylated WISP-1 is smaller as would be anticipated. However, under non-reducing conditions both recombinant proteins appear unchanged, suggesting they are monomeric and also that multimerisation is independent of glycosylation status.
Fig. 7.
Natural WISP-1 is a high molecular weight oligomer. Western blotting for WISP-1 was performed under either reduced (a) or non-reduced (b) conditions using conditioned media derived from human cut lung slices or recombinant WISP-1 produced in the baculovirus expression system
Discussion
In our present study we demonstrate for the first time that different domains of the WISP-1 protein play different functional roles in important cellular functions such as adhesion, angiogenesis and Wnt pathway activation. Data presented here support the hypothesis that WISP-1 is a pleiotropic protein and that the multiple effects reported in the literature can be attributed to the ability of the protein to interact with different cellular partners in different settings resulting in the activation of distinct signalling pathways. We found unique gene signatures on WISP-1 stimulation in fibroblasts and epithelial cells and evidence that the interaction between WISP-1 and these cells rely on different receptors. WISP-1 driven adhesion is observed in A549 cells but not NRK-49F cells and likewise, WISP-1 does not activate the β-catenin pathway or CXCL3 secretion in A549 cells (data not shown). This may be due to adhesion being an integrin-dependent event and β-catenin pathway and CXCL3 secretion being integrin independent or alternatively, the latter pathways being reliant on integrins not investigated in this study. Indeed, it is well documented that CCN1, CCN2 and CCN3 can bind at least 8 different integrins and that the functional consequences of integrin binding by CCN proteins is cell context dependent as governed by the repertoire of integrin receptor usage in different cell types (reviewed by (Chen and Lau 2009).
We demonstrate here for the first time a link between WISP-1 and activation of pro-angiogenic pathways which may be relevant for the putative roles WISP-1 plays in both cancer and fibrosis. While a similar chemokine profile has not yet been specifically attributed to other CCN family members, the pro-angiogenic effect of CCN2 (CTGF) is well documented (Holbourn et al. 2009; Yang et al. 2005) and is thought to be driven by interaction with integrins. Similarly, CCN1 and CCN3 have also been shown to have pro-angiogenic activities. Whether this effect is also driven by the chemokines we identified as downstream mediators of WISP-1 activity is yet to be determined but the shared modular structure within the family may suggest that most /all CCN family members can mediate angiogenesis through similar mechanisms.
Previous groups have shown that WISP-1 can activate the Wnt pathway through β-catenin signalling, a result we confirm in the present study. However, in our hands we saw only modest induction in our reporter gene assay. Furthermore this effect relied on a 48 h incubation period, suggesting different kinetics to activation of β-catenin by Wnt3a or possibly an indirect activation of the pathway by a secondary mediator induced by WISP-1 in fibroblasts. Such activity would also support the hypothesis that WISP-1 can act in both an autocrine and a paracrine manner, inducing the secretion of Wnt pathway regulators in adjacent cells.
Importantly, we begin to dissect the contribution of different domains of WISP-1 towards the effects we report. Our data suggest that WISP-1 mediated adhesion of A549 epithelial cells is mainly driven by the C-terminal domains of the protein with weaker activity in the N-terminal vWC domain. Regions of the protein that are involved in the activation of β-catenin signalling and CXCL3 secretion also reside within the C-terminal domains of the protein. It does not seem to be the case however that each domain can act independently or that individual functions are attributable to individual domains but rather more that the observed effects are the cumulative result of multiple domains working in concert, possibly due to the need to engage with multiple integrins recognised by several domains. A high cysteine content is common to all CCN proteins and consequently, disulphide bonding contributes to protein structure. It could therefore be argued that the truncated proteins we generated are structurally dissimilar to the same domains in the full-length protein. Whilst the crystal structure of WISP-1 is not available, modelling of other family members suggests the protein domains are discrete (Holbourn et al. 2008) although unpaired bonds exist which may drive binding to interaction partners or indeed dictate activity between domains. Ultimately the elucidation of the structure of WISP-1 is needed to answer these questions.
Furthermore there is the additional complexity of WISP-1 multimerisation to consider, the importance of which and the putative need for a high avidity interaction is pointed to by the finding that natural WISP-1 is a high order oligo. The mechanism behind the multimerisation may involve additional factors since recombinant WISP-1 produced in a baculovirus expression system is monomeric, suggesting that the multimerisation motifs in the TSP1 and vWC motifs are not sufficient to drive oligo formation in isolation. This may potentially be an artefact of the protein production system or alternatively, may indicate that binding to ECM components is needed to allow WISP-1 to multimerise in vivo. Indeed WISP-1 has been shown to interact closely with decorin and biglycan (Desnoyers et al. 2001) and the absence of these factors in vitro may account for the differences observed between recombinant WISP-1 and protein present in tissue. The ability of the truncated WISP-1 proteins to form multimers was not tested in this study but since they were generated in the same expression system as full-length WISP-1 it is anticipated that they would also prove to be monomeric.
Our results present an important first step in the functional characterization of the WISP-1 protein. Future studies will help to further delineate the complex interactions of this intriguing protein in different cell types and distinct cellular contexts.
References
- Carman CV, Springer TA. Integrin avidity regulation: are changes in affinity and conformation underemphasized? Curr Opin Cell Biol. 2003;15:547–556. doi: 10.1016/j.ceb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Cervello M, Giannitrapani L, Labbozzetta M, Notarbartolo M, D’Alessandro N, Lampiasi N, Azzolina A, Montalto G. Expression of WISPs and of their novel alternative variants in human hepatocellular carcinoma cells. Ann N Y Acad Sci. 2004;1028:432–439. doi: 10.1196/annals.1322.051. [DOI] [PubMed] [Google Scholar]
- Chen CC, Lau LF. Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol. 2009;41:771–783. doi: 10.1016/j.biocel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PC, Cheng HC, Yang SF, Lin CW, Tang CH. The CCN family proteins: modulators of bone development and novel targets in bone-associated tumors. Biomed Res Int. 2014;2014:437096. doi: 10.1155/2014/437096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang JY, Chang AC, Chiang IP, Tsai MH, Tang CH. Apoptosis signal-regulating kinase 1 is involved in WISP-1-promoted cell motility in human oral squamous cell carcinoma cells. PLoS One. 2013;8:e78022. doi: 10.1371/journal.pone.0078022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colston JT, de la Rosa SD, Koehler M, Gonzales K, Mestril R, Freeman GL, Bailey SR, Chandrasekar B. Wnt-induced secreted protein-1 is a prohypertrophic and profibrotic growth factor. Am J Physiol Heart Circ Physiol. 2007;293:H1839–H1846. doi: 10.1152/ajpheart.00428.2007. [DOI] [PubMed] [Google Scholar]
- Desnoyers L, Arnott D, Pennica D. WISP-1 binds to decorin and biglycan. J Biol Chem. 2001;276:47599–47607. doi: 10.1074/jbc.M108339200. [DOI] [PubMed] [Google Scholar]
- Holbourn KP, Acharya KR, Perbal B. The CCN family of proteins: structure-function relationships. Trends Biochem Sci. 2008;33:461–473. doi: 10.1016/j.tibs.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbourn KP, Perbal B, Ravi AK. Proteins on the catwalk: modelling the structural domains of the CCN family of proteins. J Cell Commun Signal. 2009;3:25–41. doi: 10.1007/s12079-009-0048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou CH, Tang CH, Hsu CJ, Hou SM, Liu JF. CCN4 induces IL-6 production through alphavbeta5 receptor, PI3K, Akt, and NF-kappaB singling pathway in human synovial fibroblasts. Arthritis Res Ther. 2013;15:R19. doi: 10.1186/ar4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Parsons CJ, Stefanovic B. Gene expression profile of quiescent and activated rat hepatic stellate cells implicates Wnt signaling pathway in activation. J Hepatol. 2006;45:401–409. doi: 10.1016/j.jhep.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Konigshoff M, Kramer M, Balsara N, Wilhelm J, Amarie OV, Jahn A, Rose F, Fink L, Seeger W, Schaefer L, Gunther A, Eickelberg O. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest. 2009;119:772–787. doi: 10.1172/JCI33950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JF, Hou SM, Tsai CH, Huang CY, Hsu CJ, Tang CH. CCN4 induces vascular cell adhesion molecule-1 expression in human synovial fibroblasts and promotes monocyte adhesion. Biochim Biophys Acta. 2013;1833:966–975. doi: 10.1016/j.bbamcr.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Ono M, Inkson CA, Kilts TM, Young MF. WISP-1/CCN4 regulates osteogenesis by enhancing BMP-2 activity. J Bone Miner Res. 2011;26:193–208. doi: 10.1002/jbmr.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B. Alternative splicing of CCN mRNAs .... it has been upon us. J Cell Commun Signal. 2009;3:153–157. doi: 10.1007/s12079-009-0051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimo T, Nakanishi T, Nishida T, Asano M, Kanyama M, Kuboki T, Tamatani T, Tezuka K, Takemura M, Matsumura T, Takigawa M. Connective tissue growth factor induces the proliferation, migration, and tube formation of vascular endothelial cells in vitro, and angiogenesis in vivo. J Biochem. 1999;126:137–145. doi: 10.1093/oxfordjournals.jbchem.a022414. [DOI] [PubMed] [Google Scholar]
- Su F, Overholtzer M, Besser D, Levine AJ. WISP-1 attenuates p53-mediated apoptosis in response to DNA damage through activation of the Akt kinase. Genes Dev. 2002;16:46–57. doi: 10.1101/gad.942902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang R, Wen S, McCafferty DM, Beck PL, MacNaughton WK. Nitric oxide increases Wnt-induced secreted protein-1 (WISP-1/CCN4) expression and function in colitis. J Mol Med (Berl) 2009;87:435–445. doi: 10.1007/s00109-009-0445-4. [DOI] [PubMed] [Google Scholar]
- Wang D, Dai C, Li Y, Liu Y. Canonical Wnt/beta-catenin signaling mediates transforming growth factor-beta1-driven podocyte injury and proteinuria. Kidney Int. 2011;80:1159–1169. doi: 10.1038/ki.2011.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagita T, Kubota S, Kawaki H, Kawata K, Kondo S, Takano-Yamamoto T, Tanaka S, Takigawa M. Expression and physiological role of CCN4/Wnt-induced secreted protein 1 mRNA splicing variants in chondrocytes. FEBS J. 2007;274:1655–1665. doi: 10.1111/j.1742-4658.2007.05709.x. [DOI] [PubMed] [Google Scholar]
- Yang F, Tuxhorn JA, Ressler SJ, McAlhany SJ, Dang TD, Rowley DR. Stromal expression of connective tissue growth factor promotes angiogenesis and prostate cancer tumorigenesis. Cancer Res. 2005;65:8887–8895. doi: 10.1158/0008-5472.CAN-05-1702. [DOI] [PubMed] [Google Scholar]
- Zulato E, Favaretto F, Veronese C, Campanaro S, Marshall JD, Romano S, Cabrelle A, Collin GB, Zavan B, Belloni AS, Rampazzo E, Naggert JK, Abatangelo G, Sicolo N, Maffei P, Milan G, Vettor R. ALMS1-deficient fibroblasts over-express extra-cellular matrix components, display cell cycle delay and are resistant to apoptosis. PLoS One. 2011;6:e19081. doi: 10.1371/journal.pone.0019081. [DOI] [PMC free article] [PubMed] [Google Scholar]