Epigenetic modifications are alterations in cell phenotype that are not due to change in DNA sequences; they have significant effects on gene expression and may impact the development of various diseases such as tumor growth (Dryhurst and Ausio 2014). Core histones (H2A, H2B, H3, and H4) contain DNA which is bound around an octameric histone core leading to the basic subunit of chromatin (Dryhurst and Ausio 2014). Histone lysine methylation is becoming an important signaling mechanism in eukaryotic cells; it can help indicate both transcriptional repression and activation of chromatin and is dependent upon the location, the degree and type of residue that is being methylated (Vedadi et al. 2011; Rice et al. 2003; Snowden et al. 2002). It is revealed that methylation of histones H3 lysine 4 (H3K4) and H3K36 is commonly known for active transcriptional genes while methylation of H3K9 and H3K27 are associated with condensed heterochromatin (Rosenfeld et al. 2009).
G9a and G9a-like protein (GLP) are methyltransferases that have been widely studied and have led to the development of specific inhibitors for epigenetic targets (Shinkai and Tachibana 2011; Vedadi et al. 2011; Ueda et al. 2006). G9a and GLP are methyltransferases that repress transcription by methylating the lysine at position 9 of the histone H3 subunit (H3K9) and acts as a gatekeeper for differentiation (Chang et al. 2009; Collins and Cheng 2010). These methyltransferases primarily exist as a G9a-GLP heteromeric complex (Tachibana et al. 2008). Mono- and di-methylation by G9a/GLP in H3K9 are linked to repression of certain histone and non-histone targets that are normally expressed in stem cells; G9a is also required for development of mouse embryo and differentiation of mouse embryonic stem cells (ESCs) (Wu et al. 2010; Chen et al. 2012; Fritsch et al. 2010); however, the mechanistic process of G9a/GLP methylation in H3K9 is still not well understood.
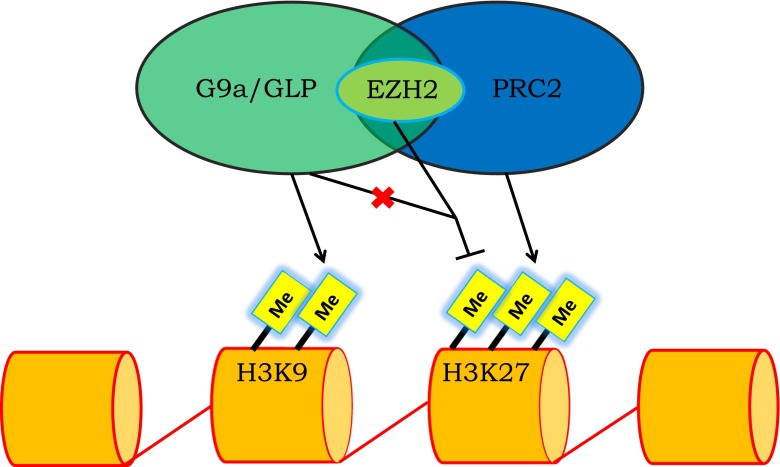
Recently, in the January 2014 edition of Molecular Cell, Mozzetta et al. highlighted the importance of enhancer of zeste homolog 2 (EZH2) regulating the interaction between G9a/GLP and polycomb repressive complex 2 (PRC2). PRC2, one of two classes of the polycomb-group (PcG) which form multimeric protein complexes, is involved in maintaining the transcriptional silencing of genes over successive cell cycles (van der Vlag and Otte 1999). PRC2 is composed of proteins SUZ12 (suppressor of zeste 12 homologue), EED (embryonic ectoderm development), RbAp48 (Rb-associated protein 48), and EZH2 (enhancer of zeste homolog 2). Of these core components, the authors found an important EZH2-mediated interaction between PRC2 and G9a/GLP. EZH2, a histone-lysine N-methyltransferase, primarily acts to silence genes in many types of cancers (Ren et al. 2012). In the study, the authors show that G9a and GLP proteins are likely to interact with PRC2 via EZH2 Fig. 1.
Fig. 1.
The diagram is a representation of the EZH2-mediated crosstalk between H3K9 and H3K27. The interaction between G9a/GLP and PRC2 are mediated by EZH2 in which methyltransferase activity of G9a/GLP is necessary in maintaining gene silencing by PRC2. More specifically, the absence of G9a/GLP suppresses EZH2 recruitment and decreases H3K27 trimethylation (The symbol X in the figure indicates absence)
The authors first demonstrated the collaboration between PRC2 and H3K9 and between the PRC2 core components and G9a/GLP methyltransferase by immunopurification analysis in human HeLa cells and mESCs. These results showed that PRC2 core members interact with the main H3K9 KMTs in cells, i.e., G9a, GLP, SETDB1 and Suv39h1. Furthermore, the PRC2 core members co-purified preferentially with G9a and GLP and, at very low levels, with SETDB1 and Suv39h1 (Shinkai and Tachibana 2011). Hence, they decided to focus on G9a and GLP. They purified a recombinant PRC2 complex, performed a pull down assay with GST-G9a/GLP at different concentrations and confirmed a strong interaction among PRC2 and G9a/GLP. They demonstrated that G9a/GLP and PRC2 both regulate common genes encoding developmental regulators. It also depressed the mESCs lacing G9a and/or GLP. Based on the interactions between the methyltransferases, the potential role of EZH2 on regulating the interaction of G9a and GLP was investigated. Mozzetta et al. compared mRNA expression levels of G9a−/−, GLP−/−, and G9a−/− GLP−/− ESCs in relation to EZH2−/− ESCs. From this comparison, they discovered that 487 genes were upregulated when there was an absence of EZH2, G9a and/or GLP; these genes are known to code proteins that regulate in cell differentiation and development (Mozzetta et al. 2014).
Mozzetta et al. showed that EZH2 and G9a/GLP share a noteworthy number of genomic targets which regulate gene expression in a division of developmental and neuronal genes through chromatin immunoprecipitation and transcriptomic analyses (Mozzetta et al. 2014). They found that the suppression of G9a and GLP reduced the recruitment of EZH2 to many of its targets. This led to an overall decrease in H3K27 trimethylation. Inversely, the re-introduction of wild-type G9a, but not the enzymatic inactive type, returned the recruitment of EZH2, trimethylation of H3K27 and inactivate PRC2 target genes. This suggests that regulation is dependent on G9a and enzymatic activity associated with PRC2 recruitment. Based on their analysis, they indicate that a direct functional cooperation occurs between PRC2 and G9a/GLP in vitro. This also suggests the role of EZH2 to ensure epigenetic gene silencing at certain chromatin regions. G9a facilitates PRC2 recruitment to a subset of target genes to promote silencing, thus establishing interaction between two important gene silencing mechanisms (Mozzetta et al. 2014).
In conclusion, the publication by Mozzetta et al. reveals that the physical interaction between G9a/GLP proteins and PRC2 from key epigenetic silencing pathways are regulated by EZH2, a gene silencer, which helps mediate the crosstalk between H3K9 and H3K27 (Tachibana et al. 2008). From this study, the authors can surmise that inhibiting EZH2 can have numerous beneficial effects by disrupting the interactions between G9a/GLP and PRC2 since G9a enzymatic activity is dependent on EZH2 recruitment. This brand of study is becoming an increasing factor in the field of epigenetic mechanisms, a process implicated in several deadly diseases such as certain forms of cancers and neurologic disorders. Hence, this study regarding the EZH2-mediated interaction between H3K9 and H3K27 may represent a basis in finding endogenous mediators that can regulate EZH2 and possibly provide therapeutic means of treating diseases such as cancer by introducing several neuronal targets for gene silencing.
Acknowledgments
NK was funded by the American Heart Association National Scientist Development Grant 09SDG2260957 and National Institutes of Health R01 HL105932 and the Joy McCann Culverhouse Endowment to the Division of Allergy and Immunology.
References
- Chang Y, Zhang X, Horton JR, Upadhyay AK, Spannhoff A, Liu J, Cheng X. Structural basis for G9a-like protein lysine methyltransferase inhibition by BIX-01294. Nat Struct Mol Biol. 2009;16(3):312–317. doi: 10.1038/nsmb.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Skutt-Kakaria K, Davison J, Ou YL, Choi E, Malik P, Paddison PJ. G9a/GLP-dependent histone H3K9me2 patterning during human hematopoietic stem cell lineage commitment. Genes Dev. 2012;26(22):2499–2511. doi: 10.1101/gad.200329.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins R, Cheng X. A case study in cross-talk: the histone lysine methyltransferases G9a and GLP. Nucleic Acids Res. 2010;38(11):3503–3511. doi: 10.1093/nar/gkq081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryhurst D, Ausio J. Histone H2A.Z deregulation in prostate cancer. Cause or effect? Cancer Metastasis Rev. 2014;33(2–3):429–439. doi: 10.1007/s10555-013-9486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch L, Robin P, Mathieu JR, Souidi M, Hinaux H, Rougeulle C, Ait-Si-Ali S. A subset of the histone H3 lysine 9 methyltransferases Suv39h1, G9a, GLP, and SETDB1 participate in a multimeric complex. Mol Cell. 2010;37(1):46–56. doi: 10.1016/j.molcel.2009.12.017. [DOI] [PubMed] [Google Scholar]
- Mozzetta C, Pontis J, Fritsch L, Robin P, Portoso M, Proux C, Ait-Si-Ali S. The histone H3 lysine 9 methyltransferases G9a and GLP regulate polycomb repressive complex 2-mediated gene silencing. Mol Cell. 2014;53(2):277–289. doi: 10.1016/j.molcel.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Ren G, Baritaki S, Marathe H, Feng J, Park S, Beach S, Yeung KC. Polycomb protein EZH2 regulates tumor invasion via the transcriptional repression of the metastasis suppressor RKIP in breast and prostate cancer. Cancer Res. 2012;72(12):3091–3104. doi: 10.1158/0008-5472.CAN-11-3546. [DOI] [PubMed] [Google Scholar]
- Rice JC, Briggs SD, Ueberheide B, Barber CM, Shabanowitz J, Hunt DF, Allis CD. Histone methyltransferases direct different degrees of methylation to define distinct chromatin domains. Mol Cell. 2003;12(6):1591–1598. doi: 10.1016/S1097-2765(03)00479-9. [DOI] [PubMed] [Google Scholar]
- Rosenfeld JA, Wang Z, Schones DE, Zhao K, DeSalle R, Zhang MQ. Determination of enriched histone modifications in non-genic portions of the human genome. BMC Genomics. 2009;10:143. doi: 10.1186/1471-2164-10-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai Y, Tachibana M. H3K9 methyltransferase G9a and the related molecule GLP. Genes Dev. 2011;25(8):781–788. doi: 10.1101/gad.2027411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden AW, Gregory PD, Case CC, Pabo CO. Gene-specific targeting of H3K9 methylation is sufficient for initiating repression in vivo. Curr Biol. 2002;12(24):2159–2166. doi: 10.1016/S0960-9822(02)01391-X. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Matsumura Y, Fukuda M, Kimura H, Shinkai Y. G9a/GLP complexes independently mediate H3K9 and DNA methylation to silence transcription. Embo j. 2008;27(20):2681–2690. doi: 10.1038/emboj.2008.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda J, Tachibana M, Ikura T, Shinkai Y. Zinc finger protein Wiz links G9a/GLP histone methyltransferases to the co-repressor molecule CtBP. J Biol Chem. 2006;281(29):20120–20128. doi: 10.1074/jbc.M603087200. [DOI] [PubMed] [Google Scholar]
- van der Vlag J, Otte AP. Transcriptional repression mediated by the human polycomb-group protein EED involves histone deacetylation. Nat Genet. 1999;23(4):474–478. doi: 10.1038/70602. [DOI] [PubMed] [Google Scholar]
- Vedadi M, Barsyte-Lovejoy D, Liu F, Rival-Gervier S, Allali-Hassani A, Labrie V, Jin J. A chemical probe selectively inhibits G9a and GLP methyltransferase activity in cells. Nat Chem Biol. 2011;7(8):566–574. doi: 10.1038/nchembio.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Min J, Lunin VV, Antoshenko T, Dombrovski L, Zeng H, Schapira M. Structural biology of human H3K9 methyltransferases. PLoS One. 2010;5(1):e8570. doi: 10.1371/journal.pone.0008570. [DOI] [PMC free article] [PubMed] [Google Scholar]