Abstract
Selected Phosphoinositide-specific Phospholipase C (PI-PLC) enzymes occupy the convergence point of the broad range of pathways that promote Rho and Ras GTPase mediated signalling, which also regulate the activation of ezrin, a member of the ezrin-radixin-moesin (ERM) proteins family involved in the metastatic osteosarcoma spread. Previous studies described that in distinct human osteosarcoma cell lines ezrin networks the PI-PLC with complex interplay controlling the expression of the PLC genes, which codify for PI-PLC enzymes. In the present study, we analyzed the expression and the sub-cellular distribution of RhoA and Rac1 respectively after ezrin silencing and after PI-PLC ε silencing, in order to investigate whether ezrin-RhoGTPAses signalling might involve one or more specific PI-PLC isoforms in cultured 143B and Hs888 human osteosarcoma cell lines. In the present experiments, both ezrin and PLCE gene silencing had different effects upon RhoA and Rac1 expression and sub-cellular localization. Displacements of Ezrin and of RhoA localization were observed, probably playing functional roles.
Keywords: Osteosarcoma, Ezrin, Phospholipase C, Ras GTPases, RhoA, Rac1
Introduction
Osteosarcoma, the most common primary bone tumour in childhood and adolescence, comprises of different clinical, radiological, and histological entities. Usually, metastatic osteosarcoma spread confers worse prognosis to the clinical outcome of patients presenting with osteosarcoma (Mirabello et al. 2009; Gatta et al. 2005).
Ezrin, the first characterized member of the ezrin-radixin-moesin (ERM) proteins family, acts as a general cross-linker of actin to the plasma membrane (Khanna et al. 2004; Ferrari et al. 2008), and was suggested to be involved in the metastasizing process (Khanna et al. 2004).
The Band 4.1, Ezrin, radixin, moesin homology (FERM) domain (PBD ID:1GC6) of ERM proteins is involved in the recognition of Phosphatydil inositol (Ferrari et al. 2008; Fievet et al. 2007) bisphosphate (PIP2), a crucial molecule belonging to the PI system (Fievet et al. 2004, 2007). The activation of ERM proteins invariably requires PIP2 local accumulation (Hartwig et al. 1995; Tolias et al. 2000; Honda et al. 1999). Two putative binding sites were identified, and PIP2 binding was demonstrated to be essential in guiding ezrin to cell surface locations (Barret et al. 2000). The reduction of PIP2 levels induces ERM protein dissociation from the membrane (Hao et al. 2009). The levels of plasma membrane PIP2 are regulated by means of the Phosphoinositide-specific Phospholipase C (PI-PLC) family (Berridge and Dupont 1994; Divecha and Irvine 1995; Hisatsune et al. 2005; Rhee 2001; Bunney and Katan 2011; Fukami et al. 2010), complex, modular, multi-domain enzymes which cover a wide spectrum of regulatory interactions, including direct binding to Ras GTPases (Rhee et al. 1991; Berridge 1993; De Smedt and Parys 1995; Nishizuka 1995; Irvine 2003; Suh et al. 2008).
In mammals, PI-PLC enzymes were divided into six sub-families on the basis of amino acid sequence, domain structure and mechanism of recruitment: β(1 − 4), γ(1, 2), δ(1, 3 and 4), ε(1), ζ(1), and η(1 and 2) (Suh et al. 2008). The presence of distinct regulatory domains renders each sub-family susceptible to different mechanisms of activation (Suh et al. 2008). Previous studies placed selected PI-PLC enzymes at the convergence point of pathways involved in the Ras GTPase mediated signalling (Hao et al. 2009), which also regulates ezrin activation.
PI-PLC ε isoform is a direct effector of the members of the Rho family (RhoGTPases), belonging to the Ras superfamily (Ridley 2001). PI-PLC ε can be directly and concomitantly activated by both RhoA and individual Ras GTPases resulting in diverse upstream control of signalling cascades downstream of PI-PLC ε by a variety of hormones, neurotransmitters, growth factors and other molecules (Suh et al. 2008; Lo Vasco et al. 2014a).
PI-PLC β subfamily enzymes can be activated by the Rho-GTPase family member Rac, comprising Rac1, Rac2, and Rac3, which interacts with the pleckstrin homology (PH) domain of PI-PLC enzymes (Hao et al. 2009). Rac1 was suggested to be involved in in actin polymerization ), cell proliferation, and in the regulation of the cell cycle (Joneson and Bar-Sagi 1997; Steffen et al. 2004; Moore et al. 1997; Juncadella et al. 2013). Moreover, Rac1 was demonstrated to induce cell transformation in vitro (Benitah et al. 2004) and malignant transformation (Gómez del Pulgar et al. 2005).
Therefore, both ezrin and some PI-PLC isoforms are regulated by Ras signalling and can bind PIP2 (Hao et al. 2009; Snyder et al. 2003). In our previous studies, the panel of expression of PI-PLC ε differed in distinct osteosarcoma cell lines, probably due to the different origin of the cells (Lo Vasco et al. 2013a). The transcription of PLCE gene was affected by Ezrin silencing (Lo Vasco et al. 2014b) and transcription of Ezrin, as well as of selected PI-PLC enzymes, was affected by PI-PLC ε silencing (Lo Vasco et al. 2014c). The levels of networking in the recruitment remained to be elucidated.
In the present study, we analyzed the expression and the sub-cellular distribution of RhoA and Rac1 in 143B and Hs888 human osteosarcoma cell lines after ezrin (Vil2 gene, OMIM *123900) silencing or after PI-PLC ε silencing, in order to investigate whether ezrin-RasGTPases signalling might involve one or more PI-PLC enzyme. Our aim was to investigate the hypothesis that ezrin-RhoGTPase-PLC pathway might exist and/or that different localization of RhoGTPases might occur after ezrin reduction.
Materials and methods
Cell cultures
Two human osteosarcoma cell lines, 143B and Hs888, were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10–15 % foetal bovine serum (FBS), 1 mM sodium pyruvate, 100 U/mL of penicillin, and 100 mg/mL of streptomycin, at 37 °C and 5 % CO2 according to producer’s recommendations. Cells were grown at 37 °C in a humidified atmosphere containing 5 % CO2 in a Forma Scientific incubator (USA). Confluent monolayer of cells was rinsed with phosphate-buffered saline (PBS) and detached using 0.25 % Trypsin/EDTA (disodium ethylene diaminetetraacetate). Cells were counted using a Neubauer haemocytometer (Weber Scientific International Ltd., Middlesex, UK), and stored at −20 °C until use.
Cells transfection for Ezrin and PLCE silencing
Cultures of 143B and Hs888 cells were transiently transfected with Vil2 or PLCE silencing RNA using METAFECTENE SI+ (Biontex Laboratories GmbH, Munich, Germany). siRNA sequences respectively targeting Vil2 or PLCE and negative control siRNA were designed and synthesized by Invitrogen (Life Technologies, Foster City, CA, USA). The siRNA was designed according to Vil2 and PLCE complementary DNA (cDNA) sequence (Vil2 Gene ID: 7430; PLCE Gene ID: 51196). Briefly, 2.2 ml cell suspension were prepared in complete cell culture medium with a concentration of 1,5 · 10^5 cells/ml of 143B cells and 3 · 10^5 cells/ml of Hs888. Cells were seeded in 6-well plates, transfected with a mixture of 150 μl of 1× SI+ buffer with previously incubated with 72 μl of METAFECTENE® SI+ and 540 pMol of RNA stock solution according to the manufacturer’s instructions. Then cells were incubated at 37 °C in CO2–containing atmosphere for 72 h. Functional siRNA was measured by reverse transcription–polymerase chain reaction (RT-PCR) and western blot analysis resepctively 24, and 48 after tranfection. Contemporarily, a growth curve was designed.
RNA extraction
Total RNA was extracted with SV Total RNA Isolation System (Promega, Madison, WI, USA) according to the manufacturer’s instructions. The cells were transferred to a microcentrifuge tube containing 175 μL of SV RNA Lysis Buffer and passed through a 20-gauge needle to shear the genomic DNA. 350 μL of SV Dilution Buffer was added in a heating block at 70 °C for 3 min. The sample was centrifuged for 10 min at 14,000×g. The lysate solution was transferred to a new microcentrifuge tube, 200 μL of 95 % ethanol were added, and the mixture was transferred to a spin column assembly, and centrifuged at 14,000×g for one minute. The liquid was discarded from the collection tube, 600 μL of SV RNA Wash Solution was added to the column, centrifuged at 14,000×g for one minute, and the collection tube was emptied. A DNase incubation mixture was prepared per sample by combining 40 μL Yellow Core Buffer (Promega), 5 μL 0.09 M MnCl2 and 5 μL of DNase I enzyme. The DNase incubation mixture was directly added, incubated for 15 min at room temperature, then 200 μL of SV DNase Stop Solution was added to the spin basket, and centrifuged at 14,000×g for 1 min. 600 μL of SV RNA Wash Solution was added and centrifuged at 14,000×g for 1 min. The collection tube was emptied, 250 μL of SV RNA Wash Solution was added, and centrifuged at 14,000×g for 2 min. The spin basket was transferred to an elution tube, 100 μL of Nuclease-Free Water was added to the membrane and centrifuged at 14,000×g for 1 min. RNA was eluted into a sterile collection tube with RNase-free water. The concentration and quality of the RNA obtained was monitored using a NanoDrop ND-1000 Spectrophotometer (Thermo Fisher Scientific, Inc. USA).
RT-PCR
RNA was reverse-transcribed into cDNA using High-Capacity cDNA Reverse Transcription Kit (Life Technologies, Foster City, CA, USA). Briefly, 2 μg RNA were incubated with the master mix (2 μl of 10 × Reverse Transcription Buffer, 0.8 μl of 25 × dNTPs (100 mM), 2 μl of 10× random primers, 1 μl of MultiScribeTM Reverse Transcriptase (50 U/μl) and 3.2 μl of DNase-free water). 10 μl of diluted RNA was then added to 20 μl final volume. The RNA mix was then amplified for 10 min at 25 °C, 120 min at 37 °C and 5 min at 85 °C in a Gene Amp® PCR System 9700 (Applied Biosystems) thermocycler.
The primer pairs (Bio Basic Inc, Amherst, New York, USA) for PI-PLC isoforms are listed in Table 1. To amplify glyceraldehyde 3 phosphate dehydrogenase (GAPDH) (Bio Basic Inc, Amherst, New York, USA) as a positive control, the following primer pair was used: forward 5′-CGAGATCCCTCCAAAATCAA-3′ reverse 5′-GTCTTCTGGGTGGCAGTGAT-3′. The following primers were used to amplify RhoA: forward, 5′-TGAAAACTATGTGGCGGATATCG; reverse, 5′-TCTGCTTCTTCAGGTTTAACCGG and Rac1: forward 5’-ATG CAG GCC ATC AAG TGT GTG GTG and reverse 3'-TTA CAA CAG CAG GCA TTT TCT CTT CC). The specificity of the primers was verified by searching in the NCBI database for possible homology to cDNAs of unrelated proteins. RNA samples were also amplified by PCR without RT to exclude possible contamination.
Table 1.
Real-time PCR results after silencing of ezrin (upper) and PLCE (lower). (T = 0) untreated; (T = 24) after 24 from silencing; (T = 48) after 48 from silencing. + = mRNA present; − = mRNA absent. > = mRNA moderately increased (not more than 30 %) with respect to T0. <= mRNA moderately decreased (not more than 30 %) with respect to T0. >> = mRNA increased (30-75 %) with respect to T0. <<=mRNA decreased (30–75 %) with respect to T0
| Ezrin silencing | ||||||
| 143B | Hs888 | |||||
| T = 0 | T = 24 h | T = 48H | T = 0 | T = 24 h | T = 48H | |
| Ezrin | + | − | − | + | − | − |
| RhoA | + | > | >> | + | < | < |
| Rac1 | + | > | < | + | << | < |
| PLCE | + | < | > | + | < | > |
| PLCG2 | + | > | > | +/− | + | + |
| PLCB1 | + | < | < | + | >> | >> |
| PLCD4 | − | − | − | + | > | = |
| PI-PLC ε silencing | ||||||
| 143B | Hs888 | |||||
| T = 0 | T = 24 h | T = 48H | T = 0 | T = 24 h | T = 48H | |
| PLCE | + | − | − | + | − | − |
| RhoA | + | < | < | + | < | < |
| Rac1 | + | < | < | + | << | << |
| Ezrin | + | < | < | + | > | = |
| PLCG2 | + | > | > | +/− | − | − |
| PLCB1 | + | < | < | + | > | >> |
| PLCD4 | − | − | − | + | < | < |
Standard analytical PCR reaction was performed with GoTaq Master Mix (Promega). Each PCR tube contained the following reagents: 5× GoTaq buffer, 0.2 μM forward primer, 0.2 μM reverse primer, 0.2 mM dNTPs, 0.5 mM MgCl2, 1.25 U GoTaq and 3,5 μl of (about 35 μg) template cDNA at a 50 μl final volume. Cycling conditions were performed with 95 °C initial denaturation step for 1 min was followed by 40 cycles consisting of 95 °C denaturation (30s), annealing (30 s) at the appropriate temperature for each primer pair and 72 °C extension (1 min) in Gene Amp® PCR System 9700 (Applied Biosystems) thermocycler. Amplified PCR products were visualized by 1.5 % TAE ethidium bromide-stained agarose gel electrophoresis for 1 h at 100 V using UV light transilluminator PC-assisted CCD camera UVB lamp (Vilber Lourmaret, France) was used for gel documentation. Gel electrophoresis of the amplification products revealed single DNA bands with nucleotide lengths as expected for all primer pairs.
Real-time PCR
The messenger RNA (mRNA) transcription of transfected 143B and Hs888 cells and normal controls was measured. Gene expression was analyzed by real-time PCR using the 7500 Real-Time PCR instrument from Applied Biosystems™. TaqMan® primers and probes for each gene, as well as the GAPDH reference gene, were obtained from Applied Biosystems™. Briefly, total RNA was extracted with a SV Total RNA Isolation System (Promega, Madison, WI, USA) according to the manufacturer’s instructions. The purity and quantity of RNA was assessed by NanoDrop ND-1000 Spectrophotometer. The RNA was reverse transcribed into cDNA with High Capacity cDNA Reverse Transcription Kit (Life Technologies, Foster City, CA, USA).
Amplification products were detected using gene-specific primers and probes labeled with reporter day FAM which yielded a predicted amplicons of 82, 84, 61, 78, 64, 93 and 62 base pairs respectively; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal standard and positive control, which yielded a predicted amplicon of 58 base pairs. Reaction mixtures for all gene expression assays contained: 5 μl TaqMan® mastermix (2×; Applied Biosystems™), 0,5 μl primer/probe mix specific for each analyzed gene and 1 μl PCR grade water. 3,5 μl of cDNA (35 ng) were added. PCR reaction was carried out in triplicate on 96-well plate with 10 uL per well using 1× TaqMan Master Mix. After 2 min incubation at 50 °C and 10 min at 95 °C, the reaction was carried out for 40 cycles at 95 °C for 15 s and 60 °C for 1 min. At the end of the reaction, the results were evaluated using the ABI PRISM 7500 software. The Ct (Cycle threshold) values for each set of three reactions were averaged for calculations. The 2^-∆∆Ct method was used to calculate relative changes in gene expression.
Western blot
Western blot analyses were conducted 24 and 48 h from transfection and in unsilenced controls. Cells were washed with cold PBS, then processed in lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 2 mM EDTA, 1 % NP-40, 2 mM sodium fluoride, 0.5 % sodium deoxycholate, and 0.1 % SDS) containing protease inhibitors. 50 μg of protein was separated by 10 % sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes (Invitrogen, Life Technologies, Foster City, CA, USA). The membranes were blocked in PBS with 5 % skim milk for 1 h and incubated overnight with the primary antibodies. Finally, membranes were visualized by the addition of anti-mouse immunoglobulin G (Jackson Immunoresearch, UK) and anti-rabbit immunoglobulin G (Jackson Immunoresearch, UK) enhanced chemiluminescence. Expression of β-actin was used as an internal control to normalize results. The densities of the bands on the membrane were scanned and analyzed with ImageJ software.
Immunofluorescence analysis of subcellular distribution of target molecules
Immunofluorescence detection of RhoA and Rac1 was performed on cultured transfected and non-transfected cells. Cells were fixed with 4 % paraformaldehyde (PFA), washed and incubated with primary antibodies (Santa Cruz Biotechnologies, CA, USA), and incubated with the Texas Red or fluorescein-conjugated specific secondary antibody (Santa Cruz), following the manufacturer’s indications. Cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) fluorescent staining. The slides (Ibidi, Munich, Germany) were visualized using an inverted microscope.
Statistical analysis
For in vitro studies, differences were determined either with two-way repeated measures analysis of variance (ANOVA) with Bonferroni’s multiple comparison test, or by student’s t test, using Prism 5.0a software (GraphPad Software, San Diego, CA, USA). A p value <0.05 was considered significant.
Results
Effectiveness of silencing
Silencing of ezrin was validated by western blot, RT-PCR and gel electrophoresis, multiliquid bioanalysis and real time PCR analysis of mRNA transcripts compared to a non-targeting control siRNA. Ezrin was absent or markedly reduced in transfected cells for 48–72 interval (Fig. 1a and b).
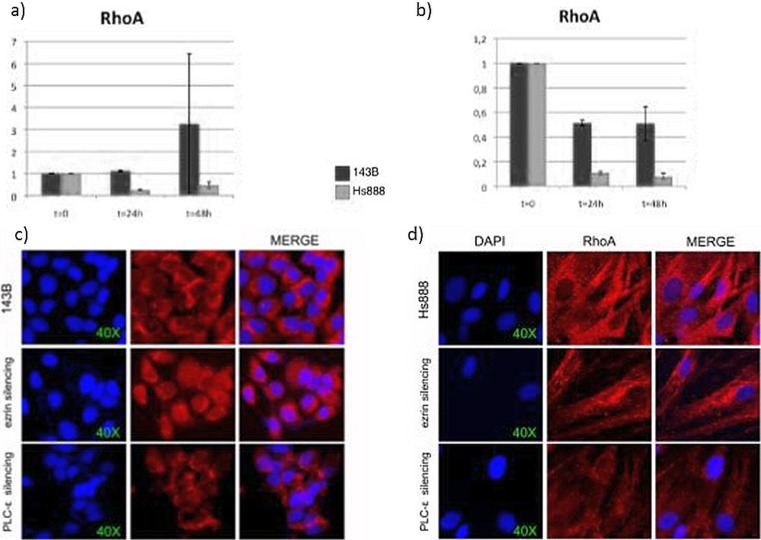
Fig. 1.
Upper: Istograms of Real-time PCR for RhoA in ). (T = 0) untreated; (T = 24) after 24 from silencing; (T = 48) after 48 from silencing (a) of ezrin and (b) of PLCE in 143B cells (dark grey) and in Hs888 cells (pale grey). Lower: Immunofluorescence imaging of 143B cells for RhoA in (T = 0) untreated; (T = 24) after 24 from silencing; (T = 48) after 48 from silencing of ezrin and of PLCE in 143B cells (c) and in Hs888 cells (d). DAPI (blue) for nuclei counterstaining, RhoA localization and merge DAPI/RhoA signals
Western blot assay showed no change in the expression of β-actin; the expression of ezrin protein was significantly decreased in ezrin siRNA transfected cells compared to the corresponding untransfected cells and cells transfected with the carrier metafectamine.
The mRNA expression levels in transfected cells was significantly reduced with respect to untreated cells (p < 0,001).
Real-Time PCR after ezrin silencing and statistics
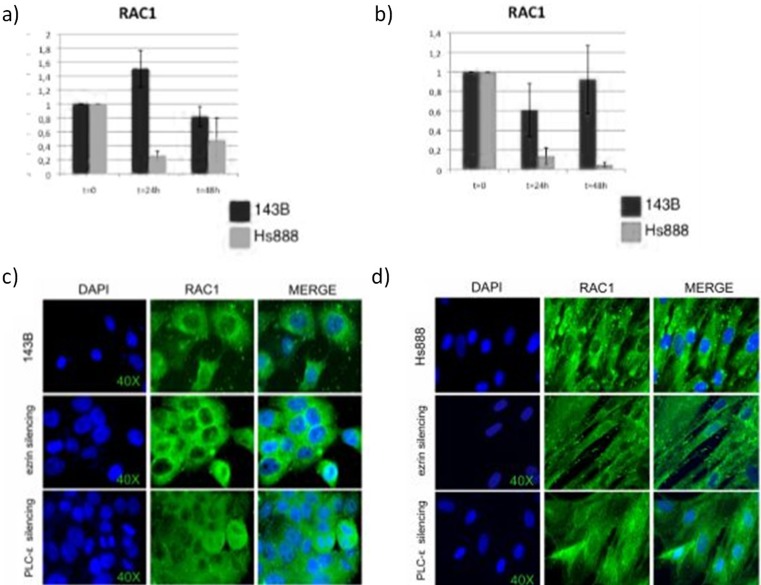
In 143B cell line, the expression of RhoA increased about 10 % after 24 h and about 3 times after 48 h; the expression of Rac1 increased 50 % after 24 h. Contemporarily PLCB1 decreased, PLCG2 slightly increased, PLCE slightly decreased after 24 h and slightly increased after 48 h. Statistically significant difference after 24 h from ezrin silencing was calculated in the mRNA expression levels of RhoA (p < 0.0005) and Rac1 (p < 0,005) (Figs. 1, 2 and 3).
Fig. 2.
Upper: Istograms of Real-time PCR for Rac1 in ). (T = 0) untreated; (T = 24) after 24 from silencing; (T = 48) after 48 from silencing (a) of ezrin and (b) of PLCE in 143B cells (dark grey) and in Hs888 cells (pale grey). Lower: Immunofluorescence imaging for Rac1 in (T = 0) untreated; (T = 24) after 24 from silencing; (T = 48) after 48 from silencing of ezrin and of PLCE in 143B cells (c) and in Hs888 cells (d). DAPI (blue) for nuclei counterstaining, RhoA localization and merge DAPI/RhoA signals
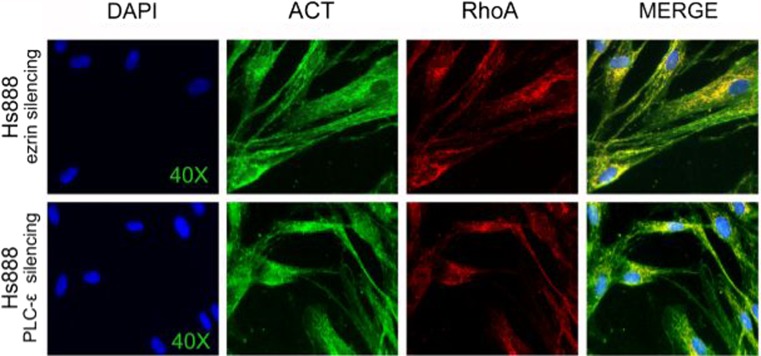
Fig. 3.
Immunofluorescence imaging of partial co-localization of actin and RhoA in HS888 cells: (upper line) after ezrin silencing and (lower line) after PI-PLC ε silencing
In Hs888 cell line, the expression of RhoA decreased about 75 % after 24 h; the expression of Rac1 decreased about 95 % after 24 h. Either molecules were reduced about 50 % after 48 h. Contemporarily PLCB1, PLCG2, and PLCD4 significantly increased after 24 h, while PLCE slightly decreased after 24 h and significantly increased after 48 h. Statistically significant difference after 24 h from ezrin silencing was calculated in the mRNA expression levels of RhoA (p < 0.0005) and Rac1 (p < 0,005) (Figs. 1 and 2).
No changes were observed in the positive control.
Real-Time PCR after PI-PLC ε silencing and statistics
In 143B cell line, the expression of RhoA decreased about 50 %. The expression of Rac1 decreased about 40 % after 24 h and 10 % after 48 h. Ezrin decreased about 70 %, PLCB1 was reduced about 65 % after 24 h and about 15 % after 48 h, and PLCG2 increased about 50 % after 24 h and 25 % after 48 h. Statistically significant differences in the mRNA expression levels of RhoA (p < 0.00125) and Rac1 (p < 0,05) was calculated in the 24–48 h interval (Figs. 1 and 2).
In Hs888 cell line after silencing of PI-PLC ε the expression of RhoA significantly decreased about 90 % in the interval 24–48 h and the expression of Rac1 significantly decreased about 95 % in the interval 24–48 h. Contemporarily, ezrin increased after 24 h and was restored at the basal levels after 48 h, PLCB1 increased two folds after 24 h and more significantly (15 folds) increased after 48 h, PLCD4 slightly decreased (about 15 %). Statistically significant differences in the mRNA expression levels of RhoA (p < 0.0125) and Rac1 (p < 0,025) was calculated in the 24–48 h interval (Figs. 1 and 2).
No changes were observed in the positive control.
Immunofluorescence
In 143B cell line ezrin was moderately present in the cytoplasm, with membrane signal enhancement. PI-PLC ε was strongly present and localized in the cytoplasm and in the perinuclear area. Ezrin and PI-PLC ε partially co-localized in the cytoplasm. RhoA was localized in perinuclear area and in the cytoplasm with strong signal intensity, RAC1 was weakly detected and localized in the perinuclear zone (Figs. 1 and 2). Ezrin and RhoA do not seem to localize in the cytoplasm.
After Ezrin silencing, a slight reduction of PI-PLC ε staining was observed in the cytoplasm. The signal intensity of RhoA did not apparently change, although the protein was displaced in the nucleus (Fig. 1). Strong increase of Rac1 was observed, localized in the perinuclear area (Fig. 2).
After PLCE silencing, Ezrin, slightly decreased, moved from the plasma membrane to the perinuclear area. Moderate reduction of RhoA signal intensity in the cytoplasma (Fig. 1) and marked reduction of the Rac1 perinuclear signal intensity was detected (Fig. 2).
In Hs888 cell line ezrin was mildly present and localized in the cytoplasm. PI-PLC ε was also weakly localized in cytoplasm. Ezrin and PI-PLC ε partially co-localized in the cytoplasm. RhoA and Rac1 were strongly localized in the perinuclear area and cytoplasm. After Ezrin silencing, the signal intensity of RhoA was weaker, the cytoplasmic signal of Rac1 moderately decreased. After ezrin silencing RhoA and actin colocalized in the cytoplasm (Fig. 3). PI-PLC ε silencing induced marked reduction of RhoA and moderate reduction of Rac1 in the cytoplasm. The signal intensity for ezrin slightly decreased in the cytoplasm and a more evident perinuclear staining was observed, with loss of membrane staining (Figs. 1 and 2).
Discussion
The activity of ERM proteins, essential for actin assembly (Defacque et al. 2000, 2002), largely depends on membrane PIP2 levels (Hao et al. 2009), regulated by PI-PLC enzymes. Both ezrin and PI-PLC enzymes recognize and bind the PIP2 on the plasma membrane, probably in a competitive manner. Moreover, both ezrin and selected PI-PLC enzymes, such as isoforms belonging to the PI-PLC β subfamily and PI-PLC ε, are involved in the Ras mediated signal transduction. The transcription of PLCE gene was affected by ezrin silencing (Lo Vasco et al. 2014b) and transcription of ezrin was affected by PI-PLC ε silencing (Lo Vasco et al. 2014c).
The reduction of PIP2 levels induced relocation of ezrin within the cell (Barret et al. 2000). Expression of dominant negative Rac1 reduced ezrin at adherens junctions. Inactivation of RhoA resulted in inhibition of ezrin relocation. Then, RhoA might be involved upstream in the pathway, probably masking the actin binding site (Auvinen et al. 2007) or using a common downstream effector (Auvinen et al. 2007). Our present results suggest that such an effector might be one or more PI-PLC isoform.
In the present experiments, silencing both ezrin and PI-PLC ε had distinct effects upon 143B cell line and Hs888, probably due to the different features of the cell lines, as suggested by previous reports (Lo Vasco et al. 2013a, b, c). Perinuclear relocation of ezrin contemporarily occurred to RhoA and Rac1 reduction. Both ezrin relocation and reduction of RhoA and Rac1 might be related to PI-PLC ε lacking.
In the 143B cell line, RhoA expression was modified by PI-PLC ε reduction. Ezrin silencing increased the expression of PLCG2, RhoA, Rac1, and after 48 h of PLCE. By contrast, decreased the expression of PLCB1 and after 24 h of PLCE. PLCE silencing increased the expression of PLCG2, and decreased the expression of Vil2 (OMIM *123900), the gene which codifies for ezrin, PLCB1, PLCD4, RHOA (OMIM *165390) and RAC1 (OMIM *602048). The behaviour of RhoA linearly correlates with the expression of PI-PLC ε. PI-PLC ε decreased after 24 h from both ezrin silencing and PI-PLC ε silencing, as well as RhoA. When, the transcription of PI-PLC ε increased also RhoA was significantly enhanced. Moreover, in 143B cells, RhoA was displaced in the nucleus after ezrin silencing. The nuclear distribution of RhoA is thought to represent a critical element (Li et al. 2001). Our data suggest that the expression of RhoA might be affected by the expression of PI-PLC ε. However, further studies are required in order to investigate the level of regulation networking RhoA and PI-PLC ε and the role in the nuclear displacement of RhoA. PLCE silencing also induced displacement of Ezrin, according to previous findings (Lo Vasco et al. 2014b). Rac1 was demonstrated to regulate the intracellular localization of Ezrin, with a mechanism involving RhoA and strictly related to the F-actin binding activity (Auvinen et al. 2007). Expression of dominant negative Rac1 modified ezrin localization.
In Hs888 PLCE silencing induced increase of ezrin and decrease of RhoA and Rac1, while Vil2 silencing reduced RhoA, Rac1 and PLCE. Accordingly to literature data, RhoA co-localized with actin after ezrin silencing, as well as after PI-PLC ε silencing, in a much less evident manner. Although these results cannot be considered conclusive, no direct link was substantiated for the regulation of the transcription of those molecules. On the other hand, the expression of PI-PLC β1 was significantly increased after either silencing experiments, confirming previous data (Lo Vasco et al. 2014b, c). The reduction of PI-PLC ε also increased PLCB1 and decreased PLCD4. The reduction of ezrin increased PLCB1, PLCG2, PLCD4 and decreased PLCE expression (Lo Vasco et al. 2014c). The overall results suggest that the regulation of PI-PLC β1, RhoA and Rac1 transcription might affect each other in a complex, unclear manner. That suggests a possible link between RhoA and PI-PLC β1. PI-PLC β1 was recently demonstrated to be down regulated in some cancers and to be involved in cell differentiation (Li et al. 2011; Lo Vasco 2010; Lo Vasco et al. 2004, 2012, 2013b; Kaminskas et al. 2005; Martins et al. 2014). In our present findings, decrease of ezrin, which is thought to facilitate tumour progression, is associated to increase of PLCB1 transcription and of cytoplasmic PI-PLC β1 enzyme. However, the mechanism and the ordered timing of recruitment is not clear, as well as the relationship of ezrin, PI-PLC enzymes and Ras GTPases. Further studies are required in order to verify the specific role of each molecule upon the regulation of the others.
The overall present results suggestthat suppression of the metastasis promoting Ezrin induce up-regulation of PI-PLC β1.
RhoA and Rac1 are differently expressed after Ezrin or PI-PLC ε silencing. The modulation of RhoA we observed in 143B cells fits with the expression of PI-PLC ε, according to literature data. That suggested that RhoA and PI-PLC ε participate in the same pathway, probably playing a concurring role. Unfortunately, controversial literature reports indicated both favouring and opposing roles to cancer progression for PI-PLC ε (49), probably depending on the cell/tumour type. In Hs888, the reduction of ezrin, RhoA and Rac1, supposed to play a tumour facilitating role, occurs contemporarily to increased expression of PI-PLC β1 enzyme. That suggests that the regulation of Ezrin activity is more complex in Hs888 and might involve further PI-PLC isoforms with respect to the 143B cell line, probably due to the different origins of the two cell lines.
Further studies, addressed to identify the crosstalk and the ordered timing of action of Ezrin, cell line-specific PI-PLC enzymes and RasGTPases might help to elucidate the specific role of the molecules, opening the way to novel insights in the progression of the disease, with special regard to metastatic spread inputs, and, as far as one can see, to novel therapeutic strategies.
Acknowledgments
The authors thank the ‘Serena Talarico Association’ for supporting this research and precious encouragement.
References
- Auvinen E, Kivi N, Vaheri A. Regulation of ezrin localization by Rac1 and PIPK in human epithelial cells. Exp Cell Res. 2007;313:824–833. doi: 10.1016/j.yexcr.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Barret C, Roy C, Montcourrier P, Mangeat P, Niggli V. Mutagenesis of the phosphatidylinositol 4,5-bisphosphate (PIP(2)) binding site in the NH(2)-terminal domain of ezrin correlates with its altered cellular distribution. J Cell Biol. 2000;151(5):1067–1080. doi: 10.1083/jcb.151.5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitah SA, Valeron PF, Van AL, Marshall CJ, Lacal JC. Rho GTPases in human cancer: an unresolved link to upstream and downstream transcriptional regulation. Biochim Biophys Acta. 2004;1705:121–132. doi: 10.1016/j.bbcan.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Dupont G. Spatial and temporal signalling by calcium. Curr Opin Cell Biol. 1994;6(2):267–274. doi: 10.1016/0955-0674(94)90146-5. [DOI] [PubMed] [Google Scholar]
- Bunney TD, Katan M. PLC regulation: emerging pictures for molecular mechanisms. Trends Biochem Sci. 2011;36(2):88–96. doi: 10.1016/j.tibs.2010.08.003. [DOI] [PubMed] [Google Scholar]
- De Smedt H, Parys JB. Molecular and functional diversity of inositol triphosphate-induced Ca(2+) release. Verh K Acad Geneeskd Belg. 1995;57:423–458. [PubMed] [Google Scholar]
- Defacque H, Egeberg M, Habermann A, Diakonova M, Roy C, Mangeat P, Voelter W, Marriott G, Pfannstiel J, Faulstich H, Griffiths G. Involvement of ezrin/moesin in de novo actin assembly on phagosomal membranes. EMBO J. 2000;19(2):199–212. doi: 10.1093/emboj/19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defacque H, Bos E, Garvalov B, Barret C, Roy C, Mangeat P, Shin HW, Rybin V, Griffiths G. Phosphoinositides regulate membrane-dependent actin assembly by latex bead phagosomes. Mol Biol Cell. 2002;13(4):1190–1202. doi: 10.1091/mbc.01-06-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divecha N, Irvine RF. Phospholipid signaling. Cell. 1995;80(2):269–278. doi: 10.1016/0092-8674(95)90409-3. [DOI] [PubMed] [Google Scholar]
- Ferrari S, Zanella L, Alberghini M, et al. Prognostic significance of immunohistochemical expression of ezrin in non-metastatic high-grade osteosarcoma. Pediatr Blood Cancer. 2008;50:752–756. doi: 10.1002/pbc.21360. [DOI] [PubMed] [Google Scholar]
- Fievet BT, Gautreau A, Roy C, Del Maestro L, Mangeat P, Louvard D, Arpin M. Phosphoinositide binding and phosphorylation act sequentially in the activation mechanism of ezrin. J Cell Biol. 2004;164:653–659. doi: 10.1083/jcb.200307032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fievet B, Louvard D, Arpin M. ERM proteins in epithelial cell organization and functions. Biochim Biophys Acta. 2007;1773:653–660. doi: 10.1016/j.bbamcr.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Fukami K, Inanobe S, Kanemaru K, Nakamura Y. Phospholipase C is a key enzyme regulating intracellular calcium and modulating the phosphoinositide balance. Prog Lipid Res. 2010;49(4):429–437. doi: 10.1016/j.plipres.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Gatta G, Capocaccia R, Stiller C, Kaatsch P, Berrino F, Terenziani M. EUROCARE Working Group. Childhood cancer survival trends in Europe: a EUROCARE Working Group study. J Clin Oncol. 2005;3(16):3742–3751. doi: 10.1200/JCO.2005.00.554. [DOI] [PubMed] [Google Scholar]
- Gómez del Pulgar T, Benitah SA, Valerón PF, Espina C, Lacal JC. Rho GTPase expression in tumourigenesis: evidence for a significant link. Bioessays. 2005;27(6):602–613. doi: 10.1002/bies.20238. [DOI] [PubMed] [Google Scholar]
- Hao JJ, Liu Y, Kruhlak M, Debell KE, Rellahan BL, Shaw S. Phospholipase C-mediated hydrolysis of PIP2 releases ERM proteins from lymphocyte membrane. J Cell Biol. 2009;184(3):451–462. doi: 10.1083/jcb.200807047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig JH, Bokoch GM, Carpenter CL, Janmey PA, Taylor LA, Toker A, Stossel TP. Thrombin receptor ligation and activated Rac uncap actin filament barbed ends through phosphoinositide synthesis in permeabilized human platelets. Cell. 1995;82(4):643–653. doi: 10.1016/0092-8674(95)90036-5. [DOI] [PubMed] [Google Scholar]
- Hisatsune C, Nakamura K, Kuroda Y, et al. Amplification of Ca2+ signaling by diacylglycerolmediatedinositol 1,4,5-trisphosphate production. J Biol Chem. 2005;280(12):11723–11730. doi: 10.1074/jbc.M409535200. [DOI] [PubMed] [Google Scholar]
- Honda A, Nogami M, Yokozeki T, Yamazaki M, Nakamura H, Watanabe H, Kawamoto K, Nakayama K, Morris AJ, Frohman MA, Kanaho Y. Phosphatidylinositol 4-phosphate 5-kinase alpha is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell. 1999;99(5):521–532. doi: 10.1016/S0092-8674(00)81540-8. [DOI] [PubMed] [Google Scholar]
- Irvine RF. Nuclear lipid signalling. Nat Rev Mol Cell Biol. 2003;4(5):349–360. doi: 10.1038/nrm1100. [DOI] [PubMed] [Google Scholar]
- Joneson T, Bar-Sagi D. Ras effectors and their role in mitogenesis and oncogenesis. J Mol Med (Berl) 1997;75(8):587–593. doi: 10.1007/s001090050143. [DOI] [PubMed] [Google Scholar]
- Juncadella IJ, Kadl A, Sharma AK, Shim YM, Hochreiter-Hufford A, Borish L, Ravichandran KS. Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature. 2013;493(7433):547–551. doi: 10.1038/nature11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminskas E, Farrell A, Abraham S, Baird A, Hsieh LS, Lee SL, Leighton JK, Patel H, Rahman A, Sridhara R, Wang YC, Pazdur R, FDA Approval summary: azacitidine for treatment of myelodysplastic syndrome subtypes. Clin Cancer Res. 2005;11(10):3604–3608. doi: 10.1158/1078-0432.CCR-04-2135. [DOI] [PubMed] [Google Scholar]
- Khanna C, Wan X, Bose S, et al. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med. 2004;10:182–186. doi: 10.1038/nm982. [DOI] [PubMed] [Google Scholar]
- Li W, Chong H, Guan KL. Function of the Rho family GTPases in Ras-stimulated Raf activation. J Biol Chem. 2001;276(37):34728–34737. doi: 10.1074/jbc.M103496200. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen Y, Xu J. Factors influencing RhoA protein distribution in the nucleus. Mol Med Rep. 2011;4(6):1115–1119. doi: 10.3892/mmr.2011.556. [DOI] [PubMed] [Google Scholar]
- Lo Vasco VR. Signaling in the genomic era. J Cell Commun Signal. 2010;4(3):115–117. doi: 10.1007/s12079-010-0091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Vasco VR, Calabrese G, Manzoli L, Palka GD, Spadano A, Morizio E, Guanciali-Franchi P, Fantasia D, Cocco L. Inositide-specific Phospholipase C β1 gene deletion in the progression of Myelodisplastic Syndrome to Acute Myeloid Leukemia. Leukemia. 2004;18(6):1122–1126. doi: 10.1038/sj.leu.2403368. [DOI] [PubMed] [Google Scholar]
- Lo Vasco VR, Leopizzi M, Chiappetta C, Businaro R, Polonia P, Della Rocca C, Litta P. Expression of Phosphoinositide-specific Phospholipase C enzymes in normal endometrium and in endometriosis. Fertil Steril. 2012;98(2):410–414. doi: 10.1016/j.fertnstert.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Lo Vasco VR, Leopizzi M, Puggioni C, Della RC. Ezrin silencing remodulates the expression of Phosphoinositide-specific Phospholipase C signal transduction pathway in human osteosarcoma cell lines. J Cell Commun Signal. 2013;7(2):141–150. doi: 10.1007/s12079-013-0194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Vasco VR, Leopizzi M, Chiappetta C, Puggioni C, Della Rocca C, Businaro R. Lypopolysaccharide down-regulates the expression of selected phospholipase C genes in cultured endothelial cells. Inflammation. 2013;36(4):862–868. doi: 10.1007/s10753-013-9613-3. [DOI] [PubMed] [Google Scholar]
- Lo Vasco VR, Leopizzi M, Puggioni C, Della Rocca C, Businaro R. Fibroblast growth factor acts upon the transcription of phospholipase C genes in human umbilical vein endothelial cells. Mol Cell Biochem. 2014;388(1):51–59. doi: 10.1007/s11010-013-1898-x. [DOI] [PubMed] [Google Scholar]
- Lo Vasco VR, Leopizzi M, Stoppoloni D, Della RC. Silencing of Phosphoinositide-specific Phospholipase C ε enzyme remodulates the expression of Phosphoinositide signal transduction pathway in human osteosarcoma cell lines. Anticancer Res. 2014;34(8):4069–4075. [PubMed] [Google Scholar]
- Lo Vasco VR, Leopizzi M, Puggioni C, Della RC. Ezrin silencing remodulates the expression of Phosphoinositide-specific Phospholipase C signal transduction pathway in human osteosarcoma cell lines. J Cell Commun Signal. 2014;8(3):219–229. doi: 10.1007/s12079-014-0235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins M, McCarthy A, Baxendale R, Guichard S, Magno L, Kessaris N, El-Bahrawy M, Yu P, Katan M (2014) Tumor suppressor role of phospholipase C{varepsilon} in Ras-triggered cancers. Proc Natl Acad Sci USA. Mar 3 (epub) [DOI] [PMC free article] [PubMed]
- Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer. 2009;125:229–234. doi: 10.1002/ijc.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KA, Sethi R, Doanes AM, Johnson TM, Pracyk JB, Kirby M, Irani K, Goldschmidt-Clermont PJ, Finkel T. Rac1 is required for cell proliferation and G2/M progression. Biochem J. 1997;326(Pt 1):17–20. doi: 10.1042/bj3260017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SG, Kim H, Suh PG, Choi WC. Multiple forms of phosphoinositide-specific phospholipase C and different modes of activation. Biochem Soc Trans. 1991;19(2):337–341. doi: 10.1042/bst0190337. [DOI] [PubMed] [Google Scholar]
- Ridley AJ. Rho family proteins: coordinating cell responses. Trends Cell Biol. 2001;11:471–477. doi: 10.1016/S0962-8924(01)02153-5. [DOI] [PubMed] [Google Scholar]
- Snyder JT, Singer AU, Wing MR, Harden TK, Sondek J. The Pleckstrin Homology Domain of Phospholipase C-β2 as an Effector Site for Rac. J Biol Chem. 2003;278:21099–21104. doi: 10.1074/jbc.M301418200. [DOI] [PubMed] [Google Scholar]
- Steffen A, Rottner K, Ehinger J, Innocenti M, Scita G, Wehland J, Stradal TE. Sra-1 and Nap1 link Rac to actin assembly driving lamellipodia formation. EMBO J. 2004;23(4):749–759. doi: 10.1038/sj.emboj.7600084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh PG, Park J, Manzoli L, Cocco L, Peak JC, Katan M, Fukami K, Kataoka T, Yuk S, Ryu SH. Multiple roles of phosphoinositide-specific phospholipase C isozymes. BMB Rep. 2008;41:415–434. doi: 10.5483/BMBRep.2008.41.6.415. [DOI] [PubMed] [Google Scholar]
- Tolias KF, Hartwig JH, Ishihara H, Shibasaki Y, Cantley LC, Carpenter CL. Type Ialpha phosphatidylinositol-4-phosphate 5-kinase mediates Rac-dependent actin assembly. Curr Biol. 2000;10(3):153–156. doi: 10.1016/S0960-9822(00)00315-8. [DOI] [PubMed] [Google Scholar]