Abstract
Introduction
Fat cell differentiation (FCD) potentiates adipose cell characteristics including lipid storage and insulin sensitivity. In vitro, we have demonstrated that CCN2, also known as connective tissue growth factor (CTGF), inhibits FCD in NIH3T3-L1 cells and in adipocytes isolated from mouse epididymal fat pads. The aim of this study was to determine if the CCN2 effect on FCD is dependent on TGF-β and TGF-β downstream pathway signalling.
Methods
NIH3T3-L1 cells were differentiated using standard methods with IBMX/Dex/Insulin. FCD at day 10 was confirmed by induced gene markers resistin and adiponectin and by lipid accumulation. Cells were treated at d0 with single dose active rhTGF-β1 (2 ng/mL), rhCCN2 (500 ng/mL) and/or TGF-β type 1 receptor blocker (SB431542, 5 μM). Early induction of FCD transcription factors: CCAAT/enhancer binding proteins (C/EBPs) and peroxisome proliferator-activated receptor-γ (PPAR-γ), were also determined.
Results
In an early time course from 2 h, single doses of rhTGF-β1 or rhCCN2 significantly inhibited by ~70 % the induction of C/EBP-β and -δ mRNA, and also nuclear protein levels otherwise seen during FCD, whereas only delayed effects on PPAR-γ, at 48 h, occurred. Furthermore, the CCN2 inhibition of FCD markers adiponectin and resistin and lipid accumulation by Oil red O stain were each prevented by TGF-β receptor blockade. Similar prevention was found using pan-specific anti-TGF-β neutralising antibody. CCN2 and TGF-β treatment each rapidly phosphorylated SMAD-3 signalling in early stages of FCD.
Conclusion
This work shows novel findings that CCN2 effects on FCD are both TGF-β and TGF-β pathway dependent and are related to early effects on C/EBPs.
Keywords: CCN2, TGFBeta, Adipocyte, Signaling
Introduction
The latest data from the World Health Organisation reports that overweight and obesity affects more than 1.4 billion adults and rates of obesity globally have near doubled over the last 30 years (http://www.who.int/mediacentre). At its most fundamental level, excess weight gain leading to obesity is explained by excess caloric intake, with secondary increased adipose tissue mass due to both increased size of adipocytes as adipocyte hypertrophy, and formation of new adipocytes, termed adipogenesis (Gregoire et al. 1998; Rosen and Spiegelman 2000; Tchkonia et al. 2002; Fernyhough et al. 2005; Tontonoz and Spiegelman 2008).
Adipogenesis examined in vitro is characterised by a multistep process whereby mesenchymal stem cells are progressively determined, then committed to the adipocyte lineage, and ultimately differentiate into mature adipocytes (Gregoire et al. 1998). This process occurs via a range of temporal transcriptional cues, ultimately resulting in a mature adipocyte characterised by a unilocular lipid droplet (McLennan et al. 2004; Tan et al. 2008). These morphological and functional changes result from alterations in the expression and organisation of the extracellular matrix and components of cytoskeleton in adipose tissue.
Transforming growth factor β1 (TGF-β1) is a key negative regulator of adipogenesis, working through its cellular canonical signalling factors, the SMADs (Choy and Derynck 2003). Increased TGF-β1 mRNA levels are observed in animal and human obesity (Ignotz and Massague 1985; Wahab et al. 2005; Secker et al. 2008; Wrighton and Feng 2008). One of the downstream mediators of TGF-β1 is the protein connective tissue growth factor (CTGF), also known as CCN2, a heparin binding 36- and 38 kDa cysteine rich protein and a member of the highly conserved CCN early response gene family of peptides (Dixon et al. 2001; Li et al. 2008). CCN2 may act via autocrine and paracrine cellular circuits to regulate cell proliferation and growth and cell differentiation in tissues such as bone and cartilage (Arnott et al. 2008). Our group have previously demonstrated that CCN2 inhibits adipocyte differentiation; administration of exogenous CCN2 protein before commitment or during differentiation results in an inhibition of adipocyte differentiation in both murine 3T3L1 and primary cultures (Tan et al. 2008). In vitro studies have shown that CCN2 is induced by TGF-β1 in several cell types including human dermal and corneal fibroblasts and renal mesangial cells (Brigstock 2003). Notably, CCN2 may not only be induced by TGF-β (Choy et al. 2000; Perbal 2004; Wahab et al. 2005; Wrighton and Feng 2008) but it may feed-forward in its effect on cells and augment TGF-βpathway signalling through multiple mechanisms (Wahab et al. 2005) including enhancing effects of exogenously added rhTGF-β1 (Abreu et al. 2002).
The CCAAT/enhancing binding proteins (C/EBPs) are a family of transcription factors, composed of six members called C/EBP α to C/EBP ζ which are involved in dimerization and DNA binding (Dixon et al. 2001; Choy and Derynck 2003; Song et al. 2006; Li et al. 2008; Tontonoz and Spiegelman 2008; Tsai et al. 2009). CEBPs play important roles in the transcriptional regulation of adipocyte differentiation with C/EBP-β and C/EBP-δ expression transiently increased at the early phase of adipocyte differentiation, which in turn and directly activates peroxisome proliferator-activated receptor-γ (PPAR-γ) leading to activation of C/EBP-α (Wrighton and Feng 2008; Sul 2009). PPAR-γ is involved in the control of cellular proliferation, growth and differentiation and its activation is critical for the differentiation of pre-adipocytes into mature adipocytes (Gregoire et al. 1998; Rosen and Spiegelman 2000; Sul 2009)
We hypothesised that CCN2 signals through TGF-β dependent cellular pathways and inhibits the early C/EBP-β and C/EBP-δ up-regulation that would otherwise occur during early fat cell differentiation. The aim of this study was to investigate whether the inhibitory effect of CCN2 on adipocyte differentiation is dependent on TGF-βand its signalling and if adipocyte transcription factors, C/EBP-β, C/EBP-δ, and PPAR-Υ are impacted by CCN2.
Methods
Cell culture and adipocyte differentiation
NIH/3 T3-L1 cells (obtained from American Type Culture Collection, ATCC, Manassas, VA, USA) were maintained in DMEM containing 4.5 g/L D-glucose, 4 mM L-glutamine and supplemented with 10 % (v/v) fetal calf serum (FCS) at 37 °C in 5 % CO2/95 % air with cells passaged prior to reaching confluence. The cells used in this study were between passages 6 and 15. Each experiment was performed three times independently in triplicate.
Cells were differentiated using standard differentiation mix. At 80 % confluence they were treated with 0.5 mM 3-isobutyl-1-methylxanthine (IBMX), 2 μM dexamethasone and 20 μM insulin in DMEM supplemented with 10 % FCS (day0). At day3, the media was replaced (10 % FCS and 20 μM insulin) and was refreshed every second day for a further seven days. The degree of differentiation was assessed by mRNA levels of differentiation markers adiponectin, resistin and Pref-1 and lipid accumulation by Oil Red O staining (ORO staining).
Quantitative real-time RT-PCR
Cells used for experiments were washed with PBS and RNA extracted with Tri-Reagent (Sigma Aldrich, MO, USA). The amount of RNA was quantified using the SmartSpecTM Plus Spectrophotometer (Bio-Rad Laboratories Inc., CA USA). Then 1,000 ng of RNA was reverse transcribed to cDNA using 10pmol Oligo (dT)12–18 Primer (Invitrogen, CA, USA) and SuperScriptTM III Reverse Transcriptase (Invitrogen). The expression of CTGF and the three differentiation markers (adiponectin, resistin and Pref-1) was determined by quantitative real-time PCR using SYBR green fluorophore (Invitrogen). All amplicons were amplified using Platinum Quantitative PCR Supermix-UDG (Invitrogen) and 20 pmol each of forward and reverse primer. The primer pairs used and their annealing temperature conditions are shown in Table 1. Plasmid standard curves ranging from 103 to 109 copies were run with the samples for each gene measured and the copy number was determined from the standard curve generated. All samples used for analysis had cycle thresholds that were on the linear part of the standard curve.
Table 1.
Primer Sequences for real-time PCR. Forward (Fw) and reverse (Rv) primers are given for each gene
| Gene | Forward and Reverse Primer (5’-3’) | Annealing T (°C) |
|---|---|---|
| Adiponectin | Fw: ATGACGGCAGCACTGGCA | 64 |
| Rv: CGGCCTTGTCCTTCTTGA | ||
| Resistin | Fw: CGCTTCCTGATGTCGGTC | 64 |
| Rv: ATGCCCACTTCGCCATCC | ||
| Pref-1 | Fw: GTCCAACCTGCGCTACAA | 60 |
| Rv: AGAGCAAACTCCACCACAAA | ||
| HMBS | Fw: CCCAAAGTTTATTGGAAAGACCC | 60 |
| Rv: GGTGCAAAATCTGGCCCACCCGG |
Oil red O staining of lipid accumulation in cells
Oil Red O is a lysochrome diazo dye used for staining neutral triglycerides and lipids. The amount of staining is directly proportional to the degree of adipocyte differentiation. As the cells change morphology from a fibroblast-like preadipocyte into mature round lipid-laden adipocytes, they begin to accumulate lipid. To determine lipid accumulation, media was removed and cells washed twice with PBS. Cells were then fixed with 10 % formalin for 10 min. The Oil Red O working solution was prepared fresh from 0.5 % (w/v) Oil Red O stock solution, which was diluted to a working solution with water at a ratio of 6:4 (Oil Red O:water). Cells were then incubated with Oil Red O for 30 min at room temperature. The stain was then removed and cells were washed gently with PBS three times to remove excess non-specific staining. The dye was extracted using isopropanol and the amount of signal present was determined by measuring the absorbance at 520 nm.
Synthesis and purification of recombinant human CCN2
Recombinant human CCN2 (rhCTCN2) was purified in-house using a recombinant adenoviral expression system exactly as previously described (Tan et al. 2008).
Nuclear and non-nuclear fractionation of cells
The NE-PER Nuclear and Cytoplasmic Extraction Reagents kit (Pierce Biotechnology, Rockford, IL) was used according to the manufacturer; in brief, 500 μl of ice-cold PBS was added to wells and scraped cells were collected, then pelleted by centrifugation at 500 g for 3 min at 4 °C, and the supernatant was removed, collecting the packed cell volume. Reagents CERI and CERII were added sequentially to the cells according to the protocol provided, the non-nuclear (supernatant) fraction to be carefully removed and stored. After addition of the NER reagent followed by vortexing, the supernatant containing the nuclear fraction was then transferred into a separate, fresh, pre-chilled tube and stored at −80 °C until further use. The protein concentration in each fraction was determined using the Bio-Rad DC Protein Assay.
The nuclear and non-nuclear lysate samples were denatured then run at 40 μg/lane on their respective SDS-PAGE gels, and Western immunoblots for C/EBP-β and -δ were performed for the total cell lysate analyses. After stripping of nitrocellulose membranes with stripping buffer, blots were probed with polyclonal anti-Hsp90 (1:400; anti-mouse Hsp90 Santa Cruz Biotechnology). Hsp90 protein was only detectable in non-nuclear fractions, indicating the nuclear protein preparation did not have cytosolic protein in it. Hsp90 protein was also used in the cytosolic fractions as a loading control.
C/EBP Western immunoblotting
In whole cell lysate, cells were treated with CCN2 and TGF-β1 up to 24 h following the addition of differentiation media. Cells were then washed with PBS and extracted according to a standard protein extraction protocol (Tan et al. 2008). Separation of the proteins was resolved on SDS-PAGE using 12.5 % separation gels and 4 % stacking gels in running buffer (3 g Tris, 14.4 g glycine, 10 g SDS). For detection of C/EBP-δ by Western immunoblot analysis, the primary antibody used was rabbit polyclonal IgG anti-C/EBP-δ (2 μg/mL) (sc-61) (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). Membranes were washed and bands were then visualized using the horseradish peroxidase-conjugated goat anti-rabbit IgG (1:10,000,Vector Laboratories) and chemiluminescence (Amersham).
Immunofluorescence studies
Cells were grown on pre-purchased chamber slides (Lab-tek 177402, Thermo Scientific), followed by rhCCN2 and TGF-β1 treatment up to 24 h post addition of differentiation media. Cells were then washed once with PBS and stained with primary antibody, rabbit polyclonal IgG anti-C/EBP- δ (2 μg/mL) (sc-61) and anti-C/EBP-β (2 μg/mL) (sc-150) (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). Photographic images were then taken using by fluorescent microscopy.
Statistical analysis
Cell culture experiments were performed at least three times independently in triplicate. All results are expressed as mean ± standard deviation. All data were compared using either unpaired two-tailed t-test or one-way ANOVA followed by post hoc comparisons using Bonferroni’s correction. P < 0.05 was considered statistically significant.
Results
Inhibitory effect of rhCCN2 and rhTGF-β1 on C/EBPs
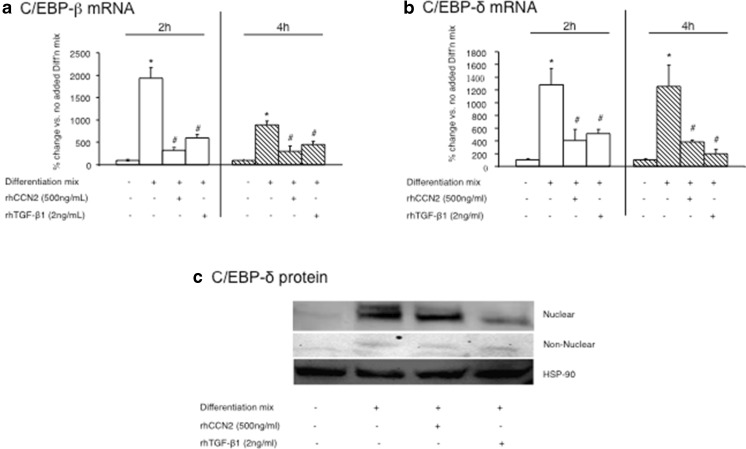
Our previous studies (McLennan et al. 2004; Tan et al. 2008) demonstrated that a single dose of rhCCN2 or active rhTGF-β1 in NIH/3T3L1 cells added to differentiation mix, resulted in a less well differentiated adipocyte phenotype than addition of differentiation mix alone. In order to examine these effects on C/EBP-β and C/EBP-δ mRNA and protein, cells were treated at day 0 of differentiation with rhCCN2 or rhTGF-β1. C/EBP-β and C/EBP-δ mRNA levels were found to be induced rapidly at 2 and 4 h after differentiation mix alone was added (Fig. 1a and b). In contrast, the addition of either rhCCN2 or rhTGF-β1 inhibited the induction of C/EBP-β and C/EBP-δ mRNA significantly compared with differentiation mix added alone (Figs. 1a to b). Higher doses of CCN2 had no greater effect than those observed for 500 ng/ml of recombinant human protein (not shown). For subsequent protein analysis, the nuclear and non-nuclear cell fractions were analyzed then quantitated using phoretix. The addition of differentiation mix alone induced C/EBP-δ protein levels at the early stages of adipocyte differentiation (Fig. 1c). As was found for the mRNA data, the addition of either rhCCN2 or rhTGF-β1 inhibited the induction of C/EBP-δ protein measured at 24 h, compared with differentiation mix added alone (Fig. 1c). This inhibition of C/EBP-δ protein induction by rhCCN2 or rhTGF-β1 was seen mainly in the nuclear fraction (Fig. 1c), and the pattern reflects that which we have previously observed and published for C/EBP-β protein regulation by rhCCN2 or rhTGF-β1 detected by Western immunoblot (Tan et al. 2008).
Fig. 1.
Regulation of CEBP-βand CEBP-δmRNA and protein by rhCCN2 or rhTGF-β1 each in the presence of differentiation mix. The mRNA levels of C/EBP-β (a) and C/EBP-δ (b) in 3T3L1 cells at 2 h and 4 h post differentiation are shown. NIH/3T3L1 cells were untreated or treated with differentiation mix alone, or differentiation mix with either rhCCN2 (500 ng/ml) or active rhTGF-β1 (2 ng/ml) respectively at time 0 h. Western immunoblot of nuclear and non-nuclear fractions for C/EBP-δ (c) protein levels are shown, with cell protein isolated 24 h post addition of differentiation mix. In some wells, rhCCN2 (500 ng/ml) or rhTGF-β1 (2 ng/ml) were added. Representative images from three independent experiments with similar data are shown. Heat shock protein 90 (HSP-90) was used as a loading control for the non-nuclear fraction and the same total protein was loaded in each lane for analysis of nuclear fractions. Data are expressed as mean ± SD *p < 0.05 vs no differentiation mix addition at the same time point; #p < 0.05 each vs differentiation mix added alone at the same time point (by ANOVA)
Nuclear localisation imaging of C/EBP-β and C/EBP-δ by immunocytochemistry
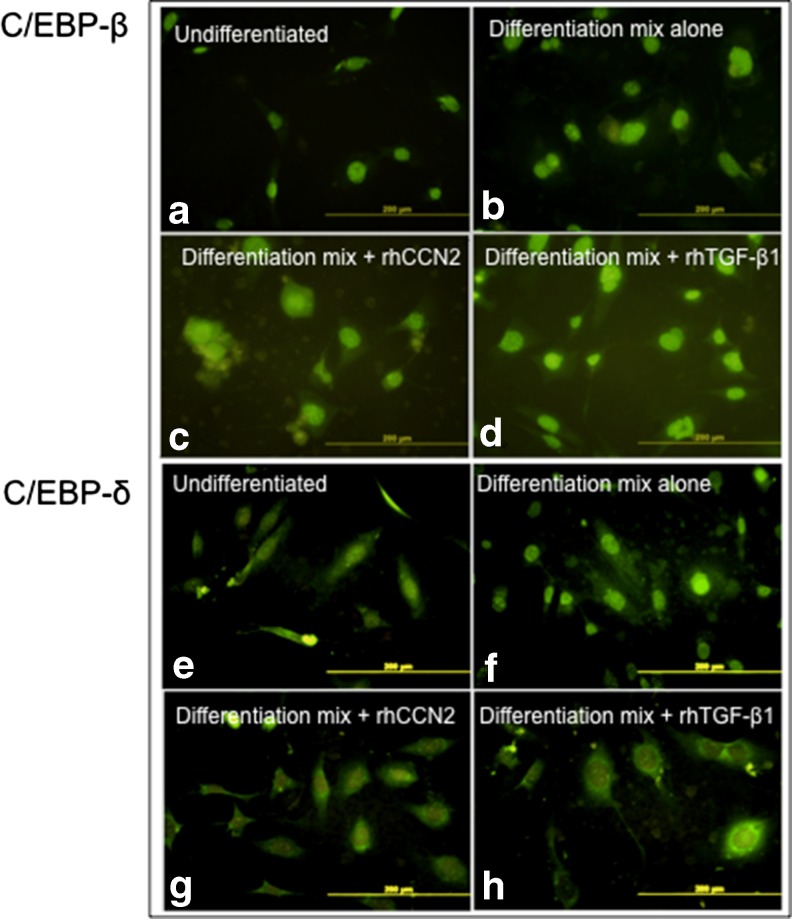
In order to complement the Western immunoblot analysis, immuno-flourescent imaging of C/EBP-β and C/EBP-δ protein were undertaken. NIH/3T3L1 cells treated with differentiation mix showed increased nuclear fluorescence at 24 h, consistent with increased nuclear localisation of C/EBP-β and C/EBP-δ (Fig. 2a and b and E and F, respectively). In parallel slides, cells treated with rhCCN2 (500 ng/ml) or rhTGF-β1 (2 ng/ml) at the time of differentiation mix addition, showed lesser nuclear localisation signal of both C/EBP-δ and C/EBP-β, especially when nuclear fluourescence is compared with that in the non-nuclear site (Fig. 2c and d and g and h, respectively). This data confirms the findings detected in the Western immunoblot studies, where each of rhCCN2 and rhTGF-β1 added during differentiation mix prevent nuclear localisation of both C/EBP-β and C/EBP-δ protein.
Fig. 2.
Nuclear compared with cytosolic localisation of C/EBP-β and C/EBP-δprotein by rhCCN2 or rhTGF-β1 each in the presence of differentiation mix. Representative immunoflourescence images of C/EBPs 24 h after addition of differentiation mix. Nuclear localisation of both C/EBP-β (a-d) and C/EBP-δ (e-h) are shown. NIH/3T3L1 cells were either non-differentiated (a, e) or they were treated with differentiation mix alone (b, f), or differentiation mix plus either added rhCCN2 (500 ng/ml) (c, g) or added active rhTGF-β1 (2 ng/ml) (d, h). Each size-bar indicates 200 μM
Secondary effects on PPAR-γ during adipocyte differentiation
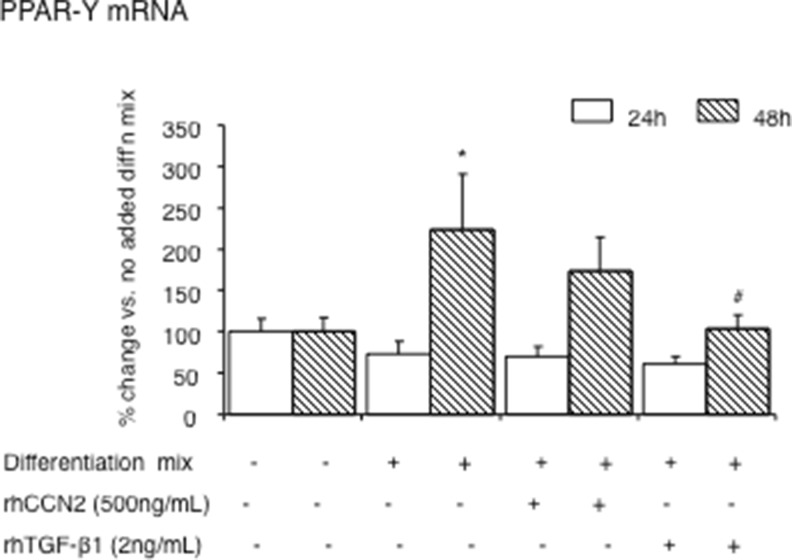
PPAR-γ is required for the differentiation of preadipocytes into mature adipocytes (Abreu et al. 2002; Brigstock 2003; Fu et al. 2003; Tan et al. 2008). Previous studies in other cell types have shown that both C/EBP-β and C/EBP-δcan activate the expression of PPAR-γ directly through trans-activating effects on the PPAR-γ promoter, which in turn then induces C/EBP-α (Dixon et al. 2001; Abreu et al. 2002; Brigstock 2003; Tan et al. 2008). In the current work, we found that induction of PPAR-γ mRNA levels is only seen 48 hours after addition of differentiation mix. Addition of rhCCN2 or active rhTGF-β1 each at time 0, showed inhibitory effects on PPAR-γ at 48 h. Thus, PPAR-γ is affected by each of CCN2 and TGF-β1 addition but it is not an immediate early target of CCN2 or TGF-β1, compared with regulation of C/EBP-β and C/EBP-δ.
Dependence of the rhCCN2 effect on endogenous TGF-β1 and TGF-β pathway signalling
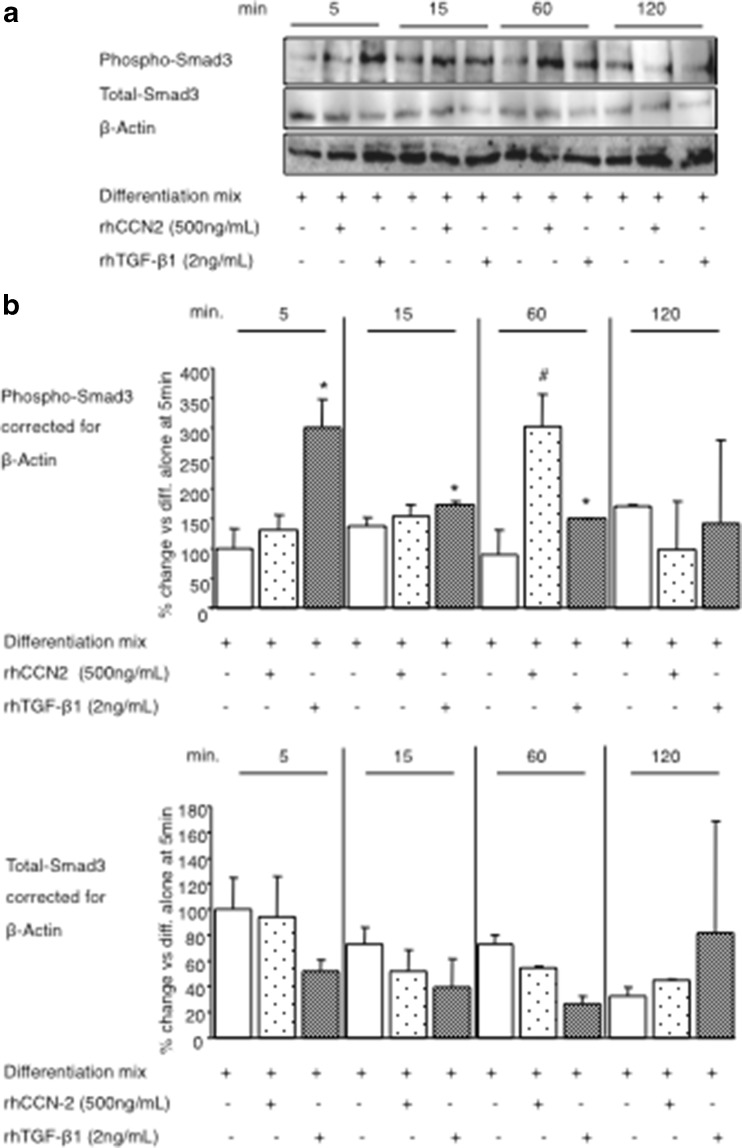
Inhibition of adipogenesis by rhTGF-β1 is largely mediated via Smad3, as Smad-3 physically associates with adipocyte transcription factors C/EBP-β and C/EBP-δ to suppress their trans-activating capacity (Abreu et al. 2002; Brigstock 2003; Fu et al. 2003; Tan et al. 2008). Since rhCCN2 and rhTGF-β1 were found to each partially inhibit the bioactivity of C/EBP-β and C/EBP-δ, we hypothesised that Smad3 bioactivity would be induced by both rhCCN2 and rhTGF-β1. Indeed, phosphorylated Smad3, as the activated form of Smad-3, was significantly increased after rhCCN2 or rhTGF-β1 treatment in differentiating cells (Fig. 4 a and b). The effect was most prominent in the first hour of the differentiation process. The addition of rhTGF-β1 reproducibly increased Phospho-Smad3 levels 5 min post treatment whereas rhCCN2 induction of Phospho-Smad-3 was only observed at 60 min. In contrast to Phospho-Smad-3 regulation, the total Smad-3 protein level did not change during the time course studied (Fig. 4a and c). This data suggests that, in the presence of differentiation mix, CCN2 regulates Smad-3 phosphorylation less directly than rhTGF-β1.Fig. 3.
Fig. 4.
Regulation of Smad-3 protein phosphorylation by rhCCN2 or rhTGF-β1 each in the presence of differentiation mix. Representative Western immunoblot images in (a) and quantitation in (b) and (c) of Smad-3 protein in NIH/3T3L1 cells after addition of differentiation mix, in some cases with either rhCCN2 (500 ng/ml) or active rhTGF-β1(2 ng/ml). Phosphorylated Smad-3 is quantiated in (b) and total Smad-3 in (C), generated from 3 independent experiments conducted in triplicate wells. Data are expressed as mean ± SD; *p < 0.05 TGF-β1 treatment vs differentiation mix alone at the respective time point; #p < 0.05 CCN2 treatment vs differentiation alone at the respective time point (by ANOVA)
Fig. 3.
PPAR-γmRNA regulation by rhCCN2 or rhTGF-β1 each in the presence of differentiation mix. PPAR-γ mRNA levels in differentiated NIH/3T3L1 cells at 24 and 48 h are shown. Cells were treated with differentiation mix alone at time 0, in some cases with added rhCCN2 (500 ng/ml) or active rhTGF-β1 (2 ng/ml). Data are expressed as mean ± SD; *p < 0.05 vs no differentiation mix added at the same time point; #p < 0.05 vs differentiation mix alone at the same time point (by ANOVA)
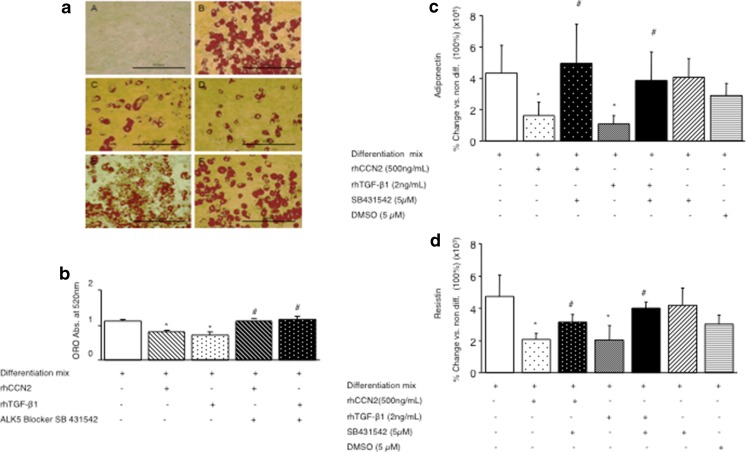
As CCN2 may augment TGF-β1 bioctivity and TGF-β pathway signaling in some cell types, in order to further investigate whether the effects of rhCCN2 to inhibit adipocyte differentiation were dependent on TGF-βand its pathway signalling, both an anti-TGF-β1 neutralising antibody and TGF-β type I receptor blocker were then examined. The induction of lipid in differentiated adipocytes measured at day 10 after addition of differentiation mix, was inhibited by addition of either rhCCN2 (500 ng/mL) or TGF-β1 (2 ng/mL) as shown in the representative lipid stain image in Fig. 5 a and as quantitated in Fig. 5B. In the presence of the TGF-β type I receptor blocker, SB431542, the inhibitory effects of rhCCN2 and rhTGF-β1 on Oil red O accumulation, were prevented (Fig. 5a and b). Other complementary end points to Oil red O accumulation to indicate adipocyte differentiation were then examined: adiponectin and resistin. As previously reported by us (Tan et al. 2008) by day 10 adiponectin and resistin steady state mRNA levels had been induced by differentiation mix addition at day 0, in the order of 106 and 103 respectively, compared with mRNA levels in undifferentiated cells (Fig. 5c and d). The inhibitory effects of rhCCN2 and TGF-β1 on these sensitive gene expression markers of adipocyte differentiation were prevented by the TGF-β receptor blocker SB431542, whereas SB431542 had no effect when added alone (Fig. 5c and d). This data demonstrates that the inhibitory effect of CCN2 on adipocyte differentiation is dependent on TGF-β signalling pathways, specifically, TGF- β type 1 receptor.
Fig. 5.
Regulation of fat cell differentiation markers by rhCCN2 or rhTGF-β1 each in the presence of differentiation mix and TGF-βreceptor blocker. (a) Representative images of Oil red O stained cells at day 0 in A, or 10 days post differentiation in B to F. Cells were treated with differentiation mix, in some cases with rhCCN2 (500 ng/ml), active rhTGF-β1 (2 ng/ml) and/or TGF-β receptor blocker SB431542 (5 μM) at day 0 as indicated, and were then cultured as described in the Methods; at day 10 cells were fixed with 10 % formalin and stained with Oil red O, then photographed. Each size-bar in (a) indicates 400 μM. In (b) Oil red O quantitative data investigating the effect of rhCCN2 (500 ng/ml), active rhTGF-β1 (2 ng/ml) and TGF-β receptor blocker SB431542 (5 μM) on adipocyte differentation are shown (b). Data are expressed as mean ± SD *p < 0.05 vs differentiation mix alone cells; #P < 0.05 each vs. the respective rhCCN2 or rhTGF-β1 treatment with differentiation mix (by ANOVA). Adiponectin (c) and Resistin (d) mRNA levels were determined at day 10. Data shown in (b) to (d) are generated from 3 independent experiments conducted in triplicate wells and are expressed as mean ± SD. DMSO was used as a vehicle control; *p < 0.05 each vs differentiation mix alone; #p < 0.05 vs added rhCCN2 or rhTGF-β1 each with differentiation mix (by ANOVA)
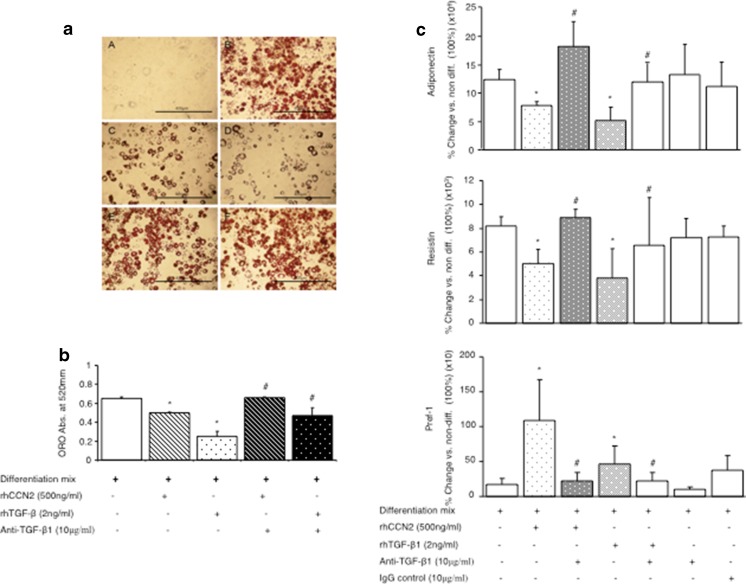
Since CCN2 may augment TGF-β1 bioactivity by facilitating TGF-β1 signaling through its cell surface receptor (Abreu et al. 2002), studies with a pan-specific anti-TGF-β antbody were then undertaken. The induction of lipid in differentiated adipocytes measured at day 10 after addition of differentiation mix, was inhibited by addition of either rhCCN2 (500 ng/mL) or TGF-β1 (2 ng/mL) as shown in the lipid stain image in Fig. 6a and quantitated in Fig. 6b. In the presence of the anti-TGF-β antibody, the inhibitory effects of rhCCN2 and rhTGF-β1 on Oil red O accumulation, were fully prevented (Fig. 6a and b). The inhibitory effects of rhCCN2 and TGF- β1 on adipocyte differentiation gene expression markers were also prevented by anti-TGF- β1 antibody, whereas neither anti-TGF- β1 antibody nor IgG control, had effect on the gene expression markers when added alone (Fig. 6c and d). The pre-adipocyte marker, Pref-1, was induced by rhCCN2 and TGF- β1, and inhibited by anti-TGF-βantibody (Fig. 6c), indicating that both inhibitory and stimulating effects of by rhCCN2 and TGF- β1 in the NIH-3 T3-L1 cells are neutralised by anti-TGF-β antibody. This data demonstrates that inhibitory effects of CCN2 on adipocyte differentiation are dependent on TGF-β signalling pathways, specifically through endogenous TGF-β.
Fig. 6.
Regulation of fat cell differentiation markers by rhCCN2 or rhTGF-β1 each in the presence of differentiation mix and anti-TGF-β neutralising antibody. (a) Representative images of Oil red O stained cells at day 0 in A, or 10 days post differentiation in B to F. Cells were treated with differentiation mix, in some cases with rhCCN2 (500 ng/ml), active rhTGF-β1 (2 ng/ml) and/or anti- TGF-βantibody (10 μg/ml) at day 0 as indicated, and were then cultured as described in the Methods; at day 10 cells were fixed with 10 % formalin and stained with Oil red O, then photographed. Each size-bar in (a) indicates 400 μM. In (b) Oil red O quantitative data investigating the effect of rhCCN2 (500 ng/ml), active rhTGF-β1 (2 ng/ml) and and/or anti- TGF-βantibody on adipocyte differentation are shown (b). IgG (10 μg/ml), was used as a loading control. Data are expressed as mean ± SD *p < 0.05 each vs. non-differentiated; #P < 0.05 vs the respective rhCCN2 or rhTGF-β1 treatment with differentiation mix (by ANOVA). Adiponectin, Resistin and Pref-1 mRNA levels were determined at day 10 as in (c). Data shown in (c) are generated from 3 independent experiments conducted in triplicate wells and are expressed as mean ± SD; *p < 0.05 each vs differentiation mix alone; #p < 0.05 vs added rhCCN2 or rhTGF-β1 each with differentiation mix (by ANOVA)
Discussion
In recent years, overweight and obesity have become increasingly common worldwide and are linked to the insulin resistant or metabolic syndrome. The metabolic syndrome is a major risk factor for many diseases including hypertension, cardiovascular disease, dyslipidaemia, type 2 diabetes mellitus, cancers, stroke (Alberti et al. 2009). One of the characteristics of the metabolic syndrome is incomplete adipocyte differentiation during adipogenesis, especially in a visceral site (Tchkonia et al. 2002). Factors that inhibit maturation of adipocytes and thus adipogenesis, in the presence of ongoing caloric excess delivery to a host may lead to ectopic organ lipid deposition and pathology, for example in the liver, myocardium, and arterial tree. Understanding mechanism of factors regulating FCD is thus important in helping to prevent disease related to obesity.
This work demonstrates that exogenously added CCN2 requires TGF-β to inhibit FCD. The data firstly shows that CCN2 requires endogenous TGF-β protein to exert its effect. Secondly, a functional TGF-β type I receptor is required. Thirdly, rhCCN2 phosporylates Smad-3. Collectively, the data suggests that endogenous TGF-β bioactivity is potentiated by TGF-β. Others have previously published, albeit in different cell types and with other end-points, that CCN2 can facilitate TGF-β binding to and activating its TGF-β type II and type I receptor complex (Abreu et al. 2002); that CCN2 may activate latent TGF-β to its active form by inducing thrombospondin1synthesis, and that CCN2 may inhibit the gene expression and protein levels of the inhibitory SMAD-7 (Wahab et al. 2005), the latter which would potentiate TGF-β pathway signalling. Amongst these potential mechanisms, the course of Smad-3 phosphorylation by rhCCN2 peaking at 60 minutes, suggests that current rather than new protein synthesis mediates the CCN2 effect to inhibit FCD. This finding combined with the evidence that the anti-TGF-β fully blocked the CCN2 effect, suggests that endogenous TGF-β is likely to be one major mechanism of the CCN2 effect to inhibit FCD in this work. Our prior studies in NIH-3 T3 L1 cells has shown that endogenous TGF-β is readily detectable in the differentiating cells (de Silva et al. 2012), providing an environment where CCN2 may act to potentiate endogenous TGF-β protein.
In previous literature, TGF-β was reported by others to mediate Smad3 signaling in differentiating fat cells and Smad3 then physically associates with adipocyte transcription factors C/EBP-β to repress trans-activating capacity in other cell types (Choy and Derynck 2003; Ignotz and Massague 1985). In the current series of experiments we found that active rhTGF-β1 not only induced Smad-3 phosphorylation and nuclear localisation of C/EBP-β, C/EBP-δ, but that it had a potent effect to largely prevent the otherwise rapid up-regulation of mRNA levels of C/EBP-β and C/EBP-δ seen by the addition of the differentiation mixture. Thus, when combined with prior reports, it appears that rhTGF-β1, and now similarly rhCCN2, may inhibit C/EBP-β and C/EBP-δ bioactivity by more than mechanism: mRNA inhibition, and preventing protein nuclear translocation. It is possible that activating Smads, especially phosphor-Smad-3 is required for bother mechanisms of effect; regulatory experiments targeting Smad-3 would be required to subsequently test this hypothesis.
Like rhTGF-β1, we have found that CCN2 inhibits adipocyte differentiation during the early stages of the differentiation process (Brigstock 2003; Tsai et al. 2009). Results in the current work from the early time course showed that a single doses of rhTGF-β1 or rhCCN2 significantly inhibited C/EBP-β and C/EBP-δ up-regulation by 50 % or more. Recently, others have found that Smad3 can less directly down-regulate C/EBP species via MAPK secong messenger activation in neuronal cells (Bhat et al. 2002). Previous studies has also demonstrated that rhTGF-β1 is also able to prevent PPAR-γ up-regulation (Zhang et al. 1998). Our data suggests that C/EBP-β and C/EBP-δ may be main targets of the rhTGF-β1 and CCN2 early effect. Our previous work has implicated the protein IGFBP-3 through negative regulatory effects on PPAR-γ bioactivity (Chan et al. 2009; Baxter and Twigg 2009), and by endogenous IGFBP-3 sensitising cells to TGF-β1 to inhibit FCD (de Silva et al. 2012), implicating multiple growth factor proteins in regulation of FCD related to TGF-β1.
Also recently, others have reported that effects of estradiol to inhibit FCD occurs through TGF-β and then downstream of this, CTGF/CCN2 (Kumar et al. 2012). This work further confirms and extends our seminal finding that CCN2 inhibits FCD (Tan et al. 2008) and it implicates a linear pathway from sex hormones to bioactive matricellular growth factors acting locally in adipose tissue. The current studies did not examine to what degree endogenous CCN2 may act downstream or otherwise of rhTGF-β1 to inhibit FCD, and based on known mechanisms of action of CCN2 it is plausible that it is both a down-stream factor of TGF-β1 action, as well as a feed-forward factor than augments TGF-β1 action and TGF-β pathway signalling.
Coordinated regulation of members of the CCN family of proteins is increasingly being recognized. In example, rhTGF-β1, acting through the TGF-β type 1 receptor, has recently been shown to induce CCN1 and CCN2, and in a reciprocal fashion to inhibit CCN3 gene expression in skin fibroblasts (Thompson et al. 2014). In some cases differing CCN family members have been shown to have balancing, and antagonistic cell and tissue effects; for example, CCN3 may suppress CCN1 and CCN2-dependent activities (Riser et al. 2009; Perbal 2013). We have previously shown that rhTGF-β1 induces CCN2 in adipocyte differentiation (Tan et al. 2008). Future studies will be required to examine whether the CCN family of proteins are differentially regulated in fat cell differentiation, including by TGF-β and its downstream pathways, and whether effects of differing CCN proteins are complementary or antagonistic with eachother in FCD.
The current work better defines cellular mechanisms of action of CCN2 to inhibit fat cell differentiation. It reflects the complexity of the interaction between TGF-β and CCN2 in these cellular processes. The in vitro data suggests that like TGF-β, CCN2 may inhibit fat cell differentiation, and thus contribute to the metabolic syndrome. It is envisaged that subsequent studies in appropriate models regulating endogenous CCN2 and also TGF-β in vivo in adipose tissue, in an environment of caloric excess, will determine related effects on FCD in obesity models and also whether CCN2 requires endogenous TGF-β in vivo to exert an inhibitory effect on FCD.
Acknowledgments
This work was supported by a National Health and Medical Research Council (NH&MRC) of Australia Project Grant #457373, to SMT, RCB and SVM.
References
- Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4(8):599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC, Jr, International Diabetes Federation Task Force on Epidemiology and Prevention. Hational Heart, Lung, and Blood Institute. American Heart Association. World Heart Federation. International Atherosclerosis Society. International Association for the Study of Obesity Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- Arnott JA, Zhang X, Sanjay A, Owen TA, Smock SL, Rehman S, DeLong WG, Safadi FF, Popoff SN. Molecular requirements for induction of CTGF expression by TGF-beta1 in primary osteoblasts. Bone. 2008;42(5):871–885. doi: 10.1016/j.bone.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter RC, Twigg SM. Actions of IGF binding proteins and related proteins in adipose tissue. Trends Endocrinol Metab. 2009;20(10):499–505. doi: 10.1016/j.tem.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Bhat N, Feinstein DL, Shen Q, Bhat AN (2002) p38 MAPK-mediated Transcriptional Activation of Inducible Nitric-oxide Synthase in Glial Cells, J Biol Chem.277, 33:29584–29592 [DOI] [PubMed]
- Brigstock DR. The CCN family: a new stimulus package. J Endocrinol. 2003;178(2):169–175. doi: 10.1677/joe.0.1780169. [DOI] [PubMed] [Google Scholar]
- Chan SS, Schedlich LJ, Twigg SM, Baxter RC. Inhibition of adipocyte differentiation by insulin-like growth factor-binding protein-3. Am J Physiol Endocrinol Metab. 2009;296(4):E654–E663. doi: 10.1152/ajpendo.90846.2008. [DOI] [PubMed] [Google Scholar]
- Choy L, Derynck R. Transforming growth factor-beta inhibits adipocyte differentiation by Smad3 interacting with CCAAT/enhancer-binding protein (C/EBP) and repressing C/EBP transactivation function. J Biol Chem. 2003;278(11):9609–9619. doi: 10.1074/jbc.M212259200. [DOI] [PubMed] [Google Scholar]
- Choy L, Skillington J, Derynck R. Roles of autocrine TGF-beta receptor and Smad signaling in adipocyte differentiation. J Cell Biol. 2000;149(3):667–682. doi: 10.1083/jcb.149.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva HC, Firth SM, Twigg SM, Baxter RC. Interaction between IGF binding protein-3 and TGFβ in the regulation of adipocyte differentiation. Endocrinology. 2012;153(10):4799–4807. doi: 10.1210/en.2011-1444. [DOI] [PubMed] [Google Scholar]
- Dixon TM, Daniel KW, Farmer SR, Collins S. CCAAT/enhancer-binding protein alpha is required for transcription of the beta 3-adrenergic receptor gene during adipogenesis. J Biol Chem. 2001;276(1):722–728. doi: 10.1074/jbc.M008440200. [DOI] [PubMed] [Google Scholar]
- Fernyhough ME, Bucci LR, Hausman GJ, Antonio J, Vierck JL, Dodson MV. Gaining a solid grip on adipogenesis. Tissue Cell. 2005;37(4):335–338. doi: 10.1016/j.tice.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Fu M, Zhang J, Lin Y, Zhu X, Zhao L, Ahmad M, Ehrengruber MU, Chen YE. Early stimulation and late inhibition of peroxisome proliferator-activated receptor gamma (PPAR gamma) gene expression by transforming growth factor beta in human aortic smooth muscle cells: role of early growth-response factor-1 (Egr-1), activator protein 1 (AP1) and Smads. Biochem J. 2003;370(Pt 3):1019–1025. doi: 10.1042/BJ20021503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78(3):783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- Ignotz RA, Massague J. Type beta transforming growth factor controls the adipogenic differentiation of 3 T3 fibroblasts. Proc Natl Acad Sci U S A. 1985;82(24):8530–8534. doi: 10.1073/pnas.82.24.8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Ruan M, Clifton K, Syed F, Khosla S, Oursler MJ. TGF-β mediates suppression of adipogenesis by estradiol through connective tissue growth factor induction. Endocrinology. 2012;153(1):254–263. doi: 10.1210/en.2011-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HX, Luo X, Liu RX, Yang YJ, Yang GS. Roles of Wnt/beta-catenin signaling in adipogenic differentiation potential of adipose-derived mesenchymal stem cells. Mol Cell Endocrinol. 2008;291(1–2):116–124. doi: 10.1016/j.mce.2008.05.005. [DOI] [PubMed] [Google Scholar]
- McLennan SV, Wang XY, Moreno V, Yue DK, Twigg SM. Connective tissue growth factor mediates high glucose effects on matrix degradation through tissue inhibitor of matrix metalloproteinase type 1: implications for diabetic nephropathy. Endocrinology. 2004;145(12):5646–5655. doi: 10.1210/en.2004-0436. [DOI] [PubMed] [Google Scholar]
- Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004;363(9402):62–64. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- Perbal B. CCN proteins: a centralized communication network. J Cell Commun Signal. 2013;7(3):169–177. doi: 10.1007/s12079-013-0193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riser BL, Najmabadi F, Perbal B, Peterson DR, Rambow JA, Riser ML, Sukowski E, Yeger H, Riser SC. CCN3 (NOV) is a negative regulator of CCN2 (CTGF) and a novel endogenous inhibitor of the fibrotic pathway in an in vitro model of renal disease. Am J Pathol. 2009;174:1725–1734. doi: 10.2353/ajpath.2009.080241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol. 2000;16:145–171. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- Secker GA, Shortt AJ, Sampson E, Schwarz QP, Schultz GS, Daniels JT. TGFbeta stimulated re-epithelialisation is regulated by CTGF and Ras/MEK/ERK signalling. Exp Cell Res. 2008;314(1):131–142. doi: 10.1016/j.yexcr.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Song K, Wang H, Krebs TL, Danielpour D. Novel roles of Akt and mTOR in suppressing TGF-beta/ALK5-mediated Smad3 activation. EMBO J. 2006;25(1):58–69. doi: 10.1038/sj.emboj.7600917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sul HS. Minireview: Pref-1: role in adipogenesis and mesenchymal cell fate. Mol Endocrinol. 2009;23(11):717–725. doi: 10.1210/me.2009-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JT, McLennan SV, Song WW, Lo LW, Bonner JG, Williams PF, Twigg SM. Connective tissue growth factor inhibits adipocyte differentiation. Am J Physiol Cell Physiol. 2008;295(3):C740–C751. doi: 10.1152/ajpcell.00333.2007. [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Giorgadze N, Pirtskhalava T, Tchoukalova Y, Karagiannides I, Forse RA, DePonte M, Stevenson M, Guo W, Han J, Waloga G, Lash TL, Jensen MD, Kirkland JL. Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am J Physiol Regul Integr Comp Physiol. 2002;282(5):R1286–R1296. doi: 10.1152/ajpregu.00653.2001. [DOI] [PubMed] [Google Scholar]
- Thompson K, Murphy-Marshman H, Leask A. ALK5 inhibition blocks TGFβ-induced CCN1 expression in human foreskin fibroblasts. J Cell Commun Signal. 2014;8(1):59–63. doi: 10.1007/s12079-014-0229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- Tsai YS, Tsai PJ, Jiang MJ, Chou TY, Pendse A, Kim HS, Maeda N. Decreased PPAR gamma expression compromises perigonadal-specific fat deposition and insulin sensitivity. Mol Endocrinol. 2009;23(11):1787–1798. doi: 10.1210/me.2009-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahab NA, Weston BS, Mason RM. Modulation of the TGFbeta/Smad signaling pathway in mesangial cells by CTGF/CCN2. Exp Cell Res. 2005;307(2):305–314. doi: 10.1016/j.yexcr.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Wrighton KH, Feng XH. To (TGF) beta or not to (TGF) beta: fine-tuning of Smad signaling via post-translational modifications. Cell Signal. 2008;20(9):1579–1591. doi: 10.1016/j.cellsig.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Feng XH, Derynck R. Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-beta-induced transcription. Nature. 1998;394(6696):909–913. doi: 10.1038/29814. [DOI] [PubMed] [Google Scholar]