Abstract
Objective
: The aim of this study was to identify gene variants of DAT1 (SLC6A3) that modulate subjective responses to acute cocaine exposure.
Methods
Non-treatment seeking volunteers with cocaine use disorders (CUDs) received a single bolus infusion of saline and cocaine (40 mg, IV) in randomized order. Subjective effects were assessed with visual analog scales administered before (-15 min) and up to 20 min after infusion. Subjective effects ratings were normalized to baseline and saline infusion values were subtracted. Data was analyzed using repeated measures ANOVA. DNA from subjects was genotyped for the DAT1 intron 8 (rs3836790) and 3’ UTR (rs28363170) variable number of tandem repeats.
Results
Participants were mostly male (~80%) and African American (~70%). No differences were found among drug use variables between groups for either polymorphism. Carriers of the 9-allele of the DAT1 3’ UTR (9,9 and 9,10) (n = 24) exhibited greater responses to cocaine for “high”, “any drug effect”, “anxious”, and “stimulated” (all p-values < 0.001) compared to individuals homozygous for the 10-allele (n = 33). For the intron 8 polymorphism, individuals homozygous for the 6 allele exhibited greater responses for “anxious” than carriers of the 5 allele (p < 0.001). Individuals possessing the genotype pattern of 10,10 and at least one 5-allele reported lower responses to “good effects”, “bad effects”, “depressed”, and “anxious” (all p-values < 0.01).
Conclusions
The data presented here support the hypothesis that genetic differences of DAT1 contribute to variation of subjective responses to cocaine among participants with CUDs.
Keywords: cocaine use disorder, dopamine, genetics
INTRODUCTION
According to the 2012 National Survey on Drug Use and Health, approximately 1.6 million Americans over the age of 12 have used cocaine in the past month [2]. Approximately 639,000 Americans (nearly 1,800 per day) older than 12 tried cocaine for the first time in 2012. Nearly 16% of Americans between the ages of 12 and 17 (approximately 1 out of every 6) reported that cocaine would be easily obtainable for them. Of all illicit drugs, cocaine only trailed marijuana and prescription drugs as having the highest level of past-year dependence, with 1.1 million individuals with cocaine use disorders (CUDs).
CUD is also problematic because individuals that use and abuse cocaine are also frequently users of other addictive drugs, such as marijuana, alcohol, and nicotine [3-5]. For example, estimates vary, but nearly 70-80% of individuals that use cocaine also smoke cigarettes [6-11]. The sequelae of comorbid cocaine and other substance use are beyond the scope of this paper, but are mentioned to highlight the complexities of CUDs.
The dopamine transporter (DAT) is primarily located in presynaptic nerve terminals and is the principal element responsible for the reuptake of dopamine (DA) from the synaptic cleft. Thus, it is essential for dopaminergic signaling and mediating DA levels within brain synapses. DAT is also a target of stimulant drugs, including cocaine. Cocaine binds to the DAT and inhibits the reuptake of DA, leading to an excess of DA within the synaptic cleft [12]. This excess DA present within the cleft can then lead to enhanced stimulation of downstream DA receptors.
It has been known for over 25 years that the DAT is important for the subjective and physiological effects produced by cocaine. It was reported that dopamine transport inhibition was likely responsible for the reinforcing effects of cocaine [13]. The DAT is encoded by the DAT1 gene. The DAT is essential for DA neurotransmission as stimulants such as cocaine and methamphetamine have no effect when in DAT1 (also referred to as SLC6A3) knock-out mice [14]. Two of the most common variable number of tandem repeats (VNTR) polymorphisms in DAT1 are found within the 3’-untranslated region (UTR) (rs28363170) and intron 8 (rs3836790). The 9- and 10-alleles are the most common alleles of the 40 bp 3’ UTR VNTR polymorphism and the 5- and 6-alleles are the most common alleles of the intron 8 polymorphism [15]. In cellular assays using transfected reporter constructs, the 6-repeat allele had lower basal activity, and had lower expression in the presence of cocaine, yet higher expression in the presence of KCl or KCl/forskolin than did the 5-repeat allele [16]. In postmortem midbrain, the 6-repeat allele had higher transcript levels [17]. When the DAT1 3’ UTR VNTR was examined in cellular transfection assays, the 10-repeat allele of the 3’ UTR VNTR had higher expression than the 9-repeat allele of SLC6A3 did [18-19], and a study using CHO cells reported that the 3’ UTR VNTR did not appear to have a statistically significant effect on DAT1 expression [20]. In contrast, in human studies, carriers of the 9-repeat allele had higher striatal DAT availability than did 10-repeat homozygous individuals [21]. Regarding the 3’ UTR VNTR, a new report published very recently seems to suggest however that it is indeed the 9-repeat allele that leads to increased DAT expression and binding [22].
Genetic differences within DAT1 have also been investigated for their relation to subjective effects of other stimulants as well. Two studies examined the effect DAT1 3’ UTR VNTR genotypes had on subjective effects after exposure to d-amphetamine (which is also known to evoke extracellular DA release) [23]. In the first study, individuals who consumed d-amphetamine (0, 10, or 20 mg, p.o.) were monitored for subjective and physiological responses for up to 3 hours. Individuals homozygous for the 9-repeat allele were observed to have reduced subjective responses to d-amphetamine, particularly for adjectives such as “feel drug” and “high” compared to volunteers who had either the 9,10 or 10,10 repeat [24]. The second study used a similar design, but investigated four additional polymorphisms in DAT1. In this study, individuals possessing the C-C genotype at rs460000 demonstrated a dose-dependent increase in responsiveness to d-amphetamine compared to the other genotype groups [25], although the authors later reported a failure to replicate those findings [26]. A report investigating the effect of DAT1 genotype on response to smoking cues found that individuals with a 9-allele had a greater brain and behavioral response to cigarette smoking cues than individuals homozygous for the 10-allele [27]. Last, a study investigated the effect of DAT1 genotype on response to methylphenidate in children with ADHD as measured by tests of ADHD symptoms [28]. The report found that individuals homozygous for the 9-allele did not appear to improve ADHD symptoms despite increasing doses of methylphenidate, further demonstrating that DAT1 genotypes may influence the subjective response to stimulants. To date, however, no studies have been published investigating the effect of DAT1 polymorphisms on subjective effects following acute exposure to cocaine.
It would be useful to identify a therapeutic agent that could eliminate or reduce CUD. However, individuals with CUDs are not members of a homogeneous population. It has been reported that 27-36% of the variance of cocaine use could be accounted for by genetic factors [29-30]. The consensus of the literature appears to be that individuals who carry the 3’ UTR 9-repeat allele have higher DAT binding than their counterparts. This implies greater clearance of extracellular DA, lower extracellular DA concentrations, and subsequently reduced downstream DA signaling. In lieu of this, we hypothesized that participants carrying 9-repeat alleles would have lower positive and lower negative subjective responses to cocaine than the 10,10 homozygous participants. We also hypothesized that participants carrying a 5-repeat allele of the intron 8 polymorphism would have lower positive and lower negative subjective responses to cocaine than the 6,6 homozygous participants. To test these hypotheses, we enrolled several participants into one of several ongoing studies where they received an acute infusion of cocaine and were then asked to complete a subjective effects questionnaire and have their blood pressure and heart rate monitored. These individuals were then genotyped and examined for differences in their subjective responses based on their DAT1 genotypes.
METHODS
Participants
Data for this study was collected between March 2010 and January 2013 as part of the Stimulant Addiction Research Program at Baylor College of Medicine (BCM) and the Michael E. DeBakey Veterans’ Affairs Medical Center (MEDVAMC), located in Houston, TX. Participants were recruited from the local area through print and radio advertisements, as well as word of mouth from past participants. All potential participants were screened by telephone to ensure basic eligibility, and those eligible volunteers were brought into the Research Commons of the MEDVAMC to complete an in-person screen. Basic eligibility criteria included age between 18-55 years, being currently non-treatment-seeking, no other substance use disorders except cocaine and nicotine, and for women, a negative pregnancy test. All participants met Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) criteria for CUDs, and had a history of using cocaine either via smoking or intravenously. During the in-person screen, participants were allowed to read, ask questions about, and then sign the informed consent document. The study was sponsored by the National Institute on Drug Abuse, and approved by the BCM IRB and the Research and Development Committee of the MEDVAMC. Participants were compensated with a $40 (USD) gift card for the in-person screen and $50 cash for each inpatient day of the protocol. Participants received a double blind infusion of cocaine (40 mg, I.V.) or saline, in randomized order during the same day at 10 AM and 2 PM. The infusion was continuously delivered over a period of 2 minutes, and subjective effects of cocaine were recorded along with heart rate, systolic, and diastolic blood pressure.
Subjective Effects
To assess the subjective effects produced by the infusions of cocaine, participants answered a visual analog scale (VAS), which consisted of 10 questions rated from 0 (no effect at all) to 100 (greatest effect possible) in increments of 10, plus an additional item rating the monetary value (USD) of the infusion. Participants completed a baseline VAS 15 minutes prior to each infusion, and every 5 minutes post infusion up to 20 minutes. Participants were asked if they felt: “any drug effect”, “high”, “good effects”, “bad effects”, “like infusion”, “desire cocaine”, “depressed”, “anxious”, “stimulated”, and “ likeliness to use cocaine if it was accessible”. Cardiovascular data including heart rate, systolic and diastolic blood pressure were also recorded at the same time points.
Genotyping and statistical analyses
DNA was purified from blood samples as previously described [31]. Genotypes were determined by an investigator unaware of the physiological or subjective data collected from the participant. The DAT1 40 bp VNTR polymorphism was genotyped in a multiplex assay along with the DAT1 Int8 and the serotonin transporter (SLC6A4) STin2 repeats using PCR to determine the number of repeats of each allele. PCR amplification of genomic DNA was performed using the DAT1 40 bp repeat primers 5′-AGCTCAGGCTACTGCCACTC-3′ and 5′-GCCAGGCAGAGTGTGGTCTG-3′, the DAT1 Int8 primers 5′-ATGTGTTCTTGCATGTATGAGTTTG-3′ and 5′-GCAGAAACAAGGAGGAGCAGGA-3′, and the SLC6A4 STin2 primers [32] 5′-GGGCAATGTCTGGCGCTTCCCCTACATA-3′ and 5′-TTCTGGCCTCTCAAGAGGACCTACAGC-3′. Amplifications were performed with 100 ng genomic DNA, 1 μM of each primer, 250 μM each of dATP, dCTP, dGTP, and dTTP, 50 mM KCl, 0.5 units Platinum Taq DNA Polymerase High Fidelity (Invitrogen, Carlsbad, CA), and 10 mM Tris-HCl (pH 8.3) in 25 μL. Samples were amplified at 95°C for 5 minutes, followed by 25 cycles of 95°C for 30 seconds, 60°C for 30 seconds, and 72°C for 30 seconds, with a final extension step of 72°C for 7 minutes. Size of the DNA fragments was determined by electrophoresis in a 4-20% polyacrylamide TBE gel. PCR amplification of the DAT1 40-bp repeat can yield fragment sizes ranging from 208 bp (3 repeats) to 528 bp (11 repeats), with only the 3 (208 bp), 8 (408 bp), 9 (448 bp), and 10 (488 bp) repeat polymorphisms being observed in our cohort. The gender of each subject was confirmed by a PCR assay of the sex-determining region gene on the Y chromosome and population substructure was determined using Taqman® single nucleotide polymorphism genotyping of ten ancestry informative markers (AIMs), as previously described [33]. Briefly, AIM data from our cohort was compared against that of the CEPH-HGDP samples (1,035 subjects of 51 populations).
A repeated measures analysis of variance (ANOVA) was used to analyze the subjective effects scores over time for each subject. Subjective effects values after each session were normalized to baseline, and then values from the saline sessions (0 mg cocaine) were subtracted from the active sessions (40 mg cocaine) to produce the final subjective effects scores. Values were corrected by subtracting the baseline (-15 minute time point) from each time point (5, 10, 15, and 20 minutes) to yield subjective effect scores. Covariates included in the statistical model were gender, age, and population structure. Individuals possessing a 3-, 7-, 8-, or 11-repeat were omitted from our analyses. The allele frequency distribution of these alleles is shown in Table 1. We independently compared the DAT1 40 bp 3’ UTR VNTR genotype (0=10,10 genotype, 1 = 9,9/9,10 genotypes) and the intron 8 genotype (0=6,6 genotype, 1 = 5,5/5,6 genotypes) and interactions between genotypes and time. Data from the genotype groups over time were analyzed to determine if the subjective effect scores were moderated by the DAT1 variants using R version 2.9.1. [34]. The Bonferroni correction was applied to correct for multiple testing for 10 adjectives and to evaluate experiment-wise significance. We calculated effect size as a partial eta-squared statistic using condition or SNP variance over residual variance.
Table 1.
Genotypes of DAT1 3' UTR VNTR Sample
| Race | |||||
|---|---|---|---|---|---|
| African American | Caucasian | Other | Total | ||
| Genotype | 3,9 | 1 | 0 | 0 | 1 |
| 3,10 | 2 | 0 | 0 | 2 | |
| 7,10 | 2 | 0 | 1 | 3 | |
| 8,10 | 2 | 0 | 0 | 2 | |
| 9,9 | 3 | 1 | 0 | 4 | |
| 9,10 | 13 | 5 | 2 | 20 | |
| 10,10 | 24 | 8 | 1 | 33 | |
| 9,11 | 1 | 0 | 0 | 1 | |
RESULTS AND DISCUSSION
Linkage disequilibrium (LD) analyses
LD analysis revealed that in the racial subgroup with the most subjects, the African Americans (n = 48), the DAT1 intron 8 (rs3836790) and 3’ UTR (rs28363170) VNTRs are in low LD where D′ = 0.32 (r2 = 0.02). The frequency of distribution of participants among the genotype groups varies and is as follows: DAT1 intron 8 (rs3836790) 5,5 = 0.18, 5,6 = 0.55, and 6,6 = 0.27, and 3′ UTR (rs28363170) 9,9 = 0.06, 9,10 = 0.30, 10,10 = 0.50, and other = 0.14.
DAT1 3’ UTR VNTR
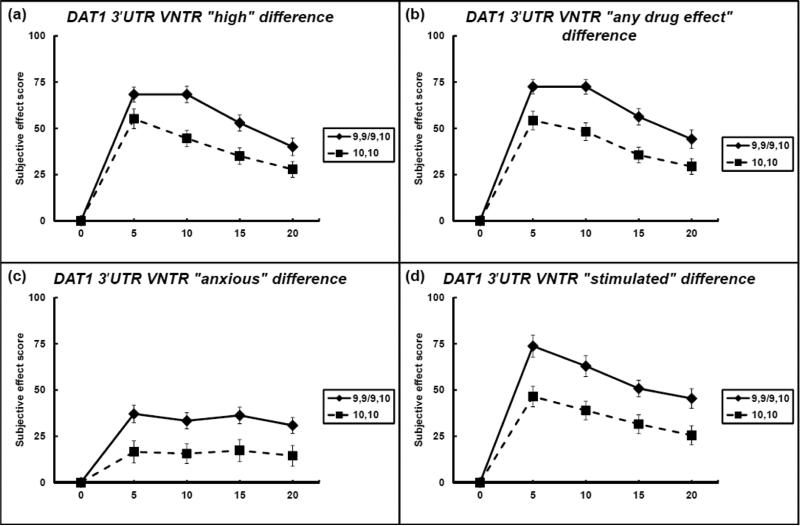
No significant differences were observed among demographic variables between the two groups. For the VAS adjective of “high”, individuals with 9,9 or 9,10 genotype, in comparison to individuals with 10,10 genotype, reported significantly greater responses for cocaine across the time course. There were significant main effects of genotype (F(1,219) = 15.20, p < 0.01, d = 0.0463) and time (F(1,219) = 19.78, p < 0.0001, d = 0.0911), but the interaction term was not significant (p > 0.05). The peak difference (largest difference between groups at any time point) between groups occurred at 10 minutes, where individuals with 9,9 or 9,10 genotype reported change from baseline scores of (mean ± s.e.m.) 68.33 ± 4.43, compared to 44.52 ± 4.41 for individuals with 10,10 genotype (Figure 1a).
Figure 1.
Difference in scores between 10,10 and 9,9/9,10 individuals from the 3′ UTR sample, all plots. Data points indicate mean, and error bars indicate standard error of mean
For the VAS adjective of “any drug effect”, individuals with 9,9 or 9,10 genotype, in comparison to individuals with 10,10 genotype, reported significantly greater responses for cocaine across the time course. There were significant main effects of genotype (F(1,219) = 13.68, p < 0.001, d = 0.063) and time (F(1,219) = 22.74, p < 0.0001, d = 0.105), but the interaction term was not significant (p > 0.05). The peak difference between groups occurred at 10 minutes, where individuals with 9,9 or 9,10 genotype reported change from baseline scores of 72.5 ± 3.91, compared to 48.18 ± 4.78 for individuals with 10,10 genotype (Figure 1b).
For the VAS adjective of “stimulated”, individuals with 9,9 or 9,10 genotype, in comparison to individuals with 10,10 genotype, reported significantly greater responses for cocaine across the time course. There were significant main effects of genotype (F(1,219) = 15.52, p < 0.001, d = 0.0715) and time (F(1,219) = 12.44, p < 0.001, d = 0.0573), but the interaction term was not significant (p > 0.05). The peak difference between groups occurred at 5 minutes, where individuals with 9,9 or 9,10 genotype reported change from baseline scores of 62.92 ± 6.01, compared to 46.45 ± 5.54 for individuals with 10,10 genotype (Figure 1c).
For the VAS adjective of “anxious”, individuals with 9,9 or 9,10 genotype, in comparison to individuals with 10,10 genotype, reported significantly greater responses for cocaine across the time course. There were significant main effects of genotype (F(1,219) = 11.81, p < 0.001, d = 0.0044) but not time nor interaction (all p-values > 0.05). The peak difference between groups occurred at 5 minutes, where individuals with 9,9 or 9,10 genotype reported change from baseline scores of 37.08 ± 4.78, compared to 16.52 ± 5.97 for individuals with 10,10 genotype (Figure 1d).
No significant differences were observed for any other VAS adjectives (all p-values > 0.05).
DAT1 Intron 8
Significant differences between individuals with 5,5 or 5,6 genotype and those with 6,6 genotype were observed for race (p < 0.01), ethnicity (p < 0.05), and years of education ( p < 0.05). Race differences were accounted for in the genetic analyses.
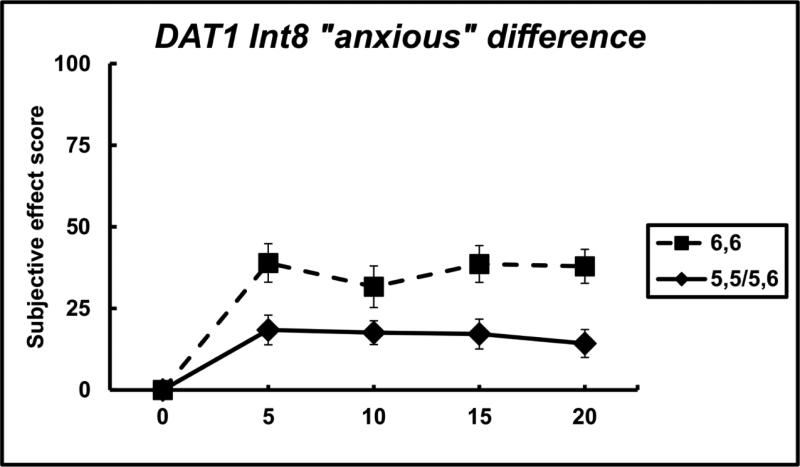
For the VAS adjective of “anxious”, individuals with 6,6 genotype, in comparison to individuals with 5,5 or 5,6 genotype, reported significantly greater responses for cocaine across the time course. There was a significant main effect of genotype (F(1,255) = 14.55, p < 0.001, d = 0.0575) but not time nor interaction (all p-values > 0.05). The peak difference between groups occurred at 20 minutes, where individuals with 6,6 genotype reported change from baseline scores of 37.89 ± 5.20, compared to 14.25 ± 4.27 for individuals with 5,5 or 5,6 genotype.
Subjective effects in African Americans: 3’ UTR VNTR and intron 8 genotypes
We analyzed the data by self-reported ethnicity to investigate if there were subjective effects within individual ethnic groups without correcting for population structure. The only group large enough for such analysis was the African Americans (n ≥ 40 for both variants). These analyses showed results similar to the previous analyses that were corrected for population structure. In particular, the analyses for the African American group for the DAT1 3’ UTR variant either remained significant (“stimulated” (F(1,149) = 8.909, p < 0.01) and “anxious” (F(1,149) = 8.537, p < 0.01)), or trended towards significance, (“high” (F(1,149) = 2.211, p = 0.1392) and “any drug effect” (F(1,149) = 2.528, p = 0.1139)). For the intron 8 variant, “anxious” remained significant (F(1,181) = 22.268, p < 0.001). This demonstrated that the genotypes in DAT1 3’ UTR and intron 8 polymorphisms showed differences or trended towards differences, in “stimulated”, “anxious”, “high”, “any drug effect”, and “anxious”, respectively. Therefore, the results with the subset of African Americans only reveal that it is unlikely that the results with the total cohort were due to population stratification.
Genotype Pattern Analysis
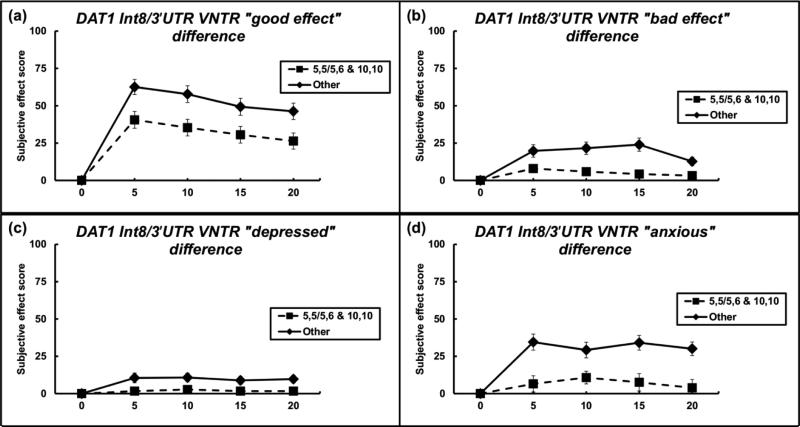
The subjective effect of “anxious” was found to be statistically significant for both the 3’ UTR and intron 8 variants, so on this basis an analysis of genotype patterns was conducted. Individuals with the lower response in both sets of groups (individuals possessing the 10,10 genotype of the 3’ UTR polymorphism and at least one 5-allele of the intron 8 polymorphism) were compared against all other genotypes (Figure 3). For the VAS adjective “anxious”, individuals with this genotype pattern reported a significantly lower response to cocaine across the time course than their cohorts. There was a significant effect of genotype pattern (F(1,217) = 21.077, p < 0.0001), but not time nor interaction (all p-values > 0.05). There were also significant differences for three other VAS adjectives between these groups. In each case, individuals possessing the genotype pattern of 10,10 and at least one 5-allele reported significantly lower responses to cocaine than their cohorts. For the VAS adjective “good effect”, there was a significant effect of genotype pattern (F(1,217) = 10.128, p < 0.01), but not time nor interaction (all p-values > 0.05). For the VAS adjective “bad effect”, there was a significant effect of genotype pattern (F(1,217) = 12.126, p < 0.001), but not time nor interaction (all p-values > 0.05). For the VAS adjective “depressed”, there was a significant effect of genotype pattern (F(1,217) = 14.955, p < 0.01), but not time nor interaction (all p-values > 0.05).
Figure 3.
Significant differences among subjective effects between groups after genotype pattern analysis. Individuals possessing DAT1 intron 8 5,5 or 5,6 and 3’ UTR 10,10 genotype are compared vs. all other genotypes. Data points indicate mean, and error bars indicate standard error of mean
The data in this report demonstrate that genetic differences within DAT1 can lead to distinct subjective responses to cocaine in individuals with CUDs. In this report, individuals with the DAT1 3’ UTR 40-bp VNTR 9,9 or 9,10 genotype exhibited significantly higher subjective responses to cocaine for multiple VAS adjectives compared to those with the 10,10 genotype. In addition, individuals with the intron 8 30-bp VNTR 6,6 genotype exhibited a higher subjective response to cocaine for the VAS adjective “anxious” compared to individuals possessing a 5-allele, although this may have been influenced by differences in race, ethnicity, or years of education. Last, individuals possessing the genotype pattern of 10,10 for the 3’ UTR polymorphism and at least one 5-allele of the intron 8 polymorphism displayed decreased subjective responses to acute cocaine than their cohorts.
Individuals reported significantly higher ratings for “high”, “stimulated”, and “any drug effect”, subjective ratings of cocaine that are generally considered to be “good” or “positive” subjective effects of cocaine, as well as a higher rating of “anxious”, which is generally considered to be a “bad” or “negative” subjective effect of cocaine. The ratings for these three positive subjective effects were all similar. As for the intron 8 polymorphism, an opposite pattern emerged. No positive subjective effects emerged with significant differences, but once again, a negative subjective effect, “anxious”, did emerge with significant differences. These results are somewhat consistent with a report that investigated the effect of SLC6A3 genotype, in combination with recent cocaine use, on cocaine cue reactivity [35]. These researchers compared individuals with CUDs (n = 73) against otherwise healthy individuals without CUDs (n = 47). The individuals with CUDs were further subdivided into individuals who had recently used cocaine (within 72 hours of the study) against those who had not. Then, a battery of four tests (event-related potentials, fMRI drug-word task, picture rating, and picture choosing) were administered to the three groups (cocaine-positive, cocaine-negative, and non-cocaine-using), and the results were analyzed in light of the individuals with the 10,10 genotype compared to those possessing a 9-allele. The authors reported that across the four tests, individuals that possessed a 9-allele were more reactive to cocaine cues than individuals with the 10,10 genotype, but only for individuals that had recently used cocaine prior to entry into the study. These results are aligned with the results of the present study, however. The results of this study and the previously mentioned study also may indicate that individuals possessing a 9-repeat allele may be more vulnerable to relapse than individuals homozygous for the 10-repeat allele.
The functional effect of genotype of the 3’ UTR VNTR and the intron 8 polymorphism on DAT continues to be controversial. One recent report found that both polymorphisms significantly affected striatal DAT density [36]. In this report, the investigators used positron emission tomography and a racially diverse sample to conclude that both polymorphisms, individually and together as a haplotype, influenced DAT levels. Specifically, they observed that the 9-repeat allele appeared to have an additive effect (i.e. 10,10 vs. 9,10 vs. 9,9) on DAT density, whereas the presence of a 5-repeat of the intron 8 polymorphism yielded higher levels than being homozygous for the 6-repeat. A more recent report found that genotype of the 3’ UTR VNTR but not intron 8 genotype was associated with increased striatal (caudate) DAT binding [37], although the authors did also report a small sample size with their intron 8 data. However, for the 3’ UTR VNTR at least, a consensus appears to be developing that the 9-repeat allele leads to increased DAT [22].
The 3’ UTR VNTR may be the more interesting of the two polymorphisms as more research and more significant findings have been published on this variant compared to the intron 8 polymorphism. These results are not intended to imply causation, but potential hypotheses to explain the results may include withdrawal [35] and/or drug priming [38]. Briefly, previous literature has reported that individuals with CUDs in various stages of withdrawal have displayed decreased striatal DA activity [39] and decreases in non-stimulated DA release [40]. This state of dysfunction in individuals possessing 9-repeat alleles could be heightened in these individuals (because of reduced tonic activity associated with the allele). Lower tonic DA levels could be expected to increase phasic DA levels upon conditioned cues [41], or in this case, the initial bolus of cocaine, especially within a state of withdrawal [37]. It should be noted that all individuals must test negative for cocaine upon entry into any of our studies, meaning they should likely be in some state of withdrawal prior to receiving cocaine in our laboratory. An alternate, but less likely potential mechanism for the observed effects of the 9-repeat allele, could be the phenomenon of drug priming, wherein exposure to an addictive drug after a period of abstinence leads to increased craving and usage [38]. However, this is a less likely potential mechanism as recent cocaine use (as verified by a positive test during screening) is an inclusion criterion for entry into all of our research studies.
Although it remains unclear as to which allele (9-repeat vs. 10-repeat) leads to increased DAT production, more evidence suggests that these polymorphisms are indeed functional. It has been reported that the 9-allele decreases tonic and increases phasic DA firing [21]. Two more studies (which both report that the 9-repeat allele leads to decreased DAT product) also suggest that this polymorphism may be functional. In the first, carriers of the 9-allele were observed to have significantly different blood-oxygen-level-dependent responses than individuals homozygous for the 10-repeat (in otherwise healthy individuals without drug use disorders) within the caudate nucleus and ventral striatum during reward anticipation, and within the lateral prefrontal cortex and midbrain during reward delivery [39]. In the second report, it was observed that the presence of the 9-repeat allele predicted approximately 10% of inter-individual variability in reward-related ventral striatum reactivity associated with self-reported impulsivity in individuals [40]. Again, these reports indicate that being homozygous for the 10-repeat allele may be considered somewhat protective of the subjective effects produced by acute cocaine exposure.
Some limitations of the current study should be considered. First, it may have been useful to compare a cohort of 9,9 individuals against 9,10 or 10,10 individuals. However, this genotype is rare, and in the present report, only 4 individuals out of 66 that were genotyped possessed both alleles. Traditionally, most researchers combine these individuals with 9,10 individuals, and compare this group to 10,10 individuals, an approach we have also taken here [41-42]. It is also worthwhile to note that despite our best efforts to recruit as heterogeneous of a sample as possible, that our sample was largely male and African American; however, given the demographics of our location, this is common for us. A similar report to this one began with intentionally using a racially homogeneous sample [21], which may be helpful in determining effects of genotype on subjective response to cocaine before expanding into more diverse populations. Last, it may have been more informative to evaluate the effects of genotype on responses to multiple doses of cocaine. Although 40 mg is a moderate dose that most cocaine users respond to in our laboratory [43], most cocaine users in our studies have historically used nearly 2 grams/day. Testing multiple doses or higher doses of cocaine may provide even more information, yet this raises possible safety concerns with the Institutional Review Board.
CONCLUSION
In conclusion, we have shown for the first time that polymorphisms present within DAT1 can lead to significant differences in subjective effects and physiological measures after acute administration of cocaine in the laboratory setting. The difference in subjective effects in response to cocaine administration may be related to the polymorphisms’ apparent ability to influence protein levels of DAT, although it is not unequivocal as to which allele leads to increased levels, or how this occurs. These results do suggest that the presence of the 9-allele within SLC6A3 may indicate vulnerability to the positive subjective effects produced by cocaine and may shed light on individual differences in subjective effects.
Figure 2.
“Anxious” scores between 6,6 and 5,5/5,6 individuals from the intron 8 sample. Data points indicate mean, and error bars indicate standard error of mean
Table 2.
Genotypes of DAT1 Intron 8 VNTR Sample
| Race | |||||
|---|---|---|---|---|---|
| African American | Caucasian | Mixed Race | Total | ||
| Genotype | 5,5 | 12 | 0 | 0 | 12 |
| 5,6 | 28 | 6 | 2 | 36 | |
| 6,6 | 8 | 8 | 2 | 18 | |
Acknowledgements
Part of this work was conducted at and supported by resources at the Michael E. DeBakey VA Medical Center.
Grant funding by NIDA to Drs. De La Garza and Newton, NIDA (DA023624), Dr. Kosten (DA018197) through MD Anderson's Cancer Center Support Grant DA026120 NIH / NIDA and from the Toomim Family Fund.
Footnotes
No conflicts of interest declared.
DSM-IV-TR diagnosis criteria for substance dependence has been shown to correlate with DSM-5 diagnostic criteria for use disorders, therefore we use DSM-5 nomenclature throughout this manuscript for consistency purposes [1]
References
- 1.Compton WM, Dawson DA, Goldstein RB, Grant BF. Crosswalk between DSM-IV dependence and DSM-5 substance use disorders for opioids, cannabis, cocaine and alcohol. Drug and Alcohol Dependence. 2013;132:387. doi: 10.1016/j.drugalcdep.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Substance Abuse and Mental Health Services Administration . Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2013. NSDUH Series H-46, HHS Publication No. (SMA) 13-4795.
- 3.Burling TA, Ziff DC. Tobacco smoking: a comparison between alcohol and drug abuse inpatients. Addict Behav. 1988;13:185. doi: 10.1016/0306-4603(88)90010-x. [DOI] [PubMed] [Google Scholar]
- 4.Burling TA, Marshall GD, Seidner AL. Smoking Cessation for Substance Abuse Inpatients. Journal of Substance Abuse. 1991;3:276. doi: 10.1016/s0899-3289(10)80011-2. [DOI] [PubMed] [Google Scholar]
- 5.Miller NS, Gold MS, Belkin BM, Klahr AL. Family history and diagnosis of alcohol dependence in cocaine dependence. Psychiatry research. 1989 Aug;29:113. doi: 10.1016/0165-1781(89)90026-7. [DOI] [PubMed] [Google Scholar]
- 6.Brewer AJ, III, Mahoney JJ, III, Nerumalla C, Newton TF, De La Garza R., II The influence of smoking cigarettes on the high and desire for cocaine among active cocaine users. Pharmacology, Biochemistry, and Behavior. 2013;106 doi: 10.1016/j.pbb.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budney AJ, Higgins ST, Hughes JR, Bickel WK. Nicotine and caffeine use in cocaine-dependent individuals. Journal of Substance Abuse. 1993;5:117. doi: 10.1016/0899-3289(93)90056-h. [DOI] [PubMed] [Google Scholar]
- 8.Gorelick DA, Simmons MS, Carriero N, Tashkin DP. Characteristics of smoked drug use among cocaine smokers. The American Journal on Addictions. 1997;6:237. [PubMed] [Google Scholar]
- 9.Patkar AA, Sterling RC, Leone FT, Lundy A, Weinstein SP. Relationship Between Tobacco Smoking and Medical Symptoms Among Cocaine-, Alcohol-, and Opiate-Dependent Patients. The American Journal on Addictions. 2002;11:209. doi: 10.1080/10550490290087974. [DOI] [PubMed] [Google Scholar]
- 10.Kalman D, Morissette SB, George TP. Co-morbidity of Smoking in Patients with Pyschiatric and Substance Use Disorders. The American Journal on Addictions. 2005;14:106. doi: 10.1080/10550490590924728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinberger AH, Sofuoglu M. The Impact of Cigarette Smoking on Stimulant Addiction. The American Journal of Drug and Alcohol Abuse. 2009;35:12. doi: 10.1080/00952990802326280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han DD, Gu HH. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacology. 2006;6:6. doi: 10.1186/1471-2210-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine Receptors on Dopamine Transporters Are Related to Self-Administration of Cocaine. Science. 1987;237:1219. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 14.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 15.Gelernter J, Kranzler HR, Satel SL, Rao PA. Genetic Association between Dopamine Transporter Protein Alleles and Cocaine-Induced Paranoia. Neuropsychopharmacology. 1994;11:195. doi: 10.1038/sj.npp.1380106. [DOI] [PubMed] [Google Scholar]
- 16.Guindalini C, Howard M, Haddley K, Laranjeira R, Collier D, Ammar N, et al. A dopamine transporter gene functional variant associated with cocaine abuse in a Brazilian sample. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:4552. doi: 10.1073/pnas.0504789103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brookes K, Neale BM, Sugden K, Khan N, Asherson P, D'Souza UM. Relationship between VNTR polymorphisms of the human dopamine transporter gene and expression in post-mortem midbrain tissue. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2007;144B:1070. doi: 10.1002/ajmg.b.30572. [DOI] [PubMed] [Google Scholar]
- 18.Fuke S, Suo S, Takahashi N, Koike H, Sasagawa N, Ishiura S. The VNTR polymorphism of the human dopamine transporter (DAT1) gene affects gene expression. The Pharmacogenomics Journal. 2001;1:152. doi: 10.1038/sj.tpj.6500026. [DOI] [PubMed] [Google Scholar]
- 19.VanNess SH, Owens MJ, Kilts CD. The variable number of tandem repeats element in DAT1 regulates in vitro dopamine transporter density. BMC genetics. 2005;6:55. doi: 10.1186/1471-2156-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinsonneault JK, et al. Dopamine transporter gene variant affecting expression in human brain is associated with bipolar disorder. Neuropsychopharmacology. 2011;36:1644. doi: 10.1038/npp.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Dyck CH, Malison RT, Jacobsen LK, Seibyl JP, Staley JK, Laruelle M, et al. Increased dopamine transporter availability associated with the 9-repeat allele of the SLC6A3 gene. The Journal of Nuclear Medicine. 2005;46:745. [PubMed] [Google Scholar]
- 22.Faraone SV, Spencer TJ, Madras BK, Zhang-James Y, Biederman J. Functional effects of dopamine transporter gene genotypes on in vivo dopamine transporter functioning: a meta-analysis. Molecular psychiatry. 2013 Sep 24; doi: 10.1038/mp.2013.126. [DOI] [PubMed] [Google Scholar]
- 23.Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011 Feb 24;69:628. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lott DC, Kim SJ, Cook EH, Jr., de Wit H. Dopamine Transporter Gene Associated with Diminished Subjective Response to Amphetamine. Neuropsychopharmacology. 2005;30:602. doi: 10.1038/sj.npp.1300637. [DOI] [PubMed] [Google Scholar]
- 25.Hamidovic A, Dlugos A, Palmer AA, de Wit H. Polymorphisms in Dopamine Transporter (SLC6A3) are Associated with Stimulant Effects of d-Amphetamine: An Exploratory Pharmacogenetic Study Using Healthy Volunteers. Behavior Genetics. 2010;40:255. doi: 10.1007/s10519-009-9331-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hart AB, de Wit H, Palmer AA. Candidate gene studies of a promising intermediate phenotype: failure to replicate. Neuropsychopharmacology. 2013;38:802. doi: 10.1038/npp.2012.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franklin TR, Lohoff FW, Wang Z, Sciortino N, Harper D, Li Y, et al. DAT Genotype Modulates Brain and Behavioral Responses Elicited by Cigarette Cues. Neuropsychopharmacology. 2009;34:717. doi: 10.1038/npp.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stein MA, Waldman ID, Sarampote CS, Seymour KE, Robb AS, Conlon C, et al. Dopamine transporter genotype and methylphenidate dose response in children with ADHD. Neuropsychopharmacology. 2005;30:1374. doi: 10.1038/sj.npp.1300718. [DOI] [PubMed] [Google Scholar]
- 29.Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, et al. Cooccurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Archives of general psychiatry. 1998 Nov;55:967. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- 30.Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. The American Journal of Psychiatry. 2003;160:687. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- 31.Spellicy CJ, Harding MJ, Hamon SC, Mahoney JJ, Reyes JA, Kosten T, Newton TF, De La Garza R, Nielsen DA. A variant in ANKK1 modulates acute subjective effects of cocaine: a preliminary study. Genes, Brain, and Behavior. 2014;2014;13:559. doi: 10.1111/gbb.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Molecular psychiatry. 2006;11:224. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- 33.Kosten TR, Wu G, Huang W, Harding MJ, Hamon SC, Lappalainen J, et al. Pharmacogenetic randomized trial for cocaine abuse: disulfiram and dopamine beta-hydroxylase. Biological Psychiatry. 2013;73:219. doi: 10.1016/j.biopsych.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.R_Development_Core_Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna: 2009. [Google Scholar]
- 35.Moeller SJ, Parvaz MA, Shumay E, Beebe-Wang N, Konova AB, Alia-Klein N, et al. Gene x abstinence effects on drug cue reactivity in addiction: multimodal evidence. The Journal of Neuroscience. 2013;33:10027. doi: 10.1523/JNEUROSCI.0695-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shumay E, Chen J, Fowler JS, Volkow ND. Genotype and ancestry modulate brain's DAT availability in healthy humans. PloS one. 2011;6:e22754. doi: 10.1371/journal.pone.0022754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spencer TJ, Biederman J, Faraone SV, Madras BK, Bonab AA, Dougherty DD, et al. Functional genomics of attention-deficit/hyperactivity disorder (ADHD) risk alleles on dopamine transporter binding in ADHD and healthy control subjects. Biological Psychiatry. 2013;74:84. doi: 10.1016/j.biopsych.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahoney JJ, 3rd, Kalechstein AD, De La Garza R, 2nd, Newton TF. A qualitative and quantitative review of cocaine-induced craving: the phenomenon of priming. Progress in neuropsychopharmacology & biological psychiatry. 2007 Apr 13;31:593. doi: 10.1016/j.pnpbp.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dreher JC, Kohn P, Kolachana B, Weinberger DR, Berman KF. Variation in dopamine genes influences responsivity of the human reward system. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:617. doi: 10.1073/pnas.0805517106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Molecular psychiatry. 2009;14:60. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacobsen LK, Staley JK, Zoghbi SS, Seibyl JP, Kosten TR, Innis RB, et al. Prediction of dopamine transporter binding availability by genotype: a preliminary report. The American Journal of Psychiatry. 2000;157:1700. doi: 10.1176/appi.ajp.157.10.1700. [DOI] [PubMed] [Google Scholar]
- 42.Martinez D, Gelernter J, Abi-Dargham A, van Dyck CH, Kegeles L, Innis RB, et al. The Variable Number of Tandem Repeats Polymorphism of the Dopamine Transporter is Not Associated with Significant Change in Dopamine Transporter Phenotype in Humans. Neuropsychopharmacology. 2001;24:553. doi: 10.1016/S0893-133X(00)00216-5. [DOI] [PubMed] [Google Scholar]
- 43.Verrico CD, Haile CN, Mahoney JJ, III, Thompson-Lake DGY, Newton TF, De La Garza R., II Treatment with modafinil and escitalopram, alone and in combination, on cocaine-induced effects: A randomized, double blind, placebo-controlled human laboratory study. Drug and Alcohol Dependence. 2014;141:72. doi: 10.1016/j.drugalcdep.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]