Abstract
Objectives
To describe utilization of 3% hypertonic saline (HTS) in hospitalized infants and to evaluate the association between HTS use and length of stay (LOS) in a real-world setting.
Study design
This multicenter retrospective cohort study included infants ≤12 months hospitalized with bronchiolitis between October 2008–September 2011 using the Pediatric Health Information System. HTS use was categorized as trial, rescue, daily, or sporadic. Differences in LOS were compared after matching daily HTS recipients and non-recipients on propensity score.
Results
There were 63,337 hospitalizations for bronchiolitis. HTS was used in 24 of 42 hospitals and 2.9% of all hospitalizations. HTS use increased from 0.4% of visits in 2008 to 9.2% of visits in 2011. There was substantial variation in HTS use across hospitals (range 0.1%–32.6%). When used, HTS was given daily during 60.6% of hospitalizations, sporadically in 10.4%, as a trial in 11.3%, and as a rescue in 17.7%. The propensity-score matched analysis of daily HTS recipients (n=953) versus non-recipients (n=953) showed no difference in mean LOS (HTS 2.3 days vs. non-recipients 2.5 days; β-coefficient −0.04; 95%CI −0.15, 0.07; p=0.5) or odds of staying longer than 1, 2, or 3 days. Daily HTS recipients had a 33% decreased odds of staying in the hospital >4 days compared with non-recipients (OR 0.67; 95%CI 0.47, 0.97; p=0.03).
Conclusions
Variation in HTS use and the lack of association between HTS and mean LOS demonstrates the need for further research to standardize HTS use and better define the infants for whom HTS will be most beneficial.
Keywords: bronchiolitis, hypertonic saline, hospitalization, health services research, infants, resource utilization, effectiveness, practice variation
Acute bronchiolitis is the most frequent lower respiratory tract infection in infants, and the most frequent cause of hospitalization in this age group.(1–3) The pathogenesis of bronchiolitis is characterized by acute inflammation, edema, and necrosis of airway epithelium, excess mucus production, and bronchospasm, ultimately leading to airway obstruction and impaired gas exchange.(4, 5) Despite high incidence of bronchiolitis and a growing understanding of its pathogenesis, currently available therapies have failed to show consistent benefit, and supportive care remains the mainstay of bronchiolitis therapy.(5) Nebulized hypertonic saline (HTS) has been proposed as a therapy that may benefit patients through reduction of airway edema, diminished plugging and improved clearance of mucus.
Over the last decade, a growing number of randomized trials suggest that early and repeated doses of nebulized HTS improve clinical outcomes in hospitalized children compared with 0.9% normal saline (NS). The most recent Cochrane Library meta-analysis examined 6 inpatient trials of 500 infants with acute bronchiolitis and concluded that nebulized 3% saline may significantly reduce the hospital length of stay (LOS) among infants hospitalized with mild-to-moderate bronchiolitis and improve post-inhalation clinical severity scores during the first 3 days of hospitalization.(6) Despite the rapid growth in literature and inconsistent benefit of HTS in children hospitalized with bronchiolitis, no official recommendations were made prior to publication of the November 2014 American Academy of Pediatrics (AAP) clinical practice guideline for bronchiolitis and little is known about how HTS is utilized in practice.(5, 7) In addition, even though the efficacy of HTS has been studied in randomized trials, there have been no studies of its effectiveness in reducing LOS in a broad population of infants hospitalized with bronchiolitis. The objectives of this study were (1) to characterize the current patterns of HTS utilization in hospitalized patients at children’s hospitals across the United States and (2) to evaluate the association between HTS use and LOS in a real-world setting.
METHODS
This multicenter retrospective observational study included inpatient visits of children diagnosed with bronchiolitis. Data were from the Pediatric Health Information System (PHIS), an administrative database of 43 not-for-profit, tertiary care pediatric hospitals in the United States affiliated with the Children’s Hospital Association (CHA, Overland Park, KS). Data quality and reliability are assured through a joint effort between CHA and participating hospitals. The database accounts for ~20% of annual pediatric hospitalizations in the United States. Hospitals provide discharge/encounter data including demographics, procedures, and diagnoses in International Classification of Diseases-9-Clinical Modification (ICD-9-CM) format; 42 of these hospitals also submit resource utilization data (e.g., pharmaceuticals, imaging, and laboratory tests) and thus were included in this study. For the current study, data were included from October 1, 2008, one year after publication of the first Cochrane meta-analysis suggesting benefit of HTS, through December 31, 2011, which were the most recent data available at time of analysis.
Patients 12 months of age and younger were eligible if they were discharged from a participating hospital between October 2008 and December 2011 with diagnosis of bronchiolitis. Bronchiolitis was defined as an ICD-9-CM discharge diagnosis code for bronchiolitis (466.11, 466.19) and an All Patient Refined-Diagnosis Related Group (APR-DRG) code for bronchiolitis (138) to minimize misclassification.(8) Children with cystic fibrosis (ICD-9-CM code, 227), spinal muscular atrophy (ICD-9-CM code, 335), or bronchiectasis (ICD-9-CM codes, 494, 748.61) were excluded, as HTS is routinely used in patients with these conditions.
Exposure and Outcome Measures
The outcome of interest for the first objective was utilization of nebulized 3% saline. Receipt of HTS was identified using PHIS-specific Clinical Transaction Classification billing codes. These codes identify if HTS was given on a particular day of hospitalization, but cannot quantify the number of times HTS was administered in a single day. Receipt of HTS was categorized into four use patterns: trial, rescue, daily and sporadic. Trial use was defined as use for a single day on day 0 or 1 of hospitalization, but no use for the remainder of the hospitalization. Rescue use was defined as initiation of HTS on the third day of hospitalization or beyond. Daily use was defined as initiation of HTS within the first two days of hospitalization and repeated administration throughout the admission. For daily use with LOS longer than 2 days, we allowed for no HTS use on the final day of hospitalization or no use for an isolated single day during the hospital stay provided that it was administered every other day consecutively. Finally, sporadic use was defined as HTS use in a random pattern that did not meet one of the first three categories. For the second objective, daily use of HTS was the primary exposure and the outcome of interest was hospital LOS.
Covariates
The following patient- and visit-level demographic covariates were included: age, sex, race, insurance payer category, season, and year. Patient severity was examined using intensive care unit admission, non-invasive positive-pressure ventilation, mechanical ventilation, supplemental oxygen, receipt of blood gas, and an APR-DRG severity subclass score of major or extreme. The APR-DRG severity score consists of four categories from mild to extreme and represent illness severity of hospitalized patients.(9, 10) Finally, several diagnostic and adjunct therapeutic resources were examined: albuterol, racemic epinephrine, corticosteroids, continuous nebulized therapies, intravenous (IV) fluids, IV antibiotics, and chest radiographs.
Statistical Analyses
Unadjusted frequency distributions were developed to explore HTS use patterns by hospital and by year. A bivariable analysis was conducted by characterizing differences in covariates and hospital LOS by pattern of HTS use across all PHIS hospitals. Percentages for categorical variables, means for age in months, and means/medians for length of stay in days were developed. To account for clustering within hospitals, SAS PROC SURVEY was used to generate tests of significance by HTS use. Similarly, general estimating equations assuming a negative binomial distribution were used to test for differences in LOS due to the highly skewed LOS data.
Because clinical trials have found benefit in using HTS in a daily fashion, infants who received HTS daily were compared with those for whom HTS was not utilized at all to examine differences in covariates and hospital LOS between these two groups. To ensure adequate numbers of patients in each hospital who received HTS daily, only hospitals with overall rates of daily HTS use in more than 5% of all patients with bronchiolitis were included in these analyses. The same bivariable analysis described above was conducted. In addition, a propensity score matched analysis was conducted to test for differences in LOS, the primary outcome, between infants receiving HTS daily or not at all in these hospitals.
Propensity scores were developed to account for potential confounding by observed baseline characteristics. A propensity score estimates the probability of receiving HTS daily given the observed set of baseline covariates. The following variables were included as risk factors for HTS receipt in a multivariable logistic regression model to generate the propensity score for HTS receipt: age, sex, race, insurance, asthma diagnosis at visit, season, year, APR-DRG, and severity score. In addition, we included a variable denoting the hospital to account for practice variation between hospitals. Finally, the management and severity variables listed above were included as indicator variables denoting whether or not they occurred within two days of hospital admission. The model’s C-statistic was 0.877, indicating that the model provided a better estimate than expected by chance alone.(11, 12)
Daily HTS recipients and non-recipients were matched one-to-one on the logit of the propensity scores using a greedy algorithm and nearest-neighbor approach to allow for matching treated and untreated individuals by closest propensity score. A matching caliper was set at 2.2, which is slightly higher than the recommended caliper of 0.2 of the standard deviation of the logit of the propensity scores.(13) This choice of caliper was made to maximize sample size, while maintaining balance on all covariates. This caliper resulted in all but 11 cases matched, while maintaining balance. Balance was assessed in the matched data by testing for differences in the covariates between daily HTS recipients and non-recipients using chi-square analysis and t-tests for categorical and continuous variables, respectively.
Differences in LOS between propensity-score matched daily HTS recipients and non-recipients were analyzed using general estimating equations to account for clustering within hospital. The negative binomial and binomial distributions were specified for the continuous and dichotomized LOS dependent variables, respectively.
RESULTS
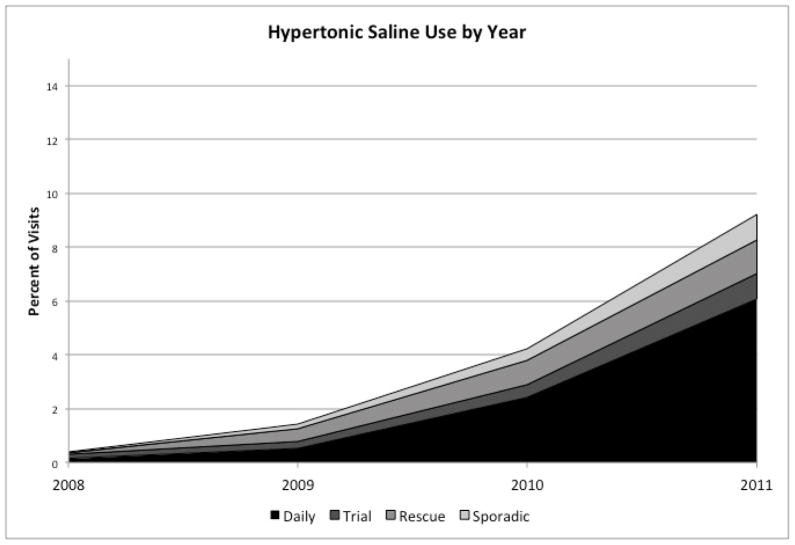
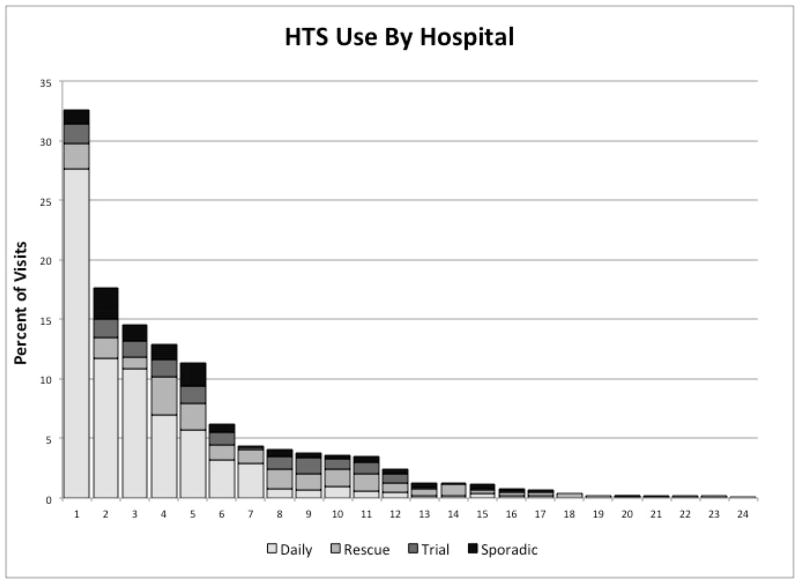
There were 63,337 hospitalizations for bronchiolitis in the 42 hospitals during the study period. Hypertonic saline was used for bronchiolitis in 24 out of 42 hospitals (57.1%), and during 1,839, or 2.9% of hospitalizations. HTS use increased more than 20-fold from 0.4% of visits in 2008 to 9.2% of visits in 2011 (Figure 1; available at www.jpeds.com). There was substantial variation in HTS use across the 24 hospitals, with hospital-level use ranging from 0.1% of bronchiolitis patients to 32.6% (Figure 2).
Figure 1. Hypertonic Saline Utilization by Year at 42 PHIS Hospitals, October 2008–December 2011.
The vertical axis represents the percent of visits overall where hypertonic saline was used at all 42 PHIS hospitals in aggregate during each year. Definitions of daily, rescue, trial and sporadic use are found in the text.
Figure 2. Hypertonic Saline Utilization by Hospital at 24 PHIS Hospitals.
Each bar represents hypertonic saline utilization at a single hospital. Each shaded box within the bar represents the percent use for each of the four patterns of use and the total height of the bar represents total hypertonic saline use at that hospital.
Of the 1,839 hospitalizations in which HTS was administered, 1,115 (60.6%) were with daily use, 207 (11.3%) with trial use, 326 (17.7%) with rescue use and 191 (10.4%) sporadically (Table I). There were significant differences in mean age, year, patient severity, and administration of medications including albuterol, racemic epinephrine, corticosteroids, continuous nebulized therapies, IV fluids and IV antibiotics. The trial, rescue and sporadic groups had higher rates of severity measures, adjunct therapies, and length of stay compared with the daily use and no use groups. There was clear variation in the patterns of HTS use across hospitals (Figure 2).
Table 1.
Patient and Visit Characteristics by Pattern of Hypertonic Saline Utilization in 42 PHIS Hospitals
| Variables | Non-Recipients (n=61498) | Daily HTS (n= 1115) | Trial HTS (n= 207) | Rescue HTS (n= 326) | Sporadic HTS (n=191) | P-value |
|---|---|---|---|---|---|---|
| Age (months), mean (SD) | 4.13 (3.40) | 3.72 (3.14) | 4.03 (3.35) | 3.44 (3.14) | 3.39 (3.11) | <0.001 |
| Male Sex, % | 58.8 | 59.6 | 62.3 | 62.3 | 59.2 | 0.51 |
| Race, % | ||||||
| Black | 22.1 | 12.6 | 25.1 | 18.1 | 15.2 | 0.05 |
| White | 54.0 | 69.3 | 55.6 | 56.8 | 67.0 | |
| Other | 18.7 | 16.3 | 18.4 | 23.0 | 16.8 | |
| Unknown | 5.2 | 1.8 | 1.0 | 2.2 | 1.0 | |
| Asthma Diagnosis, % | 6.6 | 7.0 | 10.1 | 8.3 | 6.3 | 0.45 |
| Insurance Category, % | ||||||
| Medicaid | 60.5 | 66.5 | 60.9 | 58.6 | 73.3 | 0.32 |
| Private | 24.6 | 28.1 | 29.5 | 28.8 | 20.9 | |
| Self Pay | 1.3 | 2.2 | 0.5 | 0.6 | 1.0 | |
| Other | 5.4 | 1.5 | 1.9 | 3.7 | 1.6 | |
| Unknown | 8.2 | 1.6 | 7.2 | 8.3 | 3.1 | |
| Season, % | ||||||
| Summer (June–Aug) | 3.5 | 1.8 | 2.9 | 3.1 | 4.2 | 0.46 |
| Fall (Sept–Nov) | 13.7 | 9.1 | 11.6 | 14.4 | 4.7 | |
| Winter (Dec–Feb) | 60.2 | 59.4 | 63.3 | 58.0 | 60.7 | |
| Spring (Mar–May) | 22.6 | 29.8 | 22.2 | 24.5 | 30.4 | |
| Year, % | ||||||
| 2008 | 8.1 | 0.4 | 2.4 | 0.6 | 0.5 | <0.001 |
| 2009 | 30.0 | 5.4 | 15.0 | 16.9 | 11.0 | |
| 2010 | 31.2 | 26.4 | 25.6 | 34.7 | 26.7 | |
| 2011 | 30.7 | 67.8 | 57.0 | 47.9 | 61.8 | |
| Severity, % | ||||||
| Intensive Care Unit Admission | 7.2 | 3.9 | 21.7 | 30.7 | 38.2 | <0.001 |
| Non-Invasive Positive Pressure Ventilation | 2.3 | 0.7 | 9.2 | 13.5 | 11.5 | 0.004 |
| Mechanical Ventilation | 1.0 | 0.0 | 1.9 | 0.9 | 3.7 | * |
| Blood Gas Measurement | 14.1 | 15.7 | 34.8 | 43.3 | 51.3 | <0.001 |
| Supplemental Oxygen | 45.7 | 49.7 | 64.3 | 75.2 | 86.4 | <0.001 |
| APR-DRG Severity Subclass (major/extreme) | 7.6 | 6.6 | 19.8 | 24.5 | 29.8 | <0.001 |
| Management, % | ||||||
| Racemic Epinephrine | 16.9 | 41.9 | 48.8 | 51.2 | 61.3 | 0.002 |
| Albuterol | 52.5 | 55.2 | 74.9 | 84.0 | 85.9 | <0.001 |
| Corticosteroids | 15.7 | 16.1 | 27.5 | 27.3 | 28.3 | 0.006 |
| Continuous Nebulized Therapies | 5.5 | 13.3 | 22.2 | 25.8 | 33.0 | 0.002 |
| Intravenous Fluids | 52.8 | 53.5 | 70.0 | 69.9 | 79.1 | <0.001 |
| Intravenous Antibiotics | 23.8 | 18.7 | 30.0 | 40.2 | 48.2 | 0.006 |
| Chest Radiographs | 52.6 | 46.5 | 59.9 | 76.1 | 84.8 | 0.11 |
| Length of Stay (days), mean (SD) | 2.80 (2.52) | 2.42 (1.78) | 4.05 (2.63) | 7.05 (3.47) | 7.56 (3.33) | <0.001 |
P-value not calculated due to empty cells
Before matching on propensity score, the daily HTS recipients and non-recipients in hospitals with >5% daily HTS use had no significant differences in demographic characteristics or management except that a greater proportion of non-recipients received corticosteroids compared with daily HTS recipients (Table II; available at www.jpeds.com). The mean LOS before propensity score matching in non-recipients was 2.7 days (SD, 2.4) and was 2.3 days (SD, 1.8) in the daily HTS recipients (p=0.57). A hospital-level analysis of the 5 hospitals with >5% daily HTS use revealed reduced mean LOS in one hospital after adjusting for propensity score (Non-recipients 3.1 days (95% CI, 2.3, 2.6) vs. Daily 2.4 days (95% CI 2.1, 2.8), p=0.0002). There was no significant difference in mean LOS between non-recipients and daily recipients in the 4 other hospitals (Table III; available at www.jpeds.com). In the propensity score analysis, 953 of 964 (99%) of daily HTS recipients were matched to appropriate non-recipients. The matching algorithm resulted in an acceptable level of balance between daily HTS recipients and non-recipients. All categorical variables were within ± 5 percentage points and the difference in age was 0.2 months. After propensity-score matching, there were no significant differences in demographics, severity or management variables between those who received daily HTS and non-recipients (Table IV). In the propensity-matched analysis, although there was no difference in LOS as a continuous variable or when dichotomizing LOS at longer than 1, 2 or 3 days between daily HTS recipients and non-recipients, daily HTS recipients had a 33% decreased odds of staying in the hospital longer than 4 days compared with non-recipients (OR 0.67; 95% confidence interval 0.47, 0.97; p=0.03) (Table V).
Table 2.
Characteristics of Non-Recipients and Daily HTS Recipients in Hospitals with >5% HTS Use Before Matching on Propensity Score
| Variable | Non-Recipients (n=5928) | Daily HTS (n= 964) | P-Value |
|---|---|---|---|
| Age (months), mean (SD) | 3.88 (3.31) | 3.63 (3.09) | 0.32 |
| Male Sex, % | 58.2 | 59.1 | 0.11 |
| Race | |||
| Black | 13.0 | 11.1 | 0.22 |
| White | 64.1 | 71.8 | |
| Other | 18.7 | 15.6 | |
| Unknown | 4.1 | 1.6 | |
| Asthma Diagnosis, % | 10.2 | 6.6 | 0.12 |
| Insurance Category, % | |||
| Medicaid | 68.0 | 66.1 | 0.46 |
| Private Insurance | 25.4 | 29.8 | |
| Self Pay | 0.6 | 2.4 | |
| Other | 0.9 | 1.6 | |
| Unknown | 5.2 | 0.2 | |
| Season, % | |||
| Summer (June–Aug) | 2.2 | 1.1 | 0.48 |
| Fall (Sept–Nov) | 8.7 | 6.7 | |
| Winter (Dec–Feb) | 67.0 | 62.6 | |
| Spring (Mar–May) | 22.1 | 29.6 | |
| Year, % | |||
| 2008 | 7.4 | 0.4 | <0.001 |
| 2009 | 34.0 | 3.6 | |
| 2010 | 34.8 | 26.6 | |
| 2011 | 23.8 | 69.4 | |
| Severity, % | |||
| Intensive Care Unit Admission | 5.7 | 3.2 | 0.20 |
| Non-Invasive Positive Pressure Ventilation | 0.5 | 0.2 | 0.11 |
| Mechanical Ventilation | 0.2 | 0.0 | * |
| Blood Gas | 18.1 | 14.7 | 0.54 |
| Supplemental Oxygen | 48.3 | 52.0 | 0.35 |
| APR-DRG Severity Subclass (major/extreme) | 9.3 | 6.4 | 0.11 |
| Management, % | |||
| Racemic Epinephrine | 24.8 | 42.8 | 0.09 |
| Albuterol | 63.8 | 50.4 | 0.08 |
| Corticosteroids | 23.5 | 15.8 | 0.04 |
| Continuous Nebulized Therapies | 18.2 | 14.9 | 0.55 |
| Intravenous Fluids | 51.0 | 52.4 | 0.55 |
| Intravenous Antibiotics | 23.6 | 17.3 | 0.13 |
| Chest Radiograph | 61.6 | 44.9 | 0.08 |
| Hospital, % | |||
| Hospital A | 18.3 | 7.3 | <0.001 |
| Hospital B | 18.9 | 47.6 | |
| Hospital C | 26.8 | 23.4 | |
| Hospital D | 13.4 | 10.5 | |
| Hospital E | 22.6 | 11.2 |
P-value not calculated due to empty cells
Table 3.
Hospital-Level Mean Length of Stay (with 95% confidence intervals) in Daily Hypertonic Saline Recipients Compared with Non-Recipients, adjusting for propensity score.
| Non-Recipients (n=5928) | Daily HTS (n=964) | P-Value | |
|---|---|---|---|
| Hospital A | 2.48 (2.31, 2.6) | 2.47 (1.99, 3.05) | 0.94 |
| Hospital B | 1.58 (1.48, 1.68) | 1.64 (1.47, 1.83) | 0.4 |
| Hospital C | 3.14 (3.0, 3.28) | 2.43 (2.09, 2.81) | 0.0002 |
| Hospital D | 2.94 (2.77, 3.12) | 2.77 (2.23, 3.44) | 0.57 |
| Hospital E | 3.11 (2.99, 3.23) | 3.23 (2.84, 3.67) | 0.52 |
Table 4.
Characteristics of Non-Recipients and Daily HTS Recipients Matched by Propensity Score**
| Variable | Non-Recipients (n=953) | Daily HTS (n=953) | P-Value |
|---|---|---|---|
| Age (months), mean (SD) | 3.82 (3.22) | 2.33 (1.76) | 0.52 |
| Male Sex, % | 59.0 | 59.4 | 0.70 |
| Race, % | |||
| Black | 13.1 | 11.1 | 0.61 |
| White | 71.6 | 71.7 | |
| Other | 13.2 | 15.6 | |
| Unknown | 2.1 | 1.6 | |
| Asthma Diagnosis, % | 7.9 | 6.6 | 0.53 |
| Insurance Category, % | |||
| Medicaid | 68.2 | 66.5 | 0.77 |
| Private Insurance | 28.8 | 29.5 | |
| Self Pay | 1.4 | 2.2 | |
| Other | 1.4 | 1.6 | |
| Unknown | 0.3 | 0.2 | |
| Season, % | |||
| Summer (June–Aug) | 2.0 | 1.2 | 0.66 |
| Fall (Sept–Nov) | 6.8 | 6.7 | |
| Winter (Dec–Feb) | 63.9 | 62.8 | |
| Spring (Mar–May) | 27.3 | 29.4 | |
| Year, % | |||
| 2008 | 0.3 | 0.4 | 0.20 |
| 2009 | 2.9 | 3.7 | |
| 2010 | 32.2 | 26.9 | |
| 2011 | 64.5 | 69.0 | |
| Severity, % | |||
| Intensive Care Unit Admission | 4.5 | 3.3 | 0.37 |
| Non-Invasive Positive Pressure Ventilation | 0.1 | 0.2 | 0.41 |
| Mechanical Ventilation | 0.1 | 0.0 | * |
| Blood Gas | 17.6 | 14.9 | 0.12 |
| Supplemental Oxygen | 49.7 | 51.6 | 0.66 |
| APR-DRG severity subclass (major/extreme) | 9.4 | 6.5 | 0.11 |
| Management, % | |||
| Racemic Epinephrine | 42.7 | 42.5 | 0.99 |
| Albuterol | 55.0 | 50.8 | 0.39 |
| Corticosteroids | 17.9 | 15.7 | 0.12 |
| Continuous Nebulized Therapies | 17.1 | 15.0 | 0.65 |
| Intravenous Fluids | 51.2 | 52.2 | 0.85 |
| Intravenous Antibiotics | 18.4 | 17.4 | 0.64 |
| Chest Radiograph | 46.4 | 45.3 | 0.52 |
| Hospital, % | |||
| Hospital A | 7.4 | 7.4 | 0.58 |
| Hospital B | 45.7 | 47.1 | |
| Hospital C | 22.7 | 23.7 | |
| Hospital D | 10.5 | 10.5 | |
| Hospital E | 13.9 | 11.3 |
P-value not calculated due to empty cells
Limited to visits at hospitals with >5% overall HTS use
Table 5.
Length of Stay in Daily HTS Recipients Compared with Non-Recipients: Propensity Score Matched Analysis
| Non-Recipients (n=953) | Daily HTS (n=953) | β | 95% CI | P-Value | |
|---|---|---|---|---|---|
| LOS, mean | 2.45 days | 2.33 days | −0.0396 | − 0.15, 0.07 | 0.48 |
| Non-Recipients (n=953) | Daily (n=953) | OR* | 95% CI | P-Value | |
|---|---|---|---|---|---|
| LOS > 1 day | 53.2% | 55.9% | 1.17 | 0.98, 1.40 | 0.09 |
| LOS > 2 days | 32.5% | 35.9% | 1.19 | 0.90, 1.59 | 0.22 |
| LOS > 3 days | 22.4% | 18.4% | 0.81 | 0.58, 1.14 | 0.23 |
| LOS > 4 days | 13.7% | 9.4% | 0.67 | 0.47, 0.97 | 0.03 |
LOS = Length of Stay, OR = Odds Ratio, CI = Confidence Interval
REF=Non-Recipients
DISCUSSION
This large multicenter study describes the utilization of nebulized 3% saline and its association with length of stay in the United States. HTS was utilized for acute bronchiolitis in just over half of the 42 pediatric hospitals. Although overall rates of HTS use increased from 0.4% in 2008 to 9.2% in 2011, there was substantial variation in use across hospitals in overall utilization and the patterns of HTS administration. In a propensity score matched analysis, there was no difference in mean length of stay in infants who received HTS daily compared with those who did not receive HTS, although daily HTS recipients had a 33% decreased odds of staying in the hospital longer than 4 days compared with non-recipients.
A Cochrane systematic review published in 2013 included 11 trials of 3% saline compared with normal saline, 6 of which examined hospital length of stay.(6) The meta-analysis found that nebulized hypertonic saline decreased length of stay in infants with bronchiolitis by 1.15 days (95% CI 0.82, 1.49). The mean lengths of hospital stay in these 6 studies ranged from 3.5 to 7.4 days in the control groups and from 2.6 to 6 days in the intervention groups. Studies showing significant declines in LOS using HTS occurred in Canada, United Arab Emirates, Israel, China and Italy.(14–17) The overall mean LOS in infants with bronchiolitis in the 42 hospitals that provided data to PHIS was 2.8 days during our study period. In those where HTS was used daily in >5% of all bronchiolitis patients, the mean LOS was 2.7 days in those not receiving HTS and 2.3 days in those receiving it daily. Thus, overall hospital length of stay and LOS in daily recipients and non-recipients is shorter in our cohort of pediatric hospitals in the United States compared with published randomized trials.
We found no difference in LOS in non-recipients compared with daily HTS recipients when examined as a continuous variable or as a dichotomous variable of LOS greater than 1, 2 or 3 days. This is consistent with the lack of a statistically significant difference in LOS found in the two trials in the Cochrane meta-analysis that had the shortest mean length of stay of 2.5 to 3.5 days.(6) In addition, the only randomized trial to examine length of stay in patients receiving HTS for bronchiolitis in the United States found no difference between hospitalized infants who received HTS (3.2 days) and those who received NS (3.9 days).(18) Also consistent with the results of the Cochrane meta-analysis, we found that daily HTS recipients had decreased odds of staying in the hospital longer than 4 days compared with non-recipients.(6) Three of the four trials that found a statistically significant difference in LOS between HTS and normal saline had mean lengths of stay 4 days or longer.(15–17) Therefore, when taking the results of these trials and our data in aggregate, there may be some benefit of HTS in those patients ill enough to remain in the hospital for more than 4 days. Given the lack of association of HTS with length of stay in our cohort of infants cared for in U.S. pediatric hospitals and the trials demonstrating limited benefit with shorter lengths of stay, HTS may not be beneficial in mild-to-moderate infants with bronchiolitis that have lengths of stay in the 0–4 day range. These findings are consistent with the 2014 AAP bronchiolitis guidelines.(7) These guidelines, recognizing that most US hospitals report a LOS of <72 hours, make a weak recommendation to consider hypertonic saline use for inpatients only if the average LOS for patients with bronchiolitis is >72 hours.
There is significant variation in HTS utilization overall and how HTS is administered across pediatric hospitals in this cohort. The studies that have shown benefit of HTS in both LOS and clinical severity score all initiated HTS at the start of hospitalization and gave multiple doses per day until discharge. This most closely mirrors the daily receipt group in our study. However, when HTS was used in our study, it was not used daily (i.e., pattern of use was trial, rescue, sporadic) in 40% of visits. There was a clear difference in severity and clinical management of patients in our study who received HTS as a trial, rescue, or sporadically when compared with those who received it daily and those who did not receive HTS. Although we cannot discern clinical severity by physical exam using PHIS, the variables that we were able to examine suggest that infants who received HTS intermittently had greater disease severity compared with those who did not. We therefore hypothesize that HTS may have been used intermittently in these more severely affected patients as a last resort or in combination with other therapies when standard supportive measures failed to provide improvement. There is no evidence that such intermittent use is of benefit in infants with bronchiolitis. In fact, seven studies performed in the emergency department setting found no short-term improvement in respiratory distress after 1–3 consecutive doses of HTS, suggesting a lack of benefit of intermittent administration.(18–24)
Our study has several limitations. We are unable to discern the true intent behind use or pattern of use of HTS in individual patients. In addition, we could only determine whether a treatment was administered on a particular day of hospitalization and we could not distinguish how many doses of HTS were given each day. We attempted to limit misclassification bias by including only those infants 12 months of age or younger to minimize inclusion of patients with reactive airways disease. We also included both ICD-9-CM and APR-DRG code in our case ascertainment to minimize misclassification. Any misclassification is likely to be non-differential, therefore biasing our results toward the null. Additionally, the comparison between daily recipients and non-recipients was based on a relatively small proportion of the overall study population for the propensity score LOS analyses. Despite this small proportion overall, only 11 daily HTS recipients, or 1%, were dropped in the propensity score matched analyses. The use of administrative data also limited the types of variables included in the propensity score. For example, we could not include laboratory or imaging results. Furthermore, as with all observational studies, there is the potential for residual confounding. We did, however, include demographic factors, severity and hospital, generating balance between the two comparison groups. Finally, our study period ended in 2011. Since that time, there have been several additional trials evaluating HTS and the publication of the 2014 American Academy of Pediatrics bronchiolitis clinical practice guideline.(7) Although our study addresses one of the key future research needs outlined by the 2014 guideline – studies of HTS in hospitals with shorter LOS – additional research is required. This is emphasized by our finding that despite 4 out of the 5 hospitals showing no difference in mean LOS between daily and non-recipients after adjusting for propensity score, one hospital did show a 0.5-day less mean LOS in daily recipients. Therefore, there may be hospital-level factors that affect LOS in HTS recipients; however, the data available do not allow for any additional analyses to explain the reasons for these results at this single hospital. This highlights the need for future research to understand the effects of HTS in hospitals with shorter lengths of stay for bronchiolitis. Future studies should be prospective and evaluate a standardized means of administering HTS based on available evidence in the outpatient setting and in hospitals with a shorter LOS with an evaluation of both efficacy and effectiveness. Our study has several key strengths. This study addresses real-world effectiveness of HTS as opposed to efficacy in a controlled randomized trial. The randomized trials also all used normal saline as a placebo, even though normal saline may not function as a true placebo.21 Our study overcomes this limitation by comparing daily recipients of HTS with those who did not receive nebulized saline.
Our results suggest that although HTS use is increasing over time, HTS is still not widely used despite the results of meta-analyses suggesting benefit in length of stay and clinical severity. When it is used, there is substantial variation in the patterns of use. We did not observe a reduction in mean length of stay associated with daily HTS use in this retrospective analysis. Further prospective trials are needed to define the role of HTS in treatment of bronchiolitis in the inpatient setting.
Acknowledgments
Supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (8 KL2 TR000078-05 [to T.F.]).
We would like to acknowledge Lilliam Ambroggio, PhD, for advice regarding propensity score methodology, in addition to a critical review of the manuscript, and Matthew Hall, PhD, for assistance with obtaining some of the PHIS data.
ABBREVIATIONS
- APR-DRG
All Patient Refined-Diagnosis Related Group
- HTS
hypertonic saline
- ICD-9-CM
International Classification of Diseases-9-Clinical Modification
- IV
intravenous
- LOS
length of stay
- NS
normal saline
- PHIS
Pediatric Health Information System
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bordley WC, Viswanathan M, King VJ, Sutton SF, Jackman AM, Sterling L, et al. Diagnosis and testing in bronchiolitis: a systematic review. Archives of pediatrics & adolescent medicine. 2004;158:119–26. doi: 10.1001/archpedi.158.2.119. [DOI] [PubMed] [Google Scholar]
- 2.King VJ, Viswanathan M, Bordley WC, Jackman AM, Sutton SF, Lohr KN, et al. Pharmacologic treatment of bronchiolitis in infants and children: a systematic review. Archives of pediatrics & adolescent medicine. 2004;158:127–37. doi: 10.1001/archpedi.158.2.127. [DOI] [PubMed] [Google Scholar]
- 3.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA: the journal of the American Medical Association. 1999;282:1440–6. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 4.Zorc JJ, Hall CB. Bronchiolitis: recent evidence on diagnosis and management. Pediatrics. 2010;125:342–9. doi: 10.1542/peds.2009-2092. [DOI] [PubMed] [Google Scholar]
- 5.American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:1774–93. doi: 10.1542/peds.2006-2223. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Mendoza-Sassi RA, Wainwright C, Klassen TP. Nebulised hypertonic saline solution for acute bronchiolitis in infants. Cochrane database of systematic reviews. 2013;7:CD006458. doi: 10.1002/14651858.CD006458.pub3. [DOI] [PubMed] [Google Scholar]
- 7.Ralston SL, Lieberthal AS, Meissner HC, Alverson BK, Baley JE, Gadomski AM, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474–502. doi: 10.1542/peds.2014-2742. [DOI] [PubMed] [Google Scholar]
- 8.Christakis DA, Cowan CA, Garrison MM, Molteni R, Marcuse E, Zerr DM. Variation in inpatient diagnostic testing and management of bronchiolitis. Pediatrics. 2005;115:878–84. doi: 10.1542/peds.2004-1299. [DOI] [PubMed] [Google Scholar]
- 9.Hughes J. 3M Health Information Systems (HIS) APR-Drug Classification Software Overview. Utah: 3M Health Information Systems; 2009. [Google Scholar]
- 10.Sedman AB, Bahl V, Bunting E, Bandy K, Jones S, Nasr SZ, et al. Clinical redesign using all patient refined diagnosis related groups. Pediatrics. 2004;114:965–9. doi: 10.1542/peds.2004-0650. [DOI] [PubMed] [Google Scholar]
- 11.Weitzen S, Lapane KL, Toledano AY, Hume AL, Mor V. Principles for modeling propensity scores in medical research: a systematic literature review. Pharmacoepidemiology and drug safety. 2004;13:841–53. doi: 10.1002/pds.969. [DOI] [PubMed] [Google Scholar]
- 12.Westreich D, Cole SR, Funk MJ, Brookhart MA, Sturmer T. The role of the c-statistic in variable selection for propensity score models. Pharmacoepidemiology and drug safety. 2011;20:317–20. doi: 10.1002/pds.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Austin PCM, Ko DT, Goeree R, Tu JV. Institute S, editor. Propensity Score Matching for Estimating Treatment Effects. In: Faries DL, AC, Haro JM, Obenshain RL, editors. Analysis of Observational Health Care Data using SAS. Cary, NC: SAS Institute; 2010. p. 55. [Google Scholar]
- 14.Mandelberg A, Tal G, Witzling M, Someck E, Houri S, Balin A, et al. Nebulized 3% hypertonic saline solution treatment in hospitalized infants with viral bronchiolitis. Chest. 2003;123:481–7. doi: 10.1378/chest.123.2.481. [DOI] [PubMed] [Google Scholar]
- 15.Luo ZLE, Luo J, Li S, Zeng F, Yang X, Fu Z. Nebulized Hypertonic Saline/Salbutamol Solution in Hospitalized Children with Mild to Moderate Bronchiolitis. Pediatrics International. 2010;52:199–202. doi: 10.1111/j.1442-200X.2009.02941.x. [DOI] [PubMed] [Google Scholar]
- 16.Luo ZFZ, Liu E, Xu X, Fu X, Peng D, Liu Y, Li S, Zeng F, Yang X. Nebulized Hypertonic Saline Treatment in Hospitalized Children with Moderate to Severe Viral Bronchiolitis. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2011;17:1829–33. doi: 10.1111/j.1469-0691.2010.03304.x. [DOI] [PubMed] [Google Scholar]
- 17.Miraglia Del Giudice M, Saitta F, Leonardi S, Capasso M, Niglio B, Chinellato I, et al. Effectiveness of nebulized hypertonic saline and epinephrine in hospitalized infants with bronchiolitis. International journal of immunopathology and pharmacology. 2012;25:485–91. doi: 10.1177/039463201202500218. [DOI] [PubMed] [Google Scholar]
- 18.Wu S, Baker C, Lang ME, Schrager SM, Liley FF, Papa C, et al. Nebulized Hypertonic Saline for Bronchiolitis: A Randomized Clinical Trial. JAMA pediatrics. 2014;168:657–63. doi: 10.1001/jamapediatrics.2014.301. [DOI] [PubMed] [Google Scholar]
- 19.Al-Ansari KSM, Davidson BL, El Sayyed R, Mahjoub H, Ibrahim K. Nebulized 5% or 3% Hypertonic or 0.9% Saline for Treating Acute Bronchiolitis in Infants. The Journal of pediatrics. 2010;157:630–4. doi: 10.1016/j.jpeds.2010.04.074. [DOI] [PubMed] [Google Scholar]
- 20.Anil AB, Anil M, Saglam AB, Cetin N, Bal A, Aksu N. High volume normal saline alone is as effective as nebulized salbutamol-normal saline, epinephrine-normal saline, and 3% saline in mild bronchiolitis. Pediatric pulmonology. 2010;45:41–7. doi: 10.1002/ppul.21108. [DOI] [PubMed] [Google Scholar]
- 21.Grewal SAS, McConnell DW, Vandermeer B, Klassen TP. 3% Hypertonic Saline with Epinephrine in the Treatment of Acute Bronchiolitis in the Emergency Department. Archives of pediatrics & adolescent medicine. 2009;163:1007–12. doi: 10.1001/archpediatrics.2009.196. [DOI] [PubMed] [Google Scholar]
- 22.Ipek IOYE, Sezer RG, Bozaykut A. The Efficacy of Nebulized Salbutamol, Hypertonic Saline and Salbutamol/Hypertonic Saline Combination in Moderate Bronchiolitis. Pulmonary pharmacology & therapeutics. 2011;24:633–7. doi: 10.1016/j.pupt.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Florin TA, Shaw KN, Kittick M, Yakscoe S, Zorc JJ. Nebulized Hypertonic Saline for Bronchiolitis in the Emergency Department: A Randomized Clinical Trial. JAMA pediatrics. 2014;168:664–70. doi: 10.1001/jamapediatrics.2013.5306. [DOI] [PubMed] [Google Scholar]
- 24.Kuzik BAFM, Kent S, Zielinski D, Kwan CW, Adeleye A, Vegsund BC, Rossi C. Effect of Inhaled Hypertonic Saline on Hospital Admission Rate in Children with Viral Bronchiolitis: A Randomized Trial. Cjem. 2010;12:477–84. doi: 10.1017/s1481803500012690. [DOI] [PubMed] [Google Scholar]