Abstract
Objective
Estrogens can act in the brain to prevent body weight gain. Tremendous research efforts have been focused on estrogen physiology in the brain in the context of body weight control; estrogen receptors and the related signals have been attractive targets for development of new obesity therapies. The objective is to review recent findings in these aspects.
Methods
We reviewed recent studies, primarily from those using the conventional and conditional knockout mouse strains, regarding the cellular and molecular mechanisms for the beneficial effects of estrogens on body weight balance. We also discuss emerging genetic tools that could further benefit the field of estrogen research, and newly developed estrogen-based regimen that produce body weight-lowering benefits.
Results
The body weight-lowering effects of estrogens are mediated by multiple forms of estrogen receptors, in different brain regions through distinct but coordinated mechanisms. Both rapid signals and “classic” nuclear receptor actions of estrogen receptors appear to contribute to estrogenic regulation on body weight.
Conclusion
Estrogen receptors and associated signal networks are potential targets for obesity treatment, and further investigations are warranted.
Introduction
Dramatic decline in circulating 17β-estradiol (E2) in post-menopausal women has been associated with development of obesity, type II diabetes and the metabolic syndrome1. While supplement of E2 may ameliorate these risks, the application of estrogen replacement therapy in post-menopausal women has been very controversial. Since E2 can act upon several forms of estrogen receptors (ERs), and these ERs are coupled with complex intracellular signals, the body weight-lowering benefits provided by E2 are often associated with increased risks of reproductive endocrine toxicity and breast cancer2. Obviously, one solution to this dilemma would be to target selective ER populations and/or ER-coupled signals that produce body weight benefits without unwanted side effects. Therefore, tremendous efforts have been focused on identifying the critical ER isoforms, the specific action sites of ERs, and the ER-coupled intracellular signals that are required for estrogenic actions on body weight control. It needs to be noted that actions of E2 both in the peripheral tissues and in the brain are important for the regulation of energy homeostasis, as demonstrated by studies using systemically or centrally administrated E23. Since the peripheral actions of E2 have been extensively reviewed elsewhere4, this review focuses on the central actions of E2 in the control of body weight.
ERα in the brain regulates multiple aspects of energy homeostasis
It has been well established that estrogens play an essential role in preventing body weight gain. For example, the withdrawal of endogenous estrogens by ovariectomy (OVX) in female animals leads to body weight gain and hyperadiposity, and these obese phenotype can be prevented by E2 replacement5–10. The estrogenic effects on body weight homeostasis are believed to be primarily mediated by estrogen receptor-α (ERα), one of the “classical” estrogen receptors. Humans or mice with mutations in the ERα (Esr1) gene are obese11, 12. Further, deletion of ERα in mice blocks the anti-obesity effects of E2 replacement7. Early studies showed that microinjections of E2 into various brain regions change animal’s feeding behavior and body weight13, 14, suggesting that ERα expressed in the brain is important for the regulation of body weight balance. This notion was further supported by recent observations from various genetic mouse models. For example, Xu and Clegg crossed mice carrying loxP-flanked ERα alleles (ERαlox/lox)15 to the Nestin-Cre transgenic mice16 to produce mice lacking ERα only in the brain17. Female mutant mice develop obesity, characteristic of increased body weight and body fat. Obesity in these mice is associated with hyperphagia, decreased energy expenditure and decreased physical activity, which may all contribute to the development of obesity17. Notably, female mice lacking ERα in the brain display significantly elevated E2 in the circulation17, presumably due to the impaired negative feedback regulation by estrogens. Given that these mice develop robust obese phenotypes despite the higher E2 in the circulation, these observations further argue that compared to ERα expressed in peripheral tissues, brain ERα plays predominant roles in the regulation of energy balance.
ERα is abundantly expressed in multiple brain regions that are implicated in the regulation of body weight balance. These include the ventrolateral portion of the ventromedial hypothalamus (VMH), the arcuate nucleus (ARC), the medial preoptic area (MPOA), and the nucleus of solitary tract (NTS)18. Thus, an important question is which ERα population(s) in the brain are critical for the regulation of energy homeostasis. To this end, several groups have used genetic approaches to dissect out the physiological roles of ERα in various brain regions in the context of body weight control.
ERα in the VMH
As previously mentioned, abundant ERα is concentrated in the ventrolateral subdivision of the VMH18. The VMH (also known as the VMN) is an important component of the neural circuits responsible for the homeostatic regulation of body weight19. Accumulating evidence indicates a significant role of ERα in the VMH in mediating estrogenic actions on body weight balance. For example, Musatov et al. used shRNA-mediated gene silencing approach to knock down ERα in the VMH, while ERα expression in the adjacent ARC and other hypothalamic regions is shown to be unaffected20. Animals with VMH-specific ERα knock-down are less sensitive to E2-induced weight loss and develop obesity characteristic of increased visceral fat20. The obese syndrome is likely caused by decreased physical activity and impaired thermogenesis, whereas food intake of these animals are not directly affected20.
In parallel, Xu and Clegg crossed ERαlox/lox mice and SF1-Cre mice, a VMH-specific Cre mouse line21, to generate mice lacking ERα only in SF1 neurons. Notably, since SF1 only co-localizes with 50% of ERα-positive neurons in the VMH, these crosses achieved deletion of 50% ERα in the VMH17. Nevertheless, female mutant mice show modest body weight gain, and significant increases in body fat with a preferential increase in the visceral fat. Interestingly, these obese phenotypes are associated with normal food intake but profound decreases in brown adipose tissue (BAT)-mediated thermogenesis.
These observations in different models highlighted a significant role of VMH ERα signaling in regulating thermogenesis. This notion is further supported by Martinez de Morentin’s recent findings that injections of E2 into the VMH promote BAT-mediated thermogenesis in a feeding-independent manner22. These authors further demonstrated that effects of E2 in the VMH on thermogenesis are mediated through inhibition of the AMP-activated protein kinase (AMPK) pathway22.
Notably, Correa et al. recently developed a mouse model with NKX2-1, a transcription factor, deleted in VMH SF1 neurons23. Deletion of NKX2-1 in SF1 neurons results in loss of 26% ERα-positive neurons in the VMH23. Interestingly, female mice carrying this mutation develop profound obesity, associated with decreases in physical activity but normal BAT-mediated thermogenesis and food intake23. Further, the authors used the Designer Receptors Exclusively Activated by Designer Drugs (DREADD) approach to show that stimulation of VMH neurons promotes physical activity in mice23. Together, these data support a possibility that at least a subset of VMH ERα neurons function to stimulate physical activity, and therefore to prevent body weight gain.
Thus, multiple studies have demonstrated a critical role of VMH ERα in preventing body weight gain in females. It is clear that actions of VMH ERα do not regulate food intake, but stimulate energy expenditure. It appears that different subsets of VMH ERα neurons regulate different components of energy expenditure. Thus, some VMH ERα neurons primarily stimulate BAT-mediated thermogenesis to burn excess energy, whereas at least a subset of VMH ERα neurons promotes physical activity to dissipate energy.
ERα in the ARC
Abundant ERα is also expressed by neurons in the ARC18. The ARC contains two distinct neural populations. These are neurons expressing pro-opiomelanocortin (POMC) and those expressing neuropeptide Y (NPY) and agouti-related peptide (AgRP). While POMC neurons synthesize and secret an anorexigenic peptide, α-melanocyte-stimulating hormone (α-MSH), to activate melanocortin receptors, NPY/AgRP neurons release orexigenic peptides, NPY and AgRP24–26. Notably, AgRP is the endogenous antagonist of the melanocortin receptors24–26. POMC and NPY/AgRP populations are believed to be the primary central regulators of energy homeostasis27, 28.
Olofsson et al. demonstrated that estrus-dependent fluctuations in circulating E2 in female mice are negatively correlated with expression of NPY and AgRP in the hypothalamus and the amount of daily food intake29. These authors further showed that central administration of E2 inhibits NPY/AgRP neurons and suppresses food intake29. Importantly, the E2-induced anorexia in female mice is blunted when NPY/AgRP neurons are selectively ablated29. This study indicates that NPY/AgRP neurons are functionally required for the inhibitory effects of E2 on food intake. However, these authors also found that NPY/AgRP neurons express none to minimal levels of ERα29. Thus, E2 may regulate these NPY/AgRP neurons indirectly via presynaptic neurons that express ERα; alternatively, E2 may directly regulate NPY/AgRP neurons through other ERs.
Notably, about 20–30% POMC neurons in the ARC co-express ERα30–32. Using electron microcopy, Gao et al. reported that E2 can increase excitatory synaptic inputs onto ARC POMC neurons, which is associated with increased miniature excitatory postsynaptic current33. Similarly, Malyala et al. reported that E2 stimulates POMC neurons by rapidly uncoupling GABAB receptors from the G-protein-gated inwardly rectifying K+ channels34. To further determine the physiological significance of ERα in POMC neurons, Xu and Clegg crossed ERαlox/lox mice and POMC-Cre mice35 to generate mice lacking ERα only in POMC neurons17. Female mice lacking ERα only in POMC neurons develop hyperphagia and modest body weight gain31. Together, these observations indicate that ERα in POMC neurons is physiologically relevant in the regulation of food intake31.
ERα in the DRN
ERα is abundantly expressed in the dorsal raphe nuclei (DRN)18. Cao et al. further demonstrated that the majority of these ERα-positive neurons in the DRN are serotonin (5-HT) neurons36. Consistent with earlier results that E2 increases neural activities (demonstrated by c-fos immunoreactivity) in the DRN37, 38, Cao et al. showed that propylpyrazole triol (PPT, a selective ERα agonist) activates identified DRN 5-HT neurons via an ERα-dependent mechanism36. Interestingly, Santollo et al. reported that microinjections of E2 into the DRN decreases food intake in rats39. To further examine the roles of ERα in DRN 5-HT neurons, Cao et al. crossed ERαlox/lox mice and TPH2-CreER to generate mice lacking ERα only in 5-HT neurons36. Interestingly, while these mutant mice show comparable basal food intake and body weight, they are resistant to estrogenic effects to suppress binge-like eating36. These results suggest that ERα expressed by DRN 5-HT neurons primarily functions to suppress binge-like eating, while its roles in the basal feeding behavior may be minor.
ERα in the NTS
ERα are also present in the brainstem, including the NTS18. Geary et al. showed that E2 replacement in wild type mice suppresses food intake and potentiates CCK-induced satiation, which are accompanied by increased activity in NTS neurons7, 40. Interestingly, these responses are all abolished in mice lacking ERα7, 40. Further, it is shown that direct administration of E2 in the NTS potentiates CCK-induced satiety signals41. Collectively, these findings support the notion that ERα in the brainstem, such as in the NTS, may be another physiologically important site to mediate the E2-induced anorexia.
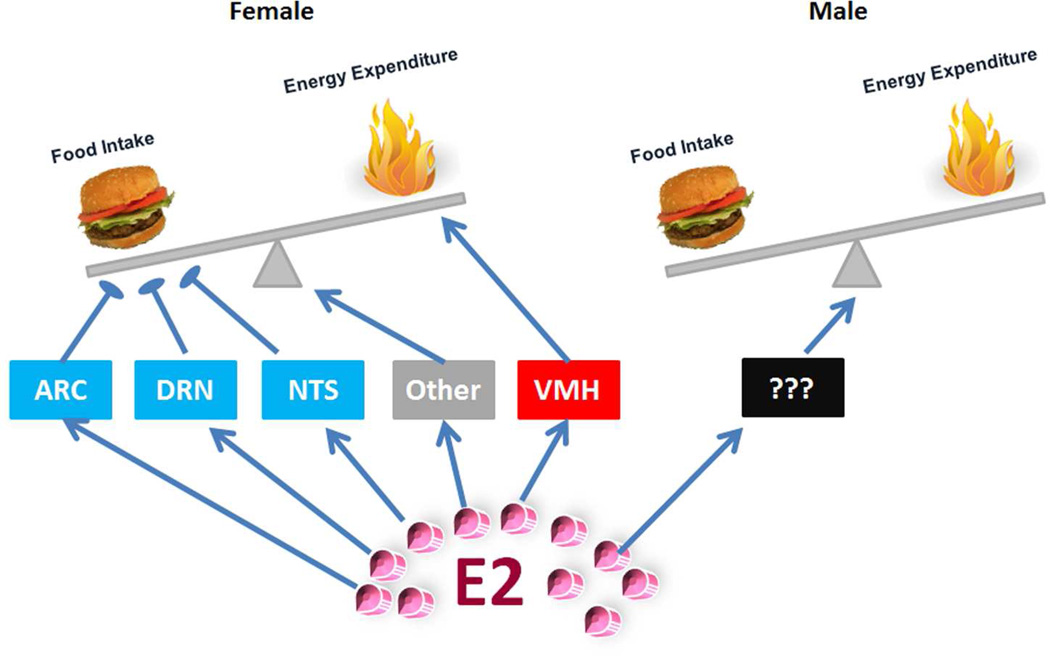
Certainly, the physiological functions of ERα in other brain regions have not been fully revealed. For example, ERα is abundantly expressed in the amygdala42. Earlier studies showed that injections of E2 into the amygdala decrease body weight in rats, effects that retain in rats with large hypothalamic lesions43, suggesting a potential roles of amygdala ERα in body weight control. In addition, Santollo et al. reported that microinjections of E2 into the MPOA decreases food intake in rats39. Further, accumulating evidence indicates that E2 regulates food-associated reward44, suggesting a role of ERα (or other ERs) expressed by brain reward centers (e.g. the nucleus accumbens and the lateral hypothalamus)18. The functions of these ERα populations (among others) warrant further validation with genetic models. Despite the incomplete genetic mapping for ERα functions in brain regions, an interesting segregation model already started to emerge (Fig. 1). Thus, ERα in the VMH enhances energy expenditure by stimulating BAT-mediated thermogenesis and/or physical activity; ERα in the ARC, DRN, NTS, and perhaps other regions, prevents body weight gain primarily by suppressing energy intake. These segregated ERα populations may function complementarily to mediate the full spectrum of estrogenic effects on female energy homeostasis. Supporting this possibility, Xu and Clegg have shown that female mice lacking ERα in both VMH SF1 neurons and in ARC POMC neurons develop hyperphagia and decreased thermogenesis which result in more robust obesity compared to modest obesity seen in mice with ERα deletion only in POMC neurons or in SF1 neurons17.
Figure 1. An emerging segregation model for brain ERα functions in female and male energy balance.
In female brains, ERα expressed by ARC, DRN and NTS neurons primarily suppresses food intake; ERα expressed by VMH neurons enhances energy expenditure; these distinct ERα populations, in addition to perhaps unidentified ERα neurons, function complementarily to mediate the full spectrum of estrogenic effects on female energy homeostasis. In males, while brain ERα is also required to maintain normal energy balance, the exact ERα sites remain to be identified.
ERα in male brains
It is clear that actions of ERα also prevent obesity in males. For example, ERα gene deficiency results in obesity in male mice12, 45 and in men46, 47. In addition, administration of E2 or its analogs reduces body weight in male mice33, 48. The major male sex hormone, testosterone, can be converted into E2 by aromatase, and both male and female aromatase knockout mice develop obesity49. Notably, abundant aromatase is expressed by the brain50, which makes it possible that ERα in male brains could be exposed to high levels of E2 despite the lack of circulating estrogens. Consistent with this notion, Xu and Clegg showed that male mice lacking ERα in the brain develop obesity17, arguing that brain ERα also regulates male energy balance as it does in females. However, deletion of ERα in VMH neurons, POMC neurons, or DRN neurons, although produces feeding and/or body weight phenotypes in females, fails to affect male energy homeostasis. Thus, it is speculated that different brain ERα population(s) may be responsible for estrogenic actions on body weight balance in males, which remain to be identified.
ERα-coupled intracellular signals
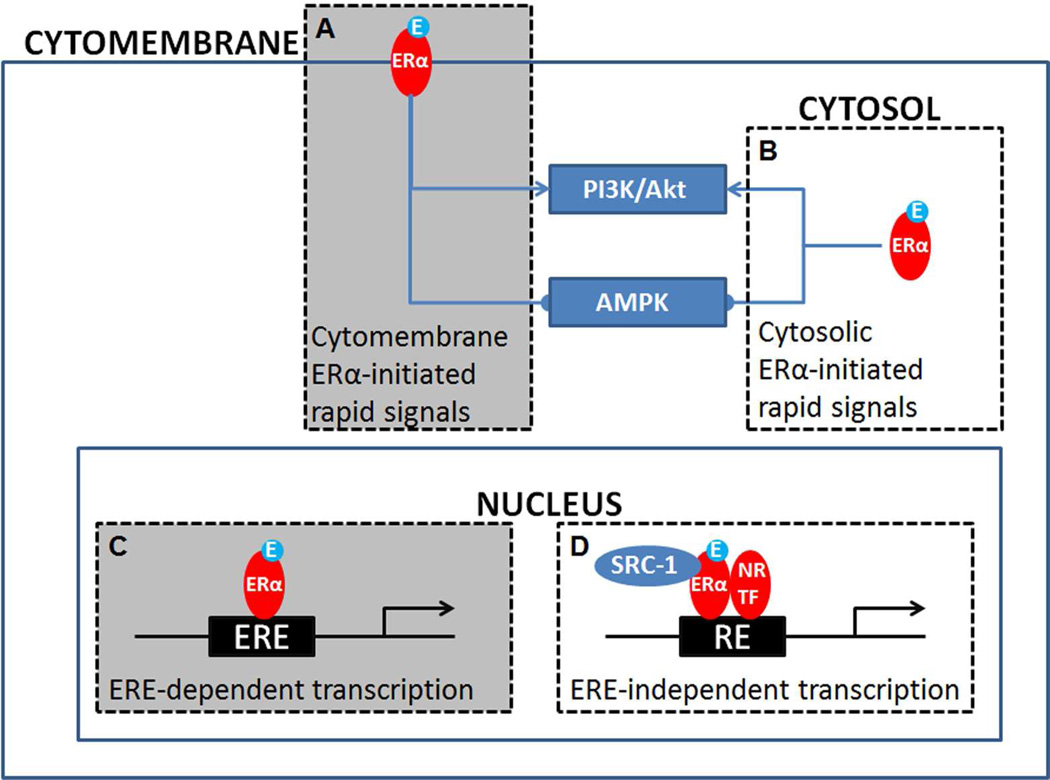
In addition to the sites of ERα actions, another major question in the field is what intracellular signals mediate ERα effects on body weight balance. ERα-coupled intracellular events can be divided into several modes. First, sub-sets of intracellular ERα are concentrated on the cytomembrane and in the cytosol, where it regulates rapid signaling pathways, including the PI3K/Akt pathway and the AMPK pathway (Fig. 2A and 2B). Park et al. found that E2 stimulates the PI3K/Akt cascade in VMH neurons51. Similarly, Malyala et al. reported that E2 activates ARC POMC neurons in an PI3K-dependent manner34. As mentioned above, Martinez de Morentin et al. found that E2 inhibits the AMPK pathway in VMH neurons via an ERα-dependent mechanism and this inhibition mediates estrogenic actions to stimulate thermogenesis22. Together, these observations support a model that ERα-initiated rapid signaling pathways, including PI3K and AMPK, mediate estrogenic actions to prevent body weight gain. However, it is worth noting that the roles of these rapid signals are not fully supported by observations from a transgenic MOER mouse model developed by Pedram et al.52 In MOER mice, the full length ERα protein is replaced by the E domain of the receptor, which only exists on the cytomembrane and retains capacity of initiating rapid signalings (e.g. PI3K)52. Importantly, no ERα activity is present in the cytosol or in the nucleus in MOER mice. Interestingly, MOER mice show similar obese phenotypes as ERα knockout mice52. Thus, these findings suggest that rapid signals initiated by cytomembrane ERα is not sufficient to mediate anti-obesity effects of estrogens52. Nevertheless, since the rapid signals initiated by cytosolic ERα are also eliminated in these MOER mice, the possible contribution of the cytosolic ERα to energy homeostasis still remains unknown (Fig. 2B).
Figure 2. Proposed models for intracellular mechanisms mediating estrogen/ERα signals to regulate energy homeostasis.
(A-B) ERα located on the cytomembrane (A) and/or ERα located in the cytosol (B) can regulate rapid signaling pathways, including activating the PI3K/Akt pathway and inhibiting the AMPK pathway. (C) ERα in the nucleus may act as a classic nuclear receptor and regulate targeted gene transcription via directly binding to the EREs. (D) Nuclear ERα can also form complex with other nuclear receptors (NR) or transcription factors (TF) to regulate gene transcription via an ERE-independent mechanism. While mechanisms described in grey boxes (A) and (C) have been excluded by observations from MOER and NERKI mouse models, respectively, contributions of cytosolic ERα-initiated rapid signals (B) and ERα-initiated ERE-independent gene transcription (D) in body weight control have not been directly tested.
As a classic nuclear receptor, ERα can also translocate to the nucleus to directly bind to the estrogen response elements (EREs) on the target genes and regulate gene transcription (Fig. 2C). These ERE-dependent actions of ERα, however, do not appear to mediate the anti-obesity effects of E2. Park et al. generated a NERKI mouse model in which E207A/G208A mutations were introduced in the DNA binding domain of ERα, which abolish ERα-ERE binding53. In these mice, metabolic phenotypes affected in ERα knockout mice including body weight, glucose homeostasis, energy expenditure, and physical activity, are restored to nearly normal levels51, suggesting that the ERE-dependent ERα functions are not required to maintain body weight.
As a nuclear receptor, ERα can also form complex with other nuclear receptors or transcription factors, which regulates gene transcription in an ERE-independent manner (Fig. 2D). Little is known, however, about whether the ERE-independent ERα functions are involved in estrogenic effects on body weight control. Zhu et al. showed that hypothalamic ERα interacts with a nuclear receptor co-activator, namely steroid receptor coactivator-1 (SRC-1), and that deletion of SRC-1 blunts effects of E2 to reduce body weight and food intake, and to stimulate energy expenditure54. These suggest that ERα’s nuclear receptor properties may still be required for estrogenic actions on energy homeostasis. Based on these observations and those from NERKI mice51, it is speculated that the ERE-independent ERα functions may contribute to estrogenic effects on body weight control. Future studies, therefore, are warranted to identify the nuclear receptors and transcription factors that form complex with ERα to mediate estrogenic actions on body weight balance.
ERβ and body weight balance
Compared to ERα, estrogen receptor-β (ERβ), another classic ER has received less attention at least in the context of body weight balance. An earlier study by Ohlsson et al. reported that chow-fed mice with global deficiency in ERβ show normal body weight and fat mass compared to wild type mice55. In addition, the authors reported that mice with compound knockout of both ERα and ERβ develop obesity with the same severity as mice only lacking ERα55. Consistent with this, both Santollo et al.56 and Roesch9 found that an ERβ agonist, diarylpropionitrile (DPN), has no effect effects on food intake and body weight in chow-fed OVX rats, while PPT (the ERα agonist) at similar doses can significantly reduce food intake and body weight. While these earlier studies suggest a minor role of ERβ in body weight control in chow-fed animals, Foryst-Ludwig et al. demonstrated that ERβ knockout mice, when fed on a high fat-diet (HFD), developed obesity compared to HFD-fed wild type mice57. This increased sensitivity to diet-induced obesity is associated with normal food intake, but increased energy expenditure and decreased fat oxidation57. Consistently, Yepuru et al. developed new selective ERβ agonists (β-LGNDs), and found these agonists attenuate HFD-induced body weight gain associated with increased energy expenditure58. Thus, the current data suggest that ERβ may play an important role in preventing obesity when animals are challenged by obesogenic diets, while ERβ’s functions in animals fed on regular chow diets appear to be minimal. Certainly, the ERβ-mediated control of energy homeostasis warrants further investigation. For example, the action sites of ERβ on energy balance remain to be confirmed, although both Foryst-Ludwig et al. and Yepuru et al. suggested a contribution from ERβ in the peripheral tissues57, 58.
GPR30 and body weight balance
GPR30 (also known as GPER) is a G protein-coupled estrogen receptor, bound to the cell membrane. In vitro studies confirmed that E2 binds to GPR30. Body weight phenotypes among several independent GPR30 knockout mouse lines are controversial. For example, both Haas et al.59 and Sharma et al.60 observed obese phenotypes in male and female GPR30 knockout mice, which were generated by Wang et al.61; however, Liu et al. reported no difference in body weight in the same GPR30 knockout stain62. Otto et al. constructed an independent GPR30 knockout line, and found no obese phenotypes in female mutants63. Interestingly, another GPR30 knockout line generated by Martensson et al. showed reduced body weight only in females, not in males64. More recently, Davis et al. carefully characterized Wang’s GPR30 knockout mice and reported that both male and female mutants are significantly heavier than wild type littermates, which appears to depend on reduced energy expenditure independent of physical activity, but not on food intake65. Importantly, body weight-lowering effects of E2 are attenuated in OVX GPR30 knockout mice compared to OVX wild type mice65. The discrepancy from these studies may be attributed to different strategies to construct the GPR30 knockout alleles, different genetic background mice were maintained on, and/or different facility environment, etc. Nevertheless, observations from Wang’s GPR30 knockout line are largely consistent and suggest a potential role of GPR30 in estrogenic regulation on body weight homeostasis. Obviously, effects of GPR30 on energy balance need further validation.
Genetic tools to dissect estrogenic actions in body weight balance
As illustrated above, our understanding about estrogenic actions on body weight balance has certainly benefited from a powerful battery of genetic mouse models. This is especially the case for ERα. The requirement of ERα functions for normal body weight balance was first validated by characterizations of the conventional ERα knockout mice12. This quickly stimulated the field to further identify the critical action sites of ERα using the Cre-loxP strategy. Development of the ERαlox/lox mouse line by Feng et al. in 2007, which was initially aimed to investigate ERα functions in the mammary glands15, has simultaneously benefited the field of obesity research. This mouse line, when combined with various Cre drivers that target distinct brain regions, has ultimately allowed the field to definitively examine the physiological roles of ERα in these various brain regions in body weight control17, 36. Of course, the ERαlox/lox mouse line has also been widely used to examine metabolic functions of ERα in peripheral tissues, including the adipose tissue66 and the liver67. Notably, an ERβlox/lox mouse line has been developed and validated by Antal et al68. With emerging evidence for a significant role of ERβ in preventing diet-induced obesity, it is expected that this ERβlox/lox mouse line will be heavily used in the field to dissect out critical ERβ populations for its beneficial effects against obesity.
Lee et al. recently developed and validated an Esr1Cre mouse line that expresses Cre recombinase in ERα-positive cells69. The authors have combined this Cre mouse line with viral vectors that express optogenetic channels, e.g. channelrhodopsin and halorhodopsin, to achieve photostimulation or photoinhibition of ERα-positive neurons selectively in the VMH. In addition to the site specificity (only targeting VMH ERα neurons), more importantly, this strategy allows manipulations of neural activity with various scales of strength and with a high temporal resolution. Taking these advantages, the authors elegantly demonstrated that while weak activation of VMH ERα neurons instantly triggers sexual behavior, strong activation of the same neurons initiates attack69. Certainly, this Esr1Cre mouse line is a long-awaited tool for the field of estrogen research. With this tool, combined with the optogenetic and pharmogenetic (DREADD) viruses, investigators can examine the roles of ERα neural activity in any given brain region in the regulation of food intake, energy expenditure, thermogenesis, and physical activity, etc. In addition, this Esr1Cre mouse can be crossed to various loxed mouse alleles to delete genes of interest only in ERα-positive cells. These would allow further dissection of intracellular signals in ERα-positive cells that may mediate estrogenic functions, including body weight control.
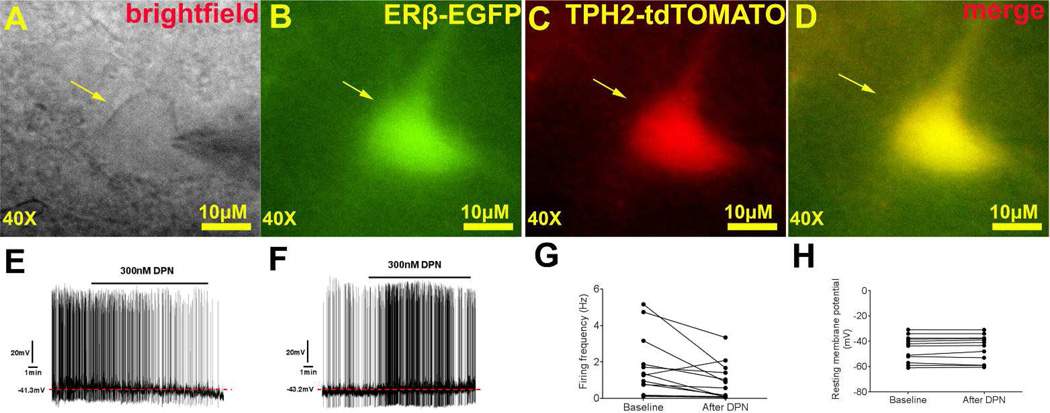
Other genetic tools that may benefit the estrogen field include a transgenic ERα-eGFP mouse line developed by Matsuda et al.70 The authors have confirmed that in these ERα-eGFP mice, an enhanced GFP protein is expressed largely in ERα-positive neurons (as indicated by endogenous ERα immunoreactivity), although a few GFP-labeled neurons are found to be ERα negative70. Similarly, Milner et al. developed and validated a transgenic ERβ-eGFP mouse line, which expresses the enhanced GFP protein in ERβ-positive cells71. Obviously, these eGFP models can replace the conventional staining procedures (e.g. immunohistochemistry or in situ hybridization) required to visualize ER-positive neurons; GFP can also be easily combined with another staining protocol to determine if ER-positive cells express other proteins or mRNAs. In addition to these histological applications in usually fixed tissues, investigators can also use GFP as a marker to directly identify ER-positive cells in un-fixed tissues. For example, flow cytometry can be used to sort out highly purified cells that express ERα or ERβ; the similar approach has been applied to purify NPY/AgRP neurons which facilitated the discovery of novel factors that regulate these neurons and food intake72. In addition, investigators can prepare fresh brain slices from ERα-eGFP or ERβ-eGFP mice, and perform electrophysiological recordings in identified ER-positive neurons. One step further is to cross these eGFP transgenes onto mice that express another fluroscent reporter in selective neural population. For example, we have crossed the ERβ-eGFP mice with TPH2-CreER/Rosa26-tdTOMATO mice36 to generate mice carrying all these 3 transgenic alleles. In the tri-genetic offspring, TPH2-CreER/Rosa26-tdTOMATO/ERβ-eGFP mice, tamoxifen injections (3 mg/injections, intraperitoneal, twice 24 hours apart) induced the strong red fluorescence (TOMATO) exclusively in TPH2-positive neurons (5-HT neurons); enhanced GFP is expressed only in ERβ-positive neurons; thus, double labelled neurons (yellow) are identified as ERβ-positive 5-HT neurons (Fig. 3A–D). With this tool, we performed electrophysiological recordings in ERβ-positive 5-HT neurons in the DRN and examined effects of DPN (the ERβ agonist) on firing properties of these neurons. We found that DPN decreased the firing frequency in 12 out of 13 neurons we recorded (see typical trace in Fig. 3E and summarized data in Fig. 3G), while the other neuron showed increased firing frequency upon the DPN treatment (see the trace in Fig. 3F and summarized data in Fig. 3G); resting membrane potential of all neurons were not altered (Fig. 3H). These findings are interesting, as the ERβ agonist inhibits most of ERβ-positive 5-HT neurons in the DRN, while the ERα agonist (PPT) has been shown to activate DRN 5-HT neurons36. More importantly, the fact that all double-labelled neurons responded to the ERβ agonist provided the proof of the principle that the ERα-eGFP and ERβ-eGFP mouse models could be used in combination with other genetically labelled reporter lines to allow experiments in highly selective neural populations. These will no doubt advance our understanding about molecular and cellular actions of these ERs.
Figure 3. Electrophysiological recordings in identified ERβ-positive 5-HT neurons in the DRN.
(A–D) Brightfield (A), fluorescence for GFP (B), for TOMATO (C) and merge (D) from a recorded neuron in the DRN of the brain slice prepared from a TPH2-CreER/Rosa26-tdTOMATO/ERβ-eGFP mouse. (E) A representative trace showing that DPN treatment (300 nM, bath perfusion) decreased firing frequency of ERβ-positive 5-HT neurons in the DRN. (F) The trace of the only ERβ-positive 5-HT neuron that showed increased firing frequency upon DPN treatment (300 nM, bath perfusion). (G–H) Summary data of the effects of DPN (300 nM) on firing frequency (G) and resting membrane potential (H) in 13 ERβ-positive 5-HT neurons in the DRN.
Therapeutic potential
While the body weight-lowering effects of estrogens have been well established in animal models, the application of estrogen replacement therapy to treat/prevent obesity in humans (including post-menopausal women) has been hampered due to increased risks of reproductive endocrine toxicity and breast cancer associated with the conventional estrogen replacement therapy2. One idea to overcome these issues is to develop estrogen analogs that may only target specific ER isoforms or specific sites of ER actions that produce body weight benefits. Indeed, Finan et al. recently developed a GLP-1-estrogen conjugate, which uses glucagon-like peptide-1 (GLP-1) as a “carrier” to deliver estrogens preferentially to GLP-1 receptor-enriched regions, including the hypothalamus48. The authors demonstrated that systemic administration of this GLP-1-estrogen conjugate, in both male and female mice with diet-induced obesity, substantially reduces body weight and food intake, and improves the glucose tolerance and insulin sensitivity48. Most importantly, the common side effects associated with estrogen replacement therapy (e.g. reproductive endocrine toxicity and breast cancer risks) are avoided in GLP-1-estrogen treated mice, presumably because estrogens are not delivered to the reproductive organs and the breast48. Further mechanistic studies indicate that the body weight-lowering effects of the conjugate stem from both GLP-1 and estrogens; interestingly, both ERα and ERβ are required for estrogen-mediated benefits since genetic deletion of either of these ERs blunts effects of the conjugate48. Subsequently, Cao et al. found that GLP-1-estrogen also delivers bioactive estrogens to the DRN and substantially suppresses binge-like eating in female mice partially through acting upon ERα expressed by DRN 5-HT neurons36. The development of this GLP-1-estrogen conjugate is certainly an exciting step forward in the field, as this conjugate itself or its modified forms could potentially become a therapy for obesity and/or binge eating. Further, these studies proved the concept that estrogens could be conjugated with other peptides or molecules to achieve target-specific delivery and therefore avoid unwanted side effects while maintaining their beneficial effects.
Another strategy is to conjugate E2 with selective estrogen receptor modulators (SERMs) which function to antagonize E2 actions selectively in the breast and reproductive organs. Kim JH et al. recently tested such a conjugate, equine estrogen (CE) and bazedoxifene (BZA)73. Oral administration of CE and BZA produces profound body weight loss through stimulating lipid oxidation and energy expenditure in HFD-fed OVX mice73. Importantly, this regimen does not cause increases in uterine weight in mice73, because BZA antagonizes estrogenic actions in breast and uterus74, 75.
The newly developed ERβ agonists (β-LGNDs) may also carry therapeutic potentials. As mentioned above, Yepuru et al. demonstrated that administration of β-LGNDs in HFD-fed mice significantly prevents body weight gain and improves glucose tolerance58. Unlike E2, these agonists do not stimulate proliferation of a human endometrial adenocarcinoma cell lines in vitro, and neither do these agonists increase uterine weight in female rats58, suggesting minimal side effects at least in the reproductive organs. While further investigations are needed to test the potential toxicities in other tissues (e.g. the breast), these newly developed ERβ agonists represent an alternative strategy: to selectively target ERβ as potential therapies for obesity.
Conclusions
The body weight-lowering effects of E2 are likely mediated by multiple forms of ERs. In particular, ERα in different brain regions regulates distinct aspects of female energy balance, and these different ERα populations may provide well-coordinated responses in food intake, thermogenesis and physical activity to ultimately prevent body weight gain. While ERα in male brains is also essential for the maintenance of normal body weight, the exact sites of actions remain to be identified. With regard to ERα-coupled intracellular signals, both rapid signals (e.g. PI3K and AMPK) and “classic” nuclear receptor actions on gene expression appear to contribute to estrogenic regulation on body weight, although the picture of this complex signal network is still not clear. Effects of other ERs (ERβ and GRP30) on body weight balance may have been under-appreciated in the past; fortunately, revisits of various knockout models started to reveal previously unrecognized roles of these receptors, and these warrant further investigations. Another area that deserves more careful investigations in future is the interactions of E2 and other sex hormones (e.g. progesterone) in the context of feeding and body weight control, as emerging evidence from human studies indicates that the interactions of E2 and progesterone, rather than either alone, influence feeding behaviors in cycling women76.
It needs to be recognized that the applications of various genetic mouse models, e.g. conventional knockout strains, ERαlox/lox, MOER and NERKI, have substantially advanced our current understanding about where and how estrogens regulate body weight balance. Further, several additional relevant genetic mouse tools, including ERβlox/lox, Esr1Cre, and eGFP reporters, can be added into our tool box. These tools are expected to facilitate our future research efforts to complete mapping critical ERα/ERβ sites for body weight controls, to identify the intracellular signals or target genes that mediate estrogenic actions on body weight, to reveal the physiological relevance of ERα neural activities in controlling feeding behavior and/or energy expenditure, and to gain molecular insights regarding how ERα/ERβ neurons may be regulated. Of course, these tools will also be extremely useful to validate the newly developed estrogen-based compounds as potential obesity therapies, and to facilitate the identification of new targets and development of new compounds.
Acknowledgements
The authors are supported by grants from the NIH (R01DK093587 and R01DK101379).
References
- 1.Allende-Vigo MZ. Women and the metabolic syndrome: an overview of its peculiarities. P R Health Sci J. 2008;27:190–195. [PubMed] [Google Scholar]
- 2.Billeci AM, Paciaroni M, Caso V, Agnelli G. Hormone replacement therapy and stroke. Curr Vasc Pharmacol. 2008;6:112–123. doi: 10.2174/157016108783955338. [DOI] [PubMed] [Google Scholar]
- 3.Yonezawa R, Wada T, Matsumoto N, et al. Central versus peripheral impact of estradiol on the impaired glucose metabolism in ovariectomized mice on a high-fat diet. Am J Physiol Endocrinol Metab. 2012;303:E445–E456. doi: 10.1152/ajpendo.00638.2011. [DOI] [PubMed] [Google Scholar]
- 4.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34:309–338. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaustein JD, Wade GN. Ovarian influences on the meal patterns of female rats. Physiol Behav. 1976;17:201–208. doi: 10.1016/0031-9384(76)90064-0. [DOI] [PubMed] [Google Scholar]
- 6.Drewett RF. Sexual behaviour and sexual motivation in the female rat. Nature. 1973;242:476–477. doi: 10.1038/242476a0. [DOI] [PubMed] [Google Scholar]
- 7.Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology. 2001;142:4751–4757. doi: 10.1210/endo.142.11.8504. [DOI] [PubMed] [Google Scholar]
- 8.Wallen WJ, Belanger MP, Wittnich C. Sex hormones and the selective estrogen receptor modulator tamoxifen modulate weekly body weights and food intakes in adolescent and adult rats. J Nutr. 2001;131:2351–2357. doi: 10.1093/jn/131.9.2351. [DOI] [PubMed] [Google Scholar]
- 9.Roesch DM. Effects of selective estrogen receptor agonists on food intake and body weight gain in rats. Physiology & behavior. 2006;87:39–44. doi: 10.1016/j.physbeh.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 10.Rogers NH, Perfield JW, 2nd, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009 doi: 10.1210/en.2008-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okura T, Koda M, Ando F, Niino N, Ohta S, Shimokata H. Association of polymorphisms in the estrogen receptor alpha gene with body fat distribution. Int J Obes Relat Metab Disord. 2003;27:1020–1027. doi: 10.1038/sj.ijo.0802378. [DOI] [PubMed] [Google Scholar]
- 12.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butera PC, Beikirch RJ. Central implants of diluted estradiol: independent effects on ingestive and reproductive behaviors of ovariectomized rats. Brain Res. 1989;491:266–273. doi: 10.1016/0006-8993(89)90062-0. [DOI] [PubMed] [Google Scholar]
- 14.Palmer K, Gray JM. Central vs. peripheral effects of estrogen on food intake and lipoprotein lipase activity in ovariectomized rats. Physiol Behav. 1986;37:187–189. doi: 10.1016/0031-9384(86)90404-x. [DOI] [PubMed] [Google Scholar]
- 15.Feng Y, Manka D, Wagner KU, Khan SA. Estrogen receptor-alpha expression in the mammary epithelium is required for ductal and alveolar morphogenesis in mice. Proc Natl Acad Sci U S A. 2007;104:14718–14723. doi: 10.1073/pnas.0706933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruning JC, Gautam D, Burks DJ, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y, Nedungadi TP, Zhu L, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14:453–465. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor alpha and beta in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J Comp Neurol. 2004;473:270–291. doi: 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- 19.King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav. 2006;87:221–244. doi: 10.1016/j.physbeh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Musatov S, Chen W, Pfaff DW, et al. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhillon H, Zigman JM, Ye C, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 22.Martinez de Morentin PB, Gonzalez-Garcia I, Martins L, et al. Estradiol Regulates Brown Adipose Tissue Thermogenesis via Hypothalamic AMPK. Cell Metab. 2014;20:41–53. doi: 10.1016/j.cmet.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Correa SM, Newstrom DW, Warne JP, et al. An estrogen-responsive module in the ventromedial hypothalamus selectively drives sex-specific activity in females. Cell Rep. 2015;10:62–74. doi: 10.1016/j.celrep.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 25.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 26.Williams DL, Schwartz MW. The melanocortin system as a central integrator of direct and indirect controls of food intake. Am J Physiol Regul Integr Comp Physiol. 2005;289:R2–R3. doi: 10.1152/ajpregu.00226.2005. [DOI] [PubMed] [Google Scholar]
- 27.Cone RD. The Central Melanocortin System and Energy Homeostasis. Trends Endocrinol Metab. 1999;10:211–216. doi: 10.1016/s1043-2760(99)00153-8. [DOI] [PubMed] [Google Scholar]
- 28.Huszar D, Lynch CA, Fairchild-Huntress V, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 29.Olofsson LE, Pierce AA, Xu AW. Functional requirement of AgRP and NPY neurons in ovarian cycle-dependent regulation of food intake. Proc Natl Acad Sci U S A. 2009;106:15932–15937. doi: 10.1073/pnas.0904747106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller MM, Tousignant P, Yang U, Pedvis S, Billiar RB. Effects of age and long-term ovariectomy on the estrogen-receptor containing subpopulations of beta-endorphin-immunoreactive neurons in the arcuate nucleus of female C57BL/6J mice. Neuroendocrinology. 1995;61:542–551. doi: 10.1159/000126878. [DOI] [PubMed] [Google Scholar]
- 31.Xu Y, Nedungadi TP, Zhu L, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011 doi: 10.1016/j.cmet.2011.08.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Souza FS, Nasif S, Lopez-Leal R, Levi DH, Low MJ, Rubinsten M. The estrogen receptor alpha colocalizes with proopiomelanocortin in hypothalamic neurons and binds to a conserved motif present in the neuron-specific enhancer nPE2. Eur J Pharmacol. 2011;660:181–187. doi: 10.1016/j.ejphar.2010.10.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao Q, Mezei G, Nie Y, et al. Anorectic estrogen mimics leptin's effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med. 2007;13:89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- 34.Malyala A, Zhang C, Bryant DN, Kelly MJ, Ronnekleiv OK. PI3K signaling effects in hypothalamic neurons mediated by estrogen. J Comp Neurol. 2008;506:895–911. doi: 10.1002/cne.21584. [DOI] [PubMed] [Google Scholar]
- 35.Balthasar N, Coppari R, McMinn J, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Cao X, Xu P, Oyola MG, et al. Estrogens stimulate serotonin neurons to inhibit binge-like eating in mice. J Clin Invest. 2014;124:4351–4362. doi: 10.1172/JCI74726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dalmasso C, Amigone JL, Vivas L. Serotonergic system involvement in the inhibitory action of estrogen on induced sodium appetite in female rats. Physiol Behav. 2011;104:398–407. doi: 10.1016/j.physbeh.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 38.Robichaud M, Debonnel G. Oestrogen and testosterone modulate the firing activity of dorsal raphe nucleus serotonergic neurones in both male and female rats. J Neuroendocrinol. 2005;17:179–185. doi: 10.1111/j.1365-2826.2005.01292.x. [DOI] [PubMed] [Google Scholar]
- 39.Santollo J, Torregrossa AM, Eckel LA. Estradiol acts in the medial preoptic area, arcuate nucleus, and dorsal raphe nucleus to reduce food intake in ovariectomized rats. Horm Behav. 2011;60:86–93. doi: 10.1016/j.yhbeh.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asarian L, Geary N. Estradiol enhances cholecystokinin-dependent lipid-induced satiation and activates estrogen receptor-alpha-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology. 2007;148:5656–5666. doi: 10.1210/en.2007-0341. [DOI] [PubMed] [Google Scholar]
- 41.Thammacharoen S, Lutz TA, Geary N, Asarian L. Hindbrain administration of estradiol inhibits feeding and activates estrogen receptor-alpha-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology. 2008;149:1609–1617. doi: 10.1210/en.2007-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor alpha and beta in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J Comp Neurol. 2004;473:270–291. doi: 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- 43.Donohoe TP, Stevens R. Modulation of food intake by amygdaloid estradiol benzoate implants in female rats. Physiol Behav. 1981;27:105–114. doi: 10.1016/0031-9384(81)90306-1. [DOI] [PubMed] [Google Scholar]
- 44.Asarian L, Geary N. Sex differences in the physiology of eating. Am J Physiol Regul Integr Comp Physiol. 2013;305:R1215–R1267. doi: 10.1152/ajpregu.00446.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Callewaert F, Venken K, Ophoff J, et al. Differential regulation of bone and body composition in male mice with combined inactivation of androgen and estrogen receptor-alpha. FASEB J. 2009;23:232–240. doi: 10.1096/fj.08-113456. [DOI] [PubMed] [Google Scholar]
- 46.Grumbach MM, Auchus RJ. Estrogen: consequences and implications of human mutations in synthesis and action. J Clin Endocrinol Metab. 1999;84:4677–4694. doi: 10.1210/jcem.84.12.6290. [DOI] [PubMed] [Google Scholar]
- 47.Smith EP, Boyd J, Frank GR, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 48.Finan B, Yang B, Ottaway N, et al. Targeted estrogen delivery reverses the metabolic syndrome. Nat Med. 2012;18:1847–1856. doi: 10.1038/nm.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones ME, Thorburn AW, Britt KL, et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci U S A. 2000;97:12735–12740. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu MV, Manoli DS, Fraser EJ, et al. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell. 2009;139:61–72. doi: 10.1016/j.cell.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park CJ, Zhao Z, Glidewell-Kenney C, et al. Genetic rescue of nonclassical ERalpha signaling normalizes energy balance in obese Eralpha-null mutant mice. J Clin Invest. 2011;121:604–612. doi: 10.1172/JCI41702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pedram A, Razandi M, Kim JK, et al. Developmental phenotype of a membrane only estrogen receptor alpha (MOER) mouse. J Biol Chem. 2008 doi: 10.1074/jbc.M806249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jakacka M, Ito M, Martinson F, Ishikawa T, Lee EJ, Jameson JL. An estrogen receptor (ER)alpha deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo. Mol Endocrinol. 2002;16:2188–2201. doi: 10.1210/me.2001-0174. [DOI] [PubMed] [Google Scholar]
- 54.Zhu L, Yang Y, Xu P, et al. Steroid receptor coactivator-1 mediates estrogenic actions to prevent body weight gain in female mice. Endocrinology. 2013;154:150–158. doi: 10.1210/en.2012-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ohlsson C, Hellberg N, Parini P, et al. Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochem Biophys Res Commun. 2000;278:640–645. doi: 10.1006/bbrc.2000.3827. [DOI] [PubMed] [Google Scholar]
- 56.Santollo J, Wiley MD, Eckel LA. Acute activation of ER alpha decreases food intake, meal size, and body weight in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2194–R2201. doi: 10.1152/ajpregu.00385.2007. [DOI] [PubMed] [Google Scholar]
- 57.Foryst-Ludwig A, Clemenz M, Hohmann S, et al. Metabolic actions of estrogen receptor beta (ERbeta) are mediated by a negative cross-talk with PPARgamma. PLoS Genet. 2008;4:e1000108. doi: 10.1371/journal.pgen.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yepuru M, Eswaraka J, Kearbey JD, et al. Estrogen receptor-{beta}-selective ligands alleviate high-fat diet- and ovariectomy-induced obesity in mice. The Journal of biological chemistry. 2010;285:31292–31303. doi: 10.1074/jbc.M110.147850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haas E, Bhattacharya I, Brailoiu E, et al. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res. 2009;104:288–291. doi: 10.1161/CIRCRESAHA.108.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma G, Hu C, Brigman JL, Zhu G, Hathaway HJ, Prossnitz ER. GPER deficiency in male mice results in insulin resistance, dyslipidemia, and a proinflammatory state. Endocrinology. 2013;154:4136–4145. doi: 10.1210/en.2013-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang C, Dehghani B, Magrisso IJ, et al. GPR30. contributes to estrogen-induced thymic atrophy. Mol Endocrinol. 2008;22:636–648. doi: 10.1210/me.2007-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu S, Le May C, Wong WP, et al. Importance of extranuclear estrogen receptor-alpha and membrane G protein-coupled estrogen receptor in pancreatic islet survival. Diabetes. 2009;58:2292–2302. doi: 10.2337/db09-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Otto C, Fuchs I, Kauselmann G, et al. GPR30. does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod. 2009;80:34–41. doi: 10.1095/biolreprod.108.071175. [DOI] [PubMed] [Google Scholar]
- 64.Martensson UE, Salehi SA, Windahl S, et al. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2009;150:687–698. doi: 10.1210/en.2008-0623. [DOI] [PubMed] [Google Scholar]
- 65.Davis KE, Carstens EJ, Irani BG, Gent LM, Hahner LM, Clegg DJ. Sexually dimorphic role of G protein-coupled estrogen receptor (GPER) in modulating energy homeostasis. Horm Behav. 2014;66:196–207. doi: 10.1016/j.yhbeh.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davis KE, M DN, Sun K, et al. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol Metab. 2013;2:227–242. doi: 10.1016/j.molmet.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu L, Brown WC, Cai Q, et al. Estrogen treatment after ovariectomy protects against fatty liver and may improve pathway-selective insulin resistance. Diabetes. 2013;62:424–434. doi: 10.2337/db11-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Antal MC, Krust A, Chambon P, Mark M. Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERbeta-null mutant. Proc Natl Acad Sci U S A. 2008;105:2433–2438. doi: 10.1073/pnas.0712029105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee H, Kim DW, Remedios R, et al. Scalable control of mounting and attack by Esr1 neurons in the ventromedial hypothalamus. Nature. 2014 doi: 10.1038/nature13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matsuda KI, Yanagisawa M, Sano K, et al. Visualisation and characterisation of oestrogen receptor alpha-positive neurons expressing green fluorescent protein under the control of the oestrogen receptor alpha promoter. Eur J Neurosci. 2013;38:2242–2249. doi: 10.1111/ejn.12227. [DOI] [PubMed] [Google Scholar]
- 71.Milner TA, Thompson LI, Wang G, et al. Distribution of estrogen receptor beta containing cells in the brains of bacterial artificial chromosome transgenic mice. Brain Res. 2010;1351:74–96. doi: 10.1016/j.brainres.2010.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ren H, Orozco IJ, Su Y, et al. FoxO1 Target Gpr17. Activates AgRP Neurons to Regulate Food Intake. Cell. 2012;149:1314–1326. doi: 10.1016/j.cell.2012.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim JH, Meyers MS, Khuder SS, et al. Tissue-selective estrogen complexes with bazedoxifene prevent metabolic dysfunction in female mice. Mol Metab. 2014;3:177–190. doi: 10.1016/j.molmet.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Komm BS, Kharode YP, Bodine PV, Harris HA, Miller CP, Lyttle CR. Bazedoxifene acetate: a selective estrogen receptor modulator with improved selectivity. Endocrinology. 2005;146:3999–4008. doi: 10.1210/en.2005-0030. [DOI] [PubMed] [Google Scholar]
- 75.Duggan ST, McKeage K. Bazedoxifene: a review of its use in the treatment of postmenopausal osteoporosis. Drugs. 2011;71:2193–2212. doi: 10.2165/11207420-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 76.Klump KL, Keel PK, Racine SE, et al. The interactive effects of estrogen and progesterone on changes in emotional eating across the menstrual cycle. J Abnorm Psychol. 2013;122:131–137. doi: 10.1037/a0029524. [DOI] [PMC free article] [PubMed] [Google Scholar]