Abstract
Purpose of review
The availability of a growing number of immunomodulatory medications over the past few years has been associated with various JC Virus (JCV) associated brain syndromes in patients with autoimmune diseases, including multiple sclerosis, Crohn’s disease and psoriasis which had not been previously recognized as predisposing factors for progressive multifocal leukoencephalopathy (PML). This review covers the three novel syndromes discovered in the last decade which are caused by JCV infection of neurons and meningeal cells.
Recent findings
For more than 30 years, JCV was thought to exclusively infect oligodendrocytes and astrocytes in the white matter of the brain of immunosuppressed individuals. We now recognize that JCV-infected glial cells are frequently located at the gray-white matter junction or exclusively within the gray matter causing demyelination in the cortex. Mutations in JCV can trigger a change in tropism leading to involvement of other cell types, such as neurons and meningeal cells, causing clinically distinct entities. These new features of JCV infection provide challenges for clinicians taking care of affected patients and investigators studying the biology of this polyomavirus, its pathogenesis, and tropism.
Summary
We hope that increasing awareness of these syndromes will lead to early diagnosis, and pave the way for new avenues of research to better understand all aspects of JCV pathogenesis and develop efficient therapies for our patients. However, we need to remain vigilant and open to the possibility that additional JC variants or yet unknown polyomaviruses may be associated with neurological diseases as well.
Keywords: Progressive multifocal leukoencephalopathy, JC virus granule cell neuronopathy, JC virus encephalopathy, JC virus meningitis
Introduction
JCV is a ubiquitous human polyomavirus that infects 50% to 86% of healthy adults without causing any disease. The virus remains quiescent in the kidney and lymphoid organs and may also remain latent in the brain [1]. In immunosuppressed individuals, including those with acquired immunodeficiency syndrome (AIDS), hematological malignancies, transplant recipients and patients with autoimmune diseases treated with immunomodulatory medications, JCV may reactivate and cause a productive and lytic infection of oligodendrocytes and astrocytes, leading to the often fatal demyelinating disease of the central nervous system (CNS) – PML.
With the growing amount of knowledge gathered since this disease was originally named 43 years ago, we have come to realize that “progressive multifocal leukoencephalopathy” has become somewhat of a misnomer. Indeed, we now understand that PML is not always progressive, may affect a single area of the brain, can involve both gray and white matter, and is sometimes associated with intense inflammation [2]. PML was initially characterized by multifocal areas of demyelination containing JCV infected oligodendrocytes, as well as reactive gliosis with enlarged, bizarre astrocytes infected by JCV. While PML lesions are predominantly localized in the subcortical white matter [3], lesions have also been found within gray matter structures.
For the first 32 years since its discovery in 1971 [4], JC virus was thought to exclusively infect oligodendrocytes and astrocytes in the brain white matter, while neurons were deemed not to be susceptible to infection [5]. We have described 3 novel syndromes caused by infection of neurons and meningeal cells. In 2003, we demonstrated productive infection of cerebellar granule cell neurons by JCV [6], and in 2005, JCV granule cell neuronopathy (JCV GCN) was characterized [7]. JCV GCN is caused by a JCV variant harboring a small deletion in the VP1 capsid protein, with specific tropism for cerebellar granule cell neurons. This infection results in cerebellar atrophy and associated dysarthria, appendicular, and gait ataxia [6-8]. In 2009, we reported a gray matter disease, JCV encephalopathy (JCVE), in one human immunodeficiency virus (HIV)-negative patient with lung cancer [9]. JCVE was found to be caused by a productive infection of cortical pyramidal neurons. Finally, in 2014, we observed a fatal case of JCV meningitis (JCVM) in an HIV-negative patient who had an extremely high CSF JC viral load and productive JCV infection of leptomeningeal cells [10]. In this review, we will discuss these syndromes in detail and how their discoveries have expanded our understanding of the pathogenesis of JCV in the CNS.
JCV Granule Cell Neuronopathy (JCV GCN)
Demyelination of the cerebellum white matter is well described in patients with PML. However, occasional focal cell loss in the granule cell layer of the cerebellum has also been reported in early cases [11, 12]. In 2000, Tagliati et al reported a syndrome of unexplained degeneration of the cerebellar granule cell layer occurring in association with HIV infection [13]. In 2003, we observed a patient with AIDS who developed PML in the subcortical white matter of both frontal lobes, and an unexplained cerebellar syndrome and cerebellar atrophy. A post mortem exam showed that in addition to classic PML lesions in the cerebral hemispheric white matter, the patient sustained a productive infection of cerebellar granule cell neurons by JCV in the absence of MRI or histologic evidence of demyelination in the cerebellum [6]. In 2005, we described isolated cerebellar atrophy caused by destruction of cerebellar granule cell neurons by JCV and named this syndrome, distinct from PML, JCV granule cell neuronopathy (JCV GCN) [14]. Since then, this condition has been reported independently throughout the world, mostly in HIV infected patients [6, 7, 15-22], but also in patients with CD40 ligand deficiency [23], sarcoidosis [12, 24], and more recently, in one patient with non-Hodgkin lymphoma treated with rituximab [25] and two Multiple Sclerosis patients treated with natalizumab [26-28].
In JCV GCN, JC virus causes productive and lytic infection of granule cell neurons in the granule cell layer of the cerebellum, but spares Purkinje cells [29]. A histologic survey of archival PML samples indicated that infection of granule cell neurons is in fact frequent and may be found in up to half of patients with PML, irrespective of whether they have concomitant demyelinating lesions of PML in the nearby cerebellar white matter. Infection of GCN was also found in 1/35 HIV-positive control samples without PML, indicating that neurons may also be the initial site of infection in the brain [30]. HIV-infected JCV GCN patients treated with cART have a median survival of at least 1.8 years as opposed to untreated patients whose median survival was only months [20].
Patients develop a subacute cerebellar syndrome characterized by truncal and appendicular ataxia, dysdiadochokinesia, dysmetria on finger to nose and heel to shin testing, and dysarthria. MRI typically shows cerebellar atrophy suggestive of neurodegeneration (Figure 1). However, additional white matter changes in the cerebellum and brainstem, particularly in the middle cerebellar peduncles and the pons, can frequently be seen [22]. A definitive diagnosis is established by PCR detection of JCV DNA in CSF or by cerebellar biopsy showing infection of granule cell neurons by JCV and immunohistochemistry (IHC) by using a neuronal marker, such as NeuN or MAP-2, together with anti-T Ag antibody v-300. The infected granule cell neurons have a hypochromatic, enlarged nucleus, and can be seen on the edges of areas of focal cell loss [7].
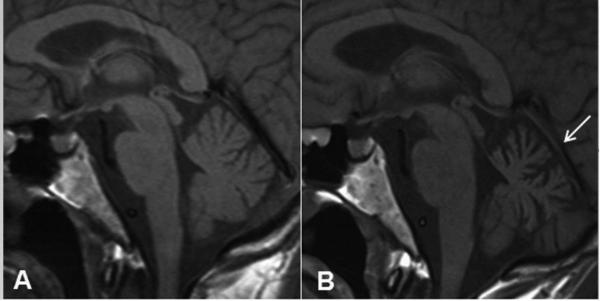
Figure 1. JC Virus Granule Cell Neuronopathy (JCV GCN).
MRI demonstrates progressive cerebellar atrophy without intra-parenchymal lesions. (A) Pre-contrast T1 sagittal image illustrating the size of the cerebellum at symptoms onset. (B) Pre-contrast T1 sagittal image done 4 months later and a week after positive CSF JC Virus PCR shows marked cerebellar atrophy (arrow).
Molecular analyses of brain and cerebrospinal fluid (CSF) samples from 6 of these JCV GCN cases showed small deletions in the C-terminal portion of the VP1 gene [8, 21, 26]. This area is responsible for linking the 72 pentamers of the VP1 protein that form the viral capsid, and is not directly exposed to the virion surface [2]. Therefore, these mutations are unlikely to cause a direct change in receptor binding but may rather affect post-entry events that may favor replication and assembly in granule cell neurons [21]. Mutated JCV strains can be found concomitant to undeleted strains in CSF and blood of PML patients, suggesting that the JCV variant that causes GCN may arise from outside the CNS. The JCV regulatory region (RR), which is required for viral expression and DNA replication, contains numerous binding sites for cellular proteins. Whereas the JCV-coding region is extremely conserved, the hypervariable non-coding RR has been associated with neurotropism and neurovirulence. We hypothesized that the RR for the JCV GCN1 variant isolated from the cerebellum of our index patient should have a uniquely different sequence allowing its growth in granule cell neurons. However, we found this to not be the case as the RR of the JCV GCN1 mutant had the same tandem-repeat pattern seen in classic PML patients suggesting that the specific tropism for neuronal cells is likely unrelated to its RR. [31, 32].
JCV Encephalopathy (JCVE)
It has long been known that demyelinating lesions of PML can extend into the cerebral gray matter [3, 33, 34], and that some neurons may contain JCV DNA, T Ag, and VP1 protein [35]. However, in 2009, we observed an HIV-negative patient who developed global cognitive decline, seizures, and aphasia. The clinical presentation differed from both classic PML and JCV GCN. Brain lesions were initially restricted to the hemispheric gray matter on MRI (Figure 2), which is the opposite of typical white matter lesions of PML. However, JCV was detected in the cortical gray matter and CSF [2]. We named this novel syndrome JCV encephalopathy (JCVE). Since this patient had a weak anti–JCV immunoglobulin M response four months after the start of her disease, JCVE may have been caused by a primary infection.
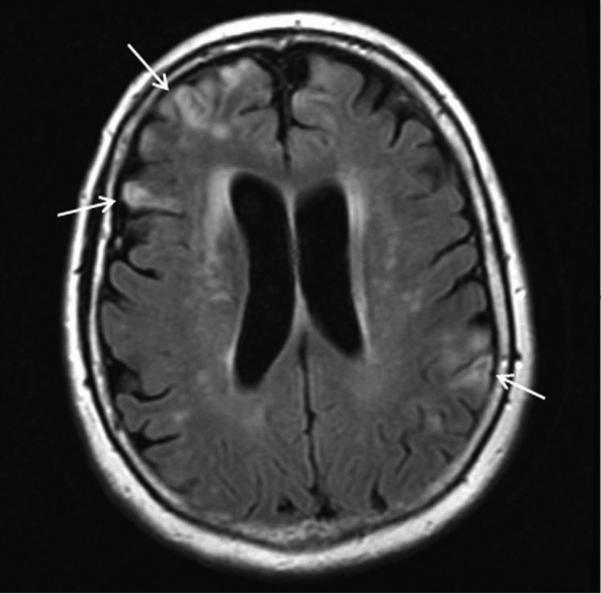
Figure 2. JC Virus Encephalopathy (JCVE).
MRI demonstrates multiple cortical lesions with hyperintense signal on fluid-attenuated inversion recovery sequence in the cerebral hemispheres bilaterally (arrows).
In JCVE, JCV infection predominantly involves cerebral pyramidal neurons and astrocytes in the cortical gray matter and gray-white junction. The presence of viral proteins in the nuclei, cytoplasm and axons of neurons suggests that JC virus may spread in the brain by migrating through axons of infected neurons [9]. More neurons were found to contain JCV T Ag, a regulatory protein expressed early in the viral life cycle, than JCV VP1 capsid protein which is produced at the time of viral assembly, suggesting the JCV infection of cortical pyramidal neurons may be abortive in those cells. We performed PCR amplification of the C terminus of the VP1 gene and sequencing to determine whether the same VP1 capsid deletion associated with JCV GCN was responsible for tropism of cortical pyramidal neurons. Interestingly, the VP1 gene was intact. However, isolation and sequencing of the JCV DNA present in the brain of this patient identified a virus with an archetype-like RR usually found in urine and kidney isolates of the virus, and a 143 base pair deletion in the agnoprotein gene [36]. The deleted agnogene encodes a 10 amino acid truncated peptide and is responsible for the majority of the JCV Cortical Pyramidal Neuron (JCVCPN) phenotype. Further analysis found that multiple forms of JCVCPN were present in the brain of this patient, and that these strains co-existed with a virus containing a full length agnogene. Compared to JCV prototype Mad-1 which readily infects glial cells, JCVCPN was not able to maintain a persistent infection in vitro, although it was capable of replicating in multiple cell lines. Experiments with chimeric viruses between JCVCPN and JCVMad-1 indicated that the agnogene deletion, rather than the kidney-type regulatory region, was the cause of this particular phenotype [37]. Further experiments demonstrated that the deletion of nucleotides 376-396 in the agnogene results in decreased levels of virus DNA replication and a lack of expression of the VP1 capsid protein [38]. Although this observation stems from a single case, these data suggest that the agnogene deletion of this particular JCV strain allowed for the virus to infect and propagate into cortical pyramidal neurons. Further studies are needed to determine whether this particular JC deletion variant actually plays a role in cortical pyramidal neuron infection in other cases.
JC Virus Meningitis (JCVM)
Although JCV is not routinely tested for in the CSF of patients with meningitis or encephalitis, several studies have documented JCV as the only pathogen present in the CSF of patients with typical meningeal signs and symptoms, such as neck stiffness and diplopia. Whether these cases result from JCV primary infection or reactivation is unclear. The exact incidence of JCV meningitis remains unknown as JCV PCR is not routinely performed in the CSF of patients with aseptic meningitis with an unremarkable MRI. In a large CSF study of patients with suspected meningitis or encephalitis, PCR showed that two HIV-negative individuals with no parenchymal brain lesions had detectable JCV DNA in their CSF. One of these patients ultimately died of cerebral lymphoma and, therefore, probably had an undocumented underlying immune suppression [39]. There are only 3 case reports of JCV meningitis (JCVM) to date. The first involved an immunocompetent girl who was diagnosed based on increasing JCV antibodies in the serum [40]. The second was a 38-year-old woman with systemic lupus erythematosus who presented with fevers, headaches, and altered mental status and was found to have JCV DNA in the CSF. She had no white matter lesions on MRI and her symptoms spontaneously resolved [41]. Recently we described a fatal case of JCVM in an HIV-seronegative patient who presented with a subacute meningoencephalitis and the classic triad of cognitive impairment, gait dysfunction, and urinary incontinence consistent with secondary normal pressure hydrocephalus. MRI also revealed FLAIR hyperintensity within the sulci but no white matter lesions, cerebellar atrophy, or cortical abnormalities (Figure 3). CSF JC viral load was extremely high, up to 48 million copies/ml. Post mortem exam showed productive JCV Infection of leptomeningeal and choroid plexus cells. Molecular analyses from the CSF JCV strain demonstrated an archetype-like RR, but no VP1 gene or agnogene mutations such as those found in cases of JCV GCN of JCVE [10]. Further studies on the role of JCV in aseptic meningitis and idiopathic hydrocephalus are warranted.
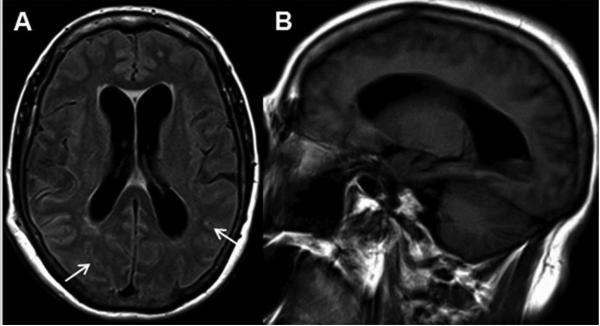
Figure 3. JC Virus Meningitis (JCVM).
MRI demonstrates hydrocephalus, abnormal signal in subarachnoid space, and no parenchymal brain lesions. (A) Axial fluid-attenuated inversion recovery sequence shows enlarged ventricles and abnormal hyperintensity in the subarachnoid space, within the sulci of the cerebral hemispheres (arrows). (B) A sagittal T1-weighted sequence demonstrates significant enlargement of the lateral ventricle.
Conclusion
It is now firmly established that JCV infection is not restricted to the CNS white matter. JCV also causes demyelination of cortical gray matter, as well as several types of gray matter disease caused by neuronal infection. Furthermore, JCV has also come out of the “gray area” by showing its ability to infect meningeal and choroid plexus cells. JCVM, JCVE, JCV GCN, and PML may occur separately or co-exist on a continuum as the virus mutates and spreads from one location to another (Figure 4). Of note, seizures, which are considered to be of cortical origin, occur in up to one-third of PML patients and have been associated with lesions located at the gray-white junction [34, 42]. Therefore, the gray matter involvement by JCV itself may provide a more direct mechanism by which this virus causes frequent seizures. In fact, seizures were associated with JCV-associated cortical demyelination, astrogliosis and infiltrates by phagocytic macrophages in a recent PML study [34].
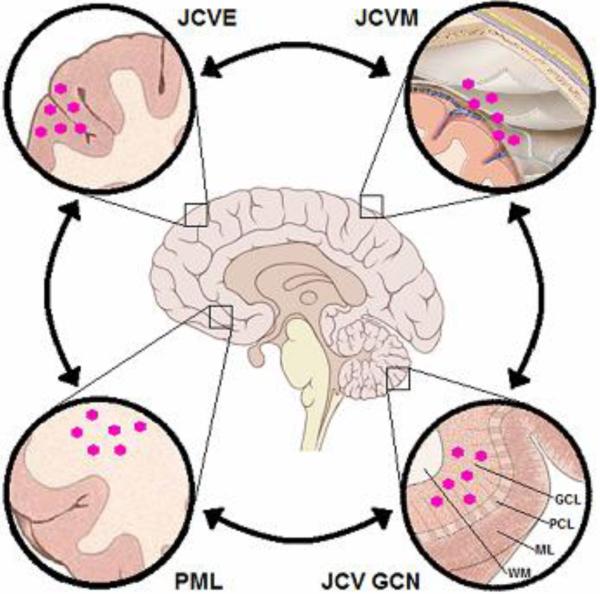
Figure 4. Continuum of CNS syndromes caused by JC Virus (JCV).
The arrows indicate that more than one syndrome may coexist. PML = progressive multifocal leukoencephalopathy; JCVE = JC Virus Encephalopathy; JCVM = JC Virus Meningitis; JCV GCN = JC Virus Granule Cell Neuronopathy; GCL = Granule Cell Layer; PCL = Purkinje Cell Layer; ML = Molecular layer; WM = white matter;  = JC virion
= JC virion
Interestingly, infection of neurons by polyomaviruses is not restricted to humans. The JCV-related simian virus 40 (SV40) can causes a fulminant and productive infection of cortical pyramidal neurons in simian-human immunodeficiency virus (SHIV)-immunosuppressed rhesus monkeys [43]. A retrospective analysis showed SV40 infection of neurons or meningeal cells in two thirds of immunosuppressed monkeys who developed a PML-like disease [44].
Due to the increasing number of patients treated with new generations of immunosuppressive drugs such as monoclonal antibodies, inhibitors of leukocyte migration, and fumarates—used for the treatment of multiple sclerosis, psoriasis, hematological malignancies, Crohn’s disease, and rheumatic diseases—a substantial increase of patients with JCV-associated brain diseases could be on the horizon [45]. Our hope is that increasing awareness of these novel syndromes will lead to early diagnosis, and pave the way for new avenues of research to better understand all aspects of JCV pathogenesis and develop efficient therapies for our patients. However, we need to remain vigilant and open to the possibility that other JCV variants or perhaps, yet unknown polyomaviruses, may causes additional shades of gray matter damage in the CNS, that are yet to be discovered.
Key points.
PML is not always progressive, multifocal, or restricted to the white matter of the brain.
Three novel syndromes associated with JC Virus (JCV) have been characterized in the last decade: JCV GCN, JCVE, and JCVM, expanding the pathogenesis of JCV and opening new areas of investigations.
JCV variant strains, co-infection with wild-type and deleted strains, and perhaps genetic predisposition all determine astroglial, neuronal, or meningeal tropism.
Acknowledgements
None.
Financial support and sponsorship:
This work has been supported in part by the National Institutes of Health (NIH) grants, R01 NS 047029 and R01 NS 074995 to IJK.
Footnotes
Conflicts of interest:
IJK has served on scientific advisory boards for Hoffmann–La Roche, GlaxoSmithKline, and Merck Serono and has received consulting fees from Bristol-Myers Squibb, Ono Pharmaceuticals, Merck Serono, Hoffmann–La Roche, Perseid Therapeutics, Vertex Pharmaceuticals, and Johnson & Johnson. I.J.K. receives royalties from UpToDate for topics on the management of HIV and CNS mass lesions and on PML.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
** of outstanding interest
- [1].Tan CS, Ellis LC, Wuthrich C, et al. JC virus latency in the brain and extraneural organs of patients with and without progressive multifocal leukoencephalopathy. J Virol. 2010;84:9200–9209. doi: 10.1128/JVI.00609-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[2].Gheuens S, Wuthrich C, Koralnik IJ. Progressive multifocal leukoencephalopathy: why gray and white matter. Annu Rev Pathol. 2013;8:189–215. doi: 10.1146/annurev-pathol-020712-164018. This review offers a more comprehensive overview of PML and other JC virus associated neurological conditions. [DOI] [PubMed] [Google Scholar]
- [3].Astrom KE, Mancall EL, Richardson EP., Jr Progressive multifocal leuko-encephalopathy; a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin's disease. Brain. 1958;81:93–111. doi: 10.1093/brain/81.1.93. [DOI] [PubMed] [Google Scholar]
- [4].Padgett BL, Walker DL, ZuRhein GM, et al. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971;1:1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- [5].Messam CA, Hou J, Gronostajski RM, Major EO. Lineage pathway of human brain progenitor cells identified by JC virus susceptibility. Ann Neurol. 2003;53:636–646. doi: 10.1002/ana.10523. [DOI] [PubMed] [Google Scholar]
- [6].Du Pasquier RA, Corey S, Margolin DH, et al. Productive infection of cerebellar granule cell neurons by JC virus in an HIV+ individual. Neurology. 2003;61:775–782. doi: 10.1212/01.wnl.0000081306.86961.33. [DOI] [PubMed] [Google Scholar]
- [7].Koralnik IJ, Wuthrich C, Dang X, et al. JC virus granule cell neuronopathy: A novel clinical syndrome distinct from progressive multifocal leukoencephalopathy. Ann Neurol. 2005;57:576–580. doi: 10.1002/ana.20431. [DOI] [PubMed] [Google Scholar]
- [8].Dang X, Koralnik IJ. A granule cell neuron-associated JC virus variant has a unique deletion in the VP1 gene. J Gen Virol. 2006;87:2533–2537. doi: 10.1099/vir.0.81945-0. [DOI] [PubMed] [Google Scholar]
- [9].Wuthrich C, Dang X, Westmoreland S, et al. Fulminant JC virus encephalopathy with productive infection of cortical pyramidal neurons. Ann Neurol. 2009;65:742–748. doi: 10.1002/ana.21619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[10].Agnihotri SP, Wuthrich C, Dang X, et al. A fatal case of JC virus meningitis presenting with hydrocephalus in a human immunodeficiency virus-seronegative patient. Ann Neurol. 2014;76:140–147. doi: 10.1002/ana.24192. This is the first case of JCV-associated meningitis in an HIV-seronegative patient who presented with hydrocephalus and meningeal signs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Richardson EP, Jr., Webster HD. Progressive multifocal leukoencephalopathy: its pathological features. Prog Clin Biol Res. 1983;105:191–203. [PubMed] [Google Scholar]
- [12].Granot R, Lawrence R, Barnett M, et al. What lies beneath the tent? JC-virus cerebellar granule cell neuronopathy complicating sarcoidosis. J Clin Neurosci. 2009;16:1091–1092. doi: 10.1016/j.jocn.2008.07.091. [DOI] [PubMed] [Google Scholar]
- [13].Tagliati M, Simpson D, Morgello S, et al. Cerebellar degeneration associated with human immunodeficiency virus infection. Neurology. 1998;50:244–251. doi: 10.1212/wnl.50.1.244. [DOI] [PubMed] [Google Scholar]
- [14].Koralnik IJ, Wuthrich C, Dang X, et al. JC virus granule cell neuronopathy: A novel clinical syndrome distinct from progressive multifocal leukoencephalopathy. Ann Neurol. 2005;57:576–580. doi: 10.1002/ana.20431. [DOI] [PubMed] [Google Scholar]
- *[15].Gonzalez-Ibarra F, Abdul W, Eivaz-Mohammadi S, et al. Cerebellar Dysfunction in a Patient with HIV. Case Rep Neurol Med. 2014;2014:180743. doi: 10.1155/2014/180743. This is a case-report of an HIV-infected patient with suspected granule cell neuronopathy who had classical symptoms of cerebellar dysfunction in the setting of high CSF JC viral load. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Roux D, Bouldouyre MA, Mercier-Delarue S, et al. JC virus variant associated with cerebellar atrophy in a patient with AIDS. J Clin Microbiol. 2011;49:2196–2199. doi: 10.1128/JCM.02057-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shang T, Delgado A, Adams D. JC virus granule cell neuronopathy and hyper-IgE in HIV disease. Neurology. 2011;76:1941–1942. doi: 10.1212/WNL.0b013e31821d751e. [DOI] [PubMed] [Google Scholar]
- [18].Otis CN, Moral LA. Images in pathology: granule cell loss in AIDS-associated progressive multifocal leukoencephalopathy. Int J Surg Pathol. 2005;13:360. doi: 10.1177/106689690501300409. [DOI] [PubMed] [Google Scholar]
- [19].Shin HW, Kang SY, Sohn YH. JC viral infection-related cerebellar degeneration as the first manifestation of AIDS. Eur Neurol. 2008;59:205–207. doi: 10.1159/000114048. [DOI] [PubMed] [Google Scholar]
- [20].Tan IL, Brew BJ. Possible JCV granular cell neuronopathy in a patient with HIV infection. Neurology. 2009;73:1598–1599. doi: 10.1212/WNL.0b013e3181c0d6cb. [DOI] [PubMed] [Google Scholar]
- [21].Dang X, Vidal JE, Oliveira AC, et al. JC virus granule cell neuronopathy is associated with VP1 C terminus mutants. J Gen Virol. 2012;93:175–183. doi: 10.1099/vir.0.037440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **[22].Wijburg MT, van Oosten BW, Murk JL, et al. Heterogeneous imaging characteristics of JC virus granule cell neuronopathy (GCN): a case series and review of the literature. J Neurol. 2014 doi: 10.1007/s00415-014-7530-5. This is a comprehensive review of the clinical, radiologic, and histopathologic findings of all 18 published cases of JCV GCN. The heterogeneous findings on imaging in JCV GCN suggests an overlap of this condition with other JCV-associated brain diseases. [DOI] [PubMed] [Google Scholar]
- [23].Hecht JH, Glenn OA, Wara DW, Wu YW. JC virus granule cell neuronopathy in a child with CD40 ligand deficiency. Pediatr Neurol. 2007;36:186–189. doi: 10.1016/j.pediatrneurol.2006.10.007. [DOI] [PubMed] [Google Scholar]
- [24].Keith J, Bilbao J, Baskind R. JC virus granular neuronopathy and rhombencephalic progressive multifocal leukoencephalopathy: case report and review of the literature. Neuropathology. 2012;32:280–284. doi: 10.1111/j.1440-1789.2011.01254.x. [DOI] [PubMed] [Google Scholar]
- *[25].Dang L, Dang X, Koralnik IJ, Todd PK. JC polyomavirus granule cell neuronopathy in a patient treated with rituximab. JAMA Neurol. 2014;71:487–489. doi: 10.1001/jamaneurol.2013.4668. This is a unique case in which the patient likely had a coinfection with both wild-type and JCVGCN5 strains before rituximab therapy, and the mutated form was responsible for the JCV GCN phenotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[26].Dang X, Koralnik IJ. Gone over to the dark side: Natalizumab-associated JC virus infection of neurons in cerebellar gray matter. Ann Neurol. 2013;74:503–505. doi: 10.1002/ana.23985. This is an editorial accompanying the first description of JCV GCN in a natalizumab-treated MS patient. This condition should be considered in patients on natalizumab presenting with progressive cerebellar symptoms and cerebellar atrophy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[27].Agnihotri SP, Dang X, Carter JL, et al. JCV GCN in a natalizumab-treated MS patient is associated with mutations of the VP1 capsid gene. Neurology. 2014;83:727–732. doi: 10.1212/WNL.0000000000000713. This is the second case of natalizumab-associated JCV GCN. Sequence analysis revealed novel GCN-type mutations in the VP1 gene. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **[28].Schippling S, Kempf C, Buchele F, et al. JC virus granule cell neuronopathy and GCN-IRIS under natalizumab treatment. Ann Neurol. 2013;74:622–626. doi: 10.1002/ana.23973. The first case of JCV GCN in a patient with MS presenting with cerebellar symptoms and atrophy after 52 months of natalizumab monotherapy. This patient developed immune reconstitution inflammatory syndrome (IRIS) after natalizumab withdrawal, but did not have contrast enhancement on MRI. [DOI] [PubMed] [Google Scholar]
- [29].Koralnik IJ. Progressive multifocal leukoencephalopathy revisited: Has the disease outgrown its name? Ann Neurol. 2006;60:162–173. doi: 10.1002/ana.20933. [DOI] [PubMed] [Google Scholar]
- [30].Wuthrich C, Cheng YM, Joseph JT, et al. Frequent infection of cerebellar granule cell neurons by polyomavirus JC in progressive multifocal leukoencephalopathy. J Neuropathol Exp Neurol. 2009;68:15–25. doi: 10.1097/NEN.0b013e3181912570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dang X, Koralnik IJ. A granule cell neuron-associated JC virus variant has a unique deletion in the VP1 gene. J Gen Virol. 2006;87:2533–2537. doi: 10.1099/vir.0.81945-0. [DOI] [PubMed] [Google Scholar]
- [32].Dang X, Vidal JE, Oliveira AC, et al. JC virus granule cell neuronopathy is associated with VP1 C terminus mutants. J Gen Virol. 2012;93:175–183. doi: 10.1099/vir.0.037440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Moll NM, Rietsch AM, Ransohoff AJ, et al. Cortical demyelination in PML and MS: Similarities and differences. Neurology. 2008;70:336–343. doi: 10.1212/01.WNL.0000284601.54436.e4. [DOI] [PubMed] [Google Scholar]
- *[34].Khoury MN, Alsop DC, Agnihotri SP, et al. Hyperintense cortical signal on magnetic resonance imaging reflects focal leukocortical encephalitis and seizure risk in progressive multifocal leukoencephalopathy. Ann Neurol. 2014;75:659–669. doi: 10.1002/ana.24144. Seizures are a frequent complication seen in approximately one-third of PML patients. Hyperintense cortical signal (HCS) is associated with seizures and immune reconstitution inflammatory syndrome (IRIS), and correlates histologically with JCV focal leukocortical encephalitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shintaku M, Matsumoto R, Sawa H, Nagashima K. Infection with JC virus and possible dysplastic ganglion-like transformation of the cerebral cortical neurons in a case of progressive multifocal leukoencephalopathy. J Neuropathol Exp Neurol. 2000;59:921–929. doi: 10.1093/jnen/59.10.921. [DOI] [PubMed] [Google Scholar]
- [36].Dang X, Wuthrich C, Gordon J, et al. JC virus encephalopathy is associated with a novel agnoprotein-deletion JCV variant. PLoS One. 2012;7:e35793. doi: 10.1371/journal.pone.0035793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[37].Ellis LC, Norton E, Dang X, Koralnik IJ. Agnogene deletion in a novel pathogenic JC virus isolate impairs VP1 expression and virion production. PLoS One. 2013;8:e80840. doi: 10.1371/journal.pone.0080840. This study analyzes the functions of the agnogene deletion and archetype-like regulatory region (RR) in determining JCVCPN phenotype in vitro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[38].Ellis LC, Koralnik IJ. JC virus nucleotides 376-396 are critical for VP1 capsid protein expression. J Neurovirol. 2014 doi: 10.1007/s13365-014-0278-y. This study indicates that deletion of nucleotides 376-396 in the agnogene impairs expression of VP1 and infectious virion production and thereby plays a crucial role in JCV replication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Behzad-Behbahani A, Klapper PE, Vallely PJ, et al. BKV-DNA and JCV-DNA in CSF of patients with suspected meningitis or encephalitis. Infection. 2003;31:374–378. doi: 10.1007/s15010-003-3078-5. [DOI] [PubMed] [Google Scholar]
- [40].Blake K, Pillay D, Knowles W, et al. JC virus associated meningoencephalitis in an immunocompetent girl. Arch Dis Child. 1992;67:956–957. doi: 10.1136/adc.67.7.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Viallard JF, Ellie E, Lazaro E, et al. JC virus meningitis in a patient with systemic lupus erythematosus. Lupus. 2005;14:964–966. doi: 10.1191/0961203305lu2229cr. [DOI] [PubMed] [Google Scholar]
- [42].Lima MA, Drislane FW, Koralnik IJ. Seizures and their outcome in progressive multifocal leukoencephalopathy. Neurology. 2006;66:262–264. doi: 10.1212/01.wnl.0000194227.16696.11. [DOI] [PubMed] [Google Scholar]
- [43].Dang X, Wuthrich C, Axthelm MK, Koralnik IJ. Productive simian virus 40 infection of neurons in immunosuppressed Rhesus monkeys. J Neuropathol Exp Neurol. 2008;67:784–792. doi: 10.1097/NEN.0b013e318180f0d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[44].Kaliyaperumal S, Dang X, Wuethrich C, et al. Frequent infection of neurons by SV40 virus in SIV-infected macaque monkeys with progressive multifocal leukoencephalopathy and meningoencephalitis. Am J Pathol. 2013;183:1910–1917. doi: 10.1016/j.ajpath.2013.08.007. The findings in this paper confirm that spontaneous SV40 neuronal infection occurs in immunosuppressed macaques, which parallels JC virus-neuronal infection in immunosuppressed patients indicating that neuronal infection may be an important aspect of both SV40 and JC virus neuropathogenesis in their respective hosts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 2010;9:425–437. doi: 10.1016/S1474-4422(10)70040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]