Abstract
An essential feature of vertebrate neural development is ensheathment of axons with myelin, an insulating membrane formed by oligodendrocytes. Not all axons are myelinated, but mechanisms directing myelination of specific axons are unknown. Using zebrafish we show that activity-dependent secretion stabilizes myelin sheath formation on select axons. When VAMP2-dependent exocytosis is silenced in single axons, oligodendrocytes preferentially ensheath neighboring axons. Nascent sheaths formed on silenced axons are shorter in length, but when activity of neighboring axons is also suppressed, inhibition of sheath growth is relieved. Using in vivo time-lapse microscopy, we show that only 25% of oligodendrocyte processes that initiate axon wrapping are stabilized during normal development, and that initiation does not require activity. Instead, oligodendrocyte processes wrapping silenced axons are retracted more frequently. We propose that axon selection for myelination results from excessive and indiscriminate initiation of wrapping followed by refinement that is biased by activity-dependent secretion from axons.
In the developing central nervous system (CNS), oligodendrocytes extend membrane processes that ensheath axons with a lipid-rich myelin membrane. Myelination enables thinner axons to transmit information more rapidly, facilitating evolution of a complex yet compact CNS in vertebrate animals. Despite this advantage, not all axons are myelinated. For instance, in the corpus callosum, a major white-matter tract connecting cerebral hemispheres, fewer than half of all axons become myelinated1. Although selective mechanisms clearly exist in vivo, cultured oligodendrocytes will myelinate fixed axons or synthetic fibers with a diameter larger than 0.4 µm2,3. If axon diameter is sufficient for indiscriminate myelination in vitro, what mechanisms enable oligodendrocytes to make stereotyped decisions and myelinate specific axons in vivo?
Numerous studies show that electrical activity promotes myelination4–9, raising the possibility that action potentials and release of axonal factors instruct nearby oligodendrocyte processes to initiate ensheathment. Unmyelinated axons secrete neurotransmitters and neurotrophic factors extrasynaptically along axons10–12, and pre-myelinating oligodendrocytes express a plethora of receptors that are poised to interpret axonal factors released in response to activity13. Consistent with this possibility, electrical stimulation of cultured neurons triggers local Ca2+ signaling in oligodendrocyte processes, which requires synaptic vesicle exocytosis and glutamate receptors7. The mechanisms mediating axon selection in vivo, and the contribution of neuronal activity, remain completely unknown.
The inability to visualize and genetically manipulate subsets of axons in vivo, while simultaneously visualizing which axons are ensheathed by oligodendrocyte wrapping processes, has precluded the discovery of axon selection mechanisms. Here, we have investigated oligodendrocyte membrane sheath formation on single, identifiable axons under normal and electrically silenced conditions in zebrafish larvae. We found that the majority of oligodendrocyte membrane processes in ventral spinal cord tracts select and ensheath phox2B+ axons. The fidelity of this axon choice was reduced when VAMP2-dependent exocytosis or electrical excitability was suppressed in single axons, indicating that axon selection is biased by activity-dependent secretion. Moreover, myelin sheaths that did form on single silenced axons were shorter in length. This activity-dependent control of sheath length is influenced by other axons, because suppressing all electrical activity restored normal sheath length. Finally, using in vivo time-lapse microscopy we found that oligodendrocytes wrapping silenced axons retract sheaths more frequently. Collectively, this study raises a new model for axon selection whereby oligodendrocytes initially form excess sheaths, detect differences in activity-dependent secretion between ensheathed axons, and preferentially maintain sheaths on select axons. These findings extend recent discoveries that social experiences and experimentally altered neuronal activity can influence oligodendrocyte proliferation, differentiation and myelin sheath thickness9,14,15 by demonstrating that neuronal activity can bias which axons become myelinated, an additional means of myelin plasticity. Additionally, our work indicates that molecular and cellular mechanisms that stabilize myelin sheaths following indiscriminate sheath initiation contribute importantly to axon selection in response to activity.
RESULTS
Activity-dependent secretion regulates axon selection
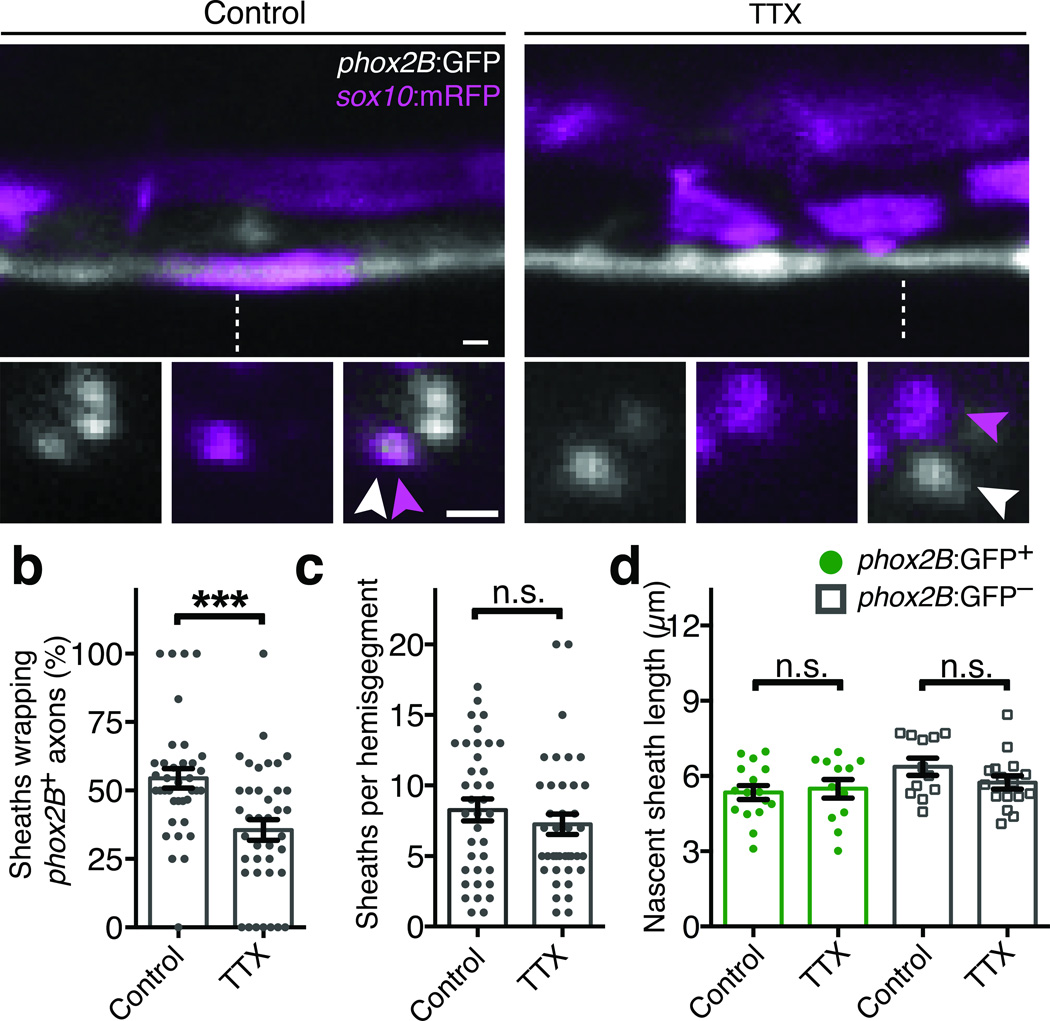
We have developed a genetically tractable system to study mechanisms of axon selection in the zebrafish spinal cord. Testing the activity-dependent myelination hypothesis in vivo necessitated transgenic reporters permitting direct observation of subsets of myelin-fated axons, and drivers enabling genetic manipulation of neuronal activity. In Tg(phox2B:GFP) zebrafish larvae (hereafter phox2B:GFP), a subset of hindbrain neurons with axons projecting into ventral spinal cord tracts express GFP (Supplementary Fig. 1). In transverse spinal cord sections, phox2B+ axons were surrounded by myelin basic protein (MBP), a marker for myelin sheaths (Supplementary Fig. 1). To observe the initial myelination of phox2B+ axons in live zebrafish, we visualized nascent sheaths by confocal microscopy using the Tg(sox10:mRFP) reporter, which expresses membrane-tethered RFP in oligodendrocyte lineage cells (hereafter sox10:mRFP). phox2B+ axons were ensheathed shortly after the onset of myelination, and a majority of nascent sheaths in the ventral spinal cord wrapped phox2B+ axons (Fig. 1a). If axon selection requires electrical activity, then suppressing activity should abrogate the selection of myelin-fated phox2B+ axons. To test this, we treated phox2B:GFP; sox10:mRFP larvae with the voltage-gated sodium channel blocker tetrodotoxin (TTX) at 48 hours post fertilization (hpf), prior to the onset of myelination. TTX treatment paralyzed zebrafish larvae for at least three days without major developmental defects or toxicity, but did not prevent myelin sheath formation in the spinal cord. We assessed axon selection in phox2B:GFP; sox10:mRFP reporter larvae and found that TTX treatment reduced the proportion of nascent sheaths wrapping phox2B+ axons (Fig. 1a,b). In contrast, TTX treatment had no effect on the overall number or length of nascent sheaths (Fig. 1c,d), or the number of spinal cord oligodendrocyte progenitor cells or oligodendrocytes (Supplementary Fig. 2). Together, these data show that TTX-sensitive activity is not required for oligodendrocyte differentiation or formation of nascent sheaths in vivo, but biases axon choice.
Figure 1. Axon selection is biased by electrical activity.
(a) Confocal images show axon wrapping in Tg(phox2B:GFP) larvae. In control larvae (left), phox2B+ axons are frequently wrapped by nascent myelin sheaths marked by sox10:mRFP. TTX treatment (right) reduces the proportion of nascent sheaths wrapping phox2B+ axons. For each condition, the upper panels are lateral spinal cord views and the lower panels show orthogonal projections, which were generated at the dashed lines. Arrowheads point to axons (white) and nascent sheaths (magenta). Scale bars, 1 µm. (b) Quantification of the proportion of sox10:mRFP sheaths wrapping phox2B+ axons. P = 0.0005, t-test. (c) Quantification of the overall number of nascent sheaths per spinal cord hemisegment. P = 0.3426, t-test. For (b–c), n =22 control larvae (283 sheaths) and 23 TTX-treated larvae (283 sheaths). (d) Quantification of nascent sheath length; n =16 control larvae (406 sheaths) and 12 TTX-treated larvae (296 sheaths). P = 0.7468 (phox2B:GFP+) and P = 0.1540 (phox2B−), t-test. For all panels, error bars show s.e.m.; ***P < 0.001; n.s., not significant.
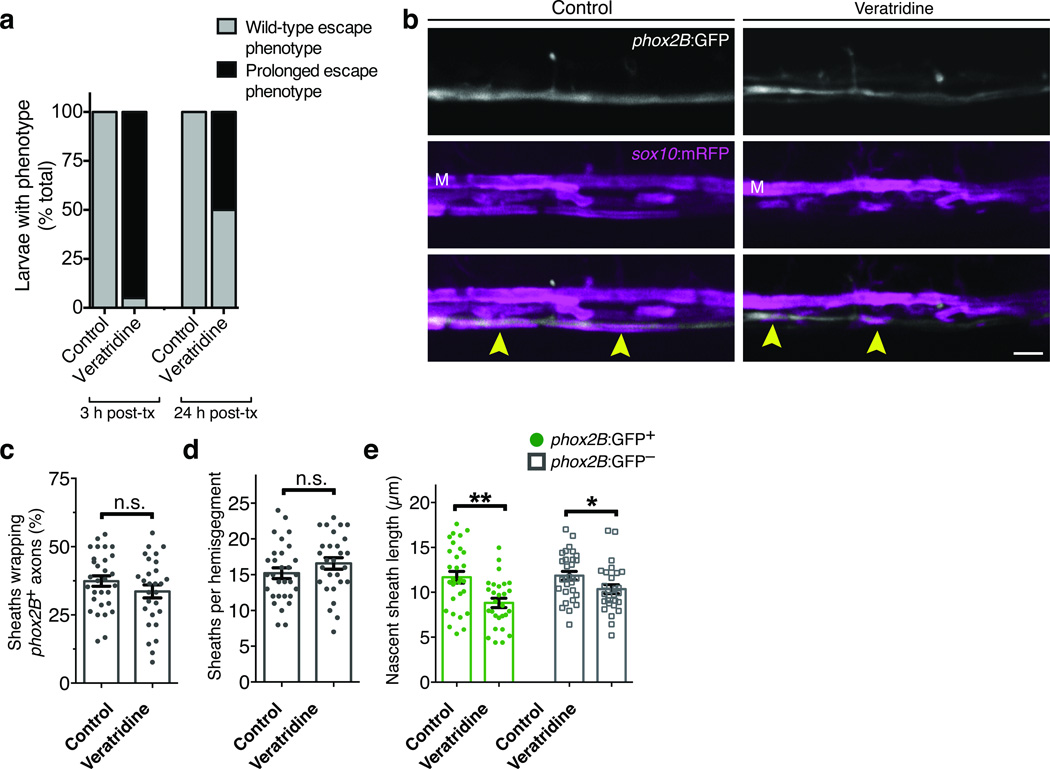
If activity is necessary for biased axon choice, can heightened activity enhance wrapping? To test this we treated embryos with the Na+ channel modulator veratridine at 72 hpf, immediately prior to the onset of myelination. Veratridine prolongs Na+ channel opening and, as expected, injected embryos showed striking and sustained behavioral phenotypes. Upon touch stimulation, control embryos initiated short bursts of swim movements (Supplementary Video 1). By contrast, veratridine-treated embryos initiated a prolonged swim response that eventually terminated in seizure-like behavior and brief paralysis (Supplementary Video 2). Although veratridine treatment had a pronounced effect on neural behavior (Fig. 2a), we observed no change within the spinal cord in the selection of phox2B+ axons or the overall level of wrapping (Fig. 2b–d). However, the length of nascent sheaths on both phox2B+ and phox2B− axons was slightly reduced in veratridine-treated embryos relative to controls (Fig. 2e). We conclude that TTX-sensitive activity is necessary for selection of axons at high fidelity but that widespread elevated neuronal activity is not sufficient to increase wrapping of spinal cord axons.
Figure 2. Veratridine reduces nascent sheath length but not sheath number or axon selection.
(a) Quantification of touch response assays on control and veratridine-treated larvae. Data shown represent the proportion of larvae exhibiting either wild-type or prolonged touch-response phenotypes (see Methods). Scoring was performed three hours after treatment and again immediately prior to confocal imaging (24 hr post-treatment). For both control and treated groups, n = 80 (3 h post-treatment) and 76 (24 h post-treatment). (b) Representative confocal images show reporter expression in control and veratridine-treated Tg(phox2B:GFP); Tg(sox10:mRFP) larvae. M indicates the position of the Mauthner axon and arrowheads point to sites of phox2B+ axon wrapping. Scale bar, 5 µm. (c–e) Summary of axon selection, sheath number, and sheath length measurements from (b). Error bars show s.e.m.; t-test; *P < 0.05, **P < 0.01; n.s., not significant. For (c–e) n = 30 control and 28 veratridine-treated larvae. P = 0.2147 (c), P = 0.2173 (d), P = 0.0011 (e, left), and P = 0.0342 (e, right); t-test.
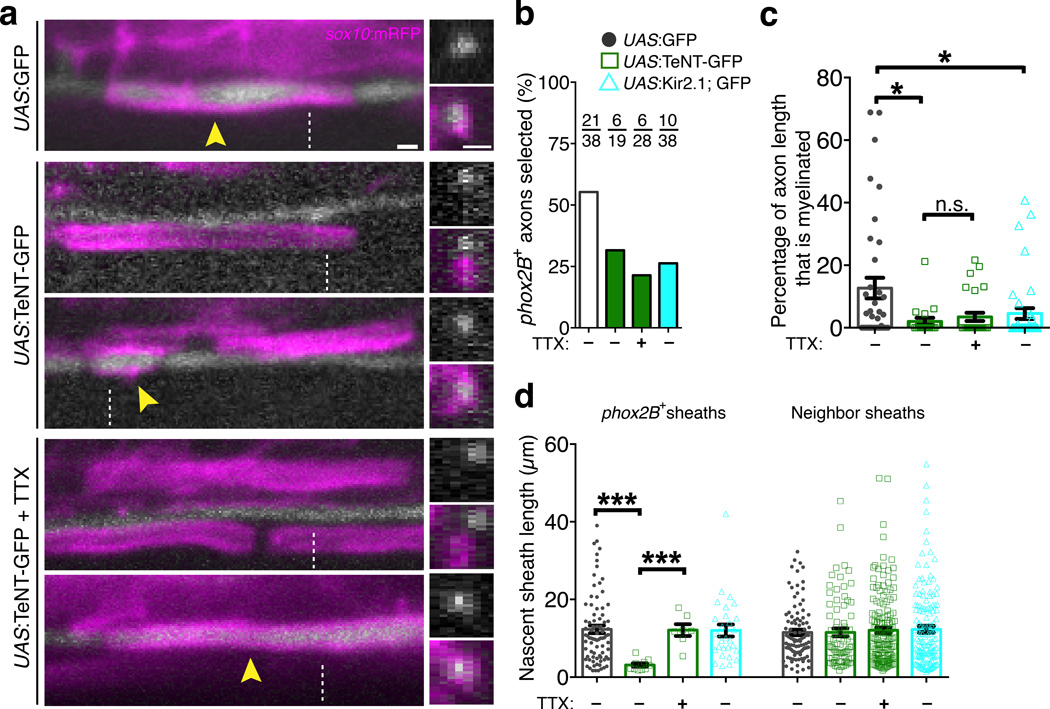
To test whether activity-dependent signals that bias axon choice originate in neurons, we next inhibited synaptic vesicle exocytosis selectively in phox2B+ axons by targeted overexpression of tetanus neurotoxin (TeNT), a protease that cleaves select Vesicle-Associated Membrane Protein (VAMP) family proteins including Synaptobrevin/VAMP2. This is a potent inhibitor of synaptic vesicle exocytosis in many animal models including zebrafish16,17. We cloned a 2.1 kb genomic fragment of the zebrafish phox2B gene and generated the Tg(phox2B:GAL4) line. When crossed to Tg(UAS:mCherry-CaaX), mCherry was faithfully co-expressed in axons marked by phox2B:GFP, validating the specificity of this new transgenic line (Supplementary Fig. 1). We injected Tg(phox2B:GAL4) embryos at early cleavage stage with DNA plasmids encoding UAS:GFP or UAS:TeNT-GFP, resulting in mosaic expression in single phox2B+ axons of 4-day larvae. When phox2B+ axons expressed GFP as a control, 55.3% were myelinated at the time of imaging, whereas only 31.6% expressing TeNT-GFP were myelinated (Fig. 3a,b). Quantitatively, we found that single TeNT-GFP+ axons were less well wrapped, because the percent length of axons ensheathed by sox10:mRFP+ sheaths was reduced (Fig. 3c). Notably, when oligodendrocytes did wrap TeNT-GFP+ axons, nascent sheaths were shorter in length (Fig. 3d).
Figure 3. Activity-dependent competition during axon selection.
(a) Confocal images show expression of UAS:GFP or UAS:TeNT-GFP in single phox2B+ axons and nascent sheaths marked by sox10:mRFP. Images on the left are lateral views and the right panel shows orthogonal projections, generated at the dashed lines. Arrowheads point to ensheathed axons. Scale bars, 1 µm. (b) Summary of the percentage of axons selected for myelination. For each condition, the number selected and overall number of axons analyzed is indicated. (c) Quantitative measurements show the wrapping efficiency of phox2B+ axons expressing from the indicated plasmids. Data are expressed as the percent of total axon length ensheathed at the time of imaging. P = 0.0096 (upper asterisk), P = 0.0294 (lower asterisk), P = 0.7257 (lower comparison, n.s.), Mann-Whitney test. (d) Quantification of nascent sheath length. Left bars show the average length of sheaths wrapping GFP+ axons, and the right bars show lengths of nascent sheaths wrapping neighboring, unmarked axons in the same larvae. P < 0.0001 (left comparison), P = 0.0002 (right comparison), Mann-Whitney test. For (c–d), n corresponds to the numbers of axons indicated in (b) derived from 17 (UAS:GFP), 17 (UAS:TeNT-GFP), 22 (UAS:TeNT-GFP + TTX), and 29 (UAS:Kir2.1; EGFP) larvae. Error bars show s.e.m.; *P < 0.05, ***P < 0.001; n.s., not significant.
Our finding that nascent sheath length is reduced on single TeNT-GFP+ axons but unaffected when all axons are silenced by TTX is reminiscent of competition mechanisms that refine topographic maps in the visual system16,18–20. To test for activity-based interactions among axons that could modulate myelination, we next asked whether the effects of blocking vesicular release are influenced, or rescued, when neighboring axons are also silenced. We treated larvae with single UAS:TeNT-GFP+ axons with TTX to suppress activity in surrounding axons. In comparison to single TeNT-GFP+ axons in a normal environment, nascent sheaths wrapping TeNT-GFP+ axons in TTX-treated larvae grew to their usual lengths (Fig. 3d). However, only 21.4% of TeNT-GFP+ axons were selected for myelination, and the overall ensheathment of phox2B+ axons remained reduced (Fig. 3b,c). These findings demonstrate that control of nascent sheath length involves vesicular release and is influenced by neighboring axons. In addition, the observation that phox2B+ axons remain selected with reduced fidelity when activity in other axons is suppressed suggests that additional activity-dependent, non-competitive forces participate in axon selection.
To test the requirement for neuronal excitability in axon selection we used targeted overexpression of the inward rectifier K+ channel Kir2.1 in single phox2B+ axons. This approach suppresses neuronal excitability in various model systems including zebrafish neurons18,21,22. In axon selection assays, UAS:Kir2.1-2A-GFP+ axons were selected for myelination less frequently than controls (Fig. 3b,c). However, unlike TeNT-GFP+ axons, nascent sheaths wrapping Kir2.1-2A-GFP+ axons were normal in length (Fig. 3d). Collectively, these data reveal distinct activity-dependent forces during axon selection. Synaptic vesicle release biases axon selection and positively regulates myelin sheath length in a manner that can be influenced by activity in other axons. Additional forces mediated by excitability, which are suppressed by TTX and Kir2.1 but not influenced by activity in other axons, are also required for axon selection. Notably, myelin sheath length is reduced on single TeNT-GFP+ axons that are excitable but cannot perform VAMP2-dependent exocytosis, whereas sheath length is normal on Kir2.1-2A-GFP+ axons with reduced excitability but normal exocytic function.
We next asked whether ectopic neuronal activity in phox2B+ is sufficient to modulate axon selection and initial ensheathment by crossing Tg(phox2B:GAL4) to the Tg(UAS:ChRWR-EGFP) line, which can induce activity of zebrafish spinal neurons in response to blue light stimulation23. Prior to optogenetic stimulation, we performed confocal microscopy to identify the specific somite at which oligodendrocytes were initiating ensheathment. After blue light stimulation (473 nm), we returned to the same position and found no change in the proportion of wrapped phox2B+ axons or the overall ensheathment of phox2B+ axons after 24 hours (Supplementary Fig. 3). These data are consistent with our pharmacologic stimulation (Fig. 2), further indicating that ectopic neuronal activity in phox2B+ axons is not sufficient to alter selection or ensheathment. However, we cannot rule out the possibility that an undetermined stimulation pattern within phox2B+ axons could bias axon selection or regulate ensheathment.
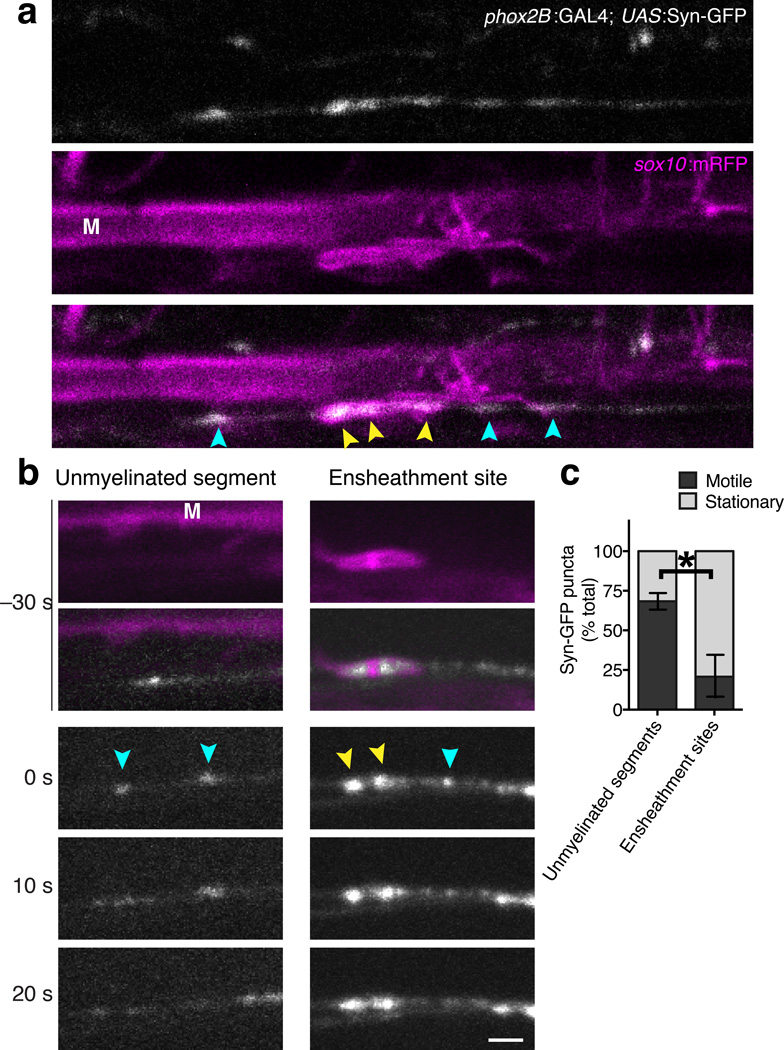
Our observation that vesicle release from axons regulates selection for myelination raises the possibility that vesicle cargo is locally secreted from axons onto oligodendrocyte processes, acting instructively to initiate or maintain nascent sheaths. We next performed confocal microscopy to directly observe the distribution and behavior of axonal vesicles at and away from sites of ensheathment. When Tg(phox2B:GAL4) was crossed to Tg(UAS:Synaptophysin-GFP) (hereafter UAS:Syn-GFP) we observed single labeled axons containing vesicles marked by GFP (Fig. 4, Supplementary Video 3). Vesicle puncta were both stationary and motile, with rates of movement consistent with microtubule-based axonal transport. When crossed to the Tg(sox10:mRFP) transgenic reporter, we frequently found accumulations of Syn-GFP+ vesicle puncta at sites of ensheathment (Fig. 4a). Syn-GFP+ puncta at ensheathment sites may be represent a stable vesicle pool, or could be undergoing transport toward or away from the synaptic terminal. To distinguish between these possibilities, we collected time-lapse images at 10 s intervals and measured the motility of vesicles at unmyelinated segments and ensheathment sites. Whereas Syn-GFP+ puncta at unmyelinated segments were frequently motile, vesicle puncta at ensheathment sites were typically stationary (Fig. 4b,c). Taken together, these data are consistent with the possibility that axon secretion at ensheathment sites participates in axon selection.
Figure 4. Accumulation of Syn-GFP vesicles at myelin ensheathment sites.
(a) Representative confocal images show Syn-GFP vesicle puncta (white) and nascent myelin sheaths marked by the sox10:mRFP reporter (magenta). (b) Representative time-lapse confocal images show stationary and motile Syn-GFP+ vesicle puncta at unmyelinated axon segments (left panels) and ensheathment sites (right panels). The upper panels show images acquired 30 s prior to the onset of time-lapse imaging, and the lower panels show time-lapse images acquired at 10 s intervals. For (a–b), Syn-GFP puncta at unmyelinated segments and ensheathment sites are indicated by blue and yellow arrowheads, respectively. Images are lateral views with dorsal up and anterior right. The Mauthner axon (M) is marked for reference. Scale bars, 2 µm. (c) Summary of vesicle motility measurements show the proportion of motile and stationary vesicles at unmyelinated segments and ensheathment sites. Error bars represent s.e.m.; n = 272 puncta at unmyelinated segments (12 larvae), 31 puncta at ensheathment sites (7 larvae); ***P = 0.0009, t-test.
Axon secretion is required for myelin sheath maintenance
What are the neuron-oligodendrocyte interactions leading to the preferential myelination of specific axons? We next aimed to determine whether axon secretion regulates myelination before or after initial ensheathment. In a preferential ensheathment model, axons without the proper excitability and secreted factors will not support initial ensheathment. Alternatively, in a preferential maintenance model, silenced axons can be initially wrapped but will not maintain sheaths. To test the contribution of activity-dependent secretion in these models, we generated the transgenic line Tg(neuroD:TeNT-GFP). In this line, the pan-neuronal driver neuroD expresses TeNT-GFP broadly in neurons but not oligodendrocytes (Supplementary Fig. 4). Consequently, Tg(neuroD:TeNT-GFP) larvae (hereafter neuroD:TeNT) show no spontaneous locomotion or touch response (Supplementary Video 4). neuroD:TeNT expression, which is initiated prior to oligodendrocyte progenitor cell specification, caused a slight reduction in oligodendrocyte progenitor cell and oligodendrocyte numbers (Supplementary Fig. 5), similar to observations describing in an accompanying manuscript (Mensch et al.). Because TTX treatments, initiated after formation of oligodendrocyte lineage cells, had no effect on cell number, we conclude that activity promotes specification. Oligodendrocytes in neuroD:TeNT+ larvae do form nascent sheaths (Figs. 5a and Supplementary Fig. 5), further supporting our conclusion that neuronal activity and synaptic vesicle exocytosis are not required for initial ensheathment of axons in vivo. Moreover, electron micrographs showed no difference in axon diameter between wild-type and Tg(neuroD:TeNT-GFP) larvae (Supplementary Fig. 6), suggesting that phenotypes resulting from TeNT-GFP overexpression are due to inhibition of exocytosis rather than a change in axon diameter.
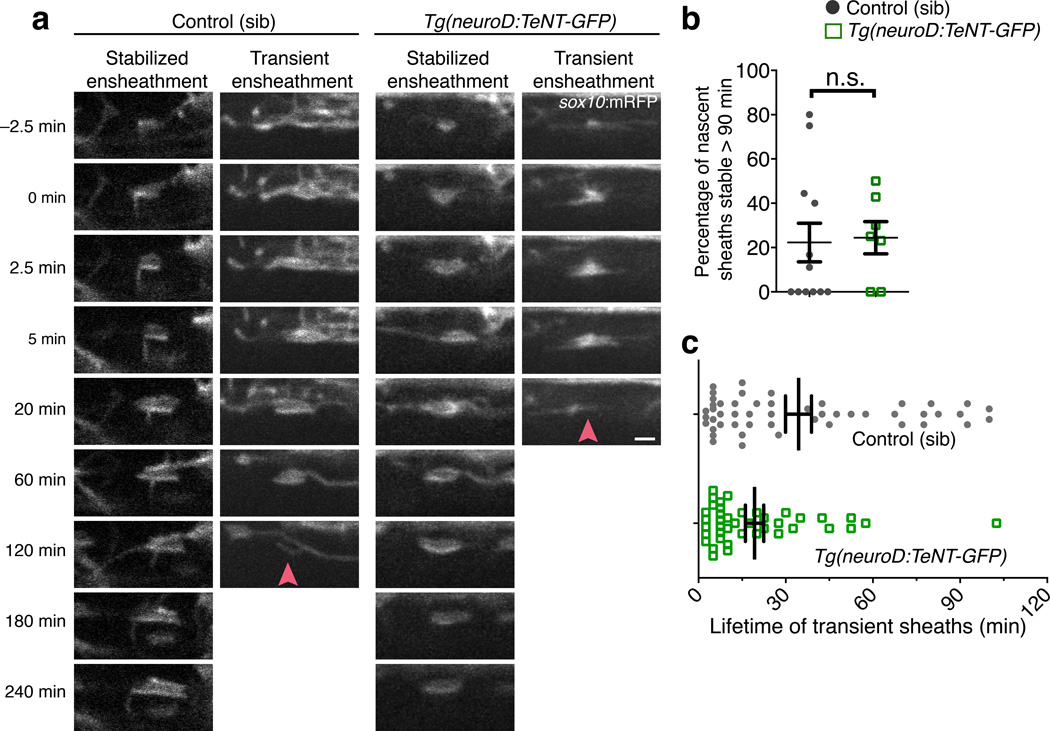
Figure 5. Activity-dependent secretion is not required for initial axon wrapping.
(a) Representative time-lapse confocal images show initiation of axon wrapping in the ventral spinal cord of sibling control (left panels) and Tg(neuroD:TeNT-GFP) larvae (right panels). Images are lateral views and time (min) relative to wrapping initiation is indicated at the left. Arrowheads point to prospective sheaths that fail to stabilize. Scale bar, 2 µm. (b) Summary of time-lapse measurements show the proportion of prospective sheaths that are stable for at least 90 min. n = 12 control (66 sheaths) and 8 TeNT-GFP larvae (57 sheaths); P = 0.5624, Mann-Whitney test. (c) Measurements of nascent sheath lifetime amongst transient ensheathments during time-lapse imaging in (a). n = 12 control (50 prospective sheaths) and 8 TeNT-GFP larvae (41 prospective sheaths); P = 0.0411, Mann-Whitney test. For (b–c), error bars show s.e.m., *P < 0.05; n.s., not significant.
To test the preferential ensheathment model, we performed time-lapse microscopy to assess oligodendrocyte membrane sheath initiation and stability in neuroD:TeNT+ larvae. We used the Tg(sox10:mRFP) reporter to observe initial axon wrapping attempts using an imaging paradigm with greater temporal resolution than previous studies. Remarkably, this revealed an unanticipated frequency of failed ensheathments in all samples (see examples in Supplementary Video 5). In both sibling control and neuroD:TeNT+ larvae, ~ 75% of wrapping attempts, or prospective sheaths, had disappeared within 90 min. Thus, 25% of prospective sheaths wrapping normal or silenced axons were initially stabilized in either condition, arguing against the preferential ensheathment model (Fig. 5a,b; Supplementary Videos 6–9). Intriguingly, although the proportion of wrapping attempts initially stabilized was unaffected, the ensheathment failure occurred more rapidly when prospective sheaths attempted to wrap silenced axons (Fig. 5c).
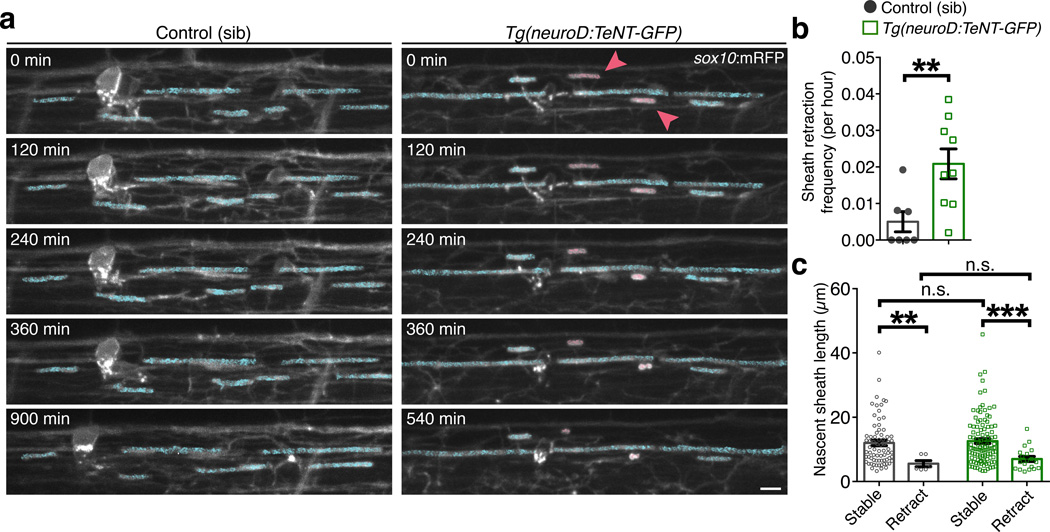
To test the preferential maintenance model, we next performed time-lapse microscopy to visualize the fate of pre-existing sheaths over an extended time period. We again utilized Tg(neuroD:TeNT-GFP); Tg(sox10:mRFP) larvae, imaging at 20 min intervals to track individual oligodendrocyte membrane sheaths over a 15-hr period. We focused on sheaths that had initiated several hours prior, and similar to previous reports24, found that existing sheaths were extremely stable. Whereas retractions were rare in control larvae, sheaths were retracted more frequently in neuroD:TeNT+ larvae (Fig. 6a,b; Supplementary Videos 10–11). In both conditions, sheaths that retracted were initially shorter than stable sheaths (Fig. 6c). Also consistent with the preferential maintenance model, and with the findings of Mensch et al. (accompanying paper), we found that by 5 dpf, singly-labeled oligodendrocytes in neuroD:TeNT+ larvae possessed fewer sheaths (Supplementary Fig. 5). Time-lapse imaging of singly-labeled oligodendrocytes in neuroD:TeNT+ larvae also supported a relationship between sheath length and retraction. Within three hours of initiation, prospective sheaths that were later maintained had extended more rapidly and were longer in length. In contrast, prospective sheaths that would be retracted extended more slowly, and never exceeded 10 µm in length before being retracted (Supplementary Fig. 7a–c). Collectively, our in vivo time-lapse imaging experiments demonstrate that the maintenance of nascent sheaths is regulated by activity-dependent secretion from axons, whereas initial axon wrapping is activity-independent.
Figure 6. Nascent myelin sheaths are stabilized by activity-dependent secretion.
(a) Representative confocal images show the retraction of existing sheaths during 15-hr time-lapse imaging in sibling control and Tg(neuroD:TeNT-GFP) larvae. Images are lateral views of the dorsal spinal cord and the time (min) relative to the start of image acquisition is indicated for each image. For demonstrative purposes, sheaths stable for the entire time-lapse are shaded in blue and retracting sheaths are shaded red. Scale bar, 5 µm. (b) Summary of time-lapse measurements show the frequency of sheath retraction. n = 7 control (99 sheaths) and 9 Tg(neuroD:TeNT-GFP) larvae (129 sheaths). P = 0.0071, Mann-Whitney test. (c) Quantitative measurements show the relationship between sheath length and stability. Bars represent the mean ± s.e.m. n = 7 control larvae (72 stable sheaths, 6 retracting sheaths) and 9 Tg(neuroD:TeNT-GFP) larvae (106 stable sheaths, 19 retracting sheaths). P = 0.0034 (left, **), P = 0.0002 (right, ***), P = 0.3737 (upper n.s.), P = 0.6105 (lower n.s.), Mann-Whitney test; For (b–c), **P < 0.01, **P < 0.001.
DISCUSSION
Taken together, our findings raise a model whereby after initial axon wrapping, activity-dependent secretion from axons promotes extension and stabilization of prospective sheaths. In the absence of this input, oligodendrocyte membrane sheaths could form but did not extend, and were retracted at a higher frequency. Oligodendrocytes sampled many axons during the first several hours after initiating myelination, but 75% of initial wrapping segments failed to form stable sheaths. In an environment in which all axons are excitable, VAMP2-dependent secretion from axons promotes myelin sheath growth, and our time-lapse imaging indicated that shorter sheaths are more susceptible to subsequent retractions. In this way, excess axon wrapping is refined, and specific axons preferentially maintain myelin sheaths. This highlights a major gap in our current knowledge. If all axons secrete neurotransmitters upon spontaneous and evoked activity, what are the specific factors that distinguish myelin-fated axons from those that will never be myelinated?
Our findings that activity and synaptic vesicle release bias axon selection provide insight into a critical facet of neural development with no previously known mechanism. In culture, oligodendrocytes can myelinate fixed axons and synthetic materials greater than 0.4 µm in diameter2,3. A minimal diameter may be permissive for myelination, but cannot explain how stereotyped decisions to myelinate occur in vivo. Does axon diameter act instructively prior to ensheathment and specify axons for myelination? Electron microscopy studies show a strong correlation between axon diameter and myelination. In the corpus callosum, 80% of myelinated axons have a diameter > 0.4 µm. Yet, there is considerable overlap between the diameters of myelinated and unmyelinated axons, suggesting that axon caliber alone is not sufficient to trigger myelination1. In the absence of causal evidence that axon diameter acts instructively, an alternative possibility is that a minimal diameter of 0.3–0.4 µm is permissive for initial ensheathment, followed by radial growth to increase axon diameter1,25,26. Because axon diameter was unaffected by TeNT-GFP overexpression, we conclude that activity-dependent secretion biases axon choice independently of axon diameter.
Our data are consistent with previous reports that oligodendrocytes have a brief window of myelinogenic potential24. The closure of this developmental window does not involve neuronal activity, because after several hours of initial axon wrapping, oligodendrocytes did not form new sheaths in Tg(neuroD:TeNT-GFP) larvae (see Supplementary Fig. 7d). Is activity-dependent refinement solely responsible for determining which axons are myelinated? Others have suggested that the amount of myelin pruning is insufficient to eliminate all excess or spurious initial wrapping, and have proposed that unidentified mechanisms may specify which axons can be wrapped prior to ensheathment27. Our data do not exclude the possibility that activity-independent forces also contribute to axon selection prior to initial ensheathment. Such mechanisms seem likely given our findings that phox2B+ axons can still be ensheathed, albeit at a lower frequency, in the presence of TTX or tetanus neurotoxin.
How might new or enhanced brain activity stimulate myelination in vivo? Mice running on a wheel with complex rung spacing increase production of oligodendrocytes28 and optogenetic stimulation, within the physiological range, promotes proliferation and maturation of oligodendrocyte lineage cells and myelin thickness9. Additionally, an accompanying study (Mensch et al.) shows that pharmacologically induced brain activity can promote formation of excess myelin sheaths by individual oligodendrocytes in zebrafish. By contrast, our optogenetic and pharmacological manipulations did not overtly change axon wrapping or axon selection bias in zebrafish spinal cord. One possible explanation is that mechanisms that mediate axon selection require highly specific activity codes that were not replicated in our gain of function experiments. Alternatively, activity might not be sufficient to direct myelination of axons that normally remain unmyelinated or to induce myelination prematurely. Because all axons are active, axon selection mechanisms that do not rely solely on activity would prevent ectopic myelination, which could be detrimental. Instead, activity might enhance myelination of predetermined axons, thereby strengthening specific neural circuits in response to experience.
Whether electrical activity and neurotransmitter release regulates myelination is a long-standing question with conflicting results6,7,29,30. Our findings are consistent with models of activity-dependent regulation of myelination and highlight specific roles in axon selection and myelin sheath maintenance. Might neuronal activity influence axon selection via mechanisms resembling synaptogenesis27? Substantial evidence supports extrasynaptic release of axonal factors such as glutamate at sites of oligodendrocyte contact10,11, and our own data show vesicle accumulation at ensheathment sites. This, coupled with evidence for membrane potential shifts and action potential-induced Ca2+ signaling in processes of oligodendrocyte-lineage cells7,31,32, supports a model where axon-oligodendrocyte interactions along the axon locally regulate myelination in response to neuronal activity.
Myelination greatly impacts action potential conduction velocity, and in principle, activity-dependent myelination has profound implications for spike timing and Hebbian learning. Therefore, myelination of specific axons, and the parameters of this myelin, may be especially important for normal brain function. Supporting this notion, many neuropsychiatric disorders have been linked to myelin genes and white matter abnormalities33. Do experiences, encoded by neuronal activity, influence myelination? Mice reared in social isolation from postnatal days 21–35 show altered prefrontal cortex myelination that corresponds with deficits in social interactions and working memory14. This critical period for experience-dependent myelination corresponds with cortical oligodendrocyte maturation and axon ensheathment, suggesting that experience and neuronal activity may alter the behavior of myelinating oligodendrocytes. Our findings that activity-dependent secretion from axons regulates myelin sheath growth, stabilization, and axon selection provide a basis for the stereotyped selection of specific axons, and how altered experience can change the myelinogenic landscape.
METHODS
Zebrafish lines and husbandry
All animal work performed in this study was approved by the Institutional Animal Care and Use Committee at the University of Colorado School of Medicine. Zebrafish embryos were raised at 28.5°C in egg water and staged according to hours post-fertilization or morphological criteria. Tg(sox10:mRFP)vu234, Tg(olig2:EGFP)vu1, Tg(phox2B:GFP)w3734, Tg(sox10:GAL4-VP16, cmlc2:Cerulean)co19, Tg(mbp:GAL4-VP16, cmlc2:Cerulean)co20, Tg(phox2B:GAL4-VP16, cmlc2:Cerulean)co21, Tg(4xnrUAS:EGFP-CaaX, cmlc2:EGFP)co18, Tg(4xnrUAS:mCherry-CaaX, clmc2:EGFP)co22, Tg(neuroD:TeNT-GFP)co23, Tg(UAS:Synaptophysin-GFP) (ref35), and Tg(UAS:ChRWR-EGFP)/js3 (ref. 23) transgenic zebrafish strains were used in this study. Tg(neuroD:TeNT-GFP)co23 zebrafish are paralyzed, unable to feed or form a swim bladder, and therefore not viable past one week. Therefore, all experiments using this line were performed in the F1 generation.
Plasmid construction and generation of new transgenic zebrafish
The GAL4 lines Tg(sox10:GAL4-VP16, cmlc2:Cerulean)co19, Tg(mbp:GAL4-VP16, cmlc2:Cerulean)co20, Tg(phox2B:GAL4-VP16, cmlc2:Cerulean)co21 were created by injecting Tol2 DNA plasmids, together with Tol2 mRNA, into one-cell embryos. GAL4 DNA plasmids were created by Gateway cloning into the Tol2 expression plasmid pCH-Tol2-Gtwy-GAL4-VP16 (gift from Michael Nonet). For pEXPR-Tol2-sox10:GAL4-VP16, cmlc2:cerulean, we used an entry plasmid containing a 7.2 kb genomic fragment of zebrafish sox10 (ref. 36). For pEXPR-Tol2-mbp:GAL4-VP16, cmlc2:cerulean, we used an entry plasmid containing a 2.6 kb genomic fragment of zebrafish mbp-a. Primers are described elsewhere37. For pEXPR-Tol2-phox2Bb:GAL4-VP16, cmlc2:cerulean, we used an entry plasmid containing a 2.1 kb genomic fragment of zebrafish phox2bb. Primer sequences were 5’ GCTGAATTTTTACAGGTTTAAAAGAGG and 5’ TCATTCAAACAGTAATATCAAAGGTTTC. We used multi-site gateway cloning to construct pEXPR-Tol2-4xnrUAS:EGFP-CaaX, cmlc2:EGFP, pEXPR-Tol2-4xnrUAS:mCherry-CaaX, cmlc2:EGFP, pEXPR-Tol2-mbp:TagRFP-T, and pEXPR-Tol2-sox10:TagRFP-T. Entry clones were derived from the Tol2kit system38. pEXPR-Tol2-neuroD:TeNT-GFP was created using multi-site gateway cloning. The 5’ entry plasmid contained a 5 kb genomic fragment of zebrafish neuroD (ref. 39). The middle entry plasmid contained TeNT-GFP fusion protein, which was subcloned from pCS2+TeNT-LC:EGFP, a gift from Martin Meyer, using EcoRI and NotI restriction enzymes. This fusion protein has been extensively characterized and potently inhibits evoked exocytosis in zebrafish neurons in vivo16.
Antibody labeling
To immunolabel MBP in Tg(phox2B:GFP) larvae, we fixed 7 dpf larvae in paraformaldehyde buffer and generated transverse sections using routine methods. Rabbit anti-MBP (1:200 dilution, generated commercially against the peptide sequence CSRSRSPPKRWSTIF, Open Biosystems)40 and goat anti-rabbit AlexaFluor 568 (10 µg/ml, Life Technologies) antibodies were applied to transverse sections. Labeling with rabbit anti-Sox10 has been previously described41.
Drug treatments
We injected 2 nL tetrodotoxin (EMD, 0.5 mM) directly into the yolk of 48 hpf larvae. This concentration consistently paralyzed larva for at least three days. Our initial experiments showed that concentrations greater than 0.5 mM compromised cardiac function, suggesting inhibition of TTX-resistant channels. We injected TTX in a buffered solution containing 0.05% phenol red, 120 mM KCl, 30 mM Hepes, pH adjusted 7.3. Control larvae were injected with a vehicle solution containing injection solution. Veratridine (Sigma-Aldrich V5754) stock was prepared by dissolving in EtOH to 10mM. We injected 2 nL veratridine (1 mM in 0.4M KCl) into the yolk of 72 hpf embryos and selected embryos with a touch response phenotype at 96 hpf for confocal imaging.
Microscopy and Image Analysis
For static imaging of single time points, we embedded larvae for lateral views of the spinal cord in 1% low-melt agarose containing tricaine. We acquired all confocal images using a Zeiss LSM 780 (Carl Zeiss) or a Zeiss Axiovert 200 microscope equipped with a PerkinElmer spinning disk confocal system (PerkinElmer Improvision) with 40× 1.3 NA oil immersion or 63× 1.2 NA water immersion objectives. We used Zen (Carl Zeiss) or Volocity (PerkinElmer) softwares for image acquisition, and ImageJ (National Institutes of Health) for image analysis and processing.
To analyze wrapping of axons marked by Tg(phox2B:GFP), we collected images of 96–100 hpf larvae at somites 27–30 and oversampled z-stacks (0.3 µm intervals) to maximize z-resolution. We measured the number and length of all nascent sheaths ventral to, but not including the Mauthner axon. Sheaths were assigned as wrapping GFP+ or GFP− axons, and we used orthogonal views to inspect all planes in order to confirm assignments. For imaging of axon selection marked by UAS-driven expression in Tg(phox2B:GAL4) larvae, we collected images of 96–100 hpf larvae at somites 21–23. Percent length measurements were made by measuring the length of all sheaths wrapping a single axon and dividing by the total length of the axon in the field of view.
For experiments measuring the number and length of myelin segments emanating from single oligodendrocytes, we injected UAS:mCherry-CaaX plasmids into Tg(mbp:GAL4) or Tg(sox10:GAL4) blastomeres. We also analyzed scatter-labeled oligodendrocytes in Tg(mbp:GAL4); Tg(UAS:mCherry-CaaX) double positive larvae. This mosaicism is most likely a consequence of UAS transgene silencing in zebrafish. To ensure that all myelin segments emanated from a single soma, and not neighboring oligodendrocytes, we only imaged and analyzed isolated oligodendrocytes with no labeled cells in adjacent segments.
To determine the number of oligodendrocyte progenitor cells (OPCs) we used the Tg(olig2:EGFP) reporter line. After chemical fixation and embedding we generated transverse sections of spinal cord. Routine immunofluorescence protocols were used to label Sox10+ spinal cord cells using rabbit anti-Sox10 and goat anti-rabbit Alexa 568 secondary antibodies. We then counted the number of olig2:EGFP+ Sox10+ cells per transverse section in the anterior spinal cord. The average OPC number per fish was generated from 10 independent sections. Cell counts were performed using a Zeiss Axiovert 200 microscope equipped with mercury arc lamp and a 20× 0.8 NA objective.
To determine the number of oligodendrocytes we used the Tg(mbp:EGFP) reporter line. Images were collected using the PerkinElmer spinning disk confocal and the number of oligodendrocytes per field was quantified within Volocity software. Only cells with fluorescence intensities two-fold greater than the mean background pixel intensity were counted in analysis.
To determine the number of OPCs and oligodendrocytes in Tg(neuroD:TeNT-GFP) larvae we crossed to either Tg(sox10:TagRFP-T) or Tg(mbp:TagRFP-T) reporter lines. Images were collected using the PerkinElmer spinning disk confocal. Confocal stacks at multiple segments were acquired and tiled to generate a large field spanning 1 mm along the anterior-posterior axis. Cell counts were performed within Volocity software.
Time-lapse imaging
All fluorescence time-lapse imaging was performed on the spinning disk confocal system equipped with a heated stage chamber to maintain larvae at 28.5°C. Larvae were paralyzed by immersion in 0.5 mg/ml α-Bungarotoxin (EMD) in 10% Hank’s solution, aided by a small incision in the caudal-most tail epidermis with a fine tungsten needle. After 10-min incubation in α-Bungarotoxin, larvae recovered in egg water without anesthetic for at least 1-hr. Paralyzed larvae were then embedded in 1% low-melt agarose, without tricaine, and immersed in egg water without tricaine. For time-lapse imaging of initial ensheathment, we collected images at 2.5 min intervals for 4 hr. Analysis of sheath initiation was performed by a blind observer. Nascent sheaths were defined morphologically by identifying two parallel sox10:mRFP+ membrane processes extending longitudinally along the anterior-posterior axis with lengths exceeding 1 µm. We acquired images at 20 min intervals for retraction assays. When time-lapse durations exceeded 18 hr, we recovered larva to egg water without tricaine, then re-embedded in low-melt agarose immediately prior to the next time point. Time-lapse videos were exported from Volocity as extended z-projection TIFF images. We used ImageJ to rotate, crop, and translate in order to correct for x-y drift. Image stacks were then exported in QuickTime (.mov) format using Sorensen 3 compression.
Swim behavior in veratridine-treated larvae
To assess behavioral phenotypes we used a standard touch assay on 3 dpf larvae. A pin tool was used to induce the touch response and any larva with movement persisting greater than 5 sec and terminating in seizure-like activity was scored as positive. We collected representative time-lapse videos using a Samsung Galaxy S3 digital camera with 8 megapixel and 30 frames per second acquisition settings. Videos were exported in .mov format within QuickTime Pro 7 using H.264 compression.
Optogenetic stimulation and analysis
We crossed Tg(phox2B:GAL4; cmlc2:Cerulean); Tg(sox10:mRFP) × Tg(UAS:ChRWR-EGFP)/js3 adults. At 72 hpf, embryos positive for EGFP+ axons and sox10:mRFP were split into control and treated groups. Blue (473 nm, 20 Hz) light was delivered using a Blues 50 laser (473 nm, 3 mW/cm2, Cobolt Inc., San Jose, CA, USA) controlled by a Uniblitz shutter (Vincent Associates, Rochester, NY, USA) and LabView software v. 2012 (National Instruments, Austin, TX, USA). We applied 30 sec pulses (20 Hz) every 2 min for a total of 3 hr. To assess selection of phox2B+ axons we performed confocal microscopy 24 hr post-stimulation. Images were acquired at the somite position corresponding to where axon wrapping was initiating at the time of treatment (as determined by confocal microscopy).
Electron Microscopy
To measure axon size, we fixed 3 dpf Tg(neuroD:TeNT-GFP) larvae and siblings from 2 clutches in 2% paraformaldehyde, 2% glutaraldehyde and 0.1M sodium cacodylate. Microwave stimulation to accelerate fixation of tissue was performed with a Biowave Pro Laboratory Microwave with ColdSpot (Ted Pella, Inc., Redding, CA, USA) maintained at 15°C. Membranes were enhanced using secondary fixation with OsO4. We collected the electron micrographs using a FEI Techai G2 BioTwin microscope and images were cropped and contrast-adjusted in Adobe Photoshop. We analyzed 4–5 cross-sections of tissue between somites 12–14 for each of 3 larvae per condition. On each cross-section, an unbiased observer randomly selected 5 to 10 axons ventral to each Mauthner axon [within a consistent area of 27 µm2] for a total of 204 sib axons and 185 axons from Tg(neuroD:TeNT-GFP) embryos. Axons were chosen without regard to myelination and were required to have a clearly defined perimeter. The axon area was measured in ImageJ and the axon diameter was calculated from the area value.
Statistical analyses
We plotted all data and performed all statistical analyses within GraphPad Prism software (v6). All data are expressed as mean ± SEM. For statistical analysis, we first performed the D’Agonstino and Pearson omnibus normality test to address normality. We used Student’s two-tailed t-test for all data with normal distribution. For all other data, we assessed statistical significance using the nonparametric Mann-Whitney test. Statistical significance is indicated as follows: *P < 0.05, **P < 0.01, ***P < 0.001. No statistical methods were used to pre-determine sample sizes but our sample sizes are similar to those reported in previous publications7,9,24. A supplementary methods checklist is available.
Supplementary Material
Acknowledgments
We are grateful to Anthony J. Treichel (Winona State University) for help with image analysis. We thank Alex Nechiporuk (Oregon Health Sciences University) for Tg(phox2B:EGFP) zebrafish, Hiromu Yawo (Tohoku University) and the National BioResource Zebrafish Project for Tg(UAS:ChRWR-EGFP) zebrafish, Martin Meyer (King’s College London) for the pCS2-TeNT-GFP plasmid, and Joseph Fetcho (Cornell University) for the UAS:hKir2.1-2A-EGFP-CaaX plasmid. We thank Mary Goll (Memorial Sloan-Kettering Cancer Center), Michael Nonet (Washington University), Chi-Bin Chien (University of Utah), and Kristen Kwan (University of Utah) for Gateway plasmids. For critical feedback on the project, we thank Matthew Rasband (Baylor University), Jason Triplett (Children’s National Medical Center), Steven Henle (Harvard University), Angie Ribera (University of Colorado School of Medicine) and Wendy Macklin (University of Colorado School of Medicine) and all members of the Appel, Ribera, and Macklin laboratories. We also thank Jamie Costabile (University of Colorado School of Medicine) and the University of Colorado Anschutz Medical Campus Optogenetics Core Facility for assistance with UAS:ChRWR-EGFP experiments. This work was supported by NIH Grant R01 NS046668 and a gift from the Gates Frontiers Fund to B.A., National Multiple Sclerosis Postdoctoral Fellowship (FG 2024-A-1), National Institutes of Health (NIMH) T32 MN015442 Fellowship to J.H.H. and National Institutes of Health (NCI) T32 5T32CA08208613 Fellowship to A.M.R. The University of Colorado Anschutz Medical Campus Zebrafish Core Facility is supported by NIH grant P30 NS048154. All DNA plasmids and transgenic zebrafish used in this study are available by request.
Footnotes
Author Contributions
JHH and BA conceived the project. JHH generated all new transgenic zebrafish lines and performed fluorescence microscopy experiments. AMR cloned the zebrafish phox2B promoter and performed veratridine and Channelrhodopsin experiments. RS performed electron microscopy experiments and assisted with cell count experiments. ES produced the Tg(UAS:Syn-GFP) transgenic line. JHH wrote and BA edited the manuscript.
References
- 1.Sturrock RR. Myelination of the mouse corpus callosum. Neuropathol. Appl. Neurobiol. 1980;6:415–420. doi: 10.1111/j.1365-2990.1980.tb00219.x. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SS, Kelland EE, Tokar E, la Torre De AR, Chan JR. The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation. Proc Natl Acad Sci USA. 2008;105:14662–14667. doi: 10.1073/pnas.0805640105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee S, et al. A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat Methods. 2012;9:917–922. doi: 10.1038/nmeth.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gyllensten L, Malmfors T. Myelinization of the optic nerve and its dependence on visual function--a quantitative investigation in mice. J Embryol Exp Morphol. 1963;11:255–266. [PubMed] [Google Scholar]
- 5.Tauber H, Waehneldt TV, Neuhoff V. Myelination in rabbit optic nerves is accelerated by artificial eye opening. Neurosci Lett. 1980;16:235–238. doi: 10.1016/0304-3940(80)90003-8. [DOI] [PubMed] [Google Scholar]
- 6.Demerens C, et al. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci USA. 1996;93:9887–9892. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wake H, Lee PR, Fields RD. Control of local protein synthesis and initial events in myelination by action potentials. Science. 2011;333:1647–1651. doi: 10.1126/science.1206998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barres BA, Raff MC. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 1993;361:258–260. doi: 10.1038/361258a0. [DOI] [PubMed] [Google Scholar]
- 9.Gibson EM, et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344:1252304–1252304. doi: 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kukley MM, Capetillo-Zarate EE, Dietrich DD. Vesicular glutamate release from axons in white matter. Nat Neurosci. 2007;10:311–320. doi: 10.1038/nn1850. [DOI] [PubMed] [Google Scholar]
- 11.Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci. 2007;10:321–330. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng BKB, Chen LL, Mandemakers WW, Cosgaya JMJ, Chan JRJ. Anterograde transport and secretion of brain-derived neurotrophic factor along sensory axons promote Schwann cell myelination. Journal of Neuroscience. 2007;27:7597–7603. doi: 10.1523/JNEUROSCI.0563-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Káradóttir R, Attwell D. Neurotransmitter receptors in the life and death of oligodendrocytes. Neuroscience. 2007;145:1426–1438. doi: 10.1016/j.neuroscience.2006.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makinodan M, Rosen KM, Ito S, Corfas G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science. 2012;337:1357–1360. doi: 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci. 2012;15:1621–1623. doi: 10.1038/nn.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben Fredj N, et al. Synaptic activity and activity-dependent competition regulates axon arbor maturation, growth arrest, and territory in the retinotectal projection. J Neurosci. 2010;30:10939–10951. doi: 10.1523/JNEUROSCI.1556-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agetsuma MM, et al. The habenula is crucial for experience-dependent modification of fear responses in zebrafish. Nat Neurosci. 2010;13:1354–1356. doi: 10.1038/nn.2654. [DOI] [PubMed] [Google Scholar]
- 18.Hua JY, Smear MC, Baier H, Smith SJ. Regulation of axon growth in vivo by activity-based competition. Nature. 2005;434:1022–1026. doi: 10.1038/nature03409. [DOI] [PubMed] [Google Scholar]
- 19.McLaughlin T, Torborg CL, Feller MB, O'Leary DDM. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron. 2003;40:1147–1160. doi: 10.1016/s0896-6273(03)00790-6. [DOI] [PubMed] [Google Scholar]
- 20.Nicol X, et al. cAMP oscillations and retinal activity are permissive for ephrin signaling during the establishment of the retinotopic map. Nat Neurosci. 2007;10:340–347. doi: 10.1038/nn1842. [DOI] [PubMed] [Google Scholar]
- 21.Plazas PV, Nicol X, Spitzer NC. Activity-dependent competition regulates motor neuron axon pathfinding via PlexinA3. Proc Natl Acad Sci USA. 2013;110:1524–1529. doi: 10.1073/pnas.1213048110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kishore S, Fetcho JR. Homeostatic regulation of dendritic dynamics in a motor map in vivo. Nat Commun. 2013;4:2086. doi: 10.1038/ncomms3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umeda K, et al. Targeted expression of a chimeric channelrhodopsin in zebrafish under regulation of Gal4-UAS system. Neurosci Res. 2013;75:69–75. doi: 10.1016/j.neures.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Czopka T, ffrench-Constant C, Lyons DA. Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths in vivo. Dev Cell. 2013;25:599–609. doi: 10.1016/j.devcel.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colello RJ, Pott U, Schwab ME. The role of oligodendrocytes and myelin on axon maturation in the developing rat retinofugal pathway. Journal of Neuroscience. 1994;14:2594–2605. doi: 10.1523/JNEUROSCI.14-05-02594.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sánchez I, Hassinger L, Paskevich PA, Shine HD, Nixon RA. Oligodendroglia regulate the regional expansion of axon caliber and local accumulation of neurofilaments during development independently of myelin formation. Journal of Neuroscience. 1996;16:5095–5105. doi: 10.1523/JNEUROSCI.16-16-05095.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almeida RG, Lyons DA. On the resemblance of synapse formation and CNS myelination. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.08.062. [DOI] [PubMed] [Google Scholar]
- 28.McKenzie IA, et al. Motor skill learning requires active central myelination. Science. 2014;346:318–322. doi: 10.1126/science.1254960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shrager P, Novakovic SD. Control of myelination, axonal growth, and synapse formation in spinal cord explants by ion channels and electrical activity. Brain Res Dev Brain Res. 1995;88:68–78. doi: 10.1016/0165-3806(95)00081-n. [DOI] [PubMed] [Google Scholar]
- 30.De Biase LM, et al. NMDA receptor signaling in oligodendrocyte progenitors is not required for oligodendrogenesis and myelination. J Neurosci. 2011;31:12650–12662. doi: 10.1523/JNEUROSCI.2455-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Káradóttir R, Hamilton NB, Bakiri Y, Attwell D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci. 2008;11:450–456. doi: 10.1038/nn2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chittajallu R, Aguirre A, Gallo V. NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J Physiol (Lond) 2004;561:109–122. doi: 10.1113/jphysiol.2004.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nechiporuk A, Linbo T, Poss KD, Raible DW. Specification of epibranchial placodes in zebrafish. Development. 2007;134:611–623. doi: 10.1242/dev.02749. [DOI] [PubMed] [Google Scholar]
- 35.Heap LA, Goh CC, Kassahn KS, Scott EK. Cerebellar output in zebrafish: an analysis of spatial patterns and topography in eurydendroid cell projections. Front Neural Circuits. 2013;7:53–53. doi: 10.3389/fncir.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kucenas S, et al. CNS-derived glia ensheath peripheral nerves and mediate motor root development. Nat Neurosci. 2008;11:143–151. doi: 10.1038/nn2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jung S-H, et al. Visualization of myelination in GFP-transgenic zebrafish. Dev Dyn. 2010;239:592–597. doi: 10.1002/dvdy.22166. [DOI] [PubMed] [Google Scholar]
- 38.Kwan KM, et al. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn. 2007;236:3088–3099. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- 39.Mo W, Nicolson T. Both pre- and postsynaptic activity of Nsf prevents degeneration of hair-cell synapses. PLoS ONE. 2011;6:e27146. doi: 10.1371/journal.pone.0027146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kucenas S, Wang W-D, Knapik EW, Appel B. A selective glial barrier at motor axon exit points prevents oligodendrocyte migration from the spinal cord. J Neurosci. 2009;29:15187–15194. doi: 10.1523/JNEUROSCI.4193-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park H-C, Boyce J, Shin J, Appel B. Oligodendrocyte specification in zebrafish requires notch-regulated cyclin-dependent kinase inhibitor function. J Neurosci. 2005;25:6836–6844. doi: 10.1523/JNEUROSCI.0981-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.