Abstract
Introduction
Multidrug resistant tuberculosis (MDR-TB) presents a great challenge to public health, especially for developing countries. Some nontuberculous mycobacteria (NTM) cause the similar clinical and radiological characteristics with tuberculosis. We aimed to identify the frequency of NTM infections among subjects who were suspected to have MDR-TB due to lack of response to anti-TB treatment.
Methods
This retrospective study evaluated patients with suspected MDR-TB due to lack of sputum conversion after 2–3 months therapy with first line anti-TB treatment from 2009 through 2014. Cultures for mycobacteria were performed and identification was done to species level by phenotypic and molecular tests. The outcome of the patients with NTM disease and related risk factors for poor outcome were evaluated.
Results
Out of 117 consecutive strains isolated from suspected MDR-TB subjects, 35 (30%) strains were identified as NTM by using conventional and molecular approaches. Of these patients with positive NTM cultures, 32 (27%) patients met ATS/IDSA diagnostic criteria. Out of 32, 29 (90%) individuals with confirmed NTM diseases had underlying disorders including 8 subjects with malignancy, 5 with organ transplantations, and 4 with the human immunodeficiency virus. No known underlying disorder was found in 3 (9%) subjects.
Treatment outcomes were available for 27 subjects, 17 (63%) of whom were cured and 10 (37%) had poor outcome including 6 (60%) who failed and 4 (40%) who died during treatment.
Conclusion
The high costs to the patient and society should lead health care providers to consider NTM in all patients suspected of having TB.
Keywords: MDR-TB, tuberculosis, NTM, nontuberculous mycobacteria
Introduction
Multidrug resistant tuberculosis presents a great challenge to public health, especially for developing countries [1]. Treatment requires great effort and cost to the health care system and to the individual patient. In many settings, tuberculosis alone is a formidable stigma; having a nearly incurable form of the disease that is contagious to friends and neighbors can ruin affected individuals, even if they are cured.
Because of the gravity of multidrug-resistant tuberculosis, making an accurate diagnosis is critical. Mistaking persons with nontuberculous mycobacteria (NTM) for multidrug resistant tuberculosis (MDR-TB) is a serious error. Yet, all mycobacteria are acid-fast; many environmental species cause lung disease that is often indistinguishable from tuberculosis; diagnosis of tuberculosis in much of the world is still only by sputum smear, and disease caused by nontuberculous mycobacteria usually does not respond to antituberculous medication.
The genus mycobacteria include more than 150 species that are found in every country of the world as recent reports have emphasized [2]. Multidrug resistant tuberculosis has been reported to be confused with disease caused by NTM in up to 11% of cases [3, 4].
In this study, we aimed to identify the frequency of NTM infections among subjects labeled with MDR-TB in the Southwestern of Iran.
Material and methods
This study was approved by the Institutional Review Board of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (approval number: ajums.REC.1391.56).
Study Design and Patient Data
This retrospective study evaluated patients referred to Infectious and Tropical Diseases Research Center of Ahvaz Jundishapur University of Medical Sciences, Ahvaz Iran, from 2009 through 2014. This research center is located in the Southwest of Iran and acts as referral center for diagnosis and treatment of infectious diseases for the Southwest region of Iran with about 10 million populations. While the outcome of MDR-TB is well studied, the outcome of the patients with NTM disease and its related risk factors for poor outcome remains unclear.
Clinical sample cultures for mycobacteria were performed for persons suspected of having MDR-TB (defined as continuation of sputum positivity beyond 2–3 months of first line anti-TB treatment). All patients were received standard anti-TB therapy including rifampin, isoniazid, pyrazinamide and ethambutol (RIPE) per Iranian National TB Program recommendations.
Mycobacteria were identified to species level by phenotypic and molecular tests. Demographic and clinical information of patients with positive cultures were collected from the medical records including 1) age and gender, 2) underlying malignancy defined as having any neoplasm, except basal cell carcinoma, during the 12 months previous to the diagnosis, 3) cavitary lesions confirmed by chest radiograph or chest CT-scan, and 4) treatment outcome, where “cure” was defined as improvement of clinical symptoms and radiologic images on appropriate antibiotics and mycobacterial culture conversion to negative and staying negative for at least 12 months. “Poor outcome” was defined as failure if clinical symptoms and radiologic images worsened with appropriate antibiotics regimen and death which was recorded for any reason during first year of treatment. “Confirmed cases” of disease caused by NTM met the definition of the American Thoracic Society and Infectious Disease Society of America (ATS/IDSA) criteria [5].
Identification of isolates
Phenotypic identification
All mycobacterial isolates were examined for growth rate, macroscopic and microscopic morphological features, growth at different temperatures, and biochemical tests including nitrate reduction, niacin production, and semi-quantitative catalase production according to standard procedures [6]. The susceptibility tests were performed using the National Committee for Clinical Laboratory Standards proportional method on Löwenstein-Jensen medium containing rifampin 40 mg/L, isoniazid 0.2 mg/L, ethambutol 2 mg/L and streptomycin 40 mg/L [6, 7].
Molecular assignment of isolates to M. tuberculosis complex group
Chromosomal DNA was extracted using QIAamp DNA Mini Kit (QIAGEN, USA) according to kit instruction. A 245-base-pair segment of a repetitive sequence of IS6110 in the chromosome of M. tuberculosis was used as a target and amplified by polymerase chain reaction (PCR) using the primers INS-1 and INS-2 [8]. M. tuberculosis H37Rv (ATCC 27294) and M. bovis BCG Pasteur 1173P2 were included as controls in all phenotypic and molecular assays.
Molecular assignment to species level
The two ways were used to speciate mycobacteria, 16S rRNA and rpoB gene sequencing.
16S rRNA gene sequencing
Nearly the full length of the 16S rRNA genes (1500-bp) from isolates were amplified using primers pA (5′-AGAGTTTGATCCTGGCTCAG-3′) and pI (5′-TGCACACAGGCCACAAGGGA-3′) as described previously [9]. The amplified PCR products of 16S rRNA gene for each isolate were purified with the GeneJET Gel Extraction Kit (Thermo Scientific) as described in the manufacturer’s instructions. The sequences of the products were determined using an ABI PRISM® 7700 Sequence Detection System (Applied Biosystems, Foster City, California) according to the standard protocol of the supplier. The sequences obtained for each isolate from 16S rRNA gene were aligned separately and compared with all existing relevant sequences of mycobacteria retrieved from GenBank database using jPhydit software (available at http://chunlab.snu.ac.kr/jphydit/) [10]. A percentage of similarity between the 16S rRNA gene sequences of each isolate was determined by comparing the sequences found to an in-house database of 16S rRNA gene sequences.
rpoB gene sequencing
A 750-bp fragment of the rpoB gene was amplified and sequenced by two specific primers MycoF (5′-GGCAAGGTCACCCCGAAGGG-3′) and MycoR (5′-AGCGGCTGCTGGGTGATCATC-3′) as previously described [11]. The amplified PCR products of rpoB gene for each isolate were purified with the GeneJET™ Gel Extraction Kit (Thermo Scientific) as described in the manufacturer’s instructions. The sequences of the products were determined according to standard protocol. The sequences obtained for each isolate from the rpoB gene analysis were aligned separately and compared with all existing relevant sequences of mycobacteria retrieved from GenBank database using jPhydit software [10]. A percentage of similarity between the rpoB gene sequences of each isolate was determined by comparing sequences found to an in-house database of rpoB gene sequences.
The GenBank accession numbers of investigated NTM clinical isolates in this work were as follows: KM507874 to KM507908 for the 16S rRNA, KM507909 to KM507943 for rpoB.
To identify the clustering of mycobacterial species, phylogenetic analysis was performed. The tree was constructed with the program MEGA by neighbor-joining with Kimura two-parameter distance [12].
Statistical analysis
Categorical variables were described as counts and percentages and were evaluated by the Chi-square test. Univariate analysis was used to compare differences in demographic and clinical variables between subjects with cure vs. poor outcomes. Comparisons were unpaired, and all tests of significance were two-tailed with p<0.05. Age was compared by Student’s t-test.
Results
Out of 117 consecutive strains isolated from suspected MDR-TB subjects, 35 (30%) strains were identified as NTM by using conventional and molecular approaches. Of these patients with positive NTM cultures, 32 (27%) patients met ATS/IDSA diagnostic criteria for NTM disease (considering 3 M. gordonae as colonization) [5]. Table 1 shows their demographic and identification data. Pulmonary disease accounted for 23 (71%) cases, of which 7 cases had cavitation visible on chest radiography. The remaining 16 cases had abnormal chest imaging including nodules and consolidations. Twenty-nine (90%) individuals with confirmed NTM diseases had underlying disorders including 8 (25%) subjects with malignancy, 5 (16%) with organ transplantations, 4 (13%) with the human immunodeficiency virus (HIV) and 12 had other underlying diseases including chronic renal failure, diabetes. No known underlying disorder was found in 3 (9%) subjects.
Table 1.
The demographic and identification data of patients with NTM disease
| Variables | Cure N(%) | Poor outcome* N(%) | P-value |
|---|---|---|---|
| Number of subjects | 17(63) | 10(37) | |
| Age years (mean ± SD) | 50.5±15 | 43.9±18 | 0.317 |
| Sex | 0.156 | ||
| Female | 10(59) | 3(30) | |
| Male | 7(41) | 7(70) | |
| Underlying malignancy | 2(40) | 3(60) | 0.253 |
| NTM location | 0.594 | ||
| Pulmonary | 14(82) | 9(90) | |
| Extrapulmonary | 3(18) | 1(10) | |
| Chest X-ray | |||
| Cavitary lesion | 5(36) | 2(22) | 0.496 |
| Mycobacteriology | N/A** | ||
| M. kansasii | 6(86) | 1(14) | |
| M. abcessus | 1(16) | 5(84) | |
| Mycobacterium avium complex | 2(50) | 2(50) | |
| M. fortuitum | 2(67) | 1(33) | |
| M. chelonae | 2(67) | 1(33) | |
| M. simiae | 3(100) | 0 | |
| M. thermoresistible | 1(100) | 0 |
Poor outcome includes relapse, failure to treatment and death.
N/A: not applicable, N; number
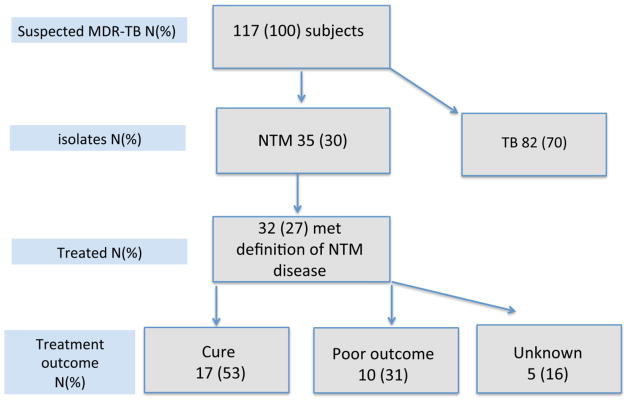
Treatment outcomes were available for 27 subjects, 17 (63%) of whom were cured and 10 (37%) had poor outcome including 6 (60%) who failed and 4 (40%) who died during treatment (Figure 1). Two subjects with M. abscessus required surgical intervention, removing skin and soft tissue infections in both.
Figure 1.
The diagram shows allocation of the study subjects.
Assignment of isolates to tuberculosis group
Out of 117 clinical isolates from patients who failed to respond to first-line treatment, 82 isolates were identified as M. tuberculosis using conventional testing along with the presence of 245-base-pair segment of a repetitive sequence of IS6110. Based on the results of in vitro drug susceptibility testing to the first-line antituberculous drugs on 82 isolates, eight isolates were susceptible to isoniazid and rifampin, four of which were mono-resistance to ethambutol or streptomycin as well. Sixty-three isolates were MDR-TB, while 23 and 18 isolates showed additional resistance to streptomycin and ethambutol respectively. Multidrug-resistance patterns defined by resistance to isoniazid and streptomycin were detected in 8 isolates. Other multidrug-resistant patterns were detected in three isolates, which had resistance to rifampin, streptomycin, and ethambutol.
Molecular assignment of NTM to species level
All clinical isolates of NTM were identified to the species level by 16S rRNA and rpoB gene sequencing. NTM were isolated from 35 clinical isolates. The first colony was detected within a week in 15 (42%) cultures and classified as rapidly growing mycobacteria (RGM). In 20 (58%) the first colony appeared longer than 2 weeks and was classified as slowly growing mycobacteria (SGM). Twenty-one isolates (60%) were confidently identified by almost full 16S rRNA gene sequencing with high bootstrap values.
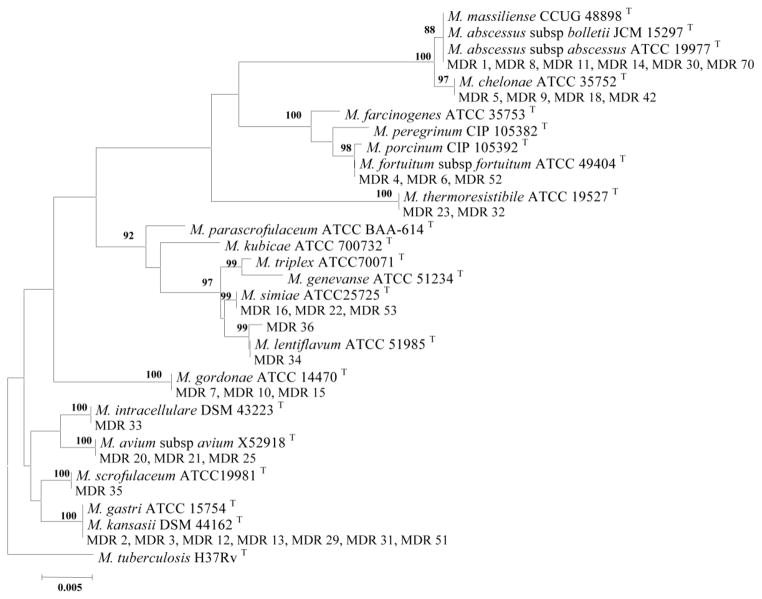
The 16S rRNA gene of isolates numbers 2, 3, 12, 13, 29, 31, and 51 showed 100% similarity to M. kansasii and M. gastri and formed single separate branch in SGM clusters (Figure 1). Isolate number 36 showed 99.8% similarity of 16S rRNA gene of to that of M. lentiflavum. The 16S rRNA gene of isolate numbers 1, 8, 11, 14, 30, and 71 shown 100% similarity to M. abscessus species and formed a single separate branch in the rapid growing mycobacterial clusters (Figure 2).
Figure 2.
Phylogenetic tree based on 16S rRNA sequences, constructed using the neighbor-joining method bootstrapped 1000 times. Bootstrap values are given at nodes. Bar, 0.005 substitutions per nucleotide position.
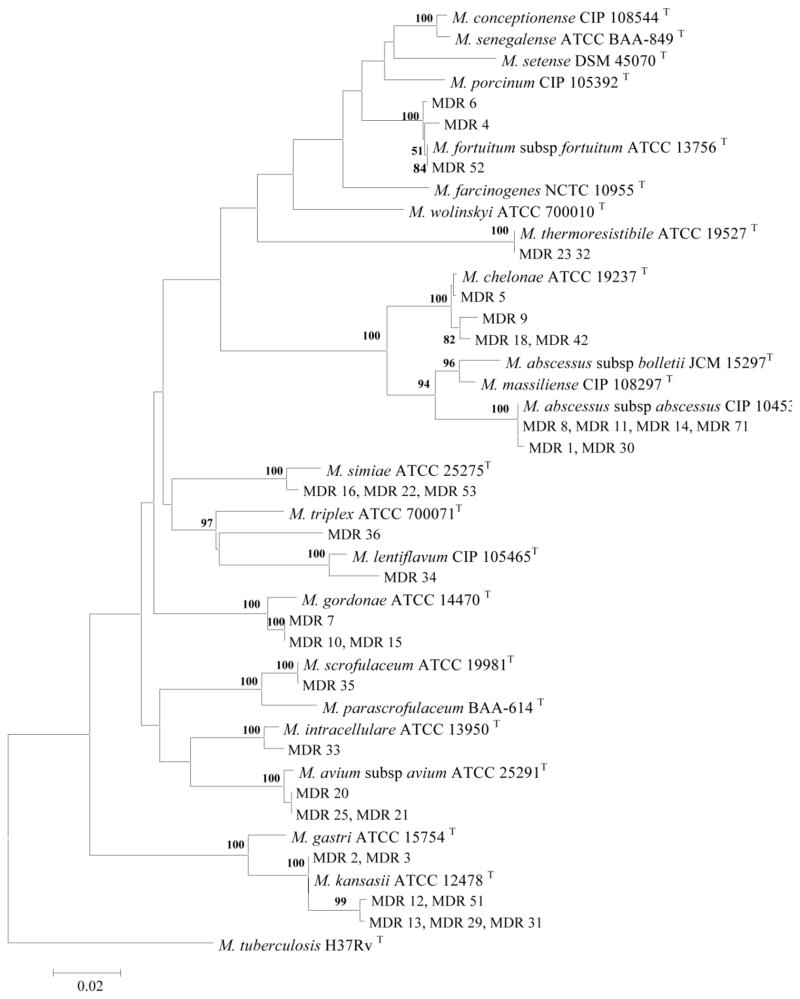
The rpoB gene sequencing of clinical isolates separated NTM into RGM and SGM groups (Figure 3). All of the isolates showed good discrimination with regard to the rpoB gene sequences. Thirty-four NTM isolates (97%) were clearly identified by rpoB gene sequencing. There was also a strong correlation and similar topology between rpoB and 16S rRNA gene sequence-derived trees (Figures 2 and 3). The resolution of the rpoB gene was higher than those obtained by 16S rRNA. The rpoB gene of isolate number 36 showed 97.7% similarity to that of M. lentiflavum and formed a distinct line in the phylogenetic tree which could classify this as a novel species. The isolates numbers 2, 3, 12, 13, 29, 31, and 51 were identified as M. kansasii. These isolates showed 98.5% to 100% and 97% similarity to the rpoB gene to M. kansasii and M. gastri respectively and formed a single separate branch in SGM clusters with M. kansasii (Figure 3). Based on rpoB gene sequence, isolates 1, 8, 11, 14, 30, and 71 were identified as M. abscessus subsp. abscessus. The isolates were showed 99.8%, 96%, and 95.3% similarity of rpoB gene to those of M. abscessus subsp. abscessus, M. abscessus subsp. bolletii, and M. massiliense respectively (Figure 2).
Figure 3.
Phylogenetic tree based on rpoB sequences, constructed using the neighbor-joining method bootstrapped 1000 times. Bootstrap values are given at nodes. Bar, 0.02 substitutions per nucleotide position.
Using the 16S rRNA and rpoB gene sequences, isolates were identified as M. kansasii (7 isolates), M. abscessus (6 isolates), M. chelonae (4 isolates), M. avium (3 isolates), M. gordonae (3 isolates), M. fortuitum (3 isolates), M. simiae (3 isolates), M. thermoresistible (2 isolate), M. intracellulare (1 isolate), M. lentiflavum (1 isolate), M. scrofulaceum (1 isolate), and one unidentifiable isolate.
Discussion
This study found that patients considered to have multidrug-resistant tuberculosis frequently had infections with environmental mycobacteria; 30% of suspected MDR-TB patients actually had NTM, and 27% of them met the NTM disease definition by the current ATS/IDSA criteria. The main organisms were M. kansasii and M. abscessus.
Diseases caused by the nontuberculous mycobacteria present with similar clinical and radiological features of tuberculosis. The NTM patients have similar risk factors including underlying pulmonary disease, immunosuppression, organ transplantation, HIV infection, and advanced age [13]. And, of course, both types of organisms are acid-fast.
In countries with high prevalence, TB is diagnosed by the clinical presentation and laboratory findings of sputum smear microscopy [14]. The World Health Organization (WHO) recommends National Tuberculosis Control Programs should obtain sputum culture only in new patients if the smear is positive in the end of month 3 of treatment [15], and even this is not followed in many resource-limited countries [3, 4, 16]. Almost 80% of TB laboratories in China are not equipped to perform mycobacterial culture and identification [17]. Although the main concern of the lack of mycobacterial culture has been the difficulty of drug-resistant TB surveillance, the point of this paper is that it misses NTM disease. Missing NTM pulmonary disease results in inappropriate treatment, high cost, and stigmatization of affected persons, with its important social and economic consequences.
The results of our study are even great than other studies that have sought to measure the rates of NTM disease. Tabarsi et al. studied 105 suspected MDR-TB subjects in the center of Iran and found 12 (11%) cases of NTM. Aliyu et al. found 69 (15%) NTM out of 444-mycobacterial positive cultures from consecutive new cases of suspected TB in Nigeria [18]. The reason for this discrepancy might be related to environmental factors or higher prevalence of underlying diseases in our study population.
Coinfection with TB and NTM also occurs, although the information about this is limited. Damaraju et al. recently showed that this coinfection occurred in 11% of TB patients in Ontario, Canada [19]. In the United States, NTM-TB coinfection has been reported to be 14% [20], and in Korea and Taiwan it was 7% [21]. We did not detect TB-NTM coinfection in our study. No treatment guidelines have been established to address this coinfection, but we treat TB first and add medication for NTM if our suspicion for it is high.
The current study has several limitations. The clinical outcomes of NTM treatment were not available on some subjects. To find the cause of death for patients stratified as poor outcomes, we relied on death certificates, which could be inaccurate. Sputum cultures were only available for 2 to 3 months after beginning antituberculous therapy. Finally, the true prevalence of NTM disease is not known because this study addresses only patients with MDR-TB. Estimating NTM prevalence would require accurate reporting of all cases in a defined area and time. Despite these limitations, the study is important because it demonstrate the problem of NTM presence in patients diagnosed and treated for TB and validate the previous study on NTM misdiagnosis among subjects with suspected TB by Tabarsi et al. [4].
The high rate of NTM in patients with suspected MDR-TB due to TB treatment failure has important implications for healthcare economics, epidemiology, antimicrobial stewardship, and especially the care and quality of life of the individual patient.
The high costs to the patient and society should lead health care providers to consider NTM in all patients suspected of having TB. Even in low resource countries where mycobacterial culture and molecular identification procedures are not routinely practiced, there could be great saving of country resources associated with correctly diagnosing and treating patients. It is another reason for National TB Programs to integrate mycobacterial culture and molecular identification procedures into routine practice.
Identifying NTM frequency and alerting physicians to this problem may increase awareness, help better understand the epidemiology, get patients on appropriate therapy sooner, and decrease the stigma and cost of this care.
Acknowledgments
Funding resources: Mehdi Mirsaeidi MD is supported by a grant from National Health Institutes (NIH Grant 5 T32 HL 82547-7).
Authors would like to thank Drs. Marybeth Allen and Ronald Smith for their great constructive comments.
List of used abbreviations
- MDR-TB
Multidrug resistant tuberculosis
- TB
Tuberculosis
- NTM
Nontuberculous mycobacteria
- HIV
Human immunodeficiency virus
- ATS/IDSA
The American Thoracic Society and Infectious Disease Society of America
- WHO
The World Health Organization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.http://www.who.int/tb/challenges/mdr/MDR_TB_FactSheet.pdf.
- 2.Tortoli E. Microbiological features and clinical relevance of new species of the genus Mycobacterium. Clinical microbiology reviews. 2014;27:727–52. doi: 10.1128/CMR.00035-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu K, Bi S, Ji Z, Hu H, Hu F, Zheng B, et al. Distinguishing nontuberculous mycobacteria from multidrug-resistant Mycobacterium tuberculosis, China. Emerging infectious diseases. 2014;20:1060–2. doi: 10.3201/eid2006.130700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabarsi P, Baghaei P, Farnia P, Mansouri N, Chitsaz E, Sheikholeslam F, et al. Nontuberculous mycobacteria among patients who are suspected for multidrug-resistant tuberculosis-need for earlier identification of nontuberculosis mycobacteria. The American journal of the medical sciences. 2009;337:182–4. doi: 10.1097/maj.0b013e318185d32f. [DOI] [PubMed] [Google Scholar]
- 5.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. American journal of respiratory and critical care medicine. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 6.Kent P, Kubica G. Public health mycobacteriology. Atlanta, GA: Centers for Disease Control, US Department of Health and Human Services; 1985. A guide for the level III laboratory. [Google Scholar]
- 7.Reller LB, Weinstein MP, Woods GL. Susceptibility testing for mycobacteria. Clinical Infectious Diseases. 2000;31:1209–15. doi: 10.1086/317441. [DOI] [PubMed] [Google Scholar]
- 8.Spigelman M, Matheson C, Lev G, Greenblatt C, Donoghue HD. Confirmation of the presence of Mycobacterium tuberculosis complex-specific DNA in three archaeological specimens. International Journal of Osteoarchaeology. 2002;12:393–401. [Google Scholar]
- 9.Rogall T, Flohr T, Böttger EC. Differentiation of Mycobacterium species by direct sequencing of amplified DNA. Journal of general microbiology. 1990;136:1915–20. doi: 10.1099/00221287-136-9-1915. [DOI] [PubMed] [Google Scholar]
- 10.Jeon Y-S, Chung H, Park S, Hur I, Lee J-H, Chun J. jPHYDIT: a JAVA-based integrated environment for molecular phylogeny of ribosomal RNA sequences. Bioinformatics. 2005;21:3171–3. doi: 10.1093/bioinformatics/bti463. [DOI] [PubMed] [Google Scholar]
- 11.Adekambi T, Colson P, Drancourt M. rpoB-based identification of nonpigmented and late-pigmenting rapidly growing mycobacteria. Journal of clinical microbiology. 2003;41:5699–708. doi: 10.1128/JCM.41.12.5699-5708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular biology and evolution. 2007;24:1596–9. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 13.Mirsaeidi M, Farshidpour M, Ebrahimi G, Aliberti S, Falkinham JO., 3rd Management of nontuberculous mycobacterial infection in the elderly. European journal of internal medicine. 2014;25:356–63. doi: 10.1016/j.ejim.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parsons LM, Somoskovi A, Gutierrez C, Lee E, Paramasivan CN, Abimiku A, et al. Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. Clinical microbiology reviews. 2011;24:314–50. doi: 10.1128/CMR.00059-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. Treatment of tuberculosis guidelines. 4. 2010. [PubMed] [Google Scholar]
- 16.Nyamogoba HD, Mbuthia G, Mining S, Kikuvi G, Biegon R, Mpoke S, et al. HIV co-infection with tuberculous and non-tuberculous mycobacteria in western Kenya: challenges in the diagnosis and management. African health sciences. 2012;12:305–11. doi: 10.4314/ahs.v12i3.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pang Y, Zhou Y, Wang S, Tan Y, Yue J, Zhao B, et al. Rapid molecular identification of mycobacterial species in positive culture isolates using the biochip test. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2011;15:1680–5. doi: 10.5588/ijtld.11.0061. [DOI] [PubMed] [Google Scholar]
- 18.Aliyu G, El-Kamary SS, Abimiku A, Brown C, Tracy K, Hungerford L, et al. Prevalence of non-tuberculous mycobacterial infections among tuberculosis suspects in Nigeria. PloS one. 2013;8:e63170. doi: 10.1371/journal.pone.0063170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Damaraju D, Jamieson F, Chedore P, Marras TK. Isolation of non-tuberculous mycobacteria among patients with pulmonary tuberculosis in Ontario, Canada. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2013;17:676–81. doi: 10.5588/ijtld.12.0684. [DOI] [PubMed] [Google Scholar]
- 20.Kendall BA, Varley CD, Hedberg K, Cassidy PM, Winthrop KL. Isolation of non-tuberculous mycobacteria from the sputum of patients with active tuberculosis. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2010;14:654–6. [PubMed] [Google Scholar]
- 21.Huang CT, Tsai YJ, Shu CC, Lei YC, Wang JY, Yu CJ, et al. Clinical significance of isolation of nontuberculous mycobacteria in pulmonary tuberculosis patients. Respiratory medicine. 2009;103:1484–91. doi: 10.1016/j.rmed.2009.04.017. [DOI] [PubMed] [Google Scholar]