Abstract
Purpose
While current osteoporosis management guidelines recommend use of pharmacologic treatment following hip fracture, the care of such patients has been suboptimal. The objective of this cross-national study is to quantify the use of and adherence to osteoporosis medication following hip fracture in three countries with different health care systems- the United States, Korea and Spain.
Methods
In three cohorts of patients aged ≥65 years hospitalized for hip fracture, we calculated the proportion receiving ≥1 osteoporosis drug after discharge. Adherence to osteoporosis treatment was measured as the proportion of days covered (PDC) during the first year following the hip fracture.
Results
We identified 86,202 patients with a hip fracture - 4,704 (U.S. Medicare), 6,700 (U.S. commercial), 57,631(Korea), and 17,167 (Spain). The mean age was 77–83 years and 74–78% were women. In the year prior to the index hip fracture, 16–18% were taking an osteoporosis medication. Within 3 months following the index hip fracture, 11% (U.S. Medicare), 13% (U.S. commercial), 39% (Korea), and 25% (Spain) of patients filled ≥1 prescription for osteoporosis medication. For those who filled one or more prescriptions for an osteoporosis medication, the mean PDC in the year following the fracture was 0.70 (U.S. Medicare), 0.67 (U.S. commercial), 0.43 (Korea) and 0.66 (Spain).
Conclusions
Regardless of differences in health care delivery systems and medication reimbursement plans, the use of osteoporosis medications for the secondary prevention of osteoporotic fracture was low. Adherence to osteoporosis treatment was also suboptimal with the PDC<0.70 in all three countries.
Keywords: osteoporotic fracture, hip fracture, bisphosphonate, cohort study, adherence
INTRODUCTION
Osteoporosis is a common and generally undertreated problem, particularly in the elderly.1, 2 It represents a major public health problem because of the disability, morbidity, mortality, and cost to which it contributes. Hip fractures are the worst consequences of osteoporosis, as the 1-year mortality of such patients is nearly 30% and it often leads to major morbidity including significant functional loss.3, 4 The economic burden related to hip fractures is also very high, with the estimated treatment cost over 10 billion dollars per year in the United States (U.S.) alone.5–7 The condition has similarly high impacts on the health care systems of other countries.
Patients who suffer their first hip fracture are at greater risk of recurrent osteoporotic fractures. In these patients, medications such as bisphosphonates can reduce the risk of recurrent osteoporotic fracture and improve survival.8–12 A previous meta-analysis of eleven randomized clinical trials of alendronate showed clinically important and statistically significant reductions in vertebral, nonvertebral, hip and wrist fractures for secondary prevention.13 In a randomized controlled trial of intravenous zoledronic acid within 3 months after surgical repair of a hip fracture also reduced a risk of recurrent clinical fracture by 35% and mortality by 28%.8 Current guidelines, therefore, recommend use of such pharmacologic treatment following hip fracture.14, 15 However, the care of patients after hip fracture has been suboptimal as less than one-third of patients suffering a hip fracture do not receive subsequent osteoporosis treatment.16–18 In 2012, the American Society for Bone and Mineral Research Task Force on Secondary Fracture Prevention emphasized the importance of secondary prevention of fragility fracture and proposed an international collaborative work using a Fracture Liaison Service to improve secondary fracture prevention.19 As access to health care is different in each country, the quality of post-fracture care may vary as well, but little is known how patterns of under-treatment vary from country to country, particularly those with very different health care delivery and reimbursement systems.
The objectives of this study were 1) to examine the use of osteoporosis medications following hip fracture, 2) to evaluate the adherence to osteoporosis medications following hip fracture, and 3) to assess time trends in the use of these medications after fracture - the U.S., South Korea and Spain- with different health care systems.
MATERIALS AND METHODS
Data Sources
The study investigators in the U.S., South Korea and Spain simultaneously conducted a retrospective cohort study using a study protocol developed by all participating investigators. For the U.S., two separate cohorts were constructed using the claims data from a U.S. government-sponsored health insurance plan (“U.S. Medicare”, 2005–2008) as well as a commercial health insurer (“U.S. commercial”, 2003–2012). The Korean cohort (“Korea”, 2007–2011) was based on complete filled prescription data from the Health Insurance Review and Assessment Service (HIRA) database, which includes the entire Korean population. The Valencia cohort (“Spain”, 2007–2012) was constructed using the claims and electronic medical records data from the Valencia Health Agency (VHA). Appendix 1 describes the data sources in detail.
Study Cohorts
We identified all patients aged 65 years or older who had a hospitalization for hip fracture based on a diagnosis code as well as a procedure code for surgical treatment of the fracture, and had 365 days of continuous health plan eligibility beforehand, to ensure adequate ascertainment of baseline characteristics. The admission date for the initial hip fracture hospitalization was defined as the index date. Patients with a diagnosis of trauma or fractures at multiple sites at the index date and those with a diagnosis of malignancy in the 365 days prior to the index date were excluded. To ensure that all patients had at least 6 months of follow-up time after the fracture, patients with less than 6 months of data after the index date were excluded. Each patient was followed from the index date to the first occurrence of insurance disenrollment, administrative end of the study, or death.
Osteoporosis Medications
The osteoporosis medications studied included alendronate, risedronate, ibandronate, etidronate, zoledronic acid, raloxifene, calcitonin, parathyroid hormone (PTH), and denosumab. Zoledronic acid, PTH and denosumab were not covered by HIRA for the Korean cohort. For the Valencia cohort, strontium ranelate was added, but data on zoledronic acid (only administered within the hospital in Spain) was not available because of lack of in-hospital medication claims data.
Outcomes
The primary outcome was the proportion of patients who filled at least 1 prescription for any osteoporosis medication any time during the first 3 and 6 months after the index date. Secondary outcomes were 1) the proportion of patients who filled at least 1 prescription for a specific category of osteoporosis medication (i.e., oral or intravenous bisphosphonates, raloxifene, and others) any time within 3 and 6 months after the index date and 2) the level of adherence to osteoporosis medications during the first 6 months and 1 year after the index hip fracture among those who filled at least 1 prescription for an osteoporosis medication. We also assessed the proportion of patients who had 1 bone mineral density test after the hip fracture (data not available in Valencia).
Covariates
Variables potentially related to the risk of hip fracture and use of osteoporosis medication were assessed using data from the 365-day baseline period before the index date. These included demographic characteristics, year of the index date, comorbidities, pre-fracture use of osteoporosis medication and other drugs including oral steroids, anticonvulsants, antipsychotics, sedatives, lipid-lowering drugs, opioids, proton-pump inhibitors, selective serotonin reuptake inhibitors, non-steroidal anti-inflammatory drugs, thyroid hormone, anti-hypertensives and diuretics, and health care utilization factors (see Table 1 for complete list).
Table 1.
Baseline characteristics of study cohorts in the 365 days prior to the index hip fracture
| U.S. Medicare (2005–2008) | U.S. Commercial (2003–2012) | Korea (2007–2011) | Spain (2007–2012) | |
|---|---|---|---|---|
| N | 4,704 | 6,700 | 57,631 | 17,167 |
| Follow-up (year) | 1.1 ± 0.8 | 1.8 ± 1.6 | 2.0 ± 1.1 | 2.3 ± 1.5 |
| Percentage or mean ± standard deviation | ||||
| Demographic | ||||
| Age | 82.1 ± 7.6 | 76.6 ± 4.2 | 78.5 ± 7.1 | 82.7 ± 6.8 |
| Female | 74.7 | 74.1 | 75.8 | 77.7 |
| Comorbidities | ||||
| Osteoporosis-related | ||||
| Osteoporosis | 30.5 | 27.5 | 31.2 | 19.8 |
| Prior fall | 9.1 | 9.2 | 0.2 | n/a |
| Prior fracture | 30.4 | 35.7 | 35.9 | 15.3 |
| Prior bone mineral density test | 9.3 | 6.9 | 15.8 | n/a |
| Other comorbidities | ||||
| Parkinson's disease | 5.8 | 5.5 | 5.0 | 6.2 |
| Dementia | 32.2 | 28.5 | 18.1 | 21.9 |
| Hypertension | 78.6 | 70.9 | 66.5 | 62.1 |
| Diabetes | 30.5 | 24.4 | 22.7 | 30.4 |
| Coronary artery disease | 36.5 | 29.0 | 16.8 | 9.8 |
| Stroke | 18.8 | 16.2 | 19.2 | 10.8 |
| Heart failure | 26.0 | 20.0 | 6.6 | 9.3 |
| Chronic lung disease | 14.8 | 13.0 | 26.6 | 11.4 |
| Chronic kidney disease | 12.7 | 9.3 | 2.9 | 4.7 |
| Inflammatory arthritis | 15.2 | 11.6 | 8.2 | 2.0 |
| Medication use | ||||
| Oral steroids | 10.8 | 11.7 | 27.7 | 8.3 |
| Anticonvulsants | 11.0 | 10.5 | 10.0 | 10.7 |
| Antipsychotics | 10.7 | 8.9 | 6.0 | 18.0 |
| Sedatives | 14.6 | 20.1 | 49.6 | 51.6 |
| Lipid-lowering drugs | 35.9 | 36.1 | 18.0 | 35.6 |
| Opioids | 30.1 | 32.8 | 2.9 | 20.6 |
| Proton pump inhibitors | 23.4 | 21.1 | 11.4 | 63.8 |
| SSRIs | 25.8 | 25.5 | 6.5 | 20.1 |
| Non-selective NSAIDs | 12.7 | 12.0 | 73.0 | 39.4 |
| Cyclooxygenase-2 | 3.7 | 6.7 | 9.0 | 8.8 |
| inhibitors | ||||
| Thyroid hormone | 17.6 | 18.5 | 1.4 | 5.4 |
| Beta-blockers | 28.9 | 28.6 | 21.1 | 14.3 |
| Calcium channel blockers | 25.2 | 24.5 | 44.8 | 24.2 |
| ACE inhibitors | 26.0 | 26.9 | 8.9 | 22.9 |
| ARBs | 12.9 | 14.2 | 27.6 | 34.6 |
| Diuretics | 40.5 | 39.8 | 36.2 | 33.7 |
| Health care utilization | ||||
| ED visits | 48.8 | 47.3 | 12.4 | 38.4 |
| Acute hospitalizations | 37.4 | 45.5 | 41.1 | 19.3 |
| No. of prescription drugs | 8.4 ± 7.1 | 8.7 ± 7.0 | 26.6 ± 18.3 | 9.4 ± 4.5 |
| No. of outpatient physician visits | 8.0 ± 7.4 | 7.8 ± 7.3 | 29.6 ± 33.2 | 12.1 ± 11.1 |
ACEI: angiotensin-converting enzyme, ARB: angiotensin receptor blocker, SSRI: selective serotonin reuptake inhibitors, NSAID: non-steroidal anti-inflammatory drug, ED: emergency department
Data Analysis
Each study team in the U.S., Korea and Spain independently ran all the pre-specified analyses and shared the results from the analyses, but not the individual-level data. We first described the baseline characteristics of patients in all four cohorts. We then calculated the proportion of patients in each population who filled a prescription for any osteoporosis medication and specific categories of osteoporosis medication in 3 and 6 months after the index hip fracture. To measure patients' adherence with their osteoporosis medications, we calculated the proportion of days covered as the number of days for which the patient had medication on hand divided by the duration of follow-up time (i.e., 6 months or 12 months). The last drug available day was estimated as the date of the last prescription dispensing plus the number of days supply in that prescription.
To evaluate time trends in the use of osteoporosis medications in general and oral bisphosphonates in particular in each cohort, we calculated the proportion of patients who received such treatment within 3 months after the index hip fracture per each quarter of calendar years. P-values for the trend in use of osteoporosis medications and oral bisphosphonates were calculated from linear regression. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC) in U.S. and Korea, and STATA version 11 (StataCorp, College Station, TX) in Spain.
Ethics
The study protocol was approved by the Institutional Review Board of the Brigham and Women's Hospital (U.S.), the Institutional Review Board of Seoul National University Hospital (Korea) and the Ethics Committee for Clinical Research of the Centre of Public Health Research – General Directorate of Public Health (Spain). Patient informed consent was not required as datasets were de-identified to protect subject confidentiality (U.S.), extracted with anonymized identifiers to protect privacy according to the Act on the Protection of Personal Information Maintained by Public Agencies (Korea) or extracted with anonymized identifiers according to Spanish laws on privacy (Act 15/1999) and patients' rights (Act 41/2002). The study protocol was approved by the Institutional Review Board of the Brigham and Women's Hospital.
RESULTS
Study Subjects
We identified a total of 69,035 patients with hospitalization for a hip fracture: 4,704 in the U.S. Medicare database, 6,700 in the U.S. commercial database, 57,631 in the Korea HIRA, and 17,167 in the Spain VHA. The mean follow-up time ranged from 1.13 (U.S. Medicare) to 2.25 years (Spain). Table 1 presents baseline characteristics of the 4 cohorts. Mean age ranged from 76.6 (U.S. commercial) to 82.7 years (Spain). About two-thirds of patients were female in all settings. At baseline, 20–30% carried a diagnosis of osteoporosis, 15–36% had prior fracture and 16–18% were on osteoporosis treatment. The proportion of patients who had a bone mineral density testing done at baseline was less than 10% in the U.S. and 15.8% in Korea. Hypertension was the most common comorbidity in all three countries, 62.1% (Spain) to 78.6% (U.S. Medicare). Dementia was also prevalent in 18.1% (Korea) to 32.2% (U.S. Medicare). Although the mean age was higher in the U.S. Medicare cohort compared to the U.S. commercial cohort, other characteristics such as comorbidities, medication and health care utilization were similar between the two U.S. cohorts.
However, pre-fracture use of different types of medications and health care utilization varied greatly between the three countries. Opioids (30–33%) and thyroid hormone (18%) were much more commonly prescribed in the U.S. compared to Korea (3% and 1%, respectively) and Spain (21% and 5%, respectively). Use of oral steroids (28%) was the most prevalent in Korea. Sedatives were far more frequently used in both Korea (50%) and Spain (52%) compared to the U.S.(15–20%). Nearly half of patients in the U.S. cohorts and 40% in Spain had at least 1 visit to an emergency department in the year prior to the index fracture, whereas 12% did in Korea. On average, patients in the U.S. and Spain used 8 to 9 unique prescription drugs of any kind and had 8–12 outpatient physician visits in the 365 days prior to the index date. By contrast, the number of prescription drugs and outpatient physician visits was much higher in Korea (see Table 1).
Use of Osteoporosis Medication After Hip Fracture
The proportion of patients who filled any prescription for an osteoporosis medication within 3 months after a non-traumatic hip fracture differed sharply across the countries studied, with rates in Korea 2- to 3 times higher than in the U.S. While medication use was suboptimal in all settings, the rate was the lowest in U.S. Medicare (11%) and the highest in Korea (39%) and Spain (24%; see Table 2). The proportion of patients who filled a prescription for an osteoporosis medication within 6 months after hip fracture remained similarly low, ranging from 16% (U.S. Medicare) to 42% (Korea). Oral bisphosphonates were the most commonly used drug category in all cohorts. After the index hip fracture, 10–11% in the U.S. and 61% in Korea underwent a bone mineral density test.
Table 2.
Proportion of patients who received pharmacologic treatment for osteoporosis at baseline and after the index hip fracture
| U.S. Medicare (2005–2008) | U.S. Commercial (2003–2012) | Korea (2007–2011) | Spain (2007–2012) | |
|---|---|---|---|---|
| N | 4,704 | 6,700 | 57,631 | 17,167 |
| At baseline | ||||
| % subjects on any osteoporosis treatment | 17.90 | 17.87 | 17.47 | 15.83 |
| Oral bisphosphonate | 14.20 | 14.16 | 14.88 | 11.59 |
| IV Bisphosphonate | 0.04 | 0.16 | 0.57 | n/a |
| Raloxifene | 1.81 | 1.70 | 1.52 | 0.64 |
| Others | 3.00 | 2.69 | 0.52 | 3.59 |
| After the index fracture | ||||
| % subjects who received at least 1 osteoporosis medication within 3 months | 11.39 | 13.40 | 38.54 | 23.96 |
| Oral bisphosphonate | 9.27 | 10.87 | 26.09 | 16.82 |
| IV Bisphosphonate | 0.09 | 0.01 | 6.36 | n/a |
| Raloxifene | 0.77 | 1.06 | 0.87 | 0.35 |
| Others | 1.64 | 1.81 | 9.56 | 6.95 |
| % subjects who received at least 1 osteoporosis medication within 6 months | 16.67 | 18.21 | 42.24 | 28.07 |
| Oral bisphosphonate | 13.58 | 14.70 | 29.07 | 19.22 |
| IV Bisphosphonate | 0.21 | 0.15 | 6.73 | n/a |
| Raloxifene | 1.15 | 1.30 | 0.95 | 0.45 |
| Others | 2.64 | 2.78 | 9.88 | 8.60 |
IV: intravenous
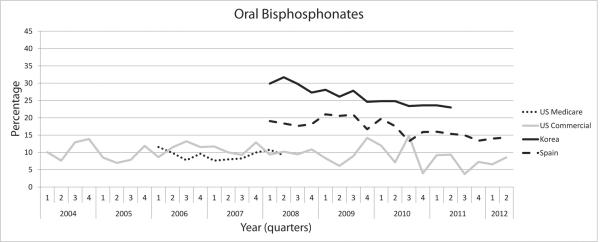
Figure 1 illustrates the time trend in the use of osteoporosis medication after hip fracture. The proportion of patients who filled a prescription for an osteoporosis medication within 3 months after hip fracture remained consistently low in U.S. Medicare and Spain, and slightly decreased over time in the U.S. commercially insured population and Korea. Use of oral bisphosphonates after hip fracture went down significantly post-fracture over the recent calendar years in Korea and Spain (p<0.0001 and p=0.0002, respectively).
Figure 1. Trend in the use of osteoporosis medication after hip fracture.
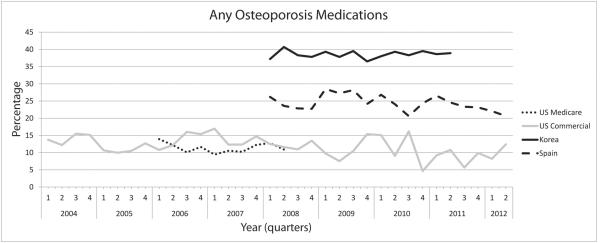
(A) Proportion of patients who filled a prescription for any osteoporosis medications within 3 months after hip fracture
(B) Proportion of patients who filled a prescription for oral bisphosphonates within 3 months after hip fracture
Adherence to Osteoporosis Medication
Among the patients who filled a first prescription for an osteoporosis medication after the index date, the mean proportion of days covered varied from 0.43 (Korea) to 0.70 (U.S. Medicare) up to 1 year of follow-up time (Table 3). Over half of patients in the U.S. and Spain had a proportion of days covered greater than or equal to 0.75 in the first year following hip fracture, as did 22% in Korea.
Table 3.
Adherence to osteoporosis treatment after the index hip fracture
| U.S. Medicare (2005–2008) | U.S. Commercial (2003–2012) | Korea (2007–2011) | Spain (2007–2012) | |
|---|---|---|---|---|
|
| ||||
| N | 4,704 | 6,700 | 57,631 | 17,167 |
|
| ||||
| PDC up to 6 months, mean ± SD | 0.73 ± 0.28 | 0.72 ± 0.29 | 0.58 ± 0.33 | 0.72 ± 0.32 |
|
| ||||
| % of patients with | ||||
| PDC ≥0.75 | 59.98 | 58.70 | 39.48 | 62.03 |
| 0.5≤ PDC <0.75 | 16.92 | 16.61 | 20.24 | 8.18 |
| 0.25≤ PDC <0.5 | 12.77 | 12.51 | 17.44 | 14.29 |
| PDC <0.25 | 10.33 | 12.18 | 22.83 | 15.50 |
|
| ||||
| PDC up to 1 year, mean ± SD | 0.70 ± 0.30 | 0.67 ± 0.31 | 0.43 ± 0.32 | 0.66 ± 0.35 |
|
| ||||
| % of patients with | ||||
| PDC ≥0.75 | 57.65 | 52.90 | 22.46 | 54.16 |
| 0.5 ≤ PDC <0.75 | 16.01 | 17.59 | 14.13 | 11.06 |
| 0.25≤ PDC <0.5 | 13.27 | 12.77 | 19.93 | 11.54 |
| PDC <0.25 | 13.07 | 16.74 | 43.47 | 23.25 |
PDC: proportion of days covered
DISCUSSION
This is to our knowledge the largest cross-national study of osteoporosis medication use in vulnerable post-fracture patients. Baseline clinical characteristics and health care utilization patterns varied greatly between the three countries with different health care systems. We found that overall, only 11% to 39% of older patients hospitalized for incident non-traumatic hip fracture were treated with an osteoporosis medication within 3 months after their fracture. Strikingly, in the U.S., the proportion of patients who received an osteoporosis medication was lower after than before the index hip fracture. Furthermore, the use of osteoporosis medication after hip fracture did not appear to increase over time in any of the three countries. In fact, use of oral bisphosphonates, the most commonly used category of the osteoporosis drugs after hip fracture, decreased significantly over the years in Korea and Spain. In patients prescribed an osteoporosis medication after hip fracture, adherence was suboptimal, with the proportion of days covered up to 1 year below 0.70 in all three countries.
The results of this study highlight multiple issues related to the care of patients following hip fracture in several countries. Understanding factors related to health care systems and medication costs, providers and patients would help close current gaps in post-fracture care. First, having a better access to health care or lower (or absent) drug copayment requirements does not seem to fully explain the problems within the U.S. and across the three countries, since many of the needed medications are available generically and/or generously covered in many of the health systems we studied. While more patients received an osteoporosis medication after fracture in Korea and Spain, where universal health coverage with low drug copayment is available, compared to the U.S., the proportions were still far below the optimum even in those countries. In addition, there was not much difference in the use of osteoporosis medications between the U.S. Medicare vs. commercial insurance plans. Other factors including patients' comorbidities, concomitant medication use, frailty and preference may partly explain this low proportion of patients who filled a prescription for osteoporosis medication after a hip fracture.
Second, it is unclear why physicians do not adhere to current management guidelines for osteoporosis. In particular, it is puzzling why the proportion of patients who received an osteoporosis medication was lower after than before the index hip fracture in the U.S. As seen in prior studies including an Italian study, the percentage of patients with fractures of the contralateral femur was 4.4% and the re-fracturing rate within a year was 2.4% among 1,183 patients with a hip fracture.20 Even though evidence from randomized clinical trials did not show adverse effects on the healing of fractures in patients treated with bisphosphonates shortly after surgical repair of a hip fracture,8, 21 some surgeons may be hesitant to initiate a bisphosphonate upon discharge after a surgery for a hip fracture. Further research should determine why health care providers, either primary care providers or specialists, do not initiate an osteoporosis medication, particularly bisphosphonates, upon hospital discharge after hip fracture or at the first follow-up visit after discharge. Third, the utility of bone mineral density testing after hip fracture should be further studied. In our study, only 10–11% of patients in the U.S. had a bone mineral density measured after hip fracture while 61% did in Korea. The proportion of patients who received an osteoporosis medication within 6 months after hip fracture was higher in Korea compared to the U.S. As non-traumatic hip fracture is a manifestation of severe osteoporosis, osteoporosis treatment should be initiated with or without bone mineral density testing. It remains to be determined whether bone mineral density testing should be done prior to initiating osteoporosis treatment in post-fracture patients and whether doing so might increase the probability of providing osteoporosis treatment after hip fracture.
Fourth, future research should investigate why use of bisphosphonates for secondary prevention has remained constantly low or decreased over time. A recent cohort study using claims data from a different U.S. commercial health plan showed that approximately 20% of women aged 65 years or older initiated any osteoporosis treatment during the year following a new osteoporotic fracture and there was a significant decrease in the rate of osteoporosis medication following hip fracture over the past decade.22 Similarly, another U.S.-based study reported that the rate of osteoporosis medication use within 12 months after a hospitalization for a hip fracture decreased from 40.2% in 2002 to 20.5% in 2011.18 Some of the decline in bisphosphonate use may be related to increasing concerns over potential side effects of bisphosphonates such as atrial fibrillation, atypical femur fracture, osteonecrosis of the jaw and esophageal cancer over past several years.23–26 Both patients and health care providers may need a better understanding of benefits versus harms related to bisphosphonates. Lastly, there is a need to improve patient adherence to medication as also seen in other studies,27 as higher medication adherence is associated with a lower risk of recurrent fracture.28, 29
This study has limitations. First, the study period varied somewhat across data sources, but there was a good overlap between 2008 and 2011 in all three countries. Second, as we used both inpatient diagnosis and procedure codes to identify patients with hip fracture in each data source, patients with hip fracture who did not undergo a surgical procedure were not included in this study. Thus, our results may be underestimates of the use of osteoporosis medication. Third, this study did not assess use of calcium and vitamin D. Fourth, this study was unable to assess whether osteoporosis medication was prescribed but patients decided not to fill a prescription (i.e. primary non-adherence). Lastly, no statistical analysis directly comparing one country to another adjusting for patients' characteristics was performed as we did not have an access to individual level data from each country.
In conclusion, regardless of differences in health care delivery systems and medication reimbursement plans, the use of osteoporosis medications for the secondary prevention of osteoporotic fracture was quite low, and did not increase over time in all three countries studied. Even after these drugs were prescribed, patient adherence to treatment was also suboptimal, despite the availability of effective, safe, and affordable medications to address this problem. This study highlights the urgent need for a better approach to improve the secondary prevention of osteoporotic fracture worldwide.
Supplementary Material
Acknowledgements
▪ SC Kim is supported by the NIH grant K23 AR059677. She received research support from Pfizer.
Footnotes
Disclosures: ▪ J Avorn, M-S Kim, G Sanfélix-Gimeno, HJ Song, L Jun, I Hurtado, S Peiró, J Lee, N-K Choi, and B-J Park have nothing to disclose.
Author Contribution ▪ All the authors were involved in study design, data interpretation and manuscript preparation. Kim drafted the manuscript. All the authors approved the final version of the manuscript.
REFERENCES
- 1.Gardner MJ, Flik KR, Mooar P, Lane JM. Improvement in the undertreatment of osteoporosis following hip fracture. J Bone Joint Surg Am. 2002;84-A(8):1342–8. doi: 10.2106/00004623-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Teng GG, Curtis JR, Saag KG. Quality health care gaps in osteoporosis: how can patients, providers, and the health system do a better job? Curr Osteoporos Rep. 2009;7(1):27–34. doi: 10.1007/s11914-009-0006-3. [DOI] [PubMed] [Google Scholar]
- 3.Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573–9. doi: 10.1001/jama.2009.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts SE, Goldacre MJ. Time trends and demography of mortality after fractured neck of femur in an English population, 1968–98: database study. BMJ. 2003;327(7418):771–5. doi: 10.1136/bmj.327.7418.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dy CJ, McCollister KE, Lubarsky DA, Lane JM. An economic evaluation of a systems-based strategy to expedite surgical treatment of hip fractures. J Bone Joint Surg Am. 2011;93(14):1326–34. doi: 10.2106/JBJS.I.01132. [DOI] [PubMed] [Google Scholar]
- 6.Nikitovic M, Wodchis WP, Krahn MD, Cadarette SM. Direct health-care costs attributed to hip fractures among seniors: a matched cohort study. Osteoporos Int. 2013;24(2):659–69. doi: 10.1007/s00198-012-2034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–33. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 8.Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic Acid in Reducing Clinical Fracture and Mortality after Hip Fracture. N Engl J Med. 2007;357 doi: 10.1056/NEJMoa074941. nihpa40967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bondo L, Eiken P, Abrahamsen B. Analysis of the association between bisphosphonate treatment survival in Danish hip fracture patients-a nationwide register-based open cohort study. Osteoporos Int. 2013;24(1):245–52. doi: 10.1007/s00198-012-2024-8. [DOI] [PubMed] [Google Scholar]
- 10.Prieto-Alhambra D, Judge A, Arden NK, et al. Fracture prevention in patients with cognitive impairment presenting with a hip fracture: secondary analysis of data from the HORIZON Recurrent Fracture Trial. Osteoporos Int. 2014;25(1):77–83. doi: 10.1007/s00198-013-2420-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ensrud KE, Black DM, Palermo L, et al. Treatment with alendronate prevents fractures in women at highest risk: results from the Fracture Intervention Trial. Arch Intern Med. 1997;157(22):2617–24. [PubMed] [Google Scholar]
- 12.Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348(9041):1535–41. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 13.Wells GA, Cranney A, Peterson J, et al. Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. 2008(1):CD001155. doi: 10.1002/14651858.CD001155.pub2. [DOI] [PubMed] [Google Scholar]
- 14.National Osteoporosis Foundation Cinician's guide to prevention and treatment of osteoporosis. 2013 [cited 2013 November 5]; Available from: http://nof.org/files/nof/public/content/resource/913/files/580.pdf.
- 15.Florence R, Allen S, Benedict L, et al. Diagnosis and Treatment of Osteoporosis. 2013 Jul; [cited 2013 November 6]; Available from: https://www.icsi.org/_asset/vnw0c3/Osteo.pdf.
- 16.Giangregorio L, Papaioannou A, Cranney A, et al. Fragility fractures and the osteoporosis care gap: an international phenomenon. Semin Arthritis Rheum. 2006;35(5):293–305. doi: 10.1016/j.semarthrit.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Rabenda V, Vanoverloop J, Fabri V, et al. Low incidence of anti-osteoporosis treatment after hip fracture. J Bone Joint Surg Am. 2008;90(10):2142–8. doi: 10.2106/JBJS.G.00864. [DOI] [PubMed] [Google Scholar]
- 18.Solomon DH, Johnston SS, Boytsov NN, et al. Osteoporosis Medication Use after Hip Fracture in U.S. Patients between 2002 and 2011. J Bone Miner Res. 2014 doi: 10.1002/jbmr.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisman JA, Bogoch ER, Dell R, et al. Making the first fracture the last fracture: ASBMR task force report on secondary fracture prevention. J Bone Miner Res. 2012;27(10):2039–46. doi: 10.1002/jbmr.1698. [DOI] [PubMed] [Google Scholar]
- 20.Scaglione M, Fabbri L, Di Rollo F, et al. The second hip fracture in osteoporotic patients: not only an orthopaedic matter. Clin Cases Miner Bone Metab. 2013;10(2):124–8. [PMC free article] [PubMed] [Google Scholar]
- 21.Colon-Emeric C, Nordsletten L, Olson S, et al. Association between timing of zoledronic acid infusion and hip fracture healing. Osteoporos Int. 2011;22(8):2329–36. doi: 10.1007/s00198-010-1473-1. [DOI] [PubMed] [Google Scholar]
- 22.Balasubramanian A, Tosi LL, Lane JM, et al. Declining rates of osteoporosis management following fragility fractures in the u.s., 2000 through 2009. J Bone Joint Surg Am. 2014;96(7):e52. doi: 10.2106/JBJS.L.01781. [DOI] [PubMed] [Google Scholar]
- 23.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809–22. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 24.Durie BG, Katz M, Crowley J. Osteonecrosis of the jaw and bisphosphonates. N Engl J Med. 2005;353(1):99–102. doi: 10.1056/NEJM200507073530120. discussion 99–102. [DOI] [PubMed] [Google Scholar]
- 25.Gedmintas L, Solomon DH, Kim SC. Bisphosphonates and risk of subtrochanteric, femoral shaft, and atypical femur fracture: a systematic review and meta-analysis. J Bone Miner Res. 2013;28(8):1729–37. doi: 10.1002/jbmr.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dixon WG, Solomon DH. Bisphosphonates and esophageal cancer--a pathway through the confusion. Nat Rev Rheumatol. 2011;7(6):369–72. doi: 10.1038/nrrheum.2011.60. [DOI] [PubMed] [Google Scholar]
- 27.Curtis JR, Yun H, Matthews R, et al. Adherence with intravenous zoledronate and intravenous ibandronate in the United States Medicare population. Arthritis Care Res (Hoboken) 2012;64(7):1054–60. doi: 10.1002/acr.21638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olsen KR, Hansen C, Abrahamsen B. Association between refill compliance to oral bisphosphonate treatment, incident fractures, and health care costs--an analysis using national health databases. Osteoporos Int. 2013;24(10):2639–47. doi: 10.1007/s00198-013-2365-y. [DOI] [PubMed] [Google Scholar]
- 29.Sampalis JS, Adachi JD, Rampakakis E, et al. Long-term impact of adherence to oral bisphosphonates on osteoporotic fracture incidence. J Bone Miner Res. 2012;27(1):202–10. doi: 10.1002/jbmr.533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


