Abstract
The detection of minority variants in mixed samples demands methods for enrichment and accurate sequencing of small genomic intervals. We describe an efficient approach based on sequential rounds of hybridization with biotinylated oligonucleotides, enabling more than one-million fold enrichment of genomic regions of interest. In conjunction with error correcting double-stranded molecular tags, our approach enables the quantification of mutations in individual DNA molecules.
Diseases such as cancer or viral infections do not manifest as a single population of cells, but rather, as a heterogeneous mixture of sub-clonal populations1. While massively parallel sequencing has made it feasible to scan whole genomes for clonal nucleotide variations, this approach cannot readily delineate the heterogeneity of mutations within a cell population. In order to detect rare, sub-clonal mutations, sequencing must be carried out to depths that can be prohibitively expensive, and at low frequencies it becomes difficult or impossible to distinguish sequencing-related errors from true variation. We overcome these challenges by coupling extensive purification of targeted sequences with highly accurate DNA sequencing.
Targeted capture approaches2 sequence large genomic regions (typically hundreds of kilobases to several megabases), limiting the depth that can be obtained. These approaches do not scale to small targets (< 50 kb) and typically result in recovery of targeted DNA sequences of 5% or less. Small targets can be amplified by methods such as PCR or molecular inversion probes3; however these methods are error-prone and generate artifactual mutations that overwhelm the detection of sub-clonal variants4.
Detection of sub-clonal and random mutations in a target gene also requires extremely accurate sequencing. Next-generation sequencing has a high error rate of 0.1%-1%, averaging one artifactual mutation in every sequencing read. Thus, millions of sequencing errors occur in every sequenced genome 5,6. These errors can be averaged to obtain a single consensus sequence for a population of cells; however due to this high error rate it is not feasible to reliably detect mutations present in fewer than 5% of cells. Molecular tagging of single-stranded DNA prior to amplification7,8,9 can reduce the frequency of erroneously called variants, but only by approximately 20-fold, since it cannot correct errors that occur in the first round of amplification and are propagated to subsequent copies10.
To overcome these limitations, we developed an alternative approach based on sequential rounds of capture with individual biotinylated DNA oligonucleotides in conjunction with Duplex Sequencing, which uses double-stranded, complementary molecular tags to separately label and amplify each of the two strands of individual duplex DNA molecules10. In Duplex Sequencing, mutations are only scored if they occur at the same position on both DNA strands, whereas amplification and sequencing errors, which only appear in one strand, are not scored.
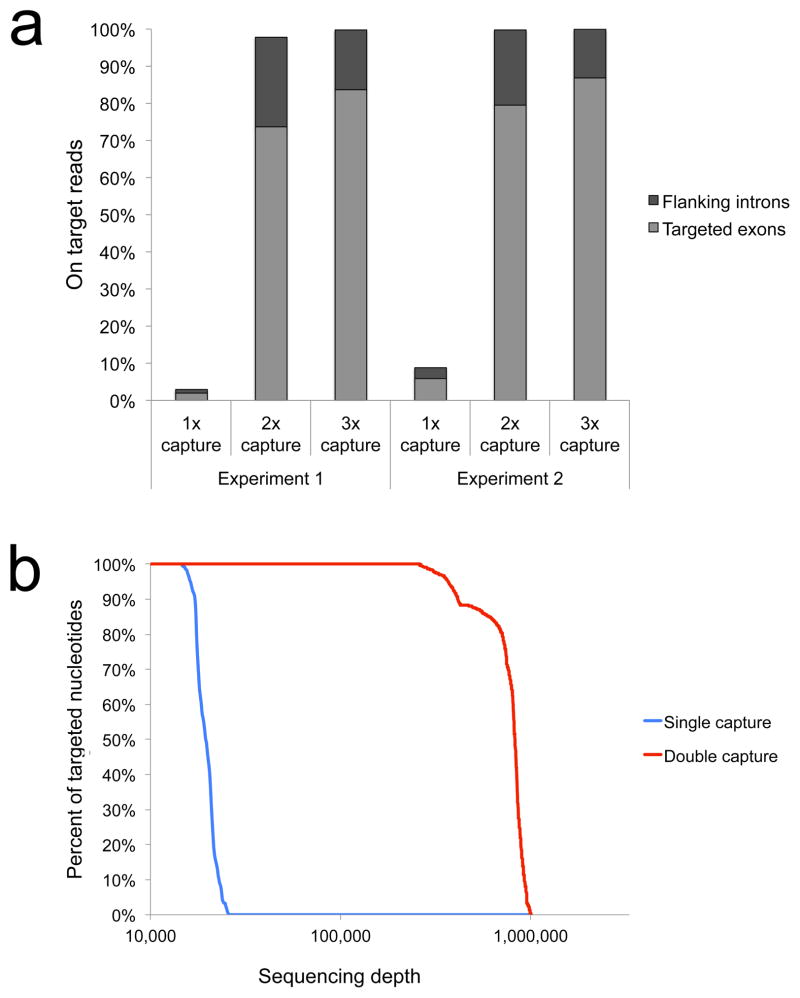
As a demonstration, we attempted to detect rare mutations in the Abl gene that confer resistance to imatinib (Gleevec) therapy of chronic myeloid leukemia (CML)11. We synthesized 5′ biotinylated DNA oligonucleotides corresponding to exons 4–7 of Abl (Supplementary Table 1). Duplex Sequencing adapters containing complementary molecular tag sequences that identify each of the two strands of individual DNA molecules were ligated to sheared human genomic DNA (Online Methods). The product was then PCR amplified and hybridized to the pooled Abl-targeting oligonucleotides followed by recovery with streptavidin beads. Elution and sequencing revealed a 50,000-fold enrichment of the target; however due to the small size of the target, this enrichment resulted in only 2%–5% of reads being on-target (Fig. 1a). The recovered DNA was then subjected to iterative rounds of PCR and capture. In two independent experiments, two rounds of capture resulted in >97% of reads mapping to the Abl gene. A third round of capture provided no further improvement (Fig. 1a).
Figure 1.
High on-target recovery with sequential rounds of capture. (a) Human genomic DNA captured with biotinylated probes targeting Abl exons 4–7 results in low on-target recovery following one round of capture, while two rounds result in >97% of reads mapping to the targeted gene. Experiment 1 was carried out with conventional blocking oligonucleotides mws60 and mws61; experiment 2 used chemically modified high-affinity blocking oligonucleotides mws58 and mws59. (b) Percent of targeted nucleotides covered at a given sequencing depth following single and double capture. Both samples were sequenced on an equivalent fraction of a HiSeq 2500 lane (5 × 106 paired-end reads, corresponding to 3% of a single lane).
The double-capture approach resulted in extremely high depth and uniformity of coverage (Fig. 1b). Conventional capture yielded a maximum on-target depth of 25,000x. In contrast, with equivalent use of sequencing capacity, double capture gave up to 1,000,000x depth, with an average and minimum depth of 830,000x and 250,000x, respectively. The Duplex Tags were then used to collapse PCR duplicates for which the two strands of individual DNA molecules were perfectly complementary into consensus sequences. This yielded an average of more than 1,000 unique DNA molecules sampled at every nucleotide position within the Abl target (Supplementary Fig. 1).
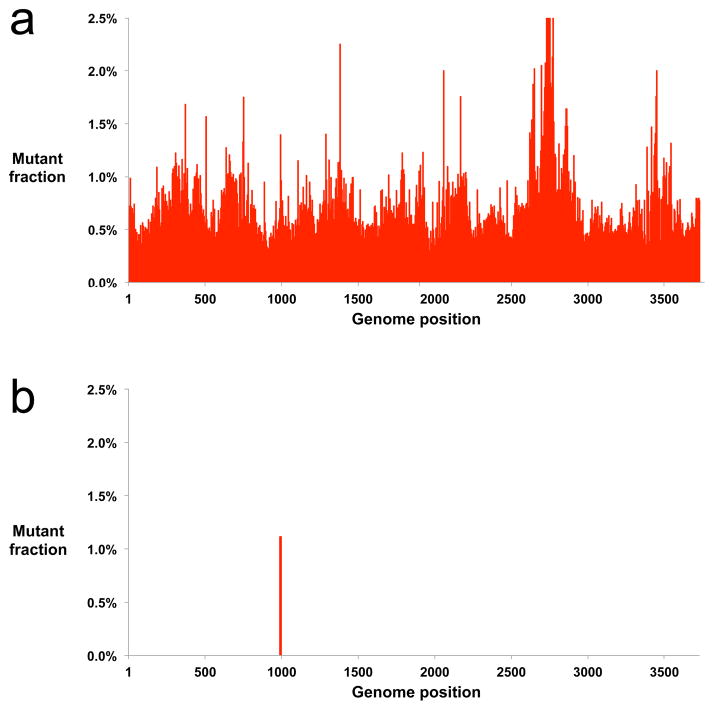
We used our protocol to sequence the Abl gene from an individual with CML at early relapse, following treatment with the targeted therapy imatinib. Conventional next-generation sequencing was unable to resolve any mutations in the sample (Fig. 2a). Even stringent quality filtering (requiring a minimum Phred quality score of 50) was unable to remove background errors, as many sequencing errors occur during PCR amplification and thus cannot be removed by quality filtering10. In contrast, Duplex Sequencing revealed a single mutation with a mutant fraction of 1% (Fig. 2b). This mutation, E279K, is known to confer imatinib resistance11.
Figure 2.
Removal of sequencing artifacts by Duplex Sequencing. (a) Exons in Abl spanning the active site of the enzyme were enriched by the double-capture protocol and sequenced conventionally on an Illumina HiSeq 2500. Despite extremely stringent quality filtering (minimum Phred score 50), and removal of end-repair artifacts by 5-nucleotide trimming from read ends, true mutations cannot be discerned among the thousands of sequencing errors that persist. (b) Duplex Sequencing of the same sample reveals a single point mutation in Abl which confers imatinib resistance. The mutation was verified by RT-PCR and Sanger sequencing.
Alternative methods to detect subclonal mutations have been described that result in multiple copies of single-stranded DNA, linked together by concatemerization12 or tagged with a molecular identifier sequence8,9. These approaches are inherently more error-prone than Duplex Sequencing, since they use information from only one of the two DNA strands and thus have less capability for error correction. To compare our double-stranded tagging approach to these methods, we re-analyzed our data using information from only one of the two tagged strands, which we refer to as “Single Strand Consensus Sequences”10. This analysis resulted in mutations at hundreds of positions in the Abl target (Supplementary Fig. 2), in contrast to the one true mutation that was found by Duplex Sequencing. The discrepancy indicates that >99% of mutations identified by the single-stranded tagging approach are artifacts.
We next determined whether our approach could scale to multiple targets. We obtained biotinylated oligonucleotides corresponding to the coding exons of the 5 human replicative DNA polymerases13 (19.4 kb total target size) and applied the double-capture approach to DNA extracted from histologically normal human prostate and colon. More than 90% of reads mapped to the targeted genes, revealing mutation frequencies of 1 × 10−7 to 4 × 10−7 (Supplementary Table 2). Among the mutations, six were in introns and two changed the coding sequence of DNA polymerase epsilon (Supplementary Table 3). The frequency of mutations is in accord with prior estimates14,15 of the spontaneous mutation rate in human cells, and thus could be the result of multiple rounds of cell division and endogenous mutagenic processes. Alternatively, these mutations could represent artifacts in our assay. However, the error frequency of Duplex Sequencing has been estimated to be < 4 × 10−10, as complementary errors would need to occur in both strands to be scored10.
Our approach allows for the study of small genomic regions, such as individual human exons or viral sequences present at low levels in human samples. Due to the high level of enrichment, substantial depth can be obtained with modest sequencing. For example, a 1 kb target can be sequenced to 100,000-fold depth with 4 × 105 paired-end 125 nucleotide reads, and thus hundreds of samples can be sequenced simultaneously on a single lane of an Illumina HiSeq 2500. Thus the approach is highly scalable and cost-effective for sequencing small targets. Duplex Sequencing on larger targets, such as whole exomes, is also possible in principle with a greater use of sequencing capacity. For example, under optimized conditions, the full exome from 100 individual cells would require approximately 2 × 1011 nucleotides of sequence capacity, which is within the output range of currently available sequencers.
Our Abl results indicate that it is possible to assay for the presence of pre-existing sub-clones encoding resistance to targeted cancer therapies, which would be expected to clonally expand in the presence of corresponding inhibitors. Armed with this knowledge, patients could be treated with drugs chosen for their lack of any detectable resistance. Targeted, high-accuracy capture has additional applications in a wide range of fields, including the detection of tumor-specific circulating DNA as a biomarker for cancer treatment16, detection of minimal residual disease in hematologic malignancies17, confirming candidate sub-clonal mutations that are found by conventional sequencing, analysis of mutational processes in cancer18, and testing for low-level resistance mutations in viral populations. Moreover, as the extreme accuracy of the approach results in a theoretical need of only 1x coverage of a genome to obtain an accurate sequence, we anticipate applications in settings where sample availability is extremely limited, such as paleogenomics, forensics, and the study of circulating tumor cells.
Online Methods
DNA isolation
Genomic DNA was extracted from peripheral blood mononuclear cells or tissue by high-salt extraction, using the Agilent DNA extraction kit #200600.
Ligation of Duplex Sequencing adapters
Duplex Sequencing was initially described with use of A-tailed adapters10,19; we have since found that T-tailed adapters result in improved ligation efficiency, and have published a detailed protocol for their synthesis and use20. In brief, DNA was sheared, end-repaired, and A-tailed, then ligated to T-tailed Duplex Sequencing adapters using a 20x molar excess of adapters relative to A-tailed DNA molecules. Following reaction cleanup with 1.0 volumes of Ampure XP beads (Agencourt), the adapter-ligated DNA was PCR amplified for 5 cycles with the KAPA Biosystems hot start high-fidelity kit, using primers mws13 and mws20. 240 nanograms of input DNA was used in each 100 microliter PCR reaction, with two to eight PCR reactions performed per sample. Due to the small amount of on-target DNA present in the starting sample, multiple PCR reactions are needed to amplify sufficient on-target DNA for capture. Each PCR reaction results in sequence data representing approximately 500 independent genomes; the number of PCR reactions performed can be adjusted depending on the sequencing coverage desired. The products from all reactions were pooled and purified with 1.2 volumes of Ampure XP beads, with a final elution volume of 50 microliters.
Targeted capture
One-third of the total amount of adapter-ligated DNA generated by PCR was combined with 5 micrograms of Cot-I DNA (Invitrogen) and 1 nanomole each of blocking oligonucleotides mws60 and mws61. The mixture was completely lyophilized, then resuspended in 2.5 microliters water, 7.5 microliters Nimblegen 2x hybridization buffer, and 3 microliters Nimblegen hybridization component A. The mixture was heated to 95°C for 10 minutes, the temperature was adjusted to 65°C, and 3 pmol of pooled 120nt biotinylated oligonucleotides were added (Integrated DNA Technologies). After 4 hours, M-270 streptavidin beads (Life Technologies) were added and washes were performed according to the IDT xGen lockdown probe protocol version 2.0. We found that the standard quantity of streptavidin beads (the IDT protocol calls for 100 microliters of beads per 50 microliter PCR reaction) can result in PCR inhibition, so the quantity of beads was decreased to 75 microliters per reaction, and the PCR reaction volume increased to 100 microliters. The product was PCR amplified for 16 cycles with primers mws13 and mws20, and purified with 1.2 volumes of Ampure XP beads. The purified DNA was combined with 2.5 micrograms Cot-I DNA and 500 picomoles each of oligonucleotides mws60 and mws61, and a second round of capture21 was performed with 1.5 picomoles of pooled biotinylated oligonucleotides. A final PCR reaction was carried out for 8–10 cycles with primers mws13 and mws21, which contains a fixed index sequence for multiplexing. After cleanup with 1.2 volumes of Ampure XP beads, the product was sequenced on an Illumina HiSeq 2500.
Data processing
Processing of Duplex Sequencing data was performed essentially as previously described20. Mutations identified by Duplex Sequencing were individually inspected in the Integrated Genome Viewer22 to verify that they were not affected by alignment errors.
Code availability
Software for Duplex Sequencing is available at https://github.com/loeblab/Duplex-Sequencing
Reverse transcription PCR of the Abl gene
Total RNA was extracted from peripheral blood using TRIzol reagent (Invitrogen). An initial RT-PCR step with nested PCR was used to amplify exons 4 to 9 (codons 199 to 507) of the Abl kinase domain, and bidirectional Sanger sequencing of the PCR product was performed, as previously described23.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institutes of Health under award numbers NCI P01-CA77852, R01-CA160674 and R33-CA181771 to L.A.L. and NCI U10-CA180861, P01-CA018029, R01-CA175008, and R01-CA175215 to J.P.R. We would like to thank Tom Walsh and Ming Lee for assistance with DNA sequencing.
Footnotes
Accession codes:
Data from this paper have been uploaded to the Sequence Read Archive under BioProject ID: PRJNA275267.
Competing Financial Interests:
The University of Washington has filed a patent application regarding Duplex Sequencing.
Author contributions:
M.W.S., E.J.F, M.J.P, K.S.R., L.D.T., J.P.R., and L.A.L. contributed to experimental design. M.W.S., E.J.F, and M.J.P. performed the experiments in the paper and analyzed data. E.J.F., L.D.T., and J.P.R. contributed patient samples. M.W.S. and L.A.L. wrote the manuscript.
Human subjects approval:
Use of human samples was approved by the Institutional Review Board at the University of Washington. Informed consent was obtained from patients who contributed samples.
References
- 1.Schmitt MW, Prindle MJ, Loeb LA. Ann N Y Acad Sci. 2012;1267:110–116. doi: 10.1111/j.1749-6632.2012.06590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mamanova L, et al. Nat Methods. 2010;7:111–118. doi: 10.1038/nmeth.1419. [DOI] [PubMed] [Google Scholar]
- 3.Hardenbol P, et al. Nat Biotechnol. 2003;21:673–678. doi: 10.1038/nbt821. [DOI] [PubMed] [Google Scholar]
- 4.Kanagawa T. J Biosci Bioeng. 2003;96:317–323. doi: 10.1016/S1389-1723(03)90130-7. [DOI] [PubMed] [Google Scholar]
- 5.Fox EJ, Bayliss-Reid KS, Emond MJ, Loeb LA. Next Generat Sequenc & Applic. 2014;1:106–109. doi: 10.4172/jngsa.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glenn TC. Mol Ecol Resour. 2011;11:759–769. doi: 10.1111/j.1755-0998.2011.03024.x. [DOI] [PubMed] [Google Scholar]
- 7.Jabara CB, Jones CD, Roach J, Anderson JA, Swanstrom R. Proc Natl Acad Sci USA. 2011;108:20166–20171. doi: 10.1073/pnas.1110064108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinde I, Wu J, Papadopoulos N, Kinzler KW, Vogelstein B. Proc Natl Acad Sci USA. 2011;108:9530–9535. doi: 10.1073/pnas.1105422108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiatt JB, Pritchard CC, Salipante SJ, O’Roak BJ, Shendure J. Genome Res. 2013;23:843–854. doi: 10.1101/gr.147686.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitt MW, et al. Proc Natl Acad Sci USA. 2012;109:14508–14513. doi: 10.1073/pnas.1208715109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soverini S, et al. Leuk Res. 2014;38:10–20. doi: 10.1016/j.leukres.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Lou DI, et al. Proc Natl Acad Sci USA. 2013;110 doi: 10.1073/pnas.1319590110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sweasy JB, Lauper JM, Eckert KA. Radiat Res. 2006;166:693–714. doi: 10.1667/RR0706.1. [DOI] [PubMed] [Google Scholar]
- 14.Albertini RJ, Nicklas JA, O’Neill JP, Robison SH. Annu Rev Genet. 1990;24:305–326. doi: 10.1146/annurev.ge.24.120190.001513. [DOI] [PubMed] [Google Scholar]
- 15.Kunkel TA. J Biol Chem. 2004;279:16895–16898. doi: 10.1074/jbc.R400006200. [DOI] [PubMed] [Google Scholar]
- 16.Esposito A, et al. Cancer Treat Rev. 2014;40:648–655. doi: 10.1016/j.ctrv.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Buckley SA, Appelbaum FR, Walter RB. Bone Marrow Transplant. 2013;48:630–641. doi: 10.1038/bmt.2012.139. [DOI] [PubMed] [Google Scholar]
- 18.Alexandrov LB, et al. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy SR, Salk JJ, Schmitt MW, Loeb LA. PLoS Genet. 2013;9:e1004794. doi: 10.1371/journal.pgen.1003794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennedy SR, et al. Nat Protoc. 2014;9:2586–2606. doi: 10.1038/nprot.2014.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green DJ, Wendt C, Kashuk M, Brockman M, Burgess D. SeqCap EZ Library: Technical notes. Roche NimbleGen, Inc; 2012. pp. 1–12. [Google Scholar]
- 22.Robinson JT, et al. Nat Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egan DN, Beppu L, Radich JP. Biol Blood Marrow Transplant. 2014;14:567–569. doi: 10.1016/j.bbmt.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.