Abstract
Emotional events are remembered better than neutral events, and this emotion advantage becomes particularly pronounced over time. The time dependent effects of emotion impact recollection rather than familiarity-based recognition, and they influence recollection of item-specific details rather than contextual details. Moreover, the amygdala, but not the hippocampus, is critical in producing these effects. Time-dependent effects of emotion have been attributed to an emotional consolidation process whereby the amygdala gradually facilitates the storage of emotional memories by other medial temporal lobe regions. However, here we propose that these effects can be better understood by an emotional binding account whereby the amygdala mediates the recollection of item-emotion bindings that are forgotten more slowly than item-context bindings supported by the hippocampus.
Keywords: emotional memory, medial temporal lobes
The effects of emotion on episodic memory
The most memorable events of our lives are often those that are emotionally arousing (e.g., an encounter with a vicious dog, viewing a photograph of a gruesome murder). It is well documented that emotional materials can attract more attention or garner more elaborative encoding than neutral materials, and that this enhanced encoding can lead emotional materials to be better remembered than neutral materials [for reviews, see 1, 2–4]. However, the beneficial effects of emotion cannot be explained solely on the basis of enhanced encoding because, as will be described below, emotional and neutral materials can often be remembered equally well shortly after they have occurred, and it is only after a delay period that the emotion advantage begins to emerge [e.g., 5, 6–9].
The reason why emotional memories are so resistant to forgetting is not yet fully understood. Although important advances have been made in developing models of episodic memory that incorporate findings from behavioral, lesion and neuroimaging studies, most of these models have focused on accounting for studies of memory for neutral materials. In the current paper we review the behavioral and neural studies examining emotion effects on episodic memory in human subjects, and we identify a number of well-established empirical regularities. Based on these results, we argue that emotional memories exhibit a time dependent memory advantage because they rely on item-emotion bindings supported by the amygdala that are forgotten more slowly than item-context bindings supported by the hippocampus.
Five empirical regularities
A majority of the existing studies examining delayed emotion effects have contrasted memory for arousing negative emotional materials such as gruesome pictures and taboo words, to neutral materials, and so our review will focus on the effects of negative emotion as measured with these types of materials. Although this reflects a rather restrictive definition of emotion, later we will return to further consider whether these findings generalize to other emotional materials such as positive arousing materials, traumatic autobiographical events, as well as fear conditioning paradigms.
The memory advantage for emotional materials increases over time
Numerous laboratory experiments have indicated that negative emotional materials are recalled and recognized better than neutral materials [1–3]. Although these effects may be due in part to enhanced encoding of emotion compared to neutral items, a number of studies have shown that the emotion effects are either absent or much smaller when memory is tested immediately and they tend to increase in magnitude after a few hours [5–13].
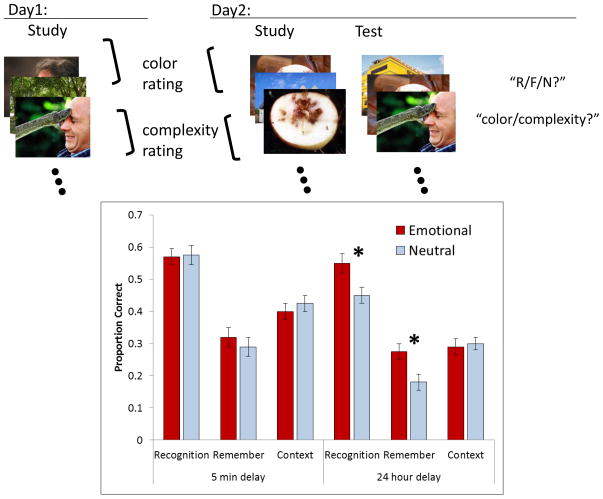
To illustrate the delayed emotion effects we describe a study [8] in which subjects were presented with a mixture of negative and neutral images. Images were divided into two lists that were studied one day apart. Immediately after exposure to the second list, participants completed a recognition memory test for all the studied images mixed with negative and neutral nonstudied images (see Figure 1). Overall recognition performance was then assessed for emotional and neutral items that had just been studied as well as those that had been studied 24 hours earlier. For the items studied and tested on the same day (i.e., the 5 minute delay condition) emotional and neutral items were recognized equally well. However, for the items studied 24 hours earlier there was a recognition memory advantage for emotional materials over neutral materials. Thus, the emotional and neutral materials were equally well encoded, but after a delay an emotion advantage emerged.
Figure 1.
Procedures and results from [8]. A) On day 1, subjects studied a mixture of negative and neutral images, half while rating visual complexity and half while rating the range of colors used in each image. On day 2, subjects studied a second list of images under similar encoding conditions, then after a 5 minute delay they received a recognition memory test containing a mixture of old items from both days and new items. For each test item subjects indicated if they could remember the occurrence of the item (“R”), if they just knew it was studied on the basis of familiarity in the absence of recollection (“F”), or if they thought it was new (“N”). In addition, they indicated whether the item was encoded in the context of the color or complexity rating task. B) The proportions of correct recognition responses are plotted for emotional and neutral materials for items tested after the 5 minute retention interval and the 24 hour retention interval. Item recognition was greater for emotional than neutral materials, but only after the longer retention interval, and this effect was due to the relative increase in remember responses. In contrast, memory for the study context task was not influenced by emotion in either delay condition.
Similar effects have been seen in other studies of recognition for words [6] and visual images [10, 13], as well as in tests of free recall [11], and these delayed emotion effects have been shown to appear even after two hours [10, 13]. The results show that emotion effects can emerge during retention, and thus cannot be attributed to enhanced encoding. This does not preclude the possibility that emotion advantages can be seen in immediate tests. For example, encoding factors such as enhanced attention or distinctiveness can contribute to emotional memory advantages even when tested immediately [e.g., 14, 15]. However, the results indicate that a simple enhanced encoding account of emotion is not sufficient to explain the long term effects of emotion [see also 4].
Emotion impacts recollection, rather than familiarity
Recognition memory judgments can be based either on the recollection of qualitative information about a study event or on assessments of familiarity [16–18]. Studies that have directly contrasted the contributions of recollection and familiarity to memory have indicated that emotion impacts recollection but has little or no effect on familiarity [7–10, 19–26]. Many of these studies have examined memory under conditions in which the relative increase in recollection may reflect better encoding of emotional compared to neutral items, but others have found that the recollection advantage for emotional materials is time dependent [7–9, 11, 13]. For example, as seen in Figure 1, the recognition memory advantage that arose in the delay condition [8] was due to the items that were recollected. That is, in that study, a remember/know procedure was used in which subjects were required to indicate if recognition was accompanied by recollection in the sense that they could remember some qualitative aspect of the study event, or if it was based on familiarity in the absence of recollection. The memory advantage for emotional materials that appeared in the delayed condition was entirely due to an increase in measures of recollection accuracy, and did not impact the items recognized on the basis of familiarity in the absence of recollection.
The results in this particular study were based on subjective reports of recollection and familiarity, but similar results have been observed using receiver operating characteristic methods [9, 22, 26]. The results show that not all forms of episodic memory benefit from emotion. Rather, emotion improves recollection, but does not benefit familiarity. Thus, any account of emotion and episodic memory needs to account for the observed selectivity of these effects.
Emotion impacts recollection of items rather than contexts
Not all forms of recollection, however, are increased by emotion. For example, as illustrated in Figure 1, although emotional materials were more accurately remembered after the delay than were the neutral items, recollection of the encoding context was similar for emotional and neutral materials for both the immediate and delayed tests [8]. That is, in that experiment, half of the images were encoded under instructions to rate the visual complexity of each image whereas the others were encoded under instructions to rate the range of colors in each image. At test, in addition to making recognition and remember/know responses, subjects were required to indicate which context question they had been asked about for each of the studied items. The ability to correctly recollect the encoding context was the same for emotional and neutral materials. Thus, over time, the emotional photos themselves were more likely to be correctly recollected than neutral photos, but this did not generalize to an increase in recollection of the study context.
These effects are consistent with a growing body of research demonstrating that emotion increases recollection for details that are intrinsic to the emotional item or object [27] or to the within-item features of emotional objects [28], while having little effect, or even negative effects, on details that are extrinsic or contextual in nature. For example, emotion has been shown to increase recollection for the colors and specific visual details of emotional objects [19, 29–36], but to have little, or even a negative, effect on memory for the type of encoding task, visual features of the background, or other items in the periphery [8, 37–45]. These effects may be due in part to the fact that the emotional items attract attention during encoding [27, 28], but in addition there is evidence that the emotional benefit for item compared to context information increases over time [25, 46].
These results further highlight the fact that emotion effects on episodic memory are quite selective, and show that emotion impacts the ability to recollect aspects of the emotional item or items, rather than increasing all aspects of the emotional event such as the contextual or peripheral features of the event. So emotion does not simply enhance memory for emotional events, but rather selectively improves recollection for the emotional item in the event rather other contextual details.
The emotion effect on episodic memory is dependent on the amygdala
Although selective amygdala lesions are rare, a number of cases have been reported in which bilateral amygdala damage either eliminates or severely reduces the normal advantage seen for emotional compared to neutral materials [47–50]. For example, in one study [48] individuals were presented with a set of slides along with an accompanying story that included neutral materials and negative arousing materials. In a subsequent recognition test one week later, patients with selective bilateral amygdala damage performed well at recognizing the neutral slides, but unlike the controls, they showed no advantage in recognizing the negative slides. A reduced memory advantage for negative materials has also been seen for verbal as well as visual materials tested with both recall and recognition [47, 49]. In addition, another patient study [50] found that in immediate tests, amygdala damage did not entirely eliminate the emotion advantage, but unlike in controls, the emotion advantage did not increase over time.
Neuroimaging results provide converging evidence that the amygdala plays a critical role in producing the emotion effects on episodic memory. Amygdala activity during learning correlates with subsequent emotional memory [e.g., 12, 51–53, see 54 for meta-analytic data], and the amygdala is more active during the retrieval of emotional relative to neutral memories [23, 55–58]. Consistent with a role in recollection, amygdala activity during encoding scales with the vividness of subsequent memory [59], and its involvement during retrieval is associated with recollection rather than familiarity processes [10, 23]. Additionally, the amygdala is selectively involved in creating and retrieving emotional memories that carry item-specific details [36, 59, 60], but not necessarily other forms of contextual information, such as memory for an accompanying background scene or the decision made at encoding [39, 59, 60]. The amygdala seems to also be involved in promoting the time-dependent emotion advantage: amygdala activity during encoding is more strongly correlated with memory performance when memory is tested after a delay (e.g., 1–2 weeks) versus immediately [9, 61, but see 62].
The lesion and imaging results show that the amygdala plays a central role in producing the emotion advantage in episodic memory. Moreover, the results further verify that the episodic advantage is selective to the recollection of the emotional item itself rather than influencing familiarity or the recollection of contextual or background information.
The emotion effect on episodic memory is not dependent on the hippocampus
A number of studies have examined emotion and memory in patients with large MTL lesions that have included the amygdala, hippocampus and surrounding perirhinal cortex, and found that these patients exhibit reduced emotion effects on memory [e.g., 11, 63, 64]. These impairments, however, appear to be due to amygdala damage rather than to damage elsewhere in the MTL, because in studies examining patients with MTL damage that does not include the amygdala, the emotional memory advantage is not disrupted [13, 65, 66]. For example, one study examined recognition memory for negative and neutral pictures and found that normal controls showed no emotion advantage in an immediate test, but after a two-hour delay they showed better memory for the emotional materials than neutral materials [13]. Patients with selective hippocampal damage were tested in the delayed condition and were found to exhibit an emotional memory advantage in recognition that was similar in magnitude to that of the controls. Another study [66] found that patients with MTL damage that did not include the amygdala exhibited a normal emotion advantage, even when controlling for overall memory performance. Similar results have been reported for story recall tests [65] and for face-emotion pair identification [64]. Finally, in temporal lobe epilepsy patients, amygdala pathology was found to be related to decreases in recollection for emotional but not neutral words, whereas hippocampal pathology was related to decreases in recollection for both emotional and neutral materials [67].
In contrast to the clear body of brain imaging evidence linking the amygdala to emotional memory processes, neuroimaging findings have been more mixed with respect to the role of other MTL regions. Some studies have found that MTL regions contribute similarly to neutral and emotional memory formation [12, 51, 68], whereas others have found that the anterior hippocampus and the rhinal cortex are more involved in emotional than neutral encoding [52, for a review see 54]. Findings have been similarly mixed with respect to the role of the MTL during emotional retrieval [c.f., 10, 23]. Although MTL activity on its own has not been a consistent predictor of the emotional memory advantage, several studies have found increased correlations between amygdala activity and other MTL regions during emotional than neutral memory encoding, including regions in the anterior hippocampus [12, 52, 68, 69], anterior parahippocampal gyrus [9, 12, 52], and posterior parahippocampal gyrus [70]. One study found that the correlation between activation in the amygdala and rhinal cortex was stronger for items remembered after a 1-week versus 20-minute delay [9], and that this effect was related to the persistence of recollection. These findings suggest that there may be important interactions between the amygdala and hippocampus at the time of encoding, but when delay effects are taken into consideration, it is the amygdala-rhinal interactions that are important for lasting memory.
In sum, the neural studies indicate that the hippocampus is not necessary for producing the delayed emotion effects. Although hippocampal damage reduces episodic memory, it reduces memory for both emotional and neutral items similarly. Moreover, hippocampal activity is related to the encoding and retrieval of both emotional and neutral materials. However, some imaging studies have suggested that the anterior hippocampus and perirhinal cortex may be more involved in emotional memory processes than posterior regions such as the parahippocampal cortex.
The emotional binding model
How can we explain the observed effects of emotion on episodic memory? Standard models of episodic memory and the MTL (Box 1) have not typically incorporated the amygdala, which, as described above, plays a critical role in supporting memory for emotional events and is richly connected to both the hippocampus and to the perirhinal cortex [71, reviewed by 72]. To account for the emotion effects on memory we propose an emotional binding account whereby the amygdala supports item-emotion bindings that are more slowly forgotten than the item-context bindings supported by the hippocampus (see Figure 2). Consistent with standard models of the MTL, we assume that the hippocampus supports the binding of item and context information, and thus plays a central role in recollection, whereas the perirhinal cortex supports item processing and so is capable of supporting familiarity-based recognition. We assume that this occurs for both neutral and emotional events. The important addition, however, is that we propose that the amygdala responds to emotional arousal and supports the binding of item and emotion information, and that these amygdala bindings, once formed, will support recollection and will exhibit relatively slow forgetting. So, for events that elicit an emotional response, the amygdala will bind that emotional response to inputs from the cortical ensembles in the perirhinal cortex that are involved in processing the emotional item or items in the event. In this way, emotional events will be more dependent on the amygdala than will neutral events, and so if the amygdala exhibits slower forgetting than the hippocampus then emotional materials will be more slowly forgotten.
Box 1. Theories of episodic memory and the MTL.
Results from lesion and neuroimaging studies of humans as well as studies of rats and nonhuman primates have led to the development of several neuroanatomical models of memory that aim to characterize the functions of the hippocampus, the perirhinal cortex, and the parahippocampal cortex, as well as the visual processing streams that feed into those regions (Figure 2A). These models share a number of core assumptions and reflect what we refer to as the standard model [Figure 2B; 87, 88–91]. In general, the hippocampus is assumed to sit at the top of a functional hierarchy, and it is involved in binding together and retrieving the items and contexts that make up an event (e.g., associating a person with a specific place or time). The perirhinal cortex is thought to process item information, which it receives from the ventral visual stream (sometimes called the ‘what stream’), whereas the parahippocampal cortex is thought to process contextual information such as spatial layout, which it receives from the dorsal processing stream (sometimes called the ‘where stream’). It is assumed that an event is coded by a representation in the hippocampus that links to the various cortical ensembles involved in processing the different aspects of the event. In this way, the presentation of an item (e.g., a person’s face) could lead to the reactivation of the hippocampal binding for an earlier experience with that item, which would then lead to the reactivation of the initial encoding context (e.g., the place in which the person was encountered). Conversely, re-presenting a spatial context might lead to the recollection of the individuals who were encountered in that context. Thus recollection can involve all three MTL regions. In contrast, the perirhinal cortex is thought to be sufficient to support familiarity-based recognition. That is, it is presumed to process repeated items more fluently than novel items and thus it can support the discrimination of familiar and new items in tests of recognition memory. The evidence for the standard model is quite extensive and has been reviewed elsewhere [see 17, 88, 89, 108–110]. Although there are some differences in the specific focus of each of these different models, there is broad consensus about the basic process distinctions between the hippocampus, perirhinal cortex and the parahippocampal cortex. However, a shortcoming of all of these models is that they have not incorporated predictions about emotional memories or the role of the amygdala.
Figure 2.
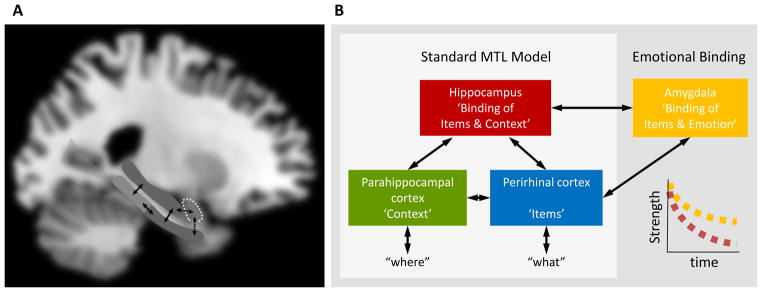
A) The medial temporal lobe (MTL) regions involved in episodic memory. B) Overview of the standard model of MTL function and the emotional binding model. In the standard model, the perirhinal cortex receives projections from the ventral ‘what’ stream and is thought to play a role in identifying and processing of the items and objects in the environment. The parahippocampal cortex receives projections from the dorsal ‘where’ stream and is thought to play a role in processing contextual information such as the ongoing spatial and temporal context. The hippocampus receives information from the perirhinal and parahippocampal cortex via entorhinal projections and binds the item and context information together to form episodic memories. The emotional binding model subsumes the standard MTL model, but in addition assumes that the amygdala forms item-emotion bindings that are forgotten more slowly than the item-context bindings supported by the hippocampus.
Why would the emotional bindings supported by the amygdala be forgotten more slowly than the bindings supported by the hippocampus? As we will describe below, this assumption is essential in accounting for the full range of empirical effects we have just described. In addition, it is consistent with results from studies of fear conditioning (Box 2), which suggest that item-emotion associations are dependent on the amygdala and are very resistant to forgetting, whereas item-context associations are dependent on the hippocampus and are forgotten more rapidly. There are also a number of theoretical reasons why emotional bindings supported by the amygdala would be forgotten more slowly than the bindings supported by the hippocampus. First, the hippocampus is unique in the sense that it has a high rate of neurogenesis and cell death [73, 74]. Relative to regions like the amygdala that do not exhibit this high level of cell turnover, the hippocampus may be subject to accelerated forgetting due to cell death or increased interference due to new cells [75]. A related account is that the hippocampus may be more susceptible to active decay processes than other brain regions [76, 77]. Such an active decay process may remove selected memories by silencing synapses that are weakly potentiated [78]. To the extent that item-emotion bindings are dependent on the amygdala, rather than the hippocampus, one may expect slower forgetting for emotional than neutral materials. Another important possibility is that the amygdala may simply be more resistant to interference because there are typically fewer competing emotional experiences outside of the experimental context, relative to neutral experiences.
Box 2. Fear conditioning studies and their relation to emotional episodic memory.
Fear conditioning refers to the process by which fear responses become associated with an intrinsically neutral stimulus, such as a tone or a context that predicts the onset of a fear-eliciting stimulus, such as a shock. Although there are a number of parallels between the emotion effects seen in episodic memory and results seen in fear conditioning studies, there are important differences across these paradigms suggesting that they reflect at least partially distinct processes. Like emotion effects on episodic memory, conditioned fear responses are amygdala-dependent [111–113] and extremely resistant to forgetting [114, 115]. In addition, however, it has been shown that contextual fear memories are affected by post-learning manipulations of glucocorticoids in the dorsal hippocampus (posterior hippocampus in humans) and noradrenaline in the basolateral amygdala [reviewed by 81, 116]. Most rodent studies linking the amygdala and hippocampus to arousal-related memory enhancements have relied on contextual fear conditioning tasks, such as the inhibitory avoidance task, in which the fear response is bound to a context representation [81]. In contrast, in episodic memory paradigms the emotion is bound directly to the item. It may be that item-emotion bindings can be supported by the amygdala alone, whereas context-emotion bindings may additionally require the hippocampus. In fact, it has been shown that contextual fear memories require both the amygdala and hippocampus, whereas other kinds of fear memories (e.g., auditorily cued fear) appear to require only the amygdala [111]—although the amygdala’s exact role has been debated [117, 118]. Other work has shown that fear responses persist for a long time, but that accompanying contextual information degrades more rapidly [119, 120], which is consistent with the notion that the amygdala supports bindings that are particularly resistant to forgetting.
However, the task demands of episodic memory and fear conditioning paradigms are quite different. For example, in human episodic memory tasks, differential forgetting effects are observed for emotional and neutral trials that are rapidly interleaved in time (i.e., the study lists typically contain a mixture of negative and neutral items presented in rapid succession). In contrast, rodent fear conditioning studies focus on the protracted neurohormonal cascade that is triggered by a single, arousal-inducing event (e.g., a shock) and its influence on memory for that event. It is unlikely that the same hormonal cascade that enhances memory for a single fearful event would also selectively enhance episodic memory for a negative image without also influencing the neutral images that immediately precede or follow it. Future research directly contrasting episodic memory and fear conditioning under conditions in which the materials and task demands are closely matched should help bridge the gap between these literatures and provide a more complete understanding of emotion and memory.
The emotional binding model naturally accounts for each of the empirical regularities described earlier. Most obviously, the fact that emotional events are forgotten more slowly over time follows directly from the assumption that emotional events depend more on the amygdala, which exhibits slower forgetting than the hippocampus. Second, the finding that the emotion effects are limited to influencing recollection rather than familiarity-based recognition arises because the recollection of item-emotion bindings is supported by the amygdala through its interactions with the perirhinal cortex. In contrast, familiarity of both neutral and emotional materials is expected to rely on the perirhinal cortex and so familiarity should show similar forgetting rates for emotional and neutral materials. Third, the binding account also explains the finding that the emotional memory advantages are limited to recollection of item specific information rather than generalizing to the recollection of contextual information. That is, slower forgetting should be seen for the item-emotion bindings that are expected to be dependent on the amygdala, rather than the item-context bindings supported by the hippocampus. Fourth, it accounts for the finding that the emotion advantage is dependent on the amygdala. Finally, according to the emotional binding account, the hippocampus should not be critical for the increasing advantage for emotional compared to neutral items over time. Because the hippocampus is assumed to support item-context associations for both emotional and neutral events, damage to this region is expected to reduce recollection, but it should do so for both emotional and neutral events.
Alternative theoretical accounts
An alternative account that has been used to explain delayed effects of emotion on memory is a modulatory emotional consolidation explanation [e.g., 2, 9, 13, 79–81]. By this account, the amygdala plays a role in identifying and responding to emotional materials, whereas the other MTL regions are assumed to be responsible for supporting episodic memory. Critically, the amygdala is thought to modulate the MTL after the initial encoding of emotional events in order to preferentially stabilize memories for these events. The consolidation account can be distinguished from the emotional binding model in two main ways. First, in the consolidation account, the amygdala facilitates long-term storage in other MTL structures but does not play a lasting role in item-emotion binding. Second, in the consolidation account, the time-dependent advantage emerges because of processes that unfold after encoding. In contrast, in the emotional binding account, once item-emotion bindings are formed, there is no need to additionally assume further modulation—the slower forgetting rate will ensure that the emotional memory advantage will emerge over time.
The consolidation account can nicely account for the fact that memory for emotional and neutral materials can be comparable immediately after encoding and that over time the emotional events will exhibit slower forgetting than neutral events. However, it does not specify which types of episodic memory should be influenced by emotion, and so it does not explain why the time-dependent emotion benefits are specific to item recollection. To resolve this issue, one might assume that the amygdala modulates some parts of the MTL more than others. If the amygdala were to selectively modulate the hippocampus, we would expect that emotion would benefit recollection of both item and context information and that hippocampal damage would reduce the time-dependent advantage. The available evidence contradicts both of these predictions. Alternatively, if the amygdala were to selectively modulate perirhinal representations, we would expect emotion to influence familiarity rather than recollection; however, this is also contradicted by the available evidence. Finally, one might assume that the amygdala modulates only the anterior portion of the hippocampus, which may be specialized for binding item and emotional information, whereas the posterior hippocampus is specialized for binding item and contextual information. However, such an account would predict that only patients with posterior hippocampal damage would exhibit normal emotion effects. In contrast, however, a number of the hippocampal patients with normal emotional memory advantages suffered from ischemic events [e.g., 13, 65], which are known to cause volumetric reductions [82–84] and cell death [e.g., 85, 86] along the entire anterior-posterior extent of the hippocampus. We conclude that there do not appear to be any immediately obvious ways of bringing the emotional consolidation model in line with the existing results.
The emotional binding model is broadly consistent with a number of other theoretical approaches to emotion and memory. Most obviously, the approach extends current MTL models of episodic memory [87–91] by incorporating emotion and the role of the amygdala (Figure 2B). The approach is also broadly consistent with models of emotion that have highlighted the fact that the effects of emotion are not expected to impact memory for all aspects of the emotional event in the same way. For example, it has been proposed that emotion leads to increased memory for the central aspects of an emotional event at the cost of poorer memory for the contextual or peripheral aspects of the event [27, 28]. According to the arousal biased competition model [92], this difference in memory arises because arousal biases perception and encoding toward “high priority” information, which typically but not necessarily includes emotional items. The current approach differs by focusing on the time dependent effects of emotion rather than on the encoding or attention processes that act on emotional materials. We emphasize, though, that although the delayed emotion effects cannot be explained by encoding processes, the emotional binding model does not preclude the possibility that other cognitive factors such as increased attention or more elaborative encoding can lead to an emotion advantage. These encoding effects would of course lead to an emotion advantage even after a brief delay.
Future directions
We believe that the emotional binding model is useful in explaining the slow forgetting of negative arousing materials seen in episodic memory, as well as some of the major functional and neural characteristics of these effects. However, we acknowledge that there are a number of important questions that the model leaves unanswered. For example, the model focuses on rather coarse neuroanatomical distinctions, such as between the amygdala and the hippocampus, but there are expected to be differences between the functions of different amygdala nuclei [93] and hippocampal subfields [94]. In addition, emerging evidence suggests that there may be functional differences within the hippocampus along the long axis of this structure [95, 96]. High-resolution fMRI may prove useful in further differentiating the functions of these sub-regions and in assessing whether they differentially contribute to episodic memory for emotional materials.
Another important question is how are the emerging effects of emotion on episodic memory related to post-encoding manipulations like stress? For example, a growing body of research indicates that when encoding is immediately followed by a stressful event that this can lead to slower forgetting compared to a no-stress control condition [22, 26, 97–99]. Post-encoding stress effects have often been attributed to a consolidation process similar to the one originally thought to produce the delayed emotion effects. However, a number of findings suggest these effects are quite distinct. For example, the emotion effects do not seem to require stress to emerge, as they can be observed even after a 2-hour no-stress delay [26]. In addition, stress can impact memory for both emotional and neutral materials, so it does not seem to preferentially slow the forgetting of emotional materials [22, 26, 97] [but see 98, 99].
We have argued that although the MTL regions might interact during encoding, in order to account for the emerging emotion advantage in memory there is no need to assume that there are any additional interactions between the amygdala and other MTL regions across the delay period. However, it is, of course, possible that under certain conditions there may be additional interactions. For example, if subjects preferentially rehearse or are reminded of the emotional events, this could lead to additional encodings of those items, slowing forgetting even further. However, in this case one would expect to see both recollection and familiarity advantages emerge over time, because both the items and the item-emotion bindings are repeated. The existing literature shows that the time dependent emotion advantage is limited to recollection, arguing against this type of reminding. We suspect, however, that with truly traumatic real-life events, this type of reminding may occur more often. Future work could test whether traumatic events are characterized by increased reminding and whether this is related to the emergence of recollection and familiarity based emotional advantages.
Concluding Remarks
The current literature indicates that emotional memories are forgotten more slowly than neutral memories. Moreover, these effects are specific to recollection of item-related information rather than familiarity or contextual recollection, and they are dependent on the amygdala but not the hippocampus. The results are consistent with an emotional binding model in which item-emotion bindings supported by the amygdala are particularly resistant to forgetting compared to the item-context bindings supported by the hippocampus. Although the emotional binding account appears to explain the emerging emotional memory effects, there are a number of questions about these effects that have not yet been fully addressed. We hope that future studies designed to test this approach will prove useful in furthering our understanding of the impact of emotion on episodic memory.
Highlights.
Emotion-related memory enhancements become particularly pronounced over time
These effects may be explained by item-emotion binding processes in the amygdala
Item-emotion bindings may be forgotten more slowly than item-context bindings
Outstanding Questions.
Can item-emotion associations be unitized? Prior studies have shown that when different features of an item or event are unitized or treated as a single object during encoding, the perirhinal cortex can support familiarity-based recognition for those associations [104, 105]. If the current model is correct, then the perirhinal cortex may support unitized item-emotion associations under encoding conditions that promote unitization. In such cases, one expects that the emotion advantage should be observed for familiarity-based recognition, and unlike the recollection advantage it should not increase across delay.
How does damage to the perirhinal cortex and parahippocampal cortex impact the delayed emotion effects? The current model predicts that perirhinal damage, but not parahippocampal damage, should reduce the emergence of the emotional advantage in memory. Selective lesions of this type are rare in humans, but volumetric studies in human patients or analogous emotional recognition paradigms in rodents may be useful for testing these predictions.
Are there conditions in which emotion enhances memory for context information, and are those effects time-dependent? The current model predicts that context bindings supported by the hippocampus should be forgotten more quickly than the item-emotion bindings supported by the amygdala. However, it is an open question whether there are other kinds of context associations (e.g., internal context) that can be supported by a non-hippocampal pathway, such as the medial prefrontal cortex.
What hormonal mechanisms are involved in producing the emotion effects on episodic memory, and are they specific to particular neuroanatomical regions of the medial temporal lobes?
Do the item-emotion bindings supported by the amygdala also support memory for emotionally positive materials like images of babies or smiling faces? Imaging studies have shown memory related amygdala activity for both positive and negative arousing materials [106, 107]. To our knowledge, however, there are no studies that have examined emotional memory for positive materials in either patients with selective hippocampal or selective amygdala lesions, nor is it yet clear whether positive emotion effects show the same delay dependent effects seen with negative emotion.
How do these laboratory results relate to memory for traumatic real-life experiences? In the existing lab studies emotion has been shown to benefit recollection rather than familiarity, but more traumatic experiences may impact both processes. For example, people may be more likely to be reminded of real-life traumatic experiences and thus re-encode those items, which would be expected to slow the forgetting of both recollection and familiarity.
Glossary
- Episodic memory
Memory of a specific event that was personally experienced at a particular time or place in the past. It is typically measured using tests of recognition or recall
- Recognition
Memory tests in which subjects must discriminate between stimuli that were earlier studied from those that are new to the experimental setting. In item recognition tests, stimuli typically include words, scenes, faces or objects. In relational recognition tests, subjects must discriminate between pairings of items or stimuli that were earlier studied from re-pairings. For example, the task may require recognizing that a particular word was studied with a particular face previously, which is sometimes referred to as associative recognition, or it may require recognizing that a particular word was encountered in a specific location, sometimes referred to as source recognition
- Recall
Memory tests in which subjects are required to generate items from a previous encoding event, such as the words or images from a previous encoding list
- Recollection
A memory process whereby subjects retrieve qualitative information about a specific study event. For example, remembering that a particular object was encountered at a specific time or location, or was associated with a particular semantic and emotional state. It is expected to play a role in free recall and in tests of recognition memory, particularly relational recognition tests
- Familiarity
A memory process whereby subjects discriminate between old and new items on the basis of perceived memory strength (sometimes referred to processing fluency or a sense of recency). It is thought to be particularly useful in tests of item recognition where old items are familiar and the new items are novel, but to be somewhat less useful in relational recognition tests or recall tests [17, 100]
- Receiver operating characteristic (ROC) procedure
A procedure that can be used to measure the contribution of recollection and familiarity to recognition performance [101]. The function describes the relationship between the proportion of correctly recognized studied items against the proportion of incorrectly recognized nonstudied items across variations in response criterion or confidence. A nonlinear model is fit to the observed function to estimate the probability of recollection and familiarity
- Remember/know procedure
A procedure that can be used to measure recollection and familiarity on the basis of introspective reports [102]. For each recognition response, subjects report whether they recognize items on the basis of remembering (i.e., recollection of qualitative information about the study event) or knowing (i.e. the item is familiar in the absence of recollection). Because subjects are instructed to respond ‘remember’ whenever they recollect a test item, the probability of a ‘remember’ response is used as an index of recollection, whereas the probability that an item is familiar is equal to the conditional probability that it received a ‘know’ response given it was not recollected [103]
- Consolidation
A process of stabilizing a memory trace after it has been encoded. Synaptic consolidation is used to refer to a set of cellular/molecular processes that are engaged to support the strengthening of the synapses in a local circuit, and it is thought to occur within the first few hours after encoding. Systems consolidation is used to refer to a process whereby hippocampus-dependent memories are transferred to the cortex over a period of weeks, months or decades. Emotional consolidation refers to the idea that after encoding, the amygdala signals the hippocampus to preferentially stabilize or protect hippocampal-dependent memories of emotional compared to neutral events
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hamann S. Cognitive and neural mechanisms of emotional memory. Trends in Cognitive Sciences. 2001;5:394–400. doi: 10.1016/s1364-6613(00)01707-1. [DOI] [PubMed] [Google Scholar]
- 2.LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- 3.Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Current Opinion in Neurobiology. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Talmi D. Enhanced emotional memory: Cognitive and neural mechanisms (vol 22, pg 430, 2013) Current Directions in Psychological Science. 2014;23:83–83. [Google Scholar]
- 5.Kleinsmith LJ, Kaplan S. Paired-associate learning as a function of arousal and interpolated interval. Journal of Experimental Psychology. 1963;65:190–193. doi: 10.1037/h0040288. [DOI] [PubMed] [Google Scholar]
- 6.Sharot T, Phelps EA. How arousal modulates memory: Disentangling the effects of attention and retention. Cognitive, Affective, & Behavioral Neuroscience. 2004;4:294–306. doi: 10.3758/cabn.4.3.294. [DOI] [PubMed] [Google Scholar]
- 7.Anderson AK, et al. Emotional memories are not all created equal: Evidence for selective memory enhancement. Learning & Memory. 2006;13:711–718. doi: 10.1101/lm.388906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharot T, Yonelinas AP. Differential time-dependent effects of emotion on recollective experience and memory for contextual information. Cognition. 2008;106:538–547. doi: 10.1016/j.cognition.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Ritchey M, et al. Role of Amygdala Connectivity in the Persistence of Emotional Memories Over Time: An Event-Related fMRI Investigation. Cerebral Cortex. 2008;18:2494–2504. doi: 10.1093/cercor/bhm262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharot T, et al. How emotion enhances the feeling of remembering. Nature Neuroscience. 2004;7:1376–1380. doi: 10.1038/nn1353. [DOI] [PubMed] [Google Scholar]
- 11.LaBar KS, Phelps EA. Arousal-mediated memory consolidation: Role of the medial temporal lobe in humans. Psychological Science. 1998;9:490–493. [Google Scholar]
- 12.Hamann SB, et al. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nature Neuroscience. 1999;2:289–293. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- 13.Sharot T, et al. How emotion strengthens the recollective experience: a time-dependent hippocampal process. PLoS ONE. 2007;2:e1068. doi: 10.1371/journal.pone.0001068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talmi D, McGarry LM. Accounting for immediate emotional memory enhancement. Journal of Memory and Language. 2012;66:93–108. [Google Scholar]
- 15.Talmi D, et al. The role of attention and relatedness in emotionally enhanced memory. Emotion. 2007;7:89–102. doi: 10.1037/1528-3542.7.1.89. [DOI] [PubMed] [Google Scholar]
- 16.Jacoby LL. A process dissociation framework: Separating automatic from intentional uses of memory. Journal of Memory and Language. 1991;30:513–541. [Google Scholar]
- 17.Yonelinas AP. The Nature of Recollection and Familiarity: A Review of 30 Years of Research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]
- 18.Mandler G. Recognizing: The judgment of previous occurrence. Psychological Review. 1980;87:252–271. [Google Scholar]
- 19.Kensinger EA, Corkin S. Memory enhancement for emotional words: Are emotional words more vividly remembered than neutral words? Memory & Cognition. 2003;31:1169–1180. doi: 10.3758/bf03195800. [DOI] [PubMed] [Google Scholar]
- 20.Dewhurst SA, Parry LA. Emotionality, distinctiveness, and recollective experience. European Journal of Cognitive Psychology. 2000;12:541–551. [Google Scholar]
- 21.Atienza M, Cantero JL. Modulatory effects of emotion and sleep on recollection and familiarity. Journal of sleep research. 2008;17:285–294. doi: 10.1111/j.1365-2869.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- 22.Yonelinas AP, et al. The effects of post-encoding stress on recognition memory: examining the impact of skydiving in young men and women. Stress. 2011;14:136–144. doi: 10.3109/10253890.2010.520376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dolcos F, et al. Remembering one year later: Role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proceedings of the National Academy of Sciences. 2005;102:2626–2631. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ochsner KN. Are affective events richly recollected or simply familiar? The experience and process of recognizing feelings past. Journal of Experimental Psychology: General. 2000;129:242–261. doi: 10.1037//0096-3445.129.2.242. [DOI] [PubMed] [Google Scholar]
- 25.Pierce BH, Kensinger EA. Effects of emotion on associative recognition: valence and retention interval matter. Emotion. 2011;11:139–144. doi: 10.1037/a0021287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCullough AM, Yonelinas AP. Cold-pressor stress after learning enhances familiarity-based recognition memory in men. Neurobiol Learn Mem. 2013;106:11–17. doi: 10.1016/j.nlm.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kensinger EA. Negative emotion enhances memory accuracy: Behavioral and neuroimaging evidence. Current Directions in Psychological Science. 2007;16:213–218. [Google Scholar]
- 28.Mather M. Emotional Arousal and Memory Binding: An Object-Based Framework. Perspectives on Psychological Science. 2007;2:33–52. doi: 10.1111/j.1745-6916.2007.00028.x. [DOI] [PubMed] [Google Scholar]
- 29.Mather M, Nesmith K. Arousal-enhanced location memory for pictures. Journal of Memory and Language. 2008;58:449–464. doi: 10.1016/j.jml.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacKay D, et al. Relations between emotion, memory, and attention: evidence from taboo stroop, lexical decision, and immediate memory tasks. Mem Cognit. 2004;32:474–488. doi: 10.3758/bf03195840. [DOI] [PubMed] [Google Scholar]
- 31.MacKay DG, Ahmetzanov MV. Emotion, memory, and attention in the taboo Stroop paradigm. Psychol Sci. 2005;16:25–32. doi: 10.1111/j.0956-7976.2005.00776.x. [DOI] [PubMed] [Google Scholar]
- 32.D’Argembeau A, Van der Linden M. Influence of affective meaning on memory for contextual information. Emotion. 2004;4:173–188. doi: 10.1037/1528-3542.4.2.173. [DOI] [PubMed] [Google Scholar]
- 33.Doerksen S, Shimamura A. Source memory enhancement for emotional words. Emotion. 2001;1:5–11. doi: 10.1037/1528-3542.1.1.5. [DOI] [PubMed] [Google Scholar]
- 34.Dougal S, et al. The role of medial temporal lobe in item recognition and source recollection of emotional stimuli. Cogn Affect Behav Neurosci. 2007;7:233–242. doi: 10.3758/cabn.7.3.233. [DOI] [PubMed] [Google Scholar]
- 35.Kensinger EA, et al. Memory for specific visual details can be enhanced by negative arousing content. Journal of Memory and Language. 2006;54:99–112. [Google Scholar]
- 36.Kensinger EA, et al. How Negative Emotion Enhances the Visual Specificity of a Memory. Journal of Cognitive Neuroscience. 2007;19:1872–1887. doi: 10.1162/jocn.2007.19.11.1872. [DOI] [PubMed] [Google Scholar]
- 37.Mather M, Knight M. The emotional harbinger effect: poor context memory for cues that previously predicted something arousing. Emotion. 2008;8:850–860. doi: 10.1037/a0014087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kensinger EA, et al. Effects of emotion on memory specificity: Memory trade-offs elicited by negative visually arousing stimuli. Journal of Memory and Language. 2007;56:575–591. [Google Scholar]
- 39.Kensinger EA, Schacter DL. Amygdala activity is associated with the successful encoding of item, but not source, information for positive and negative stimuli. Journal of Neuroscience. 2006;26:2564–2570. doi: 10.1523/JNEUROSCI.5241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yegiyan NS, Yonelinas AP. Encoding details: positive emotion leads to memory broadening. PCEM. 2011;25:1255–1262. doi: 10.1080/02699931.2010.540821. [DOI] [PubMed] [Google Scholar]
- 41.Bergmann HC, et al. The effects of valence and arousal on associative working memory and long-term memory. PLoS ONE. 2012;7:e52616. doi: 10.1371/journal.pone.0052616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cook GI, et al. Source monitoring is not always enhanced for valenced material. Memory & Cognition. 2007;35:222–230. doi: 10.3758/bf03193443. [DOI] [PubMed] [Google Scholar]
- 43.Rimmele U, et al. Emotion enhances the subjective feeling of remembering, despite lower accuracy for contextual details. Emotion. 2011;11:553–562. doi: 10.1037/a0024246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Touryan S, et al. Effect of negative emotional pictures on associative memory for peripheral information. Memory. 2007;15:154–166. doi: 10.1080/09658210601151310. [DOI] [PubMed] [Google Scholar]
- 45.Burke A, et al. Remembering Emotional Events. Memory & Cognition. 1992;20:277–290. doi: 10.3758/bf03199665. [DOI] [PubMed] [Google Scholar]
- 46.Waring JD, Kensinger EA. Effects of emotional valence and arousal upon memory tradeoffs with aging. Psychology and Aging. 2009;24:412–422. doi: 10.1037/a0015526. [DOI] [PubMed] [Google Scholar]
- 47.Adolphs R, et al. Impaired declarative memory for emotional material following bilateral amygdala damage in humans. Learning & Memory. 1997;4:291–300. doi: 10.1101/lm.4.3.291. [DOI] [PubMed] [Google Scholar]
- 48.Cahill L, et al. The amygdala and emotional memory. Nature. 1995;377:295–296. doi: 10.1038/377295a0. [DOI] [PubMed] [Google Scholar]
- 49.Markowitsch HJ, et al. The amygdala’s contribution to memory--a study on two patients with Urbach-Wiethe disease. Neuroreport. 1994;5:1349–1352. [PubMed] [Google Scholar]
- 50.Phelps EA, et al. Specifying the contributions of the human amygdala to emotional memory: A case study. Neurocase. 1998;4:527–540. [Google Scholar]
- 51.Cahill L, et al. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dolcos F, et al. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42:855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- 53.Canli T, et al. Event-related activation in the human amygdala associates with later memory for individual emotional experience. Journal of Neuroscience. 2000;20:5. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murty V, et al. fMRI studies of successful emotional memory encoding: A quantitative meta-analysis. Neuropsychologia. 2010;48:3459–3469. doi: 10.1016/j.neuropsychologia.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith AP, et al. Task and Content Modulate Amygdala-Hippocampal Connectivity in Emotional Retrieval. Neuron. 2006;49:631–638. doi: 10.1016/j.neuron.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 56.Smith A, et al. fMRI correlates of the episodic retrieval of emotional contexts. NeuroImage. 2004;22:868–878. doi: 10.1016/j.neuroimage.2004.01.049. [DOI] [PubMed] [Google Scholar]
- 57.Maratos EJ, et al. Neural activity associated with episodic memory for emotional context. Neuropsychologia. 2001;39:910–920. doi: 10.1016/s0028-3932(01)00025-2. [DOI] [PubMed] [Google Scholar]
- 58.Somerville LH, et al. Dissociable medial temporal lobe contributions to social memory. J Cogn Neurosci. 2006;18:1253–1265. doi: 10.1162/jocn.2006.18.8.1253. [DOI] [PubMed] [Google Scholar]
- 59.Kensinger EA, et al. Amygdala activity at encoding corresponds with memory vividness and with memory for select episodic details. Neuropsychologia. 2011;49:663–673. doi: 10.1016/j.neuropsychologia.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Waring JD, Kensinger EA. How emotion leads to selective memory: neuroimaging evidence. Neuropsychologia. 2011;49:1831–1842. doi: 10.1016/j.neuropsychologia.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mackiewicz KL, et al. The effect of anticipation and the specificity of sex differences for amygdala and hippocampus function in emotional memory. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:14200–14205. doi: 10.1073/pnas.0601648103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mickley Steinmetz KR, et al. The effect of emotional arousal and retention delay on subsequent-memory effects. Cognitive neuroscience. 2012;3:150–159. doi: 10.1080/17588928.2012.677421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adolphs R, et al. Impaired emotional declarative memory following unilateral amygdala damage. Learning & Memory. 2000;7:180–186. doi: 10.1101/lm.7.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Todorov A, Olson IR. Robust learning of affective trait associations with faces when the hippocampus is damaged, but not when the amygdala and temporal pole are damaged. Social Cognitive and Affective Neuroscience. 2008;3:195–203. doi: 10.1093/scan/nsn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hamann SB, et al. Intact enhancement of declarative memory for emotional material in amnesia. Learning and Memory. 1997;4:301–309. doi: 10.1101/lm.4.3.301. [DOI] [PubMed] [Google Scholar]
- 66.Hamann SB, et al. Emotional perception and memory in amnesia. Neuropsychology. 1997;11:104–113. doi: 10.1037//0894-4105.11.1.104. [DOI] [PubMed] [Google Scholar]
- 67.Richardson MP, et al. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nat Neurosci. 2004;7:278–285. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- 68.Kensinger EA, Corkin S. Two routes to emotional memory: Distinct neural processes for valence and arousal. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3310–3315. doi: 10.1073/pnas.0306408101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.St Jacques PL, et al. Effects of Aging on Functional Connectivity of the Amygdala for Subsequent Memory of Negative Pictures. Psychological Science. 2009:20. doi: 10.1111/j.1467-9280.2008.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kilpatrick L, Cahill L. Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. Neuroimage. 2003;20:2091–2099. doi: 10.1016/j.neuroimage.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 71.Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) The Journal of Comparative Neurology. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- 72.Price J. Comparative Aspects of Amygdala Connectivity. Annals of the New York Academy of Sciences. 2003;985:50–58. doi: 10.1111/j.1749-6632.2003.tb07070.x. [DOI] [PubMed] [Google Scholar]
- 73.Biebl M, et al. Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neuroscience Letters. 2000;291:17–20. doi: 10.1016/s0304-3940(00)01368-9. [DOI] [PubMed] [Google Scholar]
- 74.Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 75.Akers KG, et al. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344:598–602. doi: 10.1126/science.1248903. [DOI] [PubMed] [Google Scholar]
- 76.Hardt O, et al. Decay happens: the role of active forgetting in memory. Trends Cogn Sci. 2013;17:111–120. doi: 10.1016/j.tics.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 77.Sadeh T, et al. How we forget may depend on how we remember. Trends Cogn Sci. 2014;18:26–36. doi: 10.1016/j.tics.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 78.Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 79.Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends in Neurosciences. 1998;21:294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- 80.McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 81.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 82.Olsen RK, et al. Volumetric analysis of medial temporal lobe subregions in developmental amnesia using high-resolution magnetic resonance imaging. Hippocampus. 2013;23:855–860. doi: 10.1002/hipo.22153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vargha-Khadem F, et al. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- 84.Vargha-Khadem F, et al. Developmental amnesia: effect of age at injury. Proc Natl Acad Sci USA. 2003;100:10055–10060. doi: 10.1073/pnas.1233756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rempel-Clower NL, et al. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. The Journal of Neuroscience. 1996;16:5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zola-Morgan S, et al. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. The Journal of Neuroscience. 1986;6:2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Davachi L. Item, context and relational episodic encoding in humans. Current Opinion in Neurobiology. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 88.Diana RA, et al. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci (Regul Ed) 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 89.Eichenbaum H, et al. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mayes A, et al. Associative memory and the medial temporal lobes. Trends Cogn Sci. 2007;11:126–135. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 91.Norman KA, O’Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: A complementary-learning-systems approach. Psychological Review. 2003;110:611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- 92.Mather M, Sutherland MR. Arousal-Biased Competition in Perception and Memory. Perspectives on Psychological Science. 2011;6:114–133. doi: 10.1177/1745691611400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sah P, et al. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- 94.Kesner RP. Role of the hippocampus in mediating interference as measured by pattern separation processes. Behavioural processes. 2013;93:148–154. doi: 10.1016/j.beproc.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 95.Poppenk J, et al. Long-axis specialization of the human hippocampus. Trends Cogn Sci. 2013;17:230–240. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 96.Strange BA, et al. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 2014;15:655–669. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- 97.Andreano JM, Cahill L. Glucocorticoid release and memory consolidation in men and women. Psychol Sci. 2006;17:466–470. doi: 10.1111/j.1467-9280.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- 98.Cahill L, et al. Enhanced Human Memory Consolidation With Post-Learning Stress: Interaction With the Degree of Arousal at Encoding. Learning & Memory. 2003;10:270–274. doi: 10.1101/lm.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smeets T, et al. True or false? Memory is differentially affected by stress-induced cortisol elevations and sympathetic activity at consolidation and retrieval. Psychoneuroendocrinology. 2008;33:1378–1386. doi: 10.1016/j.psyneuen.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 100.Tulving E. Synergistic ecphory in recall and recognition. Canadian Journal of Psychology. 1982;36:130–147. [Google Scholar]
- 101.Yonelinas AP. Receiver-operating characteristics in recognition memory: evidence for a dual-process model. J Exp Psychol Learn Mem Cogn. 1994;20:1341–1354. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
- 102.Tulving E. Précis of Elements of episodic memory. Behavioral and Brain Sciences. 1984;7:223–238. [Google Scholar]
- 103.Yonelinas AP, Jacoby LL. Dissociating automatic and controlled processes in a memory-search task: beyond implicit memory. Psychological research. 1995;57:156–165. doi: 10.1007/BF00431277. [DOI] [PubMed] [Google Scholar]
- 104.Haskins AL, et al. Perirhinal cortex supports encoding and familiarity-based recognition of novel associations. Neuron. 2008;59:554–560. doi: 10.1016/j.neuron.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 105.Quamme JR, et al. Effect of unitization on associative recognition in amnesia. Hippocampus. 2007;17:192–200. doi: 10.1002/hipo.20257. [DOI] [PubMed] [Google Scholar]
- 106.Hamann SB, et al. Ecstasy and agony: activation of the human amygdala in positive and negative emotion. Psychol Sci. 2002;13:135–141. doi: 10.1111/1467-9280.00425. [DOI] [PubMed] [Google Scholar]
- 107.Anderson AK, et al. Dissociated neural representations of intensity and valence in human olfaction. Nature Neuroscience. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- 108.Yonelinas AP, et al. Recollection and familiarity: examining controversial assumptions and new directions. Hippocampus. 2010;20:1178–1194. doi: 10.1002/hipo.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behavioral and Brain Sciences. 1999;22:425–489. [PubMed] [Google Scholar]
- 110.Rugg MD, Yonelinas AP. Human recognition memory: a cognitive neuroscience perspective. Trends Cogn Sci (Regul Ed) 2003;7:313–319. doi: 10.1016/s1364-6613(03)00131-1. [DOI] [PubMed] [Google Scholar]
- 111.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 112.Bechara A, et al. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- 113.LaBar KS, et al. Impaired fear conditioning following unilateral temporal lobectomy in humans. Journal of Neuroscience. 1995;15:6846–6855. doi: 10.1523/JNEUROSCI.15-10-06846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fanselow MS. Animal Learning & Behavior. 1990. Factors governing one-trial contextual conditioning. [Google Scholar]
- 115.Gale GD, et al. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J Neurosci. 2004;24:3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Current Opinion in Neurobiology. 2002;12:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- 117.Cahill L, et al. Is the amygdala a locus of conditioned fear? Some questions and caveats. Neuron. 1999;23:227–228. doi: 10.1016/s0896-6273(00)80774-6. [DOI] [PubMed] [Google Scholar]
- 118.Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- 119.Wiltgen BJ, Silva AJ. Memory for context becomes less specific with time. Learn Mem. 2007;14:313–317. doi: 10.1101/lm.430907. [DOI] [PubMed] [Google Scholar]
- 120.Winocur G, et al. Memory consolidation or transformation: context manipulation and hippocampal representations of memory. Nat Neurosci. 2007;10:555–557. doi: 10.1038/nn1880. [DOI] [PubMed] [Google Scholar]