Abstract
Protein synthesis is a dynamic process to tune the cellular proteome to internal and external demands. Metabolic labeling approaches identify the general proteomic response but missing is a tool to visualize within cells specific newly synthesized proteins. Here we describe a technique that couples non-canonical amino acid tagging or puromycylation with the proximity-ligation assay to visualize identified newly synthesized proteins and monitor their origin, redistribution and turnover in situ.
The regulation in space and time of protein synthesis underlies the homeostasis and plasticity of all cells. Pulse-labeling with radioactive amino acids is the traditional method for visualizing newly synthesized proteins in situ. Recent advances in bioorthogonal labeling with non-canonical amino acids have transformed our access to newly synthesized proteomes. Using click chemistry, labeled newly synthesized proteins can be tagged, purified and identified using mass spectrometry1 or visualized in situ using fluorescent tags (fluorescence non-canonical amino acid tagging; FUNCAT)2. Alternatively, the newly synthesized proteome can be visualized with puromycin-labeling and puromycin antibodies3,4. One hurdle not yet scaled, however, is the ability to visualize an identified endogenous protein as newly synthesized, in situ.
To accomplish this, we developed a proximity ligation assay (PLA)5,6-based strategy that detects the spatial coincidence of two antibodies: one that identifies a newly synthesized protein tagged with either FUNCAT or puromycylation and another that identifies a specific epitope in a protein-of-interest (POI) (Fig. 1a). In FUNCAT azidohomoalanine (AHA) is taken up by cells and charged onto methionine tRNAs. During translation, AHA is incorporated into newly synthesized protein and visualized using click chemistry, a biotin-based tag, and an anti-biotin antibody (Supplementary Fig. 1a,b). Alternatively, newly synthesized proteins can be recognized using puromycylation3,4, where cells are treated with a low concentration of puromycin, a tRNA analog7, which terminates translation and releases the truncated protein which is then be recognized by an anti-puromycin antibody.
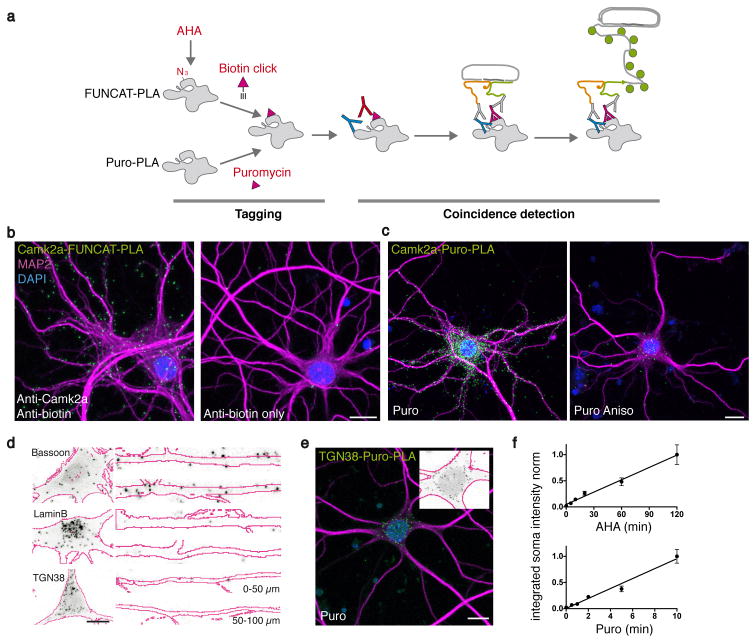
Figure 1. Labeling specific newly synthesized proteins.
(a) The working principle. Newly synthesized proteins incorporate either AHA or puromycin. AHA is biotinylated by click chemistry. Antibody (pink Y) recognition of the ‘newly synthesized’ tag (biotin or puromycin, pink triangle) and recognition of a POI by a protein-specific antibody (blue Y) detects close proximity when PLAminus and PLAplus oligonucleotides (yellow and green squiggles) coupled to secondary antibodies (grey Y) are close enough to template linker oligo ligation to a circle. Signal is amplified by binding of fluorescently-coupled detection probes (green circles). (b) Example images of FUNCAT-PLA signal (green) for newly synthesized Camk2a (2 h AHA) in cultured hippocampal neurons (left) and control without anti-Camk2a antibody (right). (c) Puro-PLA images of newly synthesized Camk2a (green) after 15 min of puromycin labeling without (left) or with (right) the protein synthesis inhibitor Anisomycin. Scale bars (b,c) = 15 μm. (d) High-magnification images of FUNCAT-PLA (grey-scale) for newly synthesized Bassoon, TGN38 or LaminB (as indicated, 2 h AHA) in somata and principal dendrites of cultured hippocampal neurons (MAP2 outlines in magenta). Scale bar = 10 μm. (e) Micrograph of newly synthesized TGN38 after 5 min puromycylation. Inset: soma signal converted as in (d). Scale bar = 20 μm. (f) Correlation of TGN38 FUNCAT-PLA and Puro-PLA signal (integrated PLA intensity in the soma) dependence on incubation time with AHA and puromycin. (R2 = 0.99 and 0.98 from two and three independent experiments, n = 20 – 27 and n = 15 – 20 respectively. Mean ± SEM and the linear regression is shown).
In order to recognize a specific POI, a second primary antibody is used. Next, respective secondary antibodies coupled to different oligonucleotides (PLAplus and PLAminus probes) are introduced; when the two probes are in proximity, linker oligonucleotides and a ligase promote formation of a “circle” subsequently amplifiable by rolling circle amplification. Ultimately, the coincidence detection (‘new’ and ‘POI’) is visualized in situ by fluorescently-labeled probes complementary to the amplified sequences, as shown for newly synthesized Camk2a in neurons (Fig. 1b,c). We extensively tested and optimized the dependence of FUNCAT-PLA and Puro-PLA on the presence of the POI, the antibodies, AHA/puromycin and intact protein synthesis (Fig. 1b,c and Supplementary Figs. 1–4).
Recently, deep-sequencing and high-resolution in situ-hybridization have led to the identification of an unexpectedly high number of mRNA species in mature neuronal processes8,9. We used FUNCAT-PLA to address whether the distribution of candidate newly synthesized proteins is consistent with their local synthesis in dendrites or axons. Cultured hippocampal neurons were treated with AHA (2 h) to pulse label a population of newly synthesized proteins and then processed as described above. The FUNCAT-PLA signal for the postsynaptic density protein Camk2a was detected throughout dendrites, consistent with the abundance of Camk2a mRNA9,10 in dendrites (Fig. 1b and Supplementary Fig. 4). Similarly, a strong FUNCAT-PLA signal for the presynaptic protein Bassoon (Bsn) was detected alongside the dendrites, most likely reflecting localization in closely-apposed presynaptic terminals (Fig. 1d and Supplementary Figs. 4 and 5). FUNCAT-PLA for other proteins such as Lamin B (Lmb1), a component of the nuclear envelope, and TGN38 (Tgoln2), a protein localized to the trans-Golgi network and recycling endosomes- two proteins whose mRNAs are not substantially enriched in dendrites - showed very low signal in dendrites as compared to the soma (Fig. 1d and Supplementary Fig. 4).
In parallel puromycylation experiments, we treated hippocampal neurons with puromycin (5–15 min) and processed for nascent Camk2a and TGN38 detection (Fig. 1c,e). Consistent with the results obtained by FUNCAT-PLA (Fig. 1b), we observed abundant newly synthesized Camk2a signal both in soma and dendrites whereas the TGN38 Puro-PLA signal was concentrated in the soma (Fig. 1c,e and Supplementary Fig. 1d).
Here both variants of metabolic labeling, FUNCAT-PLA and Puro-PLA, led to qualitatively similar results, with the major difference being the apparent extent of labeling (e.g. number of particles). While incubation times in the range of seconds to minutes are sufficient to obtain a Puro-PLA signal that is linear with time, a detectable FUNCAT-PLA signal requires longer metabolic labeling times, but is also linear over time (Fig. 1f). Using a brief methionine starvation period prior to AHA labeling allowed us to directly compare FUNCAT- and Puro-PLA for an identical metabolic labeling time (15 min) and the same POI antibody (TGN38); in this experiment we found a roughly ten-fold higher signal with Puro-PLA than with FUNCAT-PLA.
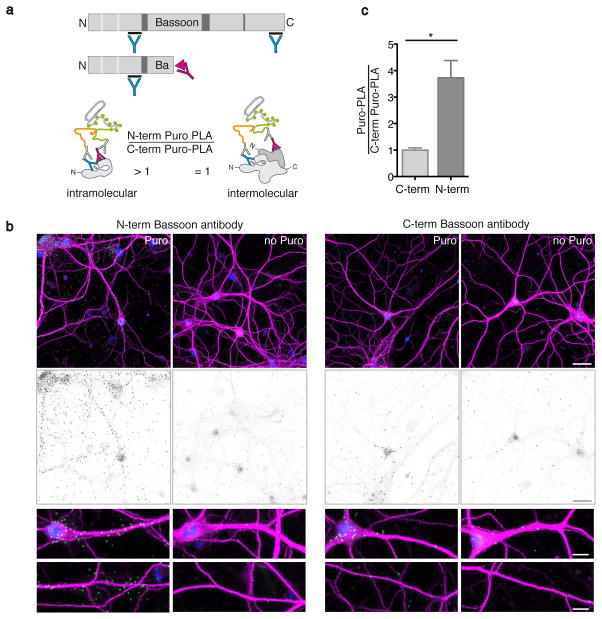
Progress in transcriptomics and proteomics have arguably delivered both what can be translated (the transcriptome)9,11,12 and the tissue-wide population of proteins that are translated in a certain time window (the proteome)1. What is clearly missing, however, is the sub-cellular resolution of the site of synthesis and the ensuing spatial redistribution of newly synthesized proteins. To explore this, we used the protein Bassoon, since it is thought to be synthesized in the soma, (despite the recent detection of Bassoon mRNA in the neuropil9,13) and then transported to presynaptic terminals by specialized transport vesicles14. To test whether, in addition to rapid transport after synthesis, a fraction of the protein might be synthesized locally we performed Bassoon Puro-PLA, labeling for just 4 min, to visualize the origin of nascent Bassoon. Consistent with a local synthesis source, some Bassoon Puro-PLA signal was detected juxtaposed to dendrites (Fig. 2b). As protein synthesis proceeds from N- to C-terminal and puromycin truncates the nascent protein chain, we reasoned that antibodies directed against the N terminus should generate more Puro-PLA labeling than C-terminal antibodies against the same protein (Fig. 2a). Indeed we found that the N-terminal Puro-PLA signal was higher than C-terminal Puro-PLA signal (Fig. 2c) (even when controlling for epitope availability) (Supplementary Fig. 5b) thus supporting the idea that the Bassoon Puro-PLA signal is primarily due to the binding of two antibodies to the same nascent polypeptide.
Figure 2. Assessing intramolecular labeling of Puro-PLA.
(a) Scheme depicting the binding sites for N- and C-terminal anti-Bassoon antibodies (blue Y, upper panel). In Puro-PLA experiments, an N-terminal Bassoon antibody and an anti-puromycin antibody (pink Y) are predicted to generate a larger signal compared to a C-terminal Bassoon and puromycin antibody pair since puromycin (pink triangle) blocks the elongation of the nascent chain. If both antibodies recognize epitopes in the same protein, the Puro-PLA signal generated with N-terminal antibodies is expected to be greater than that generated with C-terminal antibodies in contrast to binding to neighbouring proteins. (b) Representative fluorescence images and close-ups (upper panel, PLA-signal green, MAP2 magenta and DAPI-labeled nuclei blue, lower panel grey scale PLA-signal) showing Puro-PLA signal for the Bassoon protein detected after labeling with puromycin for four minutes or without puromycin, using either N- or C-terminal anti-Bassoon antibodies. Scale bar = 30 μm and 10 μm respectively. (c) Quantification of signal shown in (b). For each experiment, the background-corrected Puro-PLA density for each antibody was normalized to the mean value for the C-terminal antibody (means ± SEM from three independent experiments, n = 10 – 11). Statistical significance was tested using an unpaired Student’s t-test (*P < 0.05, P = 0.014).
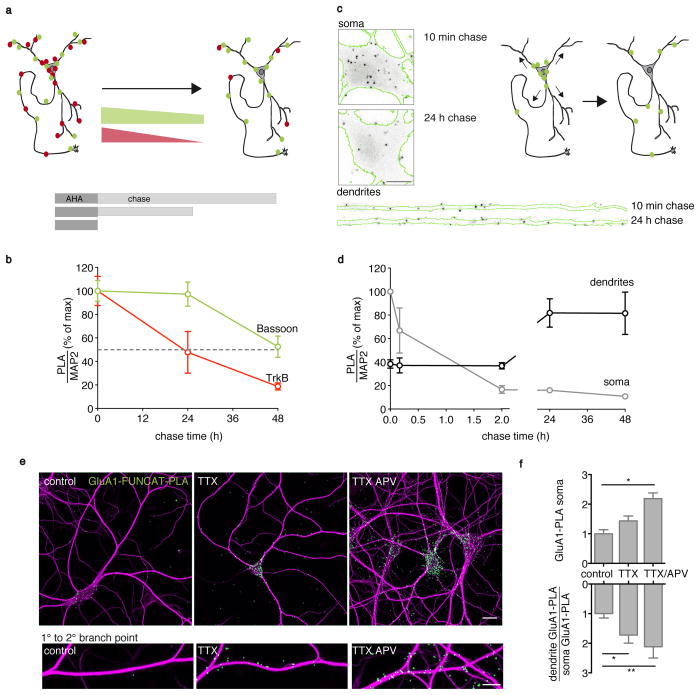
The turnover rates of specific endogenous proteins are usually determined biochemically, after incorporation of heavy isotopes and tissue solubilization, a technique that is not amendable to visualization in situ. We thus sought to visualize the turnover of individual neuronal proteins in situ with FUNCAT-PLA. We examined the turnover of two proteins with different stabilities: TrkB (Ntrk2), a neurotrophin receptor, and Bassoon, with half-lives of 0.7 and 2.6 days, respectively15. Cultured neurons were pulse-labeled with AHA (2 h) and then “chased” for different times before FUNCAT-PLA (Fig. 3a). The TrkB FUNCAT-PLA signal exhibited a steep decline over the time period examined, with 50 % of the initial signal disappearing within 24 h (Fig. 3b). In contrast, the Bassoon FUNCAT-PLA signal was much more stable, with more than 50 % of the initial signal still present after 48 h (Fig. 3b). Thus, protein stability in situ can be assessed with FUNCAT-PLA and the data are consistent with half-life values determined by biochemical means.
Figure 3. Following protein lifetime, distribution changes and synthesis rate changes with FUNCAT-PLA.
(a) Scheme depicts two hypothetical FUNCAT-PLA labeled proteins with different lifetimes. (b) Graph of Bassoon and TrkB FUNCAT-PLA signal dependence on chase time following a 2 h AHA pulse. The FUNCAT-PLA signal area overlapping with the dilated MAP2 signal was normalized to the MAP2 signal area (normalized means ± SEM from three (TrkB, n = 12 – 14) and five (Bassoon, n = 23 – 39 cells per condition) independent experiments. (c) Scheme depicting the spatio-temporal dynamics of a hypothetical FUNCAT-PLA-labeled protein population (green) and images of neuronal somata and dendrites (green MAP2 outline) labeled for Bassoon FUNCAT-PLA (grey-scale) following a 2 h AHA incubation with either a 10 min or 24 h chase. Scale bar = 10 μm; dendrite length = 135 μm. (d) Analysis graph of (c). Values displayed are percent of the maximal signal density in the respective compartment with chase time (normalized means ± SEM from three independent experiments, n = 11 – 13). (e) Micrographs showing GluA1 FUNCAT-PLA (cell overview and a corresponding dendrite below) following 3 h AHA labeling without (control) and with induction of homeostatic plasticity by blocking action potentials (TTX) alone or in combination with a block of the NMDA-component of miniature synaptic events (TTX/APV). GluA1 FUNCAT-PLA (green), MAP2 (magenta), Scale bar = 20 (overview) and 10 μm (close-ups). (f) Analysis of (e). GluA1 FUNCAT-PLA signal density normalized to control in neuronal somata (upper panel). Treatment with TTX/APV significantly enhanced the population of newly synthesized GluA1 (*P < 0.05, P = 0.0349). Lower panel: Treatment with either TTX or TTX/APV significantly enhanced the amount of newly synthesized GluA1 in the dendrites even more than in somata (*P < 0.05, **P < 0.01, normalized means ± SEM, n = 16 – 25 cells per condition from three different experiments, ANOVA, Tukey’s multiple comparisons test on raw values).
Although fluorescent protein photoswitches and other time-controlled tags have made it possible to visualize the redistribution of candidate proteins in live cells, these approaches require the addition of fluorescent tags and usually involve protein overexpression16,17. In contrast, FUNCAT-PLA allows one to address changes in the localization of a pulse-labeled population of endogenous proteins. We monitored the redistribution of Bassoon marked after a 2 h AHA pulse. After a 10 min chase, many Bassoon-FUNCAT-PLA particles were localized in the soma with some labeling also present along dendrites Over time, however, Bassoon levels in the soma declined while the population detected along dendrites increased (Fig. 3c,d and Supplementary Fig. 6a). Most Bassoon proteins made in the soma were thus exported or degraded in a compartment-specific manner. Interestingly, Bassoon FUNCAT-PLA signal measured along dendrites at early time points of the chase was relatively high (~50 % of their level at the steady state). This suggests that nascent proteins are transported very rapidly or that a substantial amount of the protein is indeed synthesized locally.
In neurons, reducing neuronal activity leads to a homeostatic increase of synaptic responses18,19. This synaptic scaling is associated with increased synaptic levels of GluA1(Gria1)-containing glutamate AMPA receptors19. We thus examined whether homeostatic plasticity elicited by blocking action potentials alone or accompanied by blockade of the NMDA-receptor component of spontaneous neurotransmission results in new synthesis of GluA1. In control neurons, metabolic labeling with AHA for 3 h resulted in a small amount of GluA1 FUNCAT-PLA signal that was primarily associated with neuronal somata and proximal dendrites (Fig. 3e,f). Homeostatic plasticity induced by inhibition of action potentials led to an increase in the newly synthesized GluA1 evident in both somata and the dendrites that was even greater when we blocked spontaneous transmission concomitantly (Fig. 3f).
Using metabolic labeling in combination with PLA we visualized with a high signal-to-noise ratio the spatial coincidence of a tag for newly synthesized and a POI epitope and provide the most sensitive method developed thus far to follow specific nascent endogenous proteins minutes to hours after their synthesis and monitor their turnover and redistribution in situ, in native cellular compartments and environments. The two methods of metabolic labeling – AHA incorporation and puromycylation – have distinct advantages and disadvantages and, as such, provide different insights (Supplementary Table 1). AHA uptake and activation by the methionyl tRNA synthetase is rate-limiting for AHA incorporation resulting in a requirement for relatively long pre-treatment times (minutes, hours). In contrast, incorporation of puromycin as a tRNA analog is considerably faster (seconds, minutes). Furthermore AHA replaces only methionine residues whereas puromycylation can occur at any residue7. AHA labeling is more efficient with a prior methionine starvation step and is ideally implemented in a methionine-free medium that might be sensed as cellular stress signal. Note, however, that an increase in overall toxicity was not observed in previous experiments1. Thus, for experiments in which brief labeling is necessary, puromycylation is preferred. On the other hand, AHA incorporation is the method of choice for pulse-labeling a fraction of proteins to follow their fate over a longer time scale (hours, days) to examine protein distribution changes, turnover or half-life. There is no indication that AHA incorporation changes a protein’s spatial fate1,2,20. In contrast, puromycin incorporation results in premature truncation of polypeptide chains and degradation of truncated proteins is enhanced21. These approaches are complemented by DiComps, a FRET-based strategy that can detect active translation of a specific mRNA following introduction of fluorophore-labeled tRNAs22. Thus, taken together FUNCAT-PLA and Puro-PLA, each with its own advantages and drawbacks (Supplementary Table 1), have the potential to answer a broad spectrum of questions related to protein synthesis rate, site and spatial fate of endogenous newly synthesized proteins within cells.
Although, in principle, the spatial proximity of the tags used could be achieved by labeling two neighboring proteins6 rather than a single protein, our data are consistent with the idea that the majority of the signal derives from a dually-labelled newly synthesized POI. Reduction of the spatial range of co-detection (e.g. by clicking a PLA-oligo directly to AHA or puromycin; PLA-oligo-coupling to POI-epitope nanobodies) might be useful in the future. In addition, multiplexing is one of the advantages of PLA detection and suggests the potential to monitor several newly synthesized proteins simultaneously.
Online methods
Cell culture
Lcells
The mouse fibroblast Lcell line (ATCC-CRL-2648) was obtained from the American Type Culture Collection (ATCC) and cultured in a humidified atmosphere at 37 °C and 5 % CO2 in DMEM-GlutaMAX-I (life technologies) supplemented with 10 % fetal bovine serum (life technologies) and 1 mM sodium pyruvate (life technologies). Generation of the stably transfected Lcell line expressing Venus-NCad was described previously23. Cells were screened and negative for mycoplasma contamination. Cells were split twice a week by trypsinizing with TrypLE Express (life technologies) for 5 min at 37 °C. The reaction was stopped by the addition of serum-containing culture medium and cells were replated in a ratio 1:5 or 1:10.
For passaging of the Lcell line expressing NCad-Venus, Geneticin (800 μg/ml, life technologies) was added to the culture medium to maintain the selection pressure. Geneticin was not present during experiments or in mixed cultures. For experiments, cells were plated onto glass bottom dishes (MatTek) and used at 90 % confluency.
Hippocampal neurons
Dissociated rat hippocampal neuron cultures were prepared and maintained essentially as described previously24,25. Briefly, we dissected hippocampi from postnatal day 0 to 1 rat pups of either sex (Sprague-Dawley strain; Charles River Laboratories), dissociated them with papain (Sigma) and plated them at a density of 40 × 103 cells/cm2 onto poly-D-lysine coated glass-bottom Petri dishes (MatTek). Hippocampal neurons were maintained and allowed to mature in a humidified atmosphere at 37 °C and 5 % CO2 in growth medium (Neurobasal-A supplemented with B27 and GlutaMAX-I, life technologies) for >18 days in vitro to ensure synapse maturation. All experiments complied with national animal care guidelines, the guidelines issued by the Max Planck Society and were approved by local authorities.
Metabolic labeling with AHA and biotin-alkyne click (FUNCAT)
The FUNCAT part of the assay was performed as described previously2,26 with the following modification: we used a biotin-alkyne (Acetylene-PEG4-Biotin, Jena Bioscience) as a tag in the copper-catalyzed [3+2] azide–alkyne cycloaddition (CuAAC) click reaction. Cells on MatTek dishes were incubated in HBS (Lcells), methionine-free HibernateA (for neurons, custom-made by BrainBits LLC) or methionine-free NeurobasalA (for neurons, custom-made by life technologies) supplemented with 4 mM AHA (prepared as described in Link et al., 2007)27 for 2 h except for the following 2 experiments: 1) investigation of the activity-dependent upregulation of GluA1 synthesis (labeling time = 3 h for detailed incubation scheme see Supplementary Fig. 6b) and 2) the direct comparison with Puro-PLA (15 min) and linearity experiments (see Fig. 1f, treated for the times indicated after preincubation with methionine-free Neurobasal A for 30 mins). In methionine control experiments AHA was replaced by 4 mM methionine (Sigma). In protein synthesis inhibitor control experiments, cells were preincubated for 30 min with 40 μM Anisomycin (Tocris) in their full conditioned medium; the same concentration of Anisomycin was present during AHA incubation. For experiments with neurons, the B27 supplement (life technologies) was present in all incubation media. After metabolic labeling cells were placed back into their original conditioned medium for 15 min (chase). Different chase periods (AHA-free) up to 48 h were used when indicated (e.g. Fig. 3a-f) and no chase was performed in linearity experiments in Figure 1f and the direct comparison with Puro-PLA. Subsequently cells were washed quickly two times with PBS-MC (1 x PBS pH 7.4, 1 mM MgCl2, 0.1 mM CaCl2) and fixed for 20 min in PFA-sucrose (4 % p-formaldehyde (Alfa Aeser), 4 % sucrose in PBS-MC) at room temperature, washed, permeabilized with 0.5 % Triton X-100 in 1 x PBS pH 7.4 for 15 min and blocked with blocking buffer (4 % goat serum in 1 x PBS) for 1 h. To optimize conditions for the CuAAC click reaction cells were equilibrated by washes with 1 x PBS pH 7.8.
For the CuAAC click reaction, to avoid copper bromide-derived precipitates, we used the reducing agent tris(2-carboxyethyl)phosphine (TCEP) in combination with CuSO4 to generate the Cu(I) catalyst for CuAAC. A click reaction mix composed of 200 μM triazole ligand Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine (TBTA), 25 μM biotin alkyne tag, 500 μM TCEP and 200 μM CuSO4 was mixed in PBS, pH 7.8, with vigorous vortexing after each addition of a reagent from stock solutions. The click reaction mixture was prepared immediately before application to the cells and CuAAC was performed overnight at room temperature. After the click reaction, cells were washed intensely with PBS and 0.5 % Triton in PBS and processed either directly for immunocytochemical detection of biotin (equivalent to regular FUNCAT) and cell markers or processed for PLA detection.
Puromycylation
For puromycylation, neurons were incubated with (or in ‘no puro’ controls without) 1–3 μM puromycin for 2 – 15 min (as indicated) in full medium at 37 °C in a humidified atmosphere with 5 % CO2. Incubation was stopped by two fast washes in prewarmed PBS-MC and cells were fixed for 20 min in PFA-sucrose. In protein synthesis inhibitor control experiments cells were pretreated with 40 μM anisomycin for 30 min before addition of puromycin to the medium. Experiments to determine site of synthesis were, in addition, carried out with a 30 min pretreatment with 355 μM cycloheximide which stalls the truncated protein at the ribosome and enhances puromycylation3. After fixation, cells were washed, permeabilized and treated as described in the sections ‘PLA’ (using puromycin antibody and protein of interest antibody as primary antibody pair for 2 h) and ‘immunocytochemistry’.
Proximity ligation assay (PLA)
Detection of newly synthesized proteins by proximity ligation was carried out using anti-biotin antibodies or anti-puromycin antibodies in combination with protein-specific antibodies and detection using Duolink reagents (Sigma) according to the manufacturer’s recommendations with slight modifications. We routinely used rb PLAplus and ms PLAminus probes as secondary antibodies and the ‘Duolink Detection reagents Red’ (Sigma) for ligation, amplification and label probe binding. PLA probes were swapped sometimes for control purposes.
Briefly, after metabolic labeling, permeabilization and washing (see sections ‘FUNCAT’ and ‘Puromycylation’) cells were blocked in blocking buffer (4 % goat serum in PBS, 1 h) and incubated with primary antibody pairs diluted in blocking buffer (1.5 h at room temperature). After washing, PLA probes were applied in 1:10 dilution in blocking buffer for 1 h at 37 °C, washed several times with wash buffer A (0.01 M Tris, 0.15 M NaCl, 0.05 % Tween20) and incubated for 30 min with the ligation reaction containing the circularization oligos and T4 ligase prepared according to the manufacturer’s recommendations (Duolink Detection reagents Red, Sigma) in a prewarmed humidified chamber at 37 °C. Amplification and label probe binding was performed after further washes with wash buffer A with the amplification reaction mixture containing Phi29 Polymerase and the fluorophore-labeled detection oligo prepared according to the manufacturer’s recommendations (Duolink Detection reagents Red, Sigma) in a prewarmed humidified chamber at 37 °C for 100 min. Amplification was stopped by three washes in 0.2 M Tris, 0.1 M NaCl pH 7.5 followed by washes in PBS pH 7.4. For better signal stability, cells were postfixed for 10 min at room temperature in PFA-sucrose, washed with PBS and processed further for immunohistochemistry with cell markers and/or DAPI nucleus stain.
Labeling of the total amount of a protein of interest with PLA was achieved by using only a single primary antibody and PLAplus and PLAminus probes against the species the antibody was developed in (see also Supplementary Fig. 2e). We didn’t detect obvious differences in the performance of monoclonal compared to polyclonal antibodies. Rather the specificity of labeling in immunocytochemistry plays a more important role. Before addressing a specific biological question in an experiment, the antibody performance and background should be determined and titrated with antibody leave-out, absence of protein of interest and in the presence of protein synthesis inhibitors.
Immunocytochemistry
After the postfixation step (FUNCAT-PLA, Puro-PLA) or alternatively after fixation and permeabilization (only immunocytochemistry) or the click reaction step (regular FUNCAT), cells were blocked with 4 % goat serum in PBS for 1 h followed by incubation with the respective antibodies for cell markers (anti-Smi312 for axons, anti-MAP2 for dendrites), washes in PBS and fluorophore-coupled secondary antibodies for 30 min. Cells were washed, counterstained with DAPI in PBS (Roth, 1 μg/ml) for 3 min and mounted with AquaPolymount (Polysciences) or imaged directly in PBS. Samples were imaged soon after the experiment and stored at 4 °C.
Sample size choice, imaging, image analysis and statistics
Sample size choice and blinding
Within one Puro-PLA or FUNCAT-PLA experiment the maximum number of MatTek dishes used was limited to twelve to ensure the concomitant processing and handling of the dishes with the same solutions and at the same time. For Lcell control experiments transfected and untransfected cells were mixed in the same dish to ensure equal treatment. For imaging neurons the experimenter selected the cells in the MAP2 channel blind to the PLA signal (but not blind to the conditions). Criteria to chose cells were healthy appearance judged by MAP2 staining (no swelling of dendrites or fragmented pattern) and homogeneous DAPI staining. No cells were taken from the outer edge of the culture dish or when completely isolated. Neurons for which soma and dendrites were to be analyzed had to show a clearly traceable dendrite. We chose only cells with apparent connections to other neurons but not hidden in a dense accumulation of cells. These criteria set the maximum number of cells imageable per experiment. All images for one experiment were acquired in close timely relationship that set further limits on the maximum number of cells per experiments. With fewer conditions per experiment the number of cells imaged per condition increased. For experiments where we analyzed only the soma signal (e.g. TGN38 Puro-PLA and FUNCAT-PLA in Fig. 1f) we did not apply the criterion of single cells with clearly traceable dendrite to enlarge the number of cells that can be analyzed per experiment but chose several fields of view selected in the MAP2 channel to contain multiple neurons. All cells with clear soma separation in a field of view were analyzed.
Imaging
Images were acquired with a LSM780 confocal microscope (Zeiss) using a 40 × 1.4 NA oil objective (Plan Apochromat 40 x/1.4 oil DIC M27) and a pinhole setting of 90 μm. All lasers were used at 2 % power. Images were acquired in 8 bit mode as Z-stacks with 1024 × 1024 pixels (or 2048 × 2048 for better visualization) xy resolution through the entire thickness of the cell with optical slice thickness set for two times oversampling to allow 3D reconstruction, pixel dwell times of 0.39 to 0.79 μs and the detector gain in each channel adjusted to cover the full dynamic range but to avoid saturated pixels. Imaging conditions were held constant within an experiment.
Image analysis
To quantify the PLA signal in Lcells multichannel maximum intensity projections of the raw images were split into single channel images in ImageJ (NIH). A manual threshold was applied to the PLA signal with the same value for controls and samples within an experiment and the puncta area measured with ImageJ was divided by the number of cells. The number of cells was determined by thresholding the DAPI channel, applying the watershed algorithm to separate overlapping nuclei and counting nuclei using the analyze particles plugin in ImageJ.
To analyze the amount of newly synthesized protein in neurons we used a similar approach but related the PLA signal to the MAP2 area representing the somato-dendritic neuronal compartment to account for the variable size and elaborate structure of neurons. Dilation of the MAP2-derived mask was performed to assure the inclusion of signal in spines or presynaptic terminals in the analysis but failed to include the majority of axonal area.
To analyze whole fields of view, PLA puncta were thresholded using the same value for all samples within one experiment. The MAP2 signal of the same cell was thresholded and dilated once using ImageJ. The sum of the PLA signal area or the integrated intensity of the PLA signal overlapping with the dilated MAP2-mask was divided by the non-dilated total MAP2 area.
To analyze the distribution of newly synthesized proteins in soma vs dendrites, for each neuron imaged, the soma was cropped and analyzed as described. From the same neuron one principal dendrite was selected in the MAP2 channel (by an experimenter “blind“ to the PLA signal) and straightened with the straighten plugin in ImageJ. The PLA signal was analyzed along the first 50 μm of dendrite as described above.
Statistical analysis
For quantification and testing of statistical significance, data are displayed as combined graphs from multiple experiments. When necessary, data were normalized to one condition to compare experiments with different treatments or time courses between multiple experiments as indicated in the figure legends. Background values from methionine controls were subtracted where indicated. Statistical significance was tested by ANOVA and Tukey’s range test on raw data or students t-test as indicated.
Image representation
For visualization, maximum intensity projections were either adjusted for brightness and contrast in ImageJ with the same settings for samples and controls, or the images were converted into outline/signal images on white background for better printability. Conversions of whole cell images in Supplementary Figure 4 were performed by overlaying MAP2 outline images and binary nucleus and PLA signal images in Adobe Photoshop CS5 (Adobe Systems Inc). The stylized images of the single channels were made by conversion of the single channel image of the maximum intensity projection into binary images in ImageJ with fixed thresholds within an experiment. The PLA signal was dilated by one pixel in the stylized whole cell images to ensure that the signal was visible. Single channel masks were outlined (MAP2) or colorized (DAPI, PLA) in Adobe Photoshop and combined. For white background closeups of somata and dendrites and for whole cell display of the Puro-PLA signal, the PLA signal was not thresholded but only adjusted for brightness and contrast with the same settings for sample and control. Where displayed, the neuron outline was created by converting the MAP2 channel into a binary image and creating an outline mask in ImageJ.
Western blot
For Western blot analysis Lcells, NCad-Venus Lcells and hippocampal neurons grown in 6 cm cell culture dishes were washed with PBS and lysed in 1.5 x LDS sample buffer (life technologies) supplemented with DTT (10 x reducing agent, life technologies) and protease inhibitors (Calbiochem). Lysate aliquots equivalent to 5 μg of total protein were loaded on 4 – 12 % BisTris NuPAGE gels (life technologies), separated by electrophoresis with MOPS running buffer (life technologies) and transferred to Immobilon-FL (Millipore) using the TurboBlot system (Biorad) at 25 V, 2.5 A for 7 min. Ponceau stain (Sigma) was used to validate homogeneous loading and transfer. Membranes were blocked for 1 h with Odyssey blocking buffer (Li-cor) and incubated overnight at 4 °C with primary antibodies in a 1 + 1 mixture of Odyssey blocking buffer and PBS containing 0.2 % Tween20. Membranes were rinsed several times with water followed by 2 × 10 min washes in PBS containing 0.2 % Tween20. Secondary antibodies coupled to IRdye680 and IRdye800 (Li-cor, 1:15.000) were applied for 1 h in a 1 + 1 mixture of Odyssey blocking buffer and PBS containing 0.2 % Tween20. Membranes were washed for 10 min in PBS containing 0.2 % Tween20 and 0.01 % SDS, 10 min in PBS containing 0.2 % Tween20 and rinsed several times in water before the signal was scanned using an Odyssey Infrared imager (Li-cor).
Antibodies and reagents
Unless noted otherwise all substances were molecular biology or cell culture grade and purchased from Sigma-Aldrich or Roth.
Antibodies were used in the following dilutions: ms-anti-biotin (Sigma, B7653, 1:1000), rb-anti-biotin monoclonal (Cell Signaling, 5597, 1:2000-1:5000), rb-anti-biotin (Bethyl, A150-109A, 1:5000), rb-anti-GFP (Abcam, ab290, 1:10.000–1:20.000), rb-Bassoon-Nterm (sap7f,28 1:2000 for Puro-PLA), rb-Bassoon-Cterm (Synaptic Systems, 141002, 1:2500 for Puro-PLA), ms-anti-Bassoon mab7f (Enzo, VAM-PS003, 1:1000 for FUNCAT-PLA), ms-anti-NCad (BD, 610921, 1:500–1:2000), rb-anti-LaminB (Abcam, ab16048, 1:1500), rb-anti-TGN38 (Sigma, 1:1000), rb-anti-Camk2a (Millipore, 04-1079, 1:1000), ms-anti-Camk2a (Thermo Scientific, MA1-048, 1:2000), ms-anti-Puromycin (Kerafast, EQ0001, 1:2500–1:3500), rb-anti-GluA1 (Abcam, ab31232, 1:500–1:1000), rb-anti-TrkB (Millipore, 07-225, 1:1000), Smi312 ms monoclonal antibody (Covance, SMI-312R, 1:5000), gp-anti-MAP2 (Synaptic Systems, 188004, 1:2000), Secondary antibodies for immunocytochemistry developed in goat were coupled to Alexa488, Cy5, Alexa594, Alexa647 or Alexa405 (life technologies) and all used 1:1000. Secondary antibodies for Western blot experiments were coupled to IRDye680 and IRDye800 (Li-cor, 1:15.000). Secondary antibodies used as PLA probes were developed in donkey and coupled to Duolink PLAplus and PLAminus oligos (Sigma; 1:5–1:10).
The following drugs and reagents were used from stock solutions: anisomycin (Tocris, stock 100 mM in DMSO, 40 μM final), cycloheximide (Sigma, stock 355 mM in MeOH prepared immediately before use, 355 μM final), puromycin dihydrochloride (Sigma, stock 90 mM in water, 1–3 μM final), TTX citrate (Tocris, 2 mM in H2O, 2 μM final), APV (Sigma, 100 mM in water, 50 μM final), DAPI (Roth, stock 1 mg/ml in water, 1 μg/ml final), TBTA (Sigma, stock 200 mM in DMSO, 200 μM final), TCEP-HCl (Thermo Scientific, 500 mM in water, freshly prepared), Biotin-alkyne (Jena Bioscience, stock 50 mM in DMSO, 25 μM final), CuSO4 (Sigma, 200 mM in water freshly prepared, 200 μM final).
Supplementary Material
Acknowledgments
We thank I. Bartnik, N. Fuerst and A. Staab for the preparation of cultured hippocampal neurons and M. Heumüller for help with analysis. E.M.S. is funded by the Max Planck Society, an Advanced Investigator award from the European Research Council, Deutsche Forschungsgemeinschaft (DFG) Collaborative Research Center (CRC) 902: Molecular Principles of RNA-based Regulation; DFG CRC 1080: Molecular and Cellular Mechanisms of Neural Homeostasis; and the DFG Cluster of Excellence for Macromolecular Complexes, Goethe University, Frankfurt and D.A.T is funded by US National Institute of Health grant R01 GM062523.
Footnotes
Author contributions
S.t.D.: designed, conducted and analyzed experiments and wrote the manuscript. L.K. designed, conducted and analyzed experiments and edited the manuscript. B.N.A.: conducted and analyzed experiments I.B.: conducted and analyzed experiments. S.B.: conducted experiments. S.G.: conducted experiments and analyzed experiments. K.M.: conducted experiments and analyzed experiments. T.M.: conducted experiments and analyzed experiments. C.H.: designed experiments and edited the manuscript D.A.T.: designed experiments and provided expertise E.M.S.: designed experiments and wrote the manuscript.
Competing financial interests
The authors declare no competing financial interests
References
- 1.Dieterich DC, Link AJ, Graumann J, Tirrell DA, Schuman EM. Proc Natl Acad Sci. 2006;103:9482–9487. doi: 10.1073/pnas.0601637103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dieterich DC, et al. Nat Neurosci. 2010;13:897–905. doi: 10.1038/nn.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.David A, et al. The Journal of cell biology. 2012;197:45–57. doi: 10.1083/jcb.201112145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt EK, Clavarino G, Ceppi M, Pierre P. Nat Methods. 2009;6:275–277. doi: 10.1038/nmeth.1314. [DOI] [PubMed] [Google Scholar]
- 5.Jarvius M, et al. Mol Cell Proteomics. 2007;6:1500–1509. doi: 10.1074/mcp.M700166-MCP200. [DOI] [PubMed] [Google Scholar]
- 6.Soderberg O, et al. Nat Methods. 2006;3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- 7.Pestka S. Annu Rev Microbiol. 1971;25:487–562. doi: 10.1146/annurev.mi.25.100171.002415. [DOI] [PubMed] [Google Scholar]
- 8.Zivraj KH, et al. J Neurosci. 2010;30:15464–15478. doi: 10.1523/JNEUROSCI.1800-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cajigas IJ, et al. Neuron. 2012;74:453–466. doi: 10.1016/j.neuron.2012.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgin KE, et al. J Neurosci. 1990;10:1788–1798. doi: 10.1523/JNEUROSCI.10-06-01788.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Femino AM, Fay FS, Fogarty K, Singer RH. Science. 1998;280:585–590. doi: 10.1126/science.280.5363.585. [DOI] [PubMed] [Google Scholar]
- 12.Battich N, Stoeger T, Pelkmans L. Nat Methods. 2013;10:1127–1133. doi: 10.1038/nmeth.2657. [DOI] [PubMed] [Google Scholar]
- 13.Gumy LF, et al. RNA. 2011;17:85–98. doi: 10.1261/rna.2386111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dresbach T, et al. J Biol Chem. 2006;281:6038–6047. doi: 10.1074/jbc.M508784200. [DOI] [PubMed] [Google Scholar]
- 15.Cohen LD, et al. PLoS One. 2013;8:e63191. doi: 10.1371/journal.pone.0063191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chudakov DM, Lukyanov S, Lukyanov KA. Nat Protoc. 2007;2:2024–2032. doi: 10.1038/nprot.2007.291. [DOI] [PubMed] [Google Scholar]
- 17.Lin MZ, Glenn JS, Tsien RY. Proc Natl Acad Sci U S A. 2008;105:7744–7749. doi: 10.1073/pnas.0803060105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- 19.Sutton MA, et al. Cell. 2006;125:785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 20.Hinz FI, Dieterich DC, Tirrell DA, Schuman EM. Acs Chemical Neuroscience. 2012;3:40–49. doi: 10.1021/cn2000876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schubert U, et al. Nature. 2000;404:770–774. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- 22.Barhoom S, et al. Nucleic Acids Res. 2013;41:e177. doi: 10.1093/nar/gkt686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SA, Tai CY, Mok LP, Mosser EA, Schuman EM. Proc Natl Acad Sci U S A. 2011;108:9857–9862. doi: 10.1073/pnas.1019003108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aakalu G, Smith WB, Nguyen N, Jiang C, Schuman EM. Neuron. 2001;30:489–502. doi: 10.1016/s0896-6273(01)00295-1. [DOI] [PubMed] [Google Scholar]
- 25.Banker G, Goslin K. Culturing nerve cells. MIT Press; 1990. [Google Scholar]
- 26.tom Dieck S, et al. Curr Protoc Cell Biol. 2012;Chapter 7(Unit7):11. doi: 10.1002/0471143030.cb0711s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Link AJ, Vink MK, Tirrell DA. Nat Protoc. 2007;2:1879–1883. doi: 10.1038/nprot.2007.268. [DOI] [PubMed] [Google Scholar]
- 28.tom Dieck S, et al. The Journal of cell biology. 1998;142:499–509. doi: 10.1083/jcb.142.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.