Abstract
In humans, circadian responses to light are thought to be mediated primarily by melanopsin-containing retinal ganglion cells, not rods or cones. Melanopsin cells are intrinsically blue-light sensitive, but also receive input from visual photoreceptors. We therefore tested in humans whether cone photoreceptors contribute to the regulation of circadian and neuroendocrine light responses. Dose-response curves for melatonin suppression and circadian phase resetting were constructed in subjects exposed to blue (460 nm) or green (555 nm) light near the onset of nocturnal melatonin secretion. At the beginning of the intervention, 555 nm light was just as effective as 460 nm light at suppressing melatonin, suggesting a significant contribution from the three-cone visual system (lambdamax 555 nm). During light exposure, however, the spectral sensitivity to 555 nm light decayed exponentially relative to 460 nm light. For phase-resetting responses, the effects of exposure to low irradiance 555 nm light were too large relative to 460 nm light to be explained solely by the activation of melanopsin. Our findings suggest that cone photoreceptors contribute substantially to non-visual responses at the beginning of a light exposure and at low irradiances, whereas melanopsin appears to be the primary circadian photopigment in response to long-duration light exposure and at high irradiances. These results are consistent with a non-redundant role for visual photoreceptors and melanopsin in mediating human non-visual photoreception and suggest that light therapy for circadian rhythm sleep disorders and other indications might be optimized by stimulating both the melanopsin- and cone-driven photoreceptor systems.
INTRODUCTION
In mammals, daily rhythms of sleepiness and alertness, physiology and metabolism, and gene expression are driven endogenously by neurons in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus. A small subset of retinal ganglion cells (RGCs) projects directly to the SCN and synchronizes the circadian timing system, ensuring that daily changes in behavior are timed appropriately with the solar cycle. Light-induced activation of SCN neurons also acutely suppresses pineal gland synthesis of the hormone melatonin, which is only released during the biological night. These non-visual light responses persist in humans with impaired or absent vision, suggesting that rod and cone photoreceptors are not required (1–4). In mice deficient in rod and cone function, non-visual light responses are mediated exclusively by intrinsically photosensitive RGCs (ipRGCs) that express the blue-light sensitive photopigment melanopsin (lambdamax ~480 nm) (5–9). In humans, circadian phase resetting, melatonin suppression, and objective measures of alertness are most sensitive to short-wavelength light, suggesting a primary role for melanopsin in regulating human non-visual light responses (10–14). Consistent with these findings, we recently reported that circadian, neuroendocrine, and neurobehavioral light responses to bright light were short-wavelength sensitive in a pair of blind individuals without rod and cone function (4). Hence, in the absence of visual photoreceptor signaling, melanopsin cells in the inner retina are sufficient to drive non-visual light responses (6,7,15–17).
In intact retinae, however, ipRGCs receive indirect synaptic input from rod and cone photoreceptors (18–20). Moreover, melanopsin null mice show intact phase resetting, melatonin suppression, and pupillary light responses; these responses are only abolished after also eliminating rod and cone signaling pathways (6,7,21,22). These findings suggest that melanopsin and visual photoreceptors are complementary in regulating non-image-forming responses. Nonetheless, in humans it is still widely assumed that cone photoreceptors play a marginal role, if any, in driving circadian and neuroendocrine light responses. Given that cone photoreceptors are more sensitive to light intensity and have more rapid, transient, response dynamics compared to the intrinsic melanopsin-driven RGC response (8,20), we hypothesized that it should be possible to determine the relative importance of the three-cone visual system by manipulating the irradiance and spectral content of light exposures. To test this hypothesis, we compared the relative effectiveness of retinal exposure to 460 nm versus 555 nm light, appearing blue and green to the normal human eye, respectively, at eliciting melatonin suppression and circadian phase-shift responses.
RESULTS
Short-wavelength shift in sensitivity for melatonin suppression in constant light
We measured melatonin suppression and phase shifting in young healthy subjects (ages 18–30 years) exposed to 6.5 h of continuous narrow-bandwidth short-wavelength (460 nm; n = 24) or longer-wavelength (555 nm; n = 24) light during the night (Fig. 1A). The 460 nm light was selected on the basis of the initially reported ~460 nm peak of spectral sensitivity for melatonin suppression in humans (10,14), whereas the 555 nm light stimulus was selected to activate the three-cone photopic visual system maximally. Fixed-irradiance light exposures were given to each individual near the onset of the melatonin rhythm using a modified Ganzfeld dome (Fig. 1B), with irradiance values spanning a 3-log unit range (half-peak bandwidth = 10–14 nm).
Fig. 1.

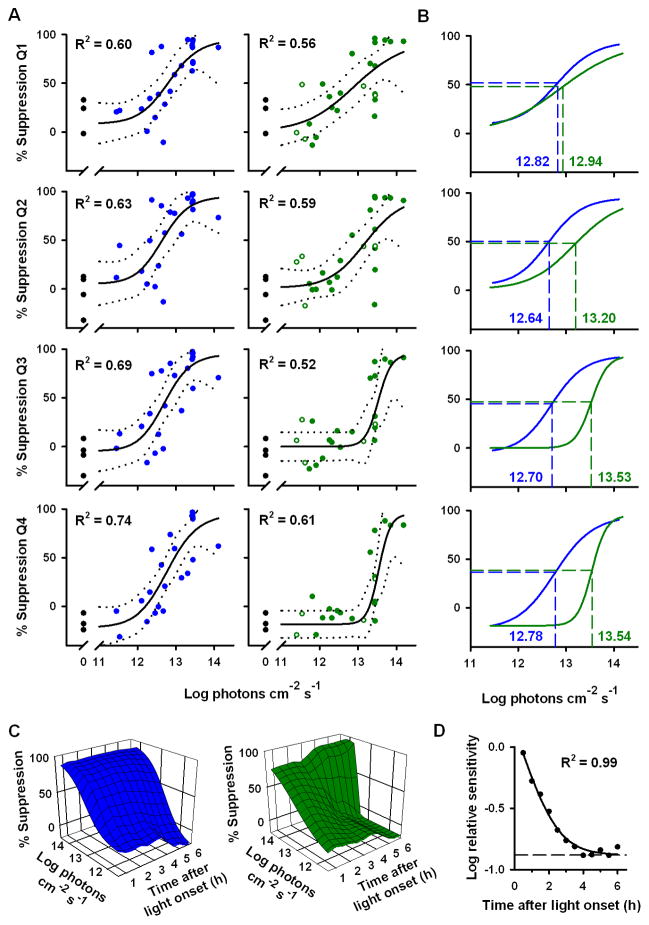
Protocol for assessing melatonin suppression and phase-shift responses. (A) Subjects participated in a 9-day inpatient protocol. White bars indicate exposure to ambient room light (<190 lux) and gray bars indicate exposure to dim ambient light (<3 lux). Black bars show scheduled sleep episodes in darkness (<0.002 lux), and the blue bar indicates the 6.5-h light intervention. (B) On the evening of day 6, each subject was exposed to 6.5 h of 460 nm or 555 nm light. The blue and green traces show the relative spectral content for a pair of representative light exposures to 460 nm and 555 nm light, respectively. The inset shows a frontal view of the modified Ganzfeld dome used to administer the light exposure. (C) Melatonin suppression and phase-shift responses are shown for two representative subjects exposed to 460 nm light (top traces), or 555 nm light (bottom traces) at 12.85 log photons cm−2 s−1. In each plot, black traces show melatonin on the day before the light exposure. In the left column, colored traces show melatonin suppression during the 6.5-h light exposure with open boxes marking the timing of the light intervention. In the right column, colored traces show the melatonin rhythm on the day after the light exposure, and drop lines indicate the timing of the dim light melatonin onset (clock time at which melatonin level exceeds 25% of the peak-to-trough fitted amplitude).
In most subjects, exposure to 460 nm light elicited a relatively constant amount of melatonin suppression during the light exposure, whereas exposure to 555 nm light elicited an initially strong suppression of melatonin which gradually recovered to baseline values even in the continued presence of light (Fig. 1C) (12). To determine the relative spectral sensitivity of melatonin suppression during the 6.5-h light intervention, we compared the log ED50 (effective dose 50%; irradiance required to elicit a half-maximal response) for the dose-response to 460 nm versus 555 nm light exposures in quarterly intervals (Fig. 2, A and B). During the first quarter, there was no difference in spectral sensitivity (Q1: F3,45 = 0.59, P = 0.62), whereas by the second quarter there was a relative decrease in sensitivity to 555 nm light compared to 460 nm light. In the third and fourth quarters of exposure, the log ED50 for the dose-response to 555 nm light was significantly higher than in response to 460 nm light (Q3, F3,45 = 6.67, P < 0.001; Q4, F3,45 = 9.21, P < 0.001) (Fig. 2B), indicating that short-wavelength light was much more effective than longer-wavelength light at suppressing melatonin in the latter half of the 6.5-h light exposure. To examine the kinetics of melatonin suppression sensitivity in greater detail, we constructed serial dose-response curves for exposure to 460 nm and 555 nm light in 30-min intervals across the 6.5-h light intervention (Fig. 2C). Compared to 460 nm light, the sensitivity of melatonin suppression to 555 nm light decayed exponentially (R2 = 0.99) with a half-life of 37.85 min (Fig. 2D). At the start of the light exposure, the log-relative sensitivity of melatonin suppression to 555 nm versus 460 nm light was essentially identical (−0.048 log units). By the fourth hour of exposure to continuous light, however, melatonin suppression sensitivity to 555 nm light was 0.88 log units lower compared to 460 nm light, which matches the predicted difference in log-relative sensitivity at these wavelengths for a vitamin A1-based photopigment with peak sensitivity to 481 nm light (23).
Fig. 2.
Melatonin suppression sensitivity to 460 nm light versus 555 nm light exposure. (A) Dose-response curves for melatonin suppression are shown in response to 460 nm light (left, blue circles) and 555 nm light (right, green circles) in quarterly intervals (Q1–Q4, top-to-bottom) across the 6.5-h light exposure. Closed and open circles show suppression of plasma or salivary melatonin, respectively, in individual subjects. Black traces show the best-fit dose-response curve with 95% confidence intervals. Black filled circles at 0 log irradiance show melatonin suppression in response to darkness. (B) The dose-response curves are overlaid, demonstrating a short-wavelength shift in spectral sensitivity during the light exposure. Horizontal dashed lines indicate the half-maximal melatonin suppression response, and vertical dashed lines show the corresponding log ED50 values which are labeled in each plot. (C) The dose-response for melatonin suppression to 460 nm light (left) remained relatively constant during the light exposure, whereas the dose-response to 555 light (right) exhibited a slow reduction in sensitivity across time (half-life = 37.85 min). (D) With increasing duration of light, the sensitivity of melatonin suppression to 555 nm light decayed exponentially relative to 460 nm light exposure. All data were analyzed in hourly bins and were plotted by midpoint of the binned data. The dashed trace at the lower asymptote (−0.88 log units) corresponds to the predicted difference in log-relative sensitivity at these wavelengths for a photoreceptor with peak sensitivity to 481 nm light.
Robust circadian phase shifting in response to longer-wavelength light
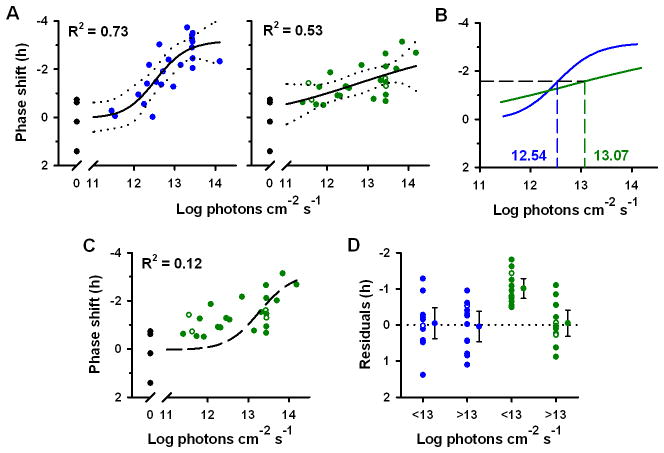
Following exposure to 460 nm light, phase shifts of the melatonin rhythm exhibited a nonlinear dose-response saturating at −3.19 h (R2 = 0.73; Fig. 3A). By comparison, the dose-response for phase resetting to 555 nm light did not appear to have the same shape (Fig. 3, A and B) and the slope was significantly different from the curve to 460 nm light (F1,48 = 10.17, P < 0.01). Given that the dose-response curves converged at lower irradiances (Fig. 3B), the log ED50 for phase shifting to 555 nm light tended to be higher than the response to 460 nm light, but the difference in log ED50 values did not reach statistical significance (0.53 log units; F1,48 = 2.94, P = 0.093). To test whether the dose-response to 555 nm light could be explained solely by a single-photoreceptor model, we fit a univariant curve to the data with the same slope as the dose-response to 460 nm light (10,14). The resulting curve-fit was poor (R2 = 0.12; Fig 3C), demonstrating that phase-shift responses to 555 nm light are better described by a dose-response curve in which the slope is not constrained (R2 = 0.53; Fig. 3B) or, perhaps, by a more complex model that incorporates combined photoreceptor drive from visual photoreceptors and melanopsin. In addition, phase-resetting responses at the 12 lowest irradiances tested (<13 log photons cm−2 s−1) were an hour greater than predicted compared to a forced univariant curve-fit (for lambdamax = 480 nm; −1.00 h ± 0.13 SEM; P < 0.001; one sample t-test), suggesting that phase-shifting responses to 555 nm light were not mediated by a single short-wavelength sensitive photopigment (Fig. 3D).
Fig. 3.
Circadian phase shifts in response to retinal exposure to 460 nm versus 555 nm light. (A) Dose-response curves for circadian phase resetting are shown in response to 6.5-h of 460 nm light (left, blue circles) versus 555 nm light (right, green circles) exposure. Closed and open circles show phase shifts of plasma or salivary melatonin, respectively, in individual subjects. Black traces show the best-fit dose-response curve with 95% confidence intervals. Black filled circles at 0 log irradiance show phase shifts in response to darkness. (B) The dose-response curves are overlaid, demonstrating a difference in relative spectral sensitivity across irradiance levels. The horizontal dashed line indicates the half-maximal phase-shift response, and vertical dashed lines show the corresponding log ED50 values, which are indicated on the plot. (C) Phase shifts in response to 555 nm light exposure did not match the best-fit univariant dose-response template (black dashed trace). (D) At low irradiances (<13 log photons cm−2 s−1; ~24 lux for 555 nm and ~2 lux for 460 nm light), phase-resetting responses to 555 nm light exposure were larger than predicted for a response mediated by melanopsin. Phase-shift residuals are shown relative to the predicted melanopsin-driven response, indicated by the dotted line. The predicted ‘melanopsin-only’ response to 555 nm light exposure was derived by translating the dose-response curve to 460 nm light by the predicted difference in log-relative sensitivity at these wavelengths for a photopigment with peak sensitivity to 480 nm light. For each group, the mean is shown with 95% confidence intervals.
DISCUSSION
Previous studies in mice demonstrated that classical visual photoreceptors are sufficient to entrain the circadian system in the absence of melanopsin (21,22). To date, however, studies of circadian photoreception in humans have failed to identify a prominent role for cone photoreceptors. We utilized the differential response of melanopsin and cone photoreceptors to the irradiance, duration, and spectral content of light to evaluate their relative roles in this process. Our data indicate that cone photoreceptors contribute substantially to circadian photoreception for short-duration or low-irradiance light exposures, whereas short-wavelength sensitive melanopsin cells dominate circadian responses to longer-duration and high-irradiance light exposures.
Cones and melanopsin contribute differentially to melatonin suppression
We found that the sensitivity of melatonin suppression to 555 nm light decayed exponentially relative to 460 nm light during the course of a 6.5-h light exposure. At the beginning of the intervention, melatonin suppression was just as sensitive to 555 nm light as 460 nm light, suggesting a substantial contribution from the photopic visual system. By the fourth quarter of light exposure, however, the difference in log-relative sensitivity at these wavelengths was consistent with a melanopsin-only response (−0.88 log units, lambdamax 481 nm). On the basis of this short-wavelength shift in spectral sensitivity, we hypothesize that cone photoreceptors provide for temporary suppression of the melatonin rhythm, whereas melanopsin signals light information continuously across long-duration exposure to light. Consistent with this interpretation, a blind individual with no detectable rod or cone function showed a constant level of melatonin suppression across a 6.5-h exposure to 460 nm light, whereas 555 nm light did not suppress melatonin at all (4). Our findings are also similar to ‘negative masking’ responses in mice in which visual photoreceptors drive temporary inhibition of locomotor activity in continuous white light, whereas melanopsin is required for sustained activity suppression throughout a 3-h light exposure (24). Parallel findings have been reported for the pupillary light reflex in humans and non-human primates in response to short-duration exposure (<10 min); visual photoreceptors contribute to pupillary constriction initially, whereas longer steady-state responses and post-stimulus constriction appear to be mediated primarily by melanopsin (25,26). Finally, in response to a short-duration light stimulus (1 min or 5 min), mice that lack mid-wavelength-sensitive cones show attenuated phase shifting compared to wild-type mice, but normal circadian responses when the light duration is increased (15 min) (27). Collectively, these studies suggest that the relative contribution of cones to non-visual light responses decreases with increasing duration of light, consistent with the present findings in humans. These data, however, are inconsistent with previous reports in humans showing that melatonin suppression is predominantly short-wavelength sensitive for light exposures ≤ 90 minutes in duration (10,14). These dissimilar findings may be due to methodological differences such as the circadian phase of the light exposure, the light conditions preceding the light intervention, the method of assessing melatonin suppression, and/or the method of fitting and comparing dose-response models.
Although our results are consistent with a gradual reduction in the contribution of cones in driving melatonin suppression, the time-course was much longer than that predicted for light adaptation of cone photoreceptors in constant light. Given that we administered the light stimulus near the onset of nocturnal melatonin secretion, the sluggish decay in sensitivity that we observed could be mediated, in part, by simultaneous phase-delay shifting of the melatonin rhythm, as this would delay the recovery of melatonin to baseline values (28,29). Other physiologic processes that could contribute to the slow time-course of recovery include light adaptation of the melanopsin cells (30,31), the spectral sensitivity and kinetics of melanopsin photoisomerase activity (see below), or complex interactions between melanopsin cells and visual photoreceptors that have yet to be fully elucidated (32). Alternatively, the decay in melatonin suppression sensitivity during the night could reflect a circadian decline in the contribution of cone photoreceptors. Therefore, in future studies it will be important to examine the kinetics of other non-visual light responses in constant light and at other circadian phases.
Similar to invertebrate opsins, melanopsin photopigment is thought to function as both a photoreceptor and photoisomerase (33–35). That is, after activation of melanopsin by light, a different portion of the light spectrum regenerates the chromophore and restores melanopsin photosensitivity. Whereas melanopsin phototransduction is most sensitive to short-wavelength light, recent studies suggest that melanopsin photoisomerase activity may be more sensitive to longer-wavelength light (26,36,37). We found, however, that exposure to 460 nm alone could maintain melatonin suppression for at least 6.5 h, despite the absence of exposure to any other wavelengths of light that could potentially interconvert ‘meta’-melanopsin back to its photosensitive form. Short-wavelength light may therefore be sufficient to elicit melanopsin photoisomerase activity, even if long-wavelength light is more efficient at restoring photoreceptor function. Alternatively, it is possible that light elicits continuous phototransduction in the melanopsin cells through another biochemical pathway, independent of photoisomerase activity (37).
Cones contribute to circadian phase shifting at low irradiances
Here, we demonstrated that dose-response curves for circadian-phase resetting to 555 nm light versus 460 nm light did not fit a univariant model (i.e., the curves were not parallel), suggesting that multiple photoreceptor classes mediate human circadian light responses. This inference is derived from the Principle of Univariance, which specifies that for a response driven by a single class of photoreceptor, dose-response curves to different wavelengths of light have the same shape, but differ in relative sensitivity (i.e., the log ED50 value) (38). Since melanopsin photopigment may exist in two spectrally distinct states, we cannot rule out the possibility that melanopsin bistability contributed to the non-univariant behavior we observed for circadian-phase resetting (Fig. 3). We consider this possibility unlikely, however, given that the action spectra for melanopsin-driven physiologic responses appear to conform to a univariant photoreceptor model in mice without rod and cone function (6,17). Our findings are consistent with an irradiance-dependent shift in spectral sensitivity of the human circadian system due to convergent input from visual photoreceptors and melanopsin. Circadian phase shifts were more sensitive to 460 nm light compared to 555 nm light at high irradiances, suggesting a primary role for short-wavelength light-sensitive melanopsin cells. As irradiance was decreased, however, dose-response curves to 555 nm and 460 nm light converged such that phase-shift responses to longer-wavelength light were much greater than would be predicted for a melanopsin-only response, suggesting that cones preferentially contribute to circadian photoreception at low irradiance levels. This interpretation is consistent with the higher sensitivity of cone photoreceptors to light compared to melanopsin-containing ganglion cells (8,20), the spectral sensitivity of SCN neurons to light pulses in the photopic range (39), the preservation of phase resetting in melanopsin null mice (6,7,22), and the long-wavelength shift in spectral sensitivity for phase-shift responses in mice with intact vision compared to animals without rod and cone function (6,40). Parallel findings have also been reported for the pupillary light reflex in mice, which is mediated primarily by melanopsin cells at high irradiances and classical visual photoreceptors at low irradiances (6,17,41). Thus, our results extend previous findings in lower mammals and establish a role for visual photoreceptors in human circadian photoreception.
Technical Considerations
In an article published while this study was in progress, the spectral sensitivity of human melanopsin cells was defined by examining sustained pupillary constriction after the offset of a light stimulus (25). The fitted peak in spectral sensitivity was 482 nm, which is consistent with the spectral tuning of melanopsin cells in the macaque and in lower mammals (8,20). Therefore, the 460 nm light stimulus that was used in the present study likely elicited strong, but sub-maximal, stimulation of the melanopsin cells. Similarly, although 555 nm light is best at stimulating the photopic visual system, which is dominated by middle- and long-wavelength-sensitive cones, short-wavelength-sensitive cones respond maximally to ~420 nm light and provide input to the melanopsin cells (20). Hence, the short-wavelength stimulus that we used does not provide for complete isolation of the melanopsin-driven response, and we cannot rule out the possibility that rod photoreceptors contributed to the responses. Likewise, the use of 555 nm light does not completely isolate cone function, as melanopsin cells can respond to longer-wavelength light when the irradiance is sufficiently high (8,20). We hypothesize that in our studies high irradiance 555 nm light (~14 log photons cm−2 s−1) was able to suppress melatonin completely near the end of the 6.5-h exposure primarily by activating melanopsin, rather than cone photoreceptors (Fig. 2). This hypothesis could be tested in blind humans with intact circadian photoreception [i.e. in the absence of rod and cone input (1,2,4)] to determine whether high intensity 555 nm light is sufficient to elicit saturating non-visual light responses.
In the present study, we did not examine the potential role of spectral opponency in regulating non-visual responses, as individual subjects were exposed to single narrow-bandwidth stimuli. Although weak spectral opponent responses have been reported for melatonin suppression (42,43), other studies suggest that polychromatic light may be as effective, if not more effective, than blue-enriched light at eliciting circadian responses (44–46). Despite opposing viewpoints on whether adding longer-wavelength light to a short-wavelength stimulus enhances or inhibits circadian responses, these studies are nonetheless consistent with the view that human circadian and neuroendocrine responses receive convergent input from cone photoreceptors. Hence, in future studies it will be important to determine how the human circadian photoreceptor system integrates and processes complex polychromatic light spectra, especially for light sources commonly used at home and in occupational settings, and for lamps used in clinical light therapy applications.
In contrast to results for circadian phase-resetting, the slopes of the dose-response curves for melatonin suppression to 460 nm versus 555 nm light did not differ significantly during any quarter of the light exposure (Fig. 2; F3,45 < 2.14, P > 0.15). That these functions can be fit by a univariant model does not contradict our hypothesis that cone photoreceptors and melanopsin contribute to melatonin suppression at the start of the light exposure. Early analytic action spectra studies suggested that phase resetting in wild-type animals was consistent with a univariant response (40,47,48), yet it is now well-established that visual photoreceptors and melanopsin contribute to circadian responses in mice (6,7). It is possible that with larger sample sizes and with adequate sampling across irradiance values, a difference in shape for dose-response curves would emerge for melatonin suppression similar to that described for pupillary constriction in rodless/coneless mice versus melanopsin knockout mice (41). Our ability to assess accurately melatonin suppression at the start of the light exposure was limited, in part, by small inter-individual differences in the timing of melatonin onset and by the sampling rate of plasma melatonin (every 20 min). This variability was minimized by binning melatonin suppression results over the first hour or more (Fig. 2), but we were unable to compare dose-response curves within the first few minutes of the light exposure when the difference in slopes would be expected to be greatest.
Implications for light therapy
Our findings may have important implications for the development and optimization of light therapies for a number of disorders, including circadian rhythm sleep disorders (49,50), seasonal affective disorder (SAD) (51,52), and dementia (53), and the use of light as an alerting stimulus to counter the sleepiness associated with misalignment of circadian phase, particularly during night shift work (11,13,54). To maximize the therapeutic potential of light therapy, our findings suggest that it may be important to manipulate the spectrum, duration, and pattern of light dynamically to best stimulate both the melanopsin- and cone-driven photoreceptor systems. Our results indicate that in order to optimize light therapy interventions, it will be critical to understand the temporal dynamics of the responses of the non-visual photoreceptor system. Doing so holds the promise of reducing light therapy duration and intensity, thus possibly improving patient compliance and safety (51,52). The optimal spectral composition of light for treating SAD and other psychiatric disorders, however, remains undetermined and it has yet to be shown whether light therapy improves mood through the same sets of photoreceptors that mediate circadian phase resetting and melatonin suppression (51,52,55).
Based on the short-wavelength sensitivity of melanopsin, it has been hypothesized that phototherapy can be optimized by using predominantly short-wavelength blue light. Our results indicate that short-duration (<90 min) retinal exposure to narrow-bandwidth 555 nm light (≤24 lux) may be as effective, if not more effective, than an equivalent photon dose of 460 nm light (≤2 lux). Hence, the use of mid-wavelength narrow-bandwidth light early in the exposure period may improve treatment response. Alternatively, assuring that longer-wavelength green light is included in white polychromatic light therapy may be important to an optimal response. Such approaches deserve comparative testing in patients known to respond to light therapy. Our data also raise the possibility that activation of cone photoreceptors in the late evening by relatively low illuminance light sources such as LCD monitors, table lamps, and dimmable lamps may delay the circadian clock and therefore contribute to the high prevalence of delayed sleep phase disorder (29,56). Finally, blocking short-wavelength light with blue-blocking goggles may not always be effective in preventing undesired circadian responses (57) based on our finding that longer-wavelength light is able to induce robust phase shift responses.
Designing light therapy to optimally activate melanopsin ganglion cells and visual photoreceptors may be particularly important in a restricted light environment where bright light may not be available, for example in submarines, during space and polar missions, or other poorly-lit control rooms, institutions, or environments. Therefore, in the context of everyday life, in which humans are exposed to diverse and variable sources of lighting that vary in irradiance, duration, and spectral content, we hypothesize that the relative contributions of cone photoreceptors and melanopsin to non-visual light responses vary depending on the nature of the light exposure. The adaptive nature of circadian and neuroendocrine photoreception appears to be analogous to other major sensory systems in mammals such as image-forming vision and touch, in which multiple receptor subtypes respond differentially to the strength, frequency, and timing of stimuli in order to ensure appropriate physiologic responses.
MATERIALS AND METHODS
Subjects
Healthy research subjects (n = 66), ages 18–30 years were enrolled in a 9-day inpatient study at the Intensive Physiologic Monitoring Unit (IPM), Brigham and Women’s Hospital (BWH; Boston, MA). Physical health was assessed by medical history, physical examination, blood biochemistry and hematology, and electrocardiogram, and mental health was evaluated by interview with a staff psychologist/psychiatrist. Normal sight was confirmed by an ophthalmologic examination and the Ishihara Test for color blindness. Sleep and circadian rhythm disorders were exclusionary. For at least two weeks prior to being admitted to the IPM, subjects were required to maintain a regular sleep-wake schedule (8 h sleep, 16 h wake), which was verified by continuous actigraphy monitoring (Actiwatch-L; Minimitter, Inc.). A comprehensive toxicology screen was performed on the day of admission to the IPM to ensure that subjects had refrained from the use of drugs. Of the 66 subjects who were enrolled, eight subjects were discontinued prior to being randomized to the experimental light exposure. Four subjects were omitted from the analysis due to equipment failure and subsequent data loss during the light intervention, and two subjects were excluded post hoc because the light exposure was administered at an inappropriate circadian phase (>3.0 h from melatonin onset). Results from 16 subjects were reported previously by Lockley et al. 2003 (12). Informed consent was obtained from all subjects, and research procedures were approved by the Institutional Review Board at BWH and were in compliance with HIPAA regulations and the Declaration of Helsinki.
Protocol Design
Subjects lived individually for 9 days (Fig. 1) in an environment free of time cues. During the first three days, subjects were scheduled to sleep and wake at their regular pre-study sleep-wake times (8 h sleep, 16 h wake). Ambient light was provided by 4100K fluorescent lamps (Philips Lighting). Subjects lived in room light (<190 lux, 0.48 W/m2 measured in the horizontal plane at 183 cm) until midway through day 3, after which the light was dimmed to <3 lux (<0.01 W/m2) for the remainder of the study. After awakening on day 4, subjects underwent a 50-h constant routine procedure consisting of wakefulness enforced by technician monitors, semi-recumbent bed rest, and consumption of hourly equi-caloric snacks (58). Following an 8-h sleep opportunity, subjects awoke in the evening and were administered a 6.5-h narrow-bandwidth light exposure in a modified Ganzfeld dome (10,12,13). For the light exposure (day 6), a between-subjects design was used in which subjects were assigned to one of two wavelength conditions (460 nm or 555 nm). In each group, subjects were randomized to 16 irradiances across a broad range of photon densities (2.52 × 1011 − 1.53 × 1014 photons cm−2 s−1). These photon densities correspond to approximate illuminances of 0.04 to 27 lux for the 460 nm stimulus and 0.6 to 375 lux for the 555 nm stimulus. Narrow-bandwidth light (10–14 nm half-peak bandwidth) was generated by a Xenon arc lamp and grating monochromator, and the wavelength and bandwidth were verified by measurement with a PR-650 SpectraColorimeter (PhotoResearch Inc.). Before the onset of the light exposure, one drop of 0.5% cyclopentolate HCl was administered in each eye to dilate the pupils (Cyclogyl; Alcon Laboratories, Inc.). Head position was fixed by a chinrest, and subjects stared at the light continuously for 90 min at a time, followed by a 10-min break during which they could look elsewhere in the otherwise dark room. Subjects were asked to refrain from photophobic behavior (e.g., squinting or closing of the eyes) and compliance was monitored by a technician. The light was measured every 30–60 min at eye-level with an IL1400 radiometer and SEL-033/F/W detector (International Light Inc.) to ensure constant irradiance throughout the light exposure. For each wavelength of light, subjects were randomized to an irradiance level just prior to administration of the light exposure. Following completion of the light exposure and an 8-h sleep opportunity, subjects underwent a second constant routine for 30 h. After recovery sleep, subjects awoke on day 9 at their habitual wake time and were discharged from the study.
Specimen collection and melatonin assays
On day 2 of the study, an indwelling intravenous catheter was inserted in a forearm vein to allow for continuous collection of blood during both sleep and wake episodes. During sleep episodes, the constant routine procedures, and the light exposure session, blood was drawn from outside the research suite through a porthole in the bedroom wall. Blood was sampled every 30 min during the constant routine procedures, and every 20 min during the 6.5-h light exposure. Saliva samples were collected hourly during the constant routines and the light intervention, and sample times were digitally time-stamped using a Termiflex system (Warner Power Termiflex, Warner, NH). Melatonin concentration was determined by double-antibody radioimmunoassay with the Kennaway G280 antiserum (59) by a laboratory blind to condition (Dr. V. Ricchiuti, BWH GCRC Core Laboratory, Boston, MA). The plasma melatonin intra-assay coefficient of variation (CV) was 10.0% at 1.9 pg ml−1 and 7.2% at 21.9 pg ml−1, and the inter-assay CV was 12.65% at 3.06 pg ml−1 and 12.12% at 22.36 pg ml−1. The saliva melatonin intra-assay coefficient of variation (CV) was 4.1% at 3.56 pg ml−1 and 4.8% at 24.2 pg ml−1, and the inter-assay CV was 12.15% at 2.37 pg ml−1 and 10.20% at 19.58 pg ml−1.
Melatonin suppression and phase-shift responses
To determine percent suppression of melatonin, the area under the curve (AUC, trapezoidal method) was calculated for melatonin during the 6.5-h light exposure (AUCLE), and compared to the AUC for the melatonin rhythm during the preceding constant routine at the same relative clock times (AUCCR1). Thus, percent melatonin suppression was calculated as [1 − (AUCLE)(AUCCR1)−1] × 100, whereby higher values indicated stronger suppression of the melatonin rhythm. In five subjects from the 555 nm group, salivary melatonin was used to determine melatonin suppression because there was an insufficient number of blood samples collected during either the constant routine or light exposure. In some subjects, a small negative percent melatonin suppression value was found, which indicated that melatonin levels during the light intervention were slightly higher than those observed during the preceding constant routine. To determine the magnitude of phase-shift responses, the pre-light exposure melatonin rhythm during the first constant routine procedure was fit by a 3-harmonic regression model to estimate the amplitude. The dim light melatonin onset (DLMOn25%) was defined as the clock time at which the melatonin rhythm crossed a threshold value of 25% of the peak-to-trough fitted amplitude (half the standard amplitude). The phase-shift of the melatonin rhythm was calculated as the difference in the timing of the DLMOn25%, measured before and after the light exposure intervention using constant routine procedures (days 5 and 7). Phase-shifts were determined from plasma melatonin in 46 subjects, and from salivary melatonin in 6 subjects (460 nm, n = 2; 555 nm, n = 4) because of blood sampling difficulties. By convention, phase delays are indicated by negative values, and phase advances by positive values.
Construction of dose-response curves
Dose-response curves were fit with a sigmoidal four-parameter logistic regression model wherein y0 is the minimum response, a is the difference between the maximum and minimum response, x0 is the irradiance that elicits a half-maximal response (the ED50 value), and b is the slope parameter:
To determine the set of dose-response curve parameters that resulted in the minimal sum of squares of the residuals, the Levenberg-Marquardt method was used (SigmaPlot 11, Systat Software, Inc.). The residuals were normally distributed, as determined by the Shapiro-Wilk test for normality (for all dose-response curves, W > 0.93 and P > 0.05). A global curve-fitting procedure was used to determine the best-fit shared maximum and minimum phase-resetting responses to 460 nm light and 555 nm light exposures. Maximum and minimum phase shifts were −3.19 h and 0.034 h, respectively. These values correspond closely to the saturating phase-shift response to bright polychromatic white light reported previously (−3.24 h) (29), and the average phase shift in response to 6.5 h of darkness measured in the present study (0.062 h; n = 4). The maximum melatonin suppression response was constrained to 95%, as in our experience melatonin suppression assessed by AUC rarely exceeds this threshold.
To examine the dose-response of melatonin suppression across time, we constructed dose-response curves in quarterly (1 quarter = 97.5 min) and 1-h bins across the 6.5-h light intervention. In the latter analysis, the onset of each bin was spaced at 30 minute intervals, resulting in 12 serial dose-response curves. Thus, successive bins overlapped by 30 min each, allowing for smoothing of the data across time. When a melatonin sample did not occur precisely at the onset or offset of a bin, the concentration of melatonin was interpolated linearly from the samples that bracketed the given time-point. For each set of dose-response curves shown in Fig. 2C, the log-relative sensitivity in Fig. 2D was determined by subtracting the log ED50 for the dose-response to 555 nm light exposure from the log ED50 for the dose-response to 460 nm light exposure. The reduction in relative sensitivity across time was modeled by a three-parameter exponential decay function, which was used to calculate the half-life of the difference in relative sensitivity for melatonin suppression in response to 555 nm versus 460 nm light exposure.
Data analysis and statistics
The extra sum-of-squares F-test was used to compare dose-response models. This F-test allows for comparison of nested models which have a different number of parameters (60). To test whether the log ED50 or slope parameter differed significantly between dose-response curves, we performed a global curve-fit in which the best-fit value for each parameter was shared for dose-response curves to 555 nm light versus 460 nm light exposures. The F-test was used to determine whether the more complicated model with more parameters (i.e., the model with unshared log ED50 or slope) resulted in a significant improvement in the difference in sum-of-squares, as compared to the simpler model with fewer parameters (i.e., the model with shared log ED50 or slope).
To test whether phase-shift responses to 555 nm light exposures were higher than expected for a response mediated by melanopsin, we first derived the predicted absorption spectrum for a vitamin A1-based photopigment with peak sensitivity to 480 nm light exposure using a nomogram procedure (23). The predicted univariant dose-response curve to 555 nm light exposure was determined by translating the dose-response curve to 460 nm light by the difference in log-relative sensitivity to 555 nm versus 460 nm light exposure for the absorption spectrum template (−0.91 log units). The observed phase-shift responses to 555 nm light exposure were compared to the predicted melanopsin-driven responses (lambdamax = 480 nm) by performing a one-sample t-test on the residuals (H0 = means of residuals = 0; HA = mean of residuals ≠ 0).
Acknowledgments
We thank research volunteers, subject recruiters, and research staff at the Division of Sleep Medicine, BWH; R. Todesco (BWH) for administrative support; J. M. Ronda, M.S. (BWH) for technical support; J. Hanifin and W. Coyle (TJU) for technical support for the generation of monochromatic light; V. Ricchiuti, Ph.D. and the Core Laboratory staff (BWH) for melatonin assays; and M. St. Hilaire (BWH) for helpful discussions of the data.
Funding: NIH grants T32-HL07901 (J.J.G.), MH45130 (C.A.C), NS36590 (G.C.B.), and AT002129 (S.W.L.). G.C.B., C.A.C., and S.W.L. are supported in part by the National Space Biomedical Research Institute through NASA NCC 9-58. The project described was supported by grant M01 RR02635, Brigham and Women’s Hospital General Clinical Research Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Competing interests
J.J.G.: conference travel support, Apollo Lighting. S.M.R.: research funding from Philips Lighting; Vanda Pharmaceuticals; ResMed Foundation; Respironics Sleep and Respiratory Research Foundation; Cephalon Inc.; and Takeda Pharmaceuticals North America. G.C.B.: research funding from Philips Lighting BV; OSRM/Sylvania; Apollo Health; process patents for use of short-wavelength light for resetting the human circadian pacemaker and improving alertness and performance, assigned to Thomas Jefferson University and Brigham and Women’s Hospital. R.E.K.: process patents relating to the use of light to reset human circadian rhythms, assigned to Brigham and Women’s Hospital. C.A.C: Funding:NASA, NIH, National Institute for Occupational Safety and Health–Centers for Disease Control and Prevention, National Space Biomedical Research Institute, and Department of Homeland Security’s Federal Emergency Management Agency; Financial relationships: consulting fees from or served as a paid member of scientific advisory boards for Actelion Ltd., Bombardier Inc., Cephalon Inc., Delta Airlines, Eli Lilly and Co., Fedex Kinko’s, Federal Motor Carrier Safety Administration, U.S. Department of Transportation; Fusion Medical Education LLC, Garda Síochána Inspectorate (Dublin, Ireland), Hypnion Inc. (acquired by Eli Lilly and Co. in April 2007), Global Ground Support, Johnson & Johnson, Koninklijke Philips Electronics, N.V., Morgan Stanley, Sanofi-Aventis Groupe, Portland Trail Blazers, Respironics Inc., Sepracor Inc., Sleep Multimedia Inc., Sleep Research Society (for which he served as president), Somnus Therapeutics Inc., Takeda Pharmaceuticals, Vanda Pharmaceuticals Inc., Vital Issues in Medicine, Warburg-Pincus, and Zeo Inc. He owns an equity interest in Lifetrac Inc.; Somnus Therapeutics Inc.; Vanda Pharmaceuticals Inc.; and Zeo Inc. and received royalties from McGraw Hill, the New York Times, and Penguin Press. He has received lecture fees from the Accreditation Council of Graduate Medical Education; Alfresa; the American Academy of Allergy, Asthma and Immunology Program Directors; American Physiological Society; Association of University Anesthesiologists; Baylor College of Medicine; Beth Israel Deaconess Medical Center; Brown Medical School–Rhode Island Hospital; Cephalon Inc.; Clinical Excellence Commission (Australia); Dalhousie University; Duke University Medical Center; Harvard School of Public Health, Harvard University; Institute of Sleep Health Promotion; London Deanery; Morehouse School of Medicine; Mount Sinai School of Medicine; National Emergency Training Center Federal Emergency Management Agency; NIH; North East Sleep Society; Osaka University School of Medicine; Partners HealthCare Inc.; Sanofi-Aventis Inc.; St. Lukes Roosevelt Hospital; Takeda; Tanabe Seiyaku Co. Ltd.; Tokyo Electric Power Company; University of Michigan; University of Pennsylvania; University of Pittsburgh; University of Tsukuba; University of Virginia Medical School; University of Washington Medical Center; University of Wisconsin Medical School; and World Federation of Sleep Research and Sleep Medicine Societies. He has also received research prizes with monetary awards from the American Academy of Sleep Medicine, American Clinical and Climatological Association, Association for Patient-Oriented Research, National Institute for Occupational Safety and Health, National Sleep Foundation, and Sleep Research Society; clinical trial research contracts from Cephalon Inc., Merck & Co. Inc., and Pfizer Inc.; and an investigator-initiated research grant from Cephalon Inc. His research laboratory at the Brigham and Women’s Hospital has received unrestricted research and education funds and/or support for research expenses from Cephalon Inc.; Koninklijke Philips Electronics, N.V.; ResMed; and the Brigham and Women’s Hospital. The Harvard Medical School Division of Sleep Medicine (HMS/DSM), which he directs, has received unrestricted research and educational gifts and endowment funds from Boehringer Ingelheim Pharmaceuticals Inc.; Cephalon Inc.; George H. Kidder, Esq.; Gerald McGinnis; GlaxoSmithKline; Herbert Lee; Hypnion; Jazz Pharmaceuticals; Jordan’s Furniture; Merck & Co. Inc.; Peter C. Farrell, Ph.D.; Pfizer; ResMed; Respironics Inc.; Sanofi-Aventis Inc.; Sealy Inc.; Sepracor Inc.; Simmons; Sleep Health Centers LLC; Spring Aire; Takeda Pharmaceuticals; and Tempur-Pedic. The HMS/DSM has received gifts from many outside organizations and individuals, including Axon Sleep Research Laboratories Inc.; Boehringer Ingelheim Pharmaceuticals Inc.; Catalyst Group; Cephalon Inc.; Clarus Ventures; Eli Lilly and Co.; Farrell Family Foundation; Fisher & Paykel Healthcare Corporation; George H. Kidder, Esq.; GlaxoSmithKline; Hypnion Inc.; Jordan’s Furniture; Merck Research Laboratories; Park Place Corporation; Respironics Inc.; Sanofi-Aventis Inc.; Select Comfort Corporation; Sepracor Inc.; Sleep Health Centers LLC; Takeda Pharmaceuticals; Tempur-Pedic Medical Division; Total Sleep Holdings; and Vanda Pharmaceuticals Inc. The HMS/DSM Sleep and Health Education Program has received Educational Grant funding from Cephalon Inc.; Takeda Pharmaceuticals; Sanofi-Aventis Inc.; and Sepracor Inc. He is the incumbent of an endowed professorship provided to Harvard University by Cephalon Inc. and holds a number of process patents in the field of sleep or circadian rhythms (for example, photic resetting of the human circadian pacemaker). Since 1985, he has also served as an expert witness on various legal cases related to sleep and/or circadian rhythms. Patents: inventor on several patents related to assessment and modification of the phase and amplitude of the endogenous circadian rhythm, apparatus for delivering high-intensity light to modify circadian rhythms, a method to modify circadian rhythms and enhance alertness and performance with short wavelength light, and a test for evaluating visual function in visually impaired people, which are assigned to Brigham and Women’s Hospital; and a patent on a wrist-worn activity monitor that monitors exposure to light of different wavelengths, which is assigned to Philips Respironics. S.W.L has received consulting fees from Apollo Lighting and holds a consulting contract with Wyle Integrated Science and Engineering (NASA) to complete an evidence review. He is/was a consultant on federally-funded projects at Brigham and Women’s Hospital, Thomas Jefferson University and Warwick Medical School. He has received lecture fees from Takeda Pharmaceuticals North America and I Slept Great/Euforma, LLC; unrestricted equipment gifts from ResMed Inc, Philips Lighting and Bionetics Corporation; an unrestricted monetary gift to support research from Swinburne University of Technology, Australia; an advance author payment from Oxford University Press, and honoraria from Servier Inc. for writing an article for Dialogues in Clinical Neuroscience and from AMO Inc., for writing an educational monograph, neither of which refer to the companies’ products; honoraria and/or travel and accommodation support for invited seminars, conference presentations or teaching from 2nd International Symposium on the Design of Artificial Environments; Apollo Lighting; Bassett Research Institute; Canadian Sleep Society; Committee of Interns and Residents; Coney Island Hospital; FASEB; Harvard University; Illinois Coalition for Responsible Outdoor Lighting; International Graduate School of Neuroscience; Japan National Institute of Occupational Safety and Health; Lightfair; National Research Council Canada; New York Academy of Sciences; North East Sleep Society; Philips Lighting; Thomas Jefferson University; University of Montreal; University of Tsukuba; University of Vermont College of Medicine; Utica College; Velux; Woolcock Institute of Medical Research; investigator-initiated research grants from Respironics Inc., Philips Lighting, Apollo Lighting and Alcon Inc. and two investigator-initiated research grants from the ResMed Foundation. Dr. Lockley holds a process patent for the use of short-wavelength light for resetting the human circadian pacemaker and improving alertness and performance which is assigned to the Brigham and Women’s Hospital per Hospital policy. He has also received revenue from a patent on the use of short-wavelength light which is assigned to the University of Surrey. Dr. Lockley has also served as a paid expert witness on behalf of two public bodies on arbitration panels related to sleep, circadian rhythms and work hours.
Footnotes
Author contributions: J.J.G.: study design, data collection, data analysis, and manuscript preparation; S.M.R.: study design, data collection, and manuscript preparation; G.B.C.: study design and manuscript preparation; R.E.K.: study design and data analysis; C.A.C.: study design and manuscript preparation; S.W.L.: study design, data collection, data analysis, and manuscript preparation.
REFERENCES AND NOTES
- 1.Czeisler CA, Shanahan TL, Klerman EB, Martens H, Brotman DJ, Emens JS, Klein T, Rizzo JF., III Suppression of melatonin secretion in some blind patients by exposure to bright light. N Engl J Med. 1995;332:6–11. doi: 10.1056/NEJM199501053320102. [DOI] [PubMed] [Google Scholar]
- 2.Klerman EB, Shanahan TL, Brotman DJ, Rimmer DW, Emens JS, Rizzo JF, III, Czeisler CA. Photic resetting of the human circadian pacemaker in the absence of conscious vision. J Biol Rhythms. 2002;17:548–555. doi: 10.1177/0748730402238237. [DOI] [PubMed] [Google Scholar]
- 3.Ruberg FL, Skene DJ, Hanifin JP, Rollag MD, English J, Arendt J, Brainard GC. Melatonin regulation in humans with color vision deficiencies. J Clin Endocrinol Metab. 1996;81:2980–2985. doi: 10.1210/jcem.81.8.8768862. [DOI] [PubMed] [Google Scholar]
- 4.Zaidi FH, Hull JT, Peirson SN, Wulff K, Aeschbach D, Gooley JJ, Brainard GC, Gregory-Evans K, Rizzo JF, III, Czeisler CA, Foster RG, Moseley MJ, Lockley SW. Short-wavelength light sensitivity of circadian, pupillary, and visual awareness in humans lacking an outer retina. Curr Biol. 2007;17:2122–2128. doi: 10.1016/j.cub.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau KW, Hattar S. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:75–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M, Kay SA, Van Gelder RN, Hogenesch JB. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- 8.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 9.Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4:1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- 10.Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cajochen C, Munch M, Kobialka S, Krauchi K, Steiner R, Oelhafen P, Orgul S, Wirz-Justice A. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005;90:1311–1316. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- 12.Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 2003;88:4502–4505. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- 13.Lockley SW, Evans EE, Scheer FAJL, Brainard GC, Czeisler CA, Aeschbach D. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29:161–168. [PubMed] [Google Scholar]
- 14.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: Evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freedman MS, Lucas RJ, Soni B, von schantz M, Muñoz M, David-Gray Z, Foster R. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:502–504. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- 16.Lucas RJ, Freedman MS, Muñoz M, Garcia-Fernandez JM, Foster RG. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:505–507. doi: 10.1126/science.284.5413.505. [DOI] [PubMed] [Google Scholar]
- 17.Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci. 2001;4:621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- 18.Wong KY, Dunn FA, Graham DM, Berson DM. Synaptic influences on rat ganglion-cell photoreceptors. J Physiol. 2007;582:279–296. doi: 10.1113/jphysiol.2007.133751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belenky MA, Smeraski CA, Provencio I, Sollars PJ, Pickard GE. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J Comp Neurol. 2003;460:380–393. doi: 10.1002/cne.10652. [DOI] [PubMed] [Google Scholar]
- 20.Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 21.Panda S, Sato TK, Castrucci AM, Rollag MD, Degrip WJ, Hogenesch JB, Provencio I, Kay SA. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–2215. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- 22.Ruby NF, Brennan TJ, Xie X, Cao V, Franken P, Heller HC, O’Hara BF. Role of melanopsin in circadian responses to light. Science. 2002;298:2211–2213. doi: 10.1126/science.1076701. [DOI] [PubMed] [Google Scholar]
- 23.Lamb TD. Photoreceptor spectral sensitivities: common shape in the long-wavelength region. Vision Res. 1995;35:3083–3091. doi: 10.1016/0042-6989(95)00114-f. [DOI] [PubMed] [Google Scholar]
- 24.Mrosovsky N, Hattar S. Impaired masking responses to light in melanopsin-knockout mice. Chronobiol Int. 2003;20:989–999. doi: 10.1081/cbi-120026043. [DOI] [PubMed] [Google Scholar]
- 25.Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007;47:946–954. doi: 10.1016/j.visres.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mure LS, Rieux C, Hattar S, Cooper HM. Melanopsin-dependent nonvisual responses: evidence for photopigment bistability in vivo. J Biol Rhythms. 2007;22:411–424. doi: 10.1177/0748730407306043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dkhissi-Benyahya O, Gronfier C, De Vanssay W, Flamant F, Cooper HM. Modeling the role of mid-wavelength cones in circadian responses to light. Neuron. 2007;53:677–687. doi: 10.1016/j.neuron.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong KY, Dunn FA, Berson DM. Photoreceptor adaptation in intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48:1001–1010. doi: 10.1016/j.neuron.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 31.Smith KA, Schoen MW, Czeisler CA. Adaptation of human pineal melatonin suppression by recent photic history. J Clin Endocrinol Metab. 2004;89:3610–3614. doi: 10.1210/jc.2003-032100. [DOI] [PubMed] [Google Scholar]
- 32.Zhang DQ, Wong KY, Sollars PJ, Berson DM, Pickard GE, McMahon DG. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proc Natl Acad Sci U S A. 2008;105:14181–14186. doi: 10.1073/pnas.0803893105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melyan Z, Tarttelin EE, Bellingham J, Lucas RJ, Hankins MW. Addition of human melanopsin renders mammalian cells photoresponsive. Nature. 2005;433:741–745. doi: 10.1038/nature03344. [DOI] [PubMed] [Google Scholar]
- 35.Panda S, Nayak SK, Campo B, Walker JR, Hogenesch JB, Jegla T. Illumination of the melanopsin signaling pathway. Science. 2005;307:600–604. doi: 10.1126/science.1105121. [DOI] [PubMed] [Google Scholar]
- 36.Koyanagi M, Kubokawa K, Tsukamoto H, Shichida Y, Terakita A. Cephalochordate melanopsin: evolutionary linkage between invertebrate visual cells and vertebrate photosensitive retinal ganglion cells. Curr Biol. 2005;15:1065–1069. doi: 10.1016/j.cub.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 37.Walker MT, Brown RL, Cronin TW, Robinson PR. Photochemistry of retinal chromophore in mouse melanopsin. Proc Natl Acad Sci U S A. 2008;105:8861–8865. doi: 10.1073/pnas.0711397105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naka KI, Rushton WA. S-potentials from colour units in the retina of fish (Cyprinidae) J Physiol. 1966;185:536–555. doi: 10.1113/jphysiol.1966.sp008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aggelopoulos NC, Meissl H. Responses of neurones of the rat suprachiasmatic nucleus to retinal illumination under photopic and scotopic conditions. J Physiol. 2000;523:211–222. doi: 10.1111/j.1469-7793.2000.t01-1-00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshimura T, Ebihara S. Spectral sensitivity of photoreceptors mediating phase-shifts of circadian rhythms in retinally degenerate CBA/J (rd/rd) and normal CBA/N (+/+) mice. J Comp Physiol [A] 1996;178:797–802. doi: 10.1007/BF00225828. [DOI] [PubMed] [Google Scholar]
- 41.Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiance in melanopsin-knockout mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- 42.Figueiro MG, Bullough JD, Bierman A, Rea MS. Demonstration of additivity failure in human circadian phototransduction. Neuro Endocrinol Lett. 2005;26:493–498. [PubMed] [Google Scholar]
- 43.Figueiro MG, Bierman A, Rea MS. Retinal mechanisms determine the subadditive response to polychromatic light by the human circadian system. Neurosci Lett. 2008;438:242–245. doi: 10.1016/j.neulet.2008.04.055. [DOI] [PubMed] [Google Scholar]
- 44.Revell VL, Skene DJ. Light-induced melatonin suppression in humans with polychromatic and monochromatic light. Chronobiol Int. 2007;24:1125–1137. doi: 10.1080/07420520701800652. [DOI] [PubMed] [Google Scholar]
- 45.Smith MR, Eastman CI. Phase delaying the human circadian clock with blue-enriched polychromatic light. Chronobiol Int. 2009;26:709–725. doi: 10.1080/07420520902927742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith MR, Revell VL, Eastman CI. Phase advancing the human circadian clock with blue-enriched polychromatic light. Sleep Med. 2009;10:287–294. doi: 10.1016/j.sleep.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Provencio I, Foster RG. Circadian rhythms in mice can be regulated by photoreceptors with cone-like characteristics. Brain Res. 1995;694:183–190. doi: 10.1016/0006-8993(95)00694-l. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi JS, DeCoursey PJ, Bauman L, Menaker M. Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature. 1984;308:186–188. doi: 10.1038/308186a0. [DOI] [PubMed] [Google Scholar]
- 49.Lack L, Wright H, Kemp K, Gibbon S. The treatment of early-morning awakening insomnia with 2 evenings of bright light. Sleep. 2005;28:616–623. doi: 10.1093/sleep/28.5.616. [DOI] [PubMed] [Google Scholar]
- 50.Rosenthal NE, Joseph-Vanderpool JR, Levendosky AA, Johnston SH, Allen R, Kelly KA, Souetre E, Schultz PM, Starz KE. Phase-shifting effects of bright morning light as treatment for delayed sleep phase syndrome. Sleep. 1990;13:354–361. [PubMed] [Google Scholar]
- 51.Anderson JL, Glod CA, Dai J, Cao Y, Lockley SW. Lux vs. wavelength in light treatment of Seasonal Affective Disorder. Acta Psychiatr Scand. 2009;120:203–212. doi: 10.1111/j.1600-0447.2009.01345.x. [DOI] [PubMed] [Google Scholar]
- 52.Glickman G, Byrne B, Pineda C, Hauck WW, Brainard GC. Light therapy for seasonal affective disorder with blue narrow-band light-emitting diodes (LEDs) Biol Psychiatry. 2006;59:502–507. doi: 10.1016/j.biopsych.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 53.Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Van Someren EJ. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA. 2008;299:2642–2655. doi: 10.1001/jama.299.22.2642. [DOI] [PubMed] [Google Scholar]
- 54.Viola AU, James LM, Schlangen LJ, Dijk DJ. Blue-enriched white light in the workplace improves self-reported alertness, performance and sleep quality. Scand J Work Environ Health. 2008;34:297–306. doi: 10.5271/sjweh.1268. [DOI] [PubMed] [Google Scholar]
- 55.Brainard GC, Sherry D, Skwerer RG, Waxler M, Kelly K, Rosenthal NE. Effects of different wavelengths in seasonal affective disorder. J Affect Disord. 1990;20:209–216. doi: 10.1016/0165-0327(90)90052-a. [DOI] [PubMed] [Google Scholar]
- 56.Zeitzer JM, Kronauer RE, Czeisler CA. Photopic transduction implicated in human circadian entrainment. Neurosci Lett. 1997;232:135–138. doi: 10.1016/s0304-3940(97)00599-5. [DOI] [PubMed] [Google Scholar]
- 57.Kayumov L, Casper RF, Hawa RJ, Perelman B, Chung SA, Sokalsky S, Shapiro CM. Blocking low-wavelength light prevents nocturnal melatonin suppression with no adverse effect on performance during simulated shift work. J Clin Endocrinol Metab. 2005;90:2755–2761. doi: 10.1210/jc.2004-2062. [DOI] [PubMed] [Google Scholar]
- 58.Duffy JF, Dijk DJ. Getting through to circadian oscillators: why use constant routines? J Biol Rhythms. 2002;17:4–13. doi: 10.1177/074873002129002294. [DOI] [PubMed] [Google Scholar]
- 59.Vaughan GM. New sensitive serum melatonin radioimmunoassay employing the Kennaway G280 antibody: Syrian hamster morning adrenergic response. J Pineal Res. 1993;15:88–103. doi: 10.1111/j.1600-079x.1993.tb00514.x. [DOI] [PubMed] [Google Scholar]
- 60.Motulsky H, Christopoulos A. Fitting Models to Biological Data Using Linear and Nonlinear Regression: A Practical Guide to Curve Fitting. Oxford University Press; New York: 2008. [Google Scholar]