Abstract
Formation of crystals in the enamel space releases protons that need to be buffered to sustain mineral accretion. We hypothesized that apical Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) in maturation ameloblasts transduces chloride into forming enamel as critical step to secrete bicarbonates. We tested this by determining the calcium, chloride and fluoride levels of developing enamel of Cftr-null mice by quantitative electron probe microanalysis. Maturation stage Cftr–null enamel contained less chloride and calcium than wild-type enamel, was more acidic when stained with pH dyes ex vivo and formed no fluorescent modulation bands after in vivo injection of the mice with calcein. To further acidify the enamel we exposed Cftr-null mice to fluoride in drinking water to stimulate proton release during formation of hypermineralized lines. In enamel of Cftr-deficient mice fluoride further lowered enamel calcium without further reducing chloride levels. The data support the view that apical Cftr in maturation ameloblasts transduces chloride into developing enamel as part of the machinery to buffer protons released during mineral accretion.
Keywords: Cystic fibrosis, enamel fluorosis, Ae2, quantitative X-ray microanalysis, ameloblast modulation
During maturation stage of amelogenesis formation of apatite crystals in the enamel space generates protons that need to be neutralized to sustain further crystal growth (1-3). It has been proposed that maturation stage ameloblasts buffer these protons by secreting bicarbonates into the forming enamel (1, 4). Support for this concept is the identification of several pH regulating proteins in plasma membranes of maturation ameloblasts, including Cystic Fibrosis Transmembrane Conductance Regulator (CFTR), anion exchanger-2 (AE2), sodium bicarbonate cotransporter-1 (NBCe1), sodium hydrogen exchanger 1 (NHE1) (5-18), in plasma membranes and carbonic anhydrases 2 and 6 in the cytosol.
A key factor in pH regulation by many ion-transporting epithelia is CFTR, a chloride channel that in ductular epithelium of the pancreas and colon epithelium transports chloride into the luminal space to enable secretion of bicarbonate by anion exchangers (19-24). In mouse maturation ameloblasts CFTR was localized in the apical (luminal) plasma membrane (13) and without functional Cftr enamel mineralization in human, mouse, pig and rat was incomplete (7, 13, 25 -27).
The function of CFTR in maturation ameloblasts is not clear. Null mutation of Cftr reduces mineralization of maturation-stage enamel suggesting that functional Cftr is critical for mineral accretion (5-8). It has been proposed that CFTR in ameloblasts transduces intracellular chloride into the forming enamel (equivalent to the luminal space of epithelial ducts) either to enable secretion of protons into the enamel (16), or secretion of bicarbonates by apical AE2 (3, 28), or other bicarbonate chloride exchangers (14, 15). There is very little evidence that CFTR in ameloblasts transduces chloride. Only two studies determined chloride and mineral content in Cftr-deficient mouse enamel but the outcome was not consistent (6, 8). In the first study both chloride and calcium were indeed lower when whole incisors were analysed but analysis of microdissected fragments of enamel free from dentin showed no differences in chloride (8). In the second study maturation stage enamel of Cftr−/− mice was less mineralized but contained normal levels of chloride, while secretory enamel contained normal levels of calcium but less chloride (6). However, in wild-type mice calcium levels of secretory-stage enamel were as high as in maturation enamel (62% vs 61 % weight percentage, respectively) suggesting that secretory stage samples were in fact maturation stage (6). Both studies were performed with both upper and lower incisors, before analysis teeth were rinsed and/or sonicated in distilled water and one study included posteruptive enamel (8) that could have taken up mineral ions and chloride from oral fluids.
We re-addressed the hypothesis that CFTR acts as chloride channel by measuring mineral density and changes in mineral- and chloride content in enamel of Cftr-null mice. We analysed defined pre-eruptive stages of enamel in lower incisors using microcomputed tomography (MicroCT) and quantified x-ray electron microscopy (11). To minimize loss of trace elements hemi-mandibles were excised as quickly as possible after sacrifice, snap-frozen in liquid nitrogen, freeze-dried and processed anhydrously. We also exposed Cftr-deficient mice to fluoride to see if ameloblasts responded to hypermineralization by enhancing bicarbonate/chloride exchange (11).
Modulation or pH cycling involves cyclic change of groups of ruffle-ended ameloblasts into groups of smooth-ended ameloblasts coinciding with changes in pH of the enamel below these cells, a process critical for full mineralization of enamel (29). We also determined ameloblast modulation in fluorotic and non-fluorotic Cftr-null enamel by examining the formation of fluorescent-labelled bands in maturation-stage enamel after injection of calcein in mice in vivo and by ex vivo staining cell-free enamel with pH indicator dyes. Modulation in both Cftr -null and fluorotic enamel has been reported to be inhibited (7) or changed (30,31)
Materials and methods
Animals and tissue preparation
Cftr-null and wild-type litter mates (FVB/N strain, 26 wk old) were bred and genotyped as reported (19). The animals were distributed in two groups of three Cftr-null mice and two groups of three wild-type mice. All four groups of mice received drinking water with Kleanprep, a laxative to prevent obstruction of intestine in the mutant mice. One Cftr-null group and one wild-type group were exposed to 100 parts per million (ppm) fluoride (given as NaF) in drinking water ad libitum for 6 wk. To examine modulation during maturation stage in a separate study 8 mice (all four groups, n=2 per group) received a single intraperitoneal injection of 20 mg/kg calcein (dissolved in physiological salt solution, 4 μl fluid/gr body weight), 30 min before sacrifice. Mice were euthanized, decapitated, trunk blood collected and frozen for fluoride measurement later. Hemi-mandibles were frozen in liquid nitrogen, freeze-dried and embedded anhydrously in methyl methacrylate (MMA) (10, 11). All procedures with mice complied with (inter)national rules and were approved by the Committee for Animal Health and Animal Care of the Vrije Universiteit and the Erasmus University in Rotterdam (The Netherlands).
Microcomputed tomography, electron probe microanalysis and serum fluoride
The MMA-embedded jaws were scanned at a resolution of 8 μm voxels using a μCT-40 high resolution scanner (Scanco Medical, Bassersdorf, Switzerland). Cross-sectioned areas were made with a rotory saw at selected areas (mid-secretion, early maturation at a reference line between M1 an M2 and late maturation near alveolar crest (1), and subsequently analysed by quantitative x-ray electron microanalysis using a Jeol Superprobe JXA-8800 (10). Serum samples from 2-3 mice were pooled to obtain sufficient volume to measure plasma fluoride which was determined by the method of TAVES (32) using a fluoride ion-selective electrode (Radiometer Analytical, type E41M017)
Staining incisor enamel with pH indicator and calcein
Bone caps around lower incisors of freeze-dried mandibles and enamel organs were removed by microdissection to expose the labial surface of the incisors. Calcein labelling was examined under blue light from a hand-held dental light-cure device (Kulzer, Translux CL, Kulzer Co Wehrheim, Germany) with an Fuji C48 orange filter and images taken using a Zeiss KL2500 LCD microscope. To visualize pH incisors were submersed for 1 min in a pH indicator solution of methyl-red or phenol-red (1 mg/mL), dissolved 1 μM NaOH solution (11).
Calculations and statistics
No significant differences between fluorotic wild-type and fluorotic heterozygous Cftr+/− mice were found, neither in serum fluoride levels, enamel structure, or enamel composition. Hence, data from fluorotic wild-type and fluorotic heterozygous Cftr+/− mice were pooled and referred to as fluorotic ‘’wild-type’’. Values are presented as mean and standard deviations and data were tested for statistical differences by Student t-tests, one-way of ANOVA (between groups of the same stage), two-factor ANOVA with replication (to test interaction between fluoride and Cftr-null mutation; Excel 2010) and correlation analysis by linear regression (Graphpad Instat 3 software; p<0.05).
Results
Changes in mineral density determined by backscattered imaging and microCT
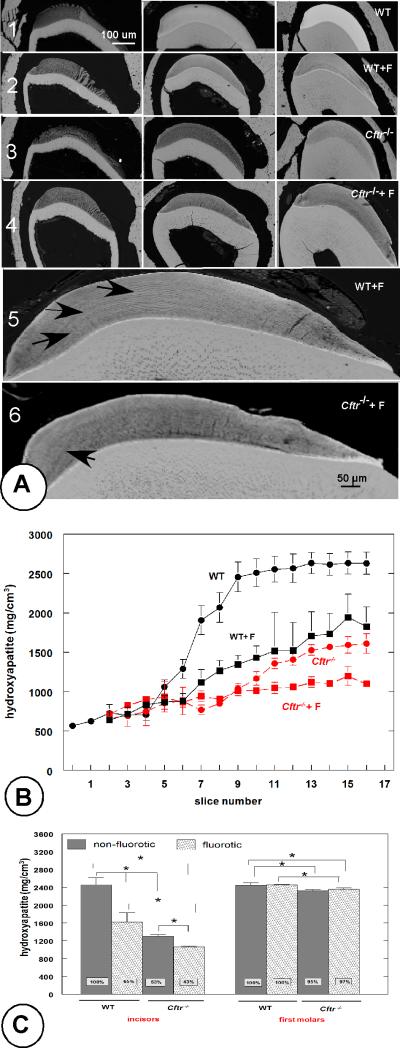
Null mutation of Cftr (Fig. 1a, row 3) severely reduced the mineral density of maturation-stage incisor enamel in comparison to wild-type enamel (Fig. 1a, row 1). Exposure to fluoride also reduced enamel mineral density (Fig. 1a, row 2) with no difference in contents between Cftr+/− heterozygous and Cftr−/− homozygous mice (not shown). In fluorotic wild-type mice the enamel contained weak hypermineralized and hypomineralized lines, a hypermineralized surface and a slightly hypomineralized subsurface (Fig. 1a, rows 2 and 5). These lines were also noted in fluorotic Cftr-null enamel, but less conspicuous (Fig.1a ; rows 4 and 6).
Fig. 1.
Fig 1a. Backscattered-electron detector (BSC) microscopy. Effect of Cftr-null mutation on enamel mineralization in lower incisors from fluorotic and nonfluorotic Cftr-null mice. Backscatter electron microscopy (Fig. 1a), and MicroCT (Fig. 1b, c). Fig. 1a. Row 1: nonfluorotic wild-type mouse; row 2: fluorotic wild-type mouse; row 3: non-fluorotic Cftr-null mouse and row 4: fluorotic Cftr-null mouse. The left column (from rows 1-4) represents secretion stages, the central column mid-maturation and the right column late maturation stages. Row 5 (fluorotic wild-type mouse) and row 6 (fluorotic Cftr-null mouse) show weak incremental lines (arrows).
Fig. 1b. Microcomputed tomography. Effect of fluoride on mineral density of incisor enamel from Cftr-null and wild-type mice as function of development (300 μm intervals). Slices 1-3 are secretory stage, from slice 4 onwards: maturation stage. Filled circles represent WT (black; solid line) and Cftr-null enamel (red, interrupted line). Filled squares (black, solid line): fluorotic WT and fluorotic Cftr-null enamel (red, interrupted line) (n=3). All density values of non-fluorotic Cftr-null and fluorotic wild-type enamel after slice 7 are significantly lower than nonfluorotic wild- type enamel.
Fig. 1c. Microcomputed tomography. Mineral density of incisor and first molar enamel from Cftr-null mice. Molar enamel is barely affected in contrast to the incisor enamel from the same animals. Fluoride exposure started when enamel formation in first molars was completed. Averages and standard deviation (slices 10-16; 3 mice/ group).* p < 0.05.
The enamel mineral density measured by microCT was progressively less in the experimental groups in comparison to the wild-type group (Fig. 1b, c), in decreasing order from fluorotic wild-type enamel (65% of control value), non-fluorotic Cftr-null enamel (53%) and fluorotic Cftr-null enamel (43%, Fig. 1b,c). The changes in density became apparent at early maturation stage. In contrast to incisor enamel mineral density of molar enamel in Cftr–null mice was hardly reduced (3-5%, Fig. 1c).
Changes in enamel composition by EPMA
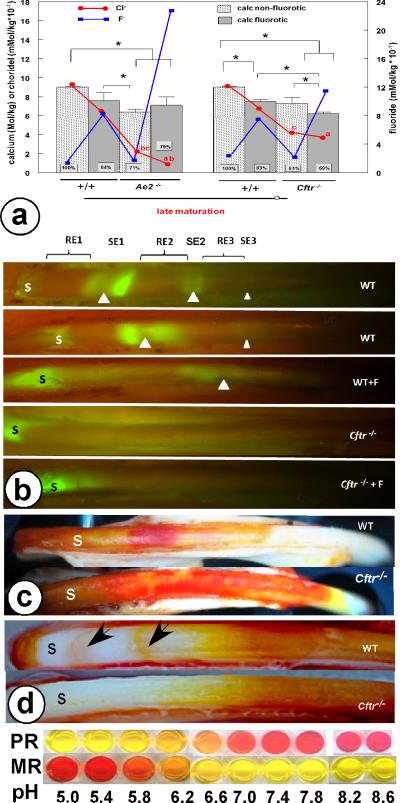
Disruption of Cftr and exposure to fluoride both significantly decreased calcium, phosphorus and chlorine content in maturation-stage but not secretion-stage incisor enamel determined by quantitative electron probe microanalysis (Table 1). Exposure of wild-type mice to fluoride decreased chloride levels in late maturation enamel to 78% and calcium to 83% of non-fluorotic wild-type controls (Fig. 2a). Non-fluorotic, maturation-stage enamel from Cftr-null mice contained far less chloride (42%) than enamel from wild-type mice. In fluorotic enamel from Cftr-null mice calcium content was significantly lower (68%) in comparison with enamel from sound wild-type mice than non-fluorotic enamel from Cftr-null mice (81%; p< 0.05) without reduction of chloride (39%, not significant from the 42% in non-fluorotic Cftr-null enamel, Table 1, Fig 2a). This contrasted the effect of fluoride on enamel formation in Ae2a,b-null mice (11) that contained slightly more calcium, much more fluoride, and significantly less chloride (from 24% to 12%, p< 0.05; Fig 2a).
TABLE 1.
Effect of Cftr-null mutation and fluoride exposure on composition of lower murine incisor enamel assessed by quantitative electron microscopical X-ray analysis (mean and standard deviation, n=3)
| Wild-type (weight %) | Fluorotic wild-type (weight %) | |||||
|---|---|---|---|---|---|---|
| secretion | mid maturation | late maturation | secretion | mid maturation | late maturation | |
| CaO | 24.7±1.5 | 45.6±2.0abc | 50.5± 0.2def | 24.8± 2.1 | 35.6± 3.9a | 4O.9±3.5d |
| P2O5 | 21.1±2.2 | 37.9±3.6hi@ | 40.7±1.7jkl | 19.5± 1.1 | 28.8± 3.5@ | 32.7±2.6j |
| Ca/P(mol/mol) | 1.49±0.13 | 1.53±0.09 | 1.57±0.06 | 1.60± 0.11 | 1.57± 0.03 | 1.58±0.02 |
| MgO×10−3 | 320±190 | 120±12 | 215±40m | 243±86 | 290±190 | 426±160 |
| SO3×10−3 | 1145±150k,l | 63± 20 | 16±11 | 880±279 | 205±155 | 49±10 |
| F×10−3 | 60±34m | 82±28p | 67±13s | 136±50 | 217±81pqr | 155±98 |
| Cl ×10−3 | 181±65 | 325±62uv | 332±18wx | 148±146 | 224±40y | 258±50z |
| Cftr-null (weight %) | Fluorotic Cftr-null (weight %) | |||||
|---|---|---|---|---|---|---|
| Secretion | mid maturation | late maturation | secretion | mid maturation | late maturation | |
| CaO | 27.9±3.2 | 27.5±1.8b | 41.9±0.9eg | 25.0±3.7 | 31.6± 4.3c | 34.8± 0.9fg |
| P2O5 | 21.3± 2.9 | 21.5±2.0h | 31.9±1.2k | 21.7±3.7 | 26.2±3.0i | 28.9± 0.9l |
| Ca/P(mol/mol) | 1.58±0.05 | 1.62±0.05 | 1.67±0.02 | 1.46± 0.05 | 1.53±0.05 | 1.52±0.04 |
| MgO×10−3 | 310±30 | 370±100 | 400±10 | 465±19k | 270±40 | 560±48m |
| SO3×10−3 | 904±75l | 643±439 | 61± 5 | 647±101 | 270±250 | 198±99 |
| F×10−3 | 56±39 | 45±18q | 51±24t | 187±24m | 179±30r | 218±45st |
| Cl×10−3 | 114±13 | 134±38u | 141±1w | 117±74 | 147±24v,y | 131± 20xz |
For each stage of development (secretory stage, early maturation or late maturation) the values were compared between the four groups. Significant differences between groups are indicated by the same character or symbol (p< 0.05; Anova, one way)
Fig 2.
Disruption of Cftr and exposure to fluoride reduces calcium and chloride content in maturation enamel (Fig. 2a), changes ameloblast modulation (Fig. 2b) and acidifies forming enamel (Fig 2c,d).
Fig 2a. Quantitative microprobe (means and SD, n=3-7). Left Y-axis: calcium (bars, * statistically significant differences) and chloride (red lines), right Y-axis: fluoride (blue lines). For the sake of clarity the standard deviations for chloride and fluoride graphs were not drawn (see table 1 and reference 11); chloride values marked by the same character are significantly different. The Ae2a,b-null data from reference 11.
Fig. 2b. Calcein labeling in non-fluorotic and fluorotic mandibular incisor enamel of wild-type (WT) and Cftr-null mice. S secretory stage. White arrow heads indicate center of smooth-ended (SE) zone between two wide bands of ruffle-ended (RE) cells. Each SE-zone is marked by an apical and incisal fluorescent label indicating the sites where the ameloblast layer is briefly leaky for calcein during change of position of tight junctions. In WT enamel the most incisal third band is extremely weak. Fig. 2c Mandibular incisor enamel of wild-type and Cftr-null mutant stained with methyl red (MR). Fig 2d: Wild-type (WT) and Cftr-null maxillary incisor stained with phenol-red (PR) revealing two neutral (light pink) SE bands.
Sulphur content (a measure for enamel matrix protein) was higher in Cftr-null enamel (Table 1). In all groups sulphur was strongly negatively correlated with calcium (not shown) suggesting that in all groups matrix was degraded with progressive mineralization.
Fluoride in sera and developing enamel
By pooling the number of serum samples became too low to draw a conclusion, but the measured values suggested no major changes in plasma fluoride levels between wild-type (1.3 and 1.2 μmoles/L, n=2) and Cftr-null mice (1.4 and 1.8 μmoles, n=2) or between fluorotic wild-type (5.3± 0.6 μmoles/L; n=6) and fluorotic Cftr- null mice (4.6 and 6.8 μmoles/L; n=2). Fluoride levels in enamel showed also no difference between non-fluorotic wild-type and Cftr null mice furthermore suggesting that Cftr null-mutation had no major influence on plasma fluoride levels (Table 1).
Interaction between fluoride exposure and disruption of Cftr was tested by two-factor ANOVA. Fluoride content only increased by exposure to fluoride (p<0.004) but not by Cftr–null mutation. Both treatments interacted strongly on reducing calcium in early maturation (p<0.005) but not in early and late maturation combined (p=0.058). There was no interaction between both treatments on decrease of chloride at late maturation (p=0.08) but interaction was significant (p=0.004) for early and late maturation combined. No interaction between treatments was found between chloride and fluoride content.
Calcein labelling and pH staining in enamel
Calcein injected into non-fluorotic wild-type mice shortly before sacrifice formed three (sets of two) narrow fluorescent bands in maturation stage enamel, the most incisal one very faint (Fig. 2b). Only one band was found in fluorotic wild-type enamel shifted more incisally (Fig. 2b) whereas no bands were noticed in the Cftr-null and fluorotic Cftr-null enamel (Fig. 2b).
Methyl-red pH indicator solution stained the entire maturation-stage enamel in Cftr-null mice pink/red (acidic) but produced in wild-type enamel 2-3 discrete red/pink bands separated by narrow yellow/white (pH neutral) stripes (Fig. 2c). Phenol-red produced in maturation stage enamel two narrow pink (neutral) stripes against yellow/white (acid) maturation enamel in wild-type enamel, not in Cftr-null enamel (Fig. 2d).
Discussion
Our data show that without functional CFTR the chloride levels in unerupted enamel at maturation stage are consistently lower, in line with the hypothesis that also in maturation ameloblasts CFTR acts as a chloride channel. Disruption of Cftr and exposure to fluoride decreased chloride levels in maturation-stage enamel and severely reduced enamel mineralization. Staining with pH indicators indicated that maturation-stage enamel of Cftr-null mice was more acidic and contained no pH-neutral bands as reported before (7). After injection of mice with calcein no fluorescent bands were formed in Cftr-null enamel at maturation-stage. Collectively, these changes demonstrate the significance of functional CFTR for maturation stage ameloblasts, suggesting that CFTR is critical for pH regulation and ameloblasts modulation.
Fluoride-ions added to solutions of apatite crystals that contain calcium- and phosphate ions stimulate mineral growth in vitro which is sustained if the pH is controlled (33). Similarly, in enamel of mice exposed to fluoride hypermineralized lines are formed in situ, a process that consequently accelerates proton release near the ameloblast membrane (11). Fluorotic enamel contains less chloride which was attributed to elevated activity of apical chloride/bicarbonate exchanger(s). In pancreatic epithelial ducts, duodenal epithelium and CFTR-expressing Xenopus oocytes several members of the SLC26A family operate in close conjunction with CFTR (20–24, 34). In developing enamel the formation of each unit hydroxyapatite (which contains 10 Ca2+) releases 8 protons and requires equimolar amounts of bicarbonate to neutralize. Since chloride levels in enamel were 2–3 magnitudes lower than calcium these data suggests that large numbers of chloride are absorbed from enamel back into the ameloblasts, possibly by apical bicarbonate/chloride exchangers of the SLC26A family (34).
It is of note that fluoride had opposite effects on enamel mineralization in Cftr-null mice than in Ae2a,b-null mice. Fluorotic Ae2a,b-null enamel contained multiple prominent fluoride-rich hypermineralization lines, fluoride levels were extremely high and calcium content was slightly higher in comparison with non-fluorotic Ae2a,b null enamel (11). In fluorotic Cftr−/− enamel calcium content was lowest of all groups, chloride levels were the same as in nonfluorotic Cftr-null enamel, hypermineralization lines were inconspicuous and fluoride content was virtually the same as in fluorotic wild-type enamel. Exposure to fluoride potentiated the negative effect of Cftr–null mutation on enamel mineralization. Consequently, these data suggest that fluorotic Cftr-null enamel is more acidic than fluorotic Ae2a,b null enamel and too acidic to sustain mineral accretion, even in the presence of fluoride.
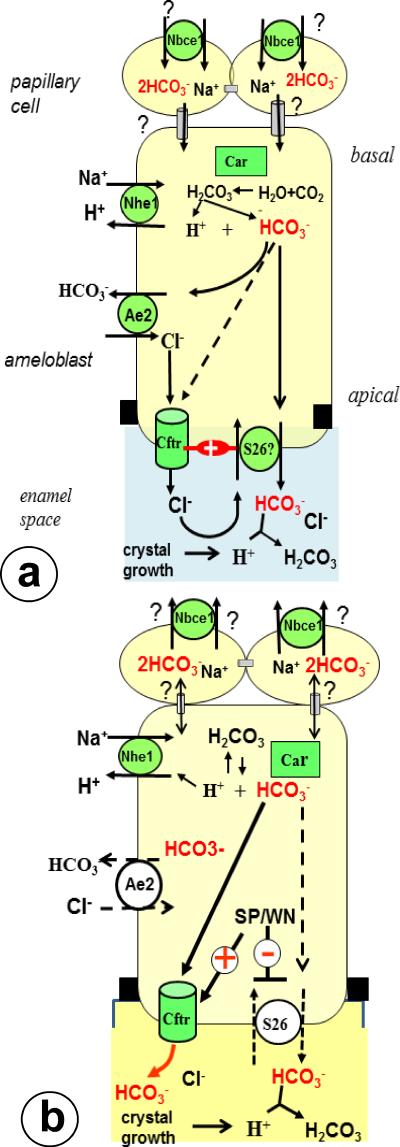
How CFTR is involved in pH regulation by ameloblasts is not yet clear. Based on published reports on ion-transport in other epithelial tissues CFTR in ameloblasts may regulate pH in enamel in three ways. (i) By electroneutrally transducing chloride into the enamel space. This enables secretion of bicarbonate in exchange for chloride by putative apical anion exchangers (Fig. 3a), possibly SLC26A family members as in pancreatic ductus epithelium (34, 35). Ameloblasts express at least one member of this family (SLC26A4, pendrin, PDS;14, 15). Pendrin-null mice however had no dental phenotype and hence was not critical for ameloblast function. (ii) By electroneutrally cotransporting (up to 20%) bicarbonate with chloride into the luminal space by a dynamical shift to conduct HCO3− (22, 35; Fig. 3a). This happens when luminal (enamel space) chloride levels become low and depends on the presence and activity of apical chloride/bicarbonate exchangers. (iii). By acting as an electrogenic conducer of bicarbonates only, independent from apical bicarbonate/chloride exchanger (Fig. 3b, 34-38). This change is triggered by low intracellular chloride levels, which activate chloride- responsive intracellular stress-related kinases (e.g. with-no-lysine kinase or WNK; oxidative stress-responsive kinase 1 or OSR1 and Sterile 20-like kinase-related proline/alanine rich kinase, SPAK) which turn CFTR into a HCO3− transporter with much higher capacity (up to 100%) than the classical way (37). Options 1 and 2 may apply to Cftr- null mutants in which chloride is normally imported via basolateral Ae2 but its secretion by Cftr is impaired. The Cftr-null mouse model we used in this study does not allow secretion of any chloride or bicarbonate. The third option potentially applies to Ae2-null mutants in which intracellular levels may be reduced by inhibition of chloride import by basolateral Ae2. Further studies with ameloblast cultures in which actual transport of chloride and bicarbonate are measured are required to give conclusive answers and how ameloblasts regulate pH.
Fig 3.
Simplified work models for buffering of enamel fluid by CFTR in maturation ameloblasts.
Fig 3a. Electroneutral secretion by CFTR and putative anion exchangers. CFTR transfers chloride to drive bicarbonate secretion by apical anion exchangers (potentially the SLC26A family, reference 36). Basolateral AE2 imports chloride that is conduced into enamel by CFTR. A minor part of chloride adsorbs to crystals in the enamel compartment, a major part is exchanged back into the cell for secretion of HCO3-. Putative apical anion exchangers depend on functional CFTR and both stimulate each others activity (“+””). CFTR itself can also transduce small amounts of bicarbonate (about 20%; stippled arrow; ref 22). NBCE1 in papillary layer may import bicarbonate and transfer it through gap junctions into ameloblasts.
Fig 3b. At low intracellular Cl- the CFTR channel switches from an electroneutral predominantly Cl conductive to an electrogenic HCO3- conductive channel activated by WNK, SPAK and OSR1 kinases.
These kinases simultaneously inhibit apical anion exchangers to prevent reabsorption of bicarbonate from the enamel space (reference 37,38). Black squares: tight junctions, gray tubes; gap junctions; Anion exchanger-2; Car carbonic anhydrase 2; NBCE1: Na Bicarbonate Cotransporter-1; NHE1 NaHydrogen Exchanger-1; S26: putative members of SLC26A family; SP Sterile 20-like kinase-related proline/alanine rich kinase (SPAK); WN with-no-lysine kinase or WNK. Not depicted: Na/K ATPase, Carbonic anhydrase 6.
Previous reports showed inconsistent reduction of chloride levels in enamel of Cftr-null mice (6,8). In the present study chloride and calcium levels were consistently lower in maturation stage Cftr-null enamel than in wild-type enamel. The likely explanation for the different outcome in our study include (i) minimizing the chance of ion-loss by rapid freezing and freeze-drying, (ii) anhydrously processing of the tissues, (iii) analysis of mandibular incisors only, (iv) selection of well-defined sample areas and (v) exclusion of posteruptive enamel to avoid uptake of ions from oral fluid or loss of ions by extensive washing that could change the composition of surface enamel, especially when enamel is poorly mineralized or porous as in Cftr-null mice. Ions from oral fluids could become trapped in mineral by posteruptive recrystallization of apatite,a process stimulated by fluoride from food or drinking water. The enamel fragments of Cftr-null enamel that had normal levels of chloride also contained significantly more fluoride (8). In the present study we found no such increase of fluoride in non-fluorosed preeruptive Cftr null enamel which argues against the idea that elevated fluoride in erupted Cftr- null enamel was incorporated during enamel development (8). The fact that levels of chloride and Ca2+ in oral fluids are elevated in cystic fibrosis patients (39, 40) and Cftr- null mice (41) could enhance posteruptive recrystallization and increase calcium and chloride content.
Molar tooth germs express CFTR (13) but surprisingly enamel of molar teeth of Cftr-null mice was hardly affected, mineral density was almost normal and enamel contained normal levels of chloride as reported (6, 8). One possible explanation why molar enamel is unaffected is that mouse molar teeth develop very early when the Cftr- deficiency or stress that comes with the null mutation in incisors is not yet severe enough to affect molar development. In children suffering from cystic fibrosis the primary teeth (comparable with molar teeth in rodents) are far less affected than the permanent teeth (25, 42). Alternatively, molar teeth in rodents develop differently than incisors in several other aspects (i.e. mouse molars do not continuously erupt or accumulate orange pigment at their surface).
In conclusion: The results are consistent with the concept that in maturation ameloblasts of mouse incisors CFTR is involved in buffering of enamel to sustain mineral accretion.
Acknowledgements
This study was supported by NIH DE13508 (PDB, AB, DL). We thank Dr. H. de Jonge for his advice (Erasmus University, Rotterdam, Netherlands). The expertise, analytical support and hospitality of Wim Lustenhouwer, Wynanda Koot (Geotechnical Laboratory, Vrije Universiteit, Amsterdam), and Saskia Kars, at the Facilities for EPMA-analyses/SEM, Vrije Universiteit and NWO, The Netherlands Organization for Scientific Research, is highly appreciated.
Footnotes
Conflicts of interest
Authors have no conflicts of interest.
References
- 1.SMITH CE, CHONG DL, BARTLETT JD, MARGOLIS JD. Mineral acquisition rates in developing enamel on maxillary and mandibular incisors of rats and mice: implications to extracellular acid loading as apatite crystals mature. J Bone Miner Res. 2005;20:240–249. doi: 10.1359/JBMR.041002. [DOI] [PubMed] [Google Scholar]
- 2.SIMMER JP, PAPAGERAKIS P, SMITH CE, FISHER DC, ROUNTREY AN, ZHENG L, HU JC. Regulation of dental enamel shape and hardness. J Dent Res. 2010;89:1024–1038. doi: 10.1177/0022034510375829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LACRUZ RS, SMITH CE, KURTZ I, HUBBARD MJ, PAINE ML. New paradigms on the transport function of maturation stage ameloblasts. J Dent Res. 2013;92:122–129. doi: 10.1177/0022034512470954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LACRUZ RS, NANCI A, KURTZ I, WRIGHT JT, PAINE ML. Regulation of pH during amelogenesis. Calcif Tissue Int. 2010;86:91–103. doi: 10.1007/s00223-009-9326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WRIGHT JT, KIEFER CL, HALL KI, GRUBB BR. Abnormal enamel development in a cystic fibrosis transgenic mouse model. J Dent Res. 1996;75:966–973. doi: 10.1177/00220345960750041101. [DOI] [PubMed] [Google Scholar]
- 6.ARQUITT CK, BOYD C, WRIGHT JT. Cystic fibrosis transmembrane regulator gene (CFTR) is associated with abnormal enamel formation. J Dent Res. 2002;81:492–496. doi: 10.1177/154405910208100712. [DOI] [PubMed] [Google Scholar]
- 7.SUI W, BOYD C, WRIGHT JT. Altered pH regulation during enamel development in the cystic fibrosis mouse incisor. J Dent Res. 2003;82:388–392. doi: 10.1177/154405910308200512. [DOI] [PubMed] [Google Scholar]
- 8.GAWENIS LR, SPENCER P, HILLMAN LS, HARLINE MC, MORRIS JS, CLARKE LL. Mineral content of calcified tissues in cystic fibrosis mice. Biol Trace Elem Res. 2001;83:69–81. doi: 10.1385/BTER:83:1:69. [DOI] [PubMed] [Google Scholar]
- 9.GAWENIS LR, BRADFORD EM, PRASAD V, LORENZ JN, SIMPSON JE, CLARKE LL, WOO AL, GRISHAM C, SANFORD LP, DOETSCHMAN T, MILLER ML, SHULL GE. Colonic anion secretory defects and metabolic acidosis in mice lacking the NBC1. J Biol Chem. 2007;282:9042–9052. doi: 10.1074/jbc.M607041200. [DOI] [PubMed] [Google Scholar]
- 10.LYARUU DM, BRONCKERS AL, MULDER L, MARDONES P, MEDINA JF, KELLOKUMPU S, OUDE ELFERINK RP, EVERTS V. The anion exchanger Ae2 is required for enamel maturation in mouse teeth. Matrix Biol. 2008;27:119–127. doi: 10.1016/j.matbio.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LYARUU DM, MEDINA JF, SARVIDE S, BERVOETS TJ, EVERTS V, DENBESTEN PK, SMITH CE, BRONCKERS ALJ. Barrier formation: potential molecular mechanisms of enamel fluorosis. J Dent Res. 2014;93:96–102. doi: 10.1177/0022034513510944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.BRONCKERS ALJJ, LYARUU DM, JANSEN ID, MEDINA JF, KELLOKUMPU S, HOEBEN KA, GAWENIS LR, OUDE ELFERINK RP, EVERTS V. Localization and function of the anion exchanger Ae2 in developing teeth and orofacial bone in rodents. J Exp Zool B Mol Dev Evol. 2009;312B:375–387. doi: 10.1002/jez.b.21267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.BRONCKERS ALJJ, KALOGERAKI L, JORNA HJ, WILKE M, BERVOETS TJ, LYARUU DM, ZANDIEH- DOULABI B, DENBESTEN P, DE JONGE H. The cystic fibrosis transmembrane conductance regulator (CFTR) is expressed in maturation stage ameloblasts, odontoblasts and bone cells. Bone. 2010;46:1188–1196. doi: 10.1016/j.bone.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.BRONCKERS ALJJ, GUO J, ZANDIEH-DOULABI B, BERVOETS TJ, LYARUU DM, LI X, WANGEMANN P, DENBESTEN P. Developmental expression of solute carrier family 26A member 4 (SLC26A4/pendrin) during amelogenesis in developing rodent teeth. Eur J Oral Sci. 2011;119(Suppl 1):185–192. doi: 10.1111/j.1600-0722.2011.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.BRONCKERS ALJJ, GUO J, LYARUU DM, DENBESTEN P, ZANDIEH-DOULABI B. Immunolocalization and western blotting of the anion exchanger pendrin in ameloblasts. Eur J Oral Sci. 2012;120:369–372. [Google Scholar]
- 16.JOSEPHSEN K, TAKANO Y, FRISCHE S, PRAETORIUS J, NIELSENS, AOBA T, FEJERSKOV O. Ion transporters in secretory and cyclically modulating ameloblasts: a new hypothesis for cellular control of pre-eruptive enamel maturation. Am J Physiol Cell Physiol. 2010;299:C1299–C1307. doi: 10.1152/ajpcell.00218.2010. [DOI] [PubMed] [Google Scholar]
- 17.LACRUZ RS, SMITH CE, CHEN YB, HUBBARD MJ, HACIA JG, PAINE ML. Gene expression analysis of early and late maturation stage rat enamel organ. Eur J Oral Sci. 2011;119(Suppl 1):149–157. doi: 10.1111/j.1600-0722.2011.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LACRUZ RS, NANCI A, WHITE SN, WEN X, WANG H, ZALZAL SF, LUONG VQ, SCHUETTER VL, CONTI PS, KURTZ I, PAINE ML. The sodium bicarbonate cotransporter (NBCe1) is essential for normal development of mouse dentition. J Biol Chem. 2010;285:24432–24428. doi: 10.1074/jbc.M110.115188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.RATTCLIFF R, EVANS MJ, CUTHBERT AW, MACVINISH LJ, FOSTER D, ANDERSON JR, COLLEDGE WH. Production of a severe cystic fibrosis mutation by gene targeting. Nature Gen. 1993;4:35–41. doi: 10.1038/ng0593-35. [DOI] [PubMed] [Google Scholar]
- 20.GREELEY T, SHUMAKER H, WANG Z, SCHWEINFEST CW, SOLEIMANI M. Downregulated in adenoma and putative anion transporter are regulated by CFTR in cultured pancreatic duct cells. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1301–1308. doi: 10.1152/ajpgi.2001.281.5.G1301. [DOI] [PubMed] [Google Scholar]
- 21.ISHIGURO H, STEWARD M, NARUSE S. Cystic fibrosis transmembrane conductance regulator and Slc26 transporters in HCO3 secretion by pancreatic duct cells. Acta Physiol Sinica. 2007;59:465–476. [PubMed] [Google Scholar]
- 22.SHCHEYNIKOV N, KIM KH, KIM K, DORWART M, KO SBH, GOTO H, NARUSE S, THOMAS PJ, MUALLEM S. Dynamic control of cystic fibrosis transmembrane conductance regulator Cl-/ HCO3- selectivity by external Cl-. J Biol Chem. 2004;279:21857–21865. doi: 10.1074/jbc.M313323200. [DOI] [PubMed] [Google Scholar]
- 23.SINGH AK, RIEDERER B, CHEN M, XIAO F, KRABBENHOEFT A, ENGELHARDT R, NYLANDER O, SOLEIMANI M, SEIDLER U. The switch of intestinal Slc26 exchangers from anion absorptive to HCO3- secretory mode is dependent on CFTR anion channel function. Am J Physiol Cell Physiol. 2010;298:C1057–1065. doi: 10.1152/ajpcell.00454.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.SINGH AK, SJOEBLOM M, ZHENG W, KRABBENHOEFT A, RIEDERER B, RAUSCH B, MANNS MP, SOLEIMANI M, SEIDLER U. CFTR and its key role in in vivo resting and luminal acid-induced duodenal HCO3- secretion. Acta Physiol (Oxf) 2008;193:357–365. doi: 10.1111/j.1748-1716.2008.01854.x. [DOI] [PubMed] [Google Scholar]
- 25.AZEVEDO TD, FEIJO GS, BEZERRA AC. Presence of developmental defects of enamel in cystic fibrosis patients. J Dent Child. 2006;73:159–163. [PubMed] [Google Scholar]
- 26.CHANG EH, LACRUZ RS, BROMAGE TG, BRINGAS P, WELSH MJ, ZABNER J, PAINE ML. Enamel pathology resulting from loss of function in the cystic fibrosis transmembrane conductance regulator in a porcine animal model. Cells Tissues Organs. 2011;194:249–254. doi: 10.1159/000324248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.TUGGLER KL, BIRKET SE, CUI X, HONG J, WARREN J, REID L, CHAMBERS A, JI D, GAMBER K, CHU KK, TEARNEY G, TANG LP, FORTENBERRY JA, DU M, CADILLAC JM, BEDWELL DM, ROWE SM, SORSCHER EJ, FANUCCHI MV. Characterization of defects in ion transport and tissue development in cystic fibrosis transmembrane conductance regulator (CFTR)-knockout rats. PLoS One. 2014;7:9. doi: 10.1371/journal.pone.0091253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.PAINE ML, SNEAD ML, WANG HJ, ABULADZE N, PUSHKIN A, LIU W, KAO LY, WALL SM, KIM YH, KURTZ I. Role of NBCe1 and AE2 in secretory ameloblasts. J Dent Res. 2008;87:391–395. doi: 10.1177/154405910808700415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.SMITH CE. Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med. 1998;9:128–161. doi: 10.1177/10454411980090020101. Review. [DOI] [PubMed] [Google Scholar]
- 30.DENBESTEN PK, CRENSHAW MA, WILSON MH. Changes in the fluoride-induced modulation of maturation stage ameloblasts of rats. J Dent Res. 1985;64:1365–1370. doi: 10.1177/00220345850640120701. [DOI] [PubMed] [Google Scholar]
- 31.SMITH CE, NANCI A, DENBESTEN PK. Effects of chronic fluoride exposure on morphometric parameters defining the stages of amelogenesis and ameloblast modulation in rat incisors. Anat Rec. 1993;237:243–258. doi: 10.1002/ar.1092370212. [DOI] [PubMed] [Google Scholar]
- 32.TAVES DR. Determinations of submicromolar concentrations of fluoride in biological samples. Talanta. 1968;15:1015–1023. doi: 10.1016/0039-9140(68)80109-2. [DOI] [PubMed] [Google Scholar]
- 33.MURA-GALELLI MJ, NARUSAWA H, SHIMADA M, IIJIMA M, AOBA T. Effects of fluoride on precipitation and hydrolysis of octacalcium phosphate in an experimental model simulating enamel mineralization during amelogenesis. Cells Mater. 1992;2:221–230. [Google Scholar]
- 34.STEWARD MC, ISHIGURO H. Molecular and cellular regulation of pancreatic duct cell function. Current Opinion Gasterol. 2009;25:447–453. doi: 10.1097/MOG.0b013e32832e06ce. [DOI] [PubMed] [Google Scholar]
- 35.KO SBH, SHCHEYNIKOV N, CHOI JY, LUO X, ISHIBASHI K, THOMAS PJ, KIM JY, KIM KH, LEE MG, NARUSE S, MUALLEM S. A molecular mechanism for aberrant CFTR-dependent HCO3 transport in cystic fibrosis. EMBO J. 2002;21:5662–5672. doi: 10.1093/emboj/cdf580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.PARK HW, NAM JH, KIM JY, NAMKUNG W, YOON JS, LEE JS, KIM KS, VENGLOVECZ V, GRAY MA, KIM KH, LEE MG. Dynamic regulation of CFTR bicarbonate permeability by [Cl-]i and its role in pancreatic bicarbonate secretion. Gastroenterology. 2010;139:620–631. doi: 10.1053/j.gastro.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 37.REDDY MM, QUINTON PM. Control of dynamic CFTR selectivity by glutamate and ATP in epithelial cells. Nature. 2003;12:423, 756–760. doi: 10.1038/nature01694. [DOI] [PubMed] [Google Scholar]
- 38.WRIGHT AM, 1, GONG X, VERDON B, LINSDELL P, MEHTA A, RIORDAN JR, ARGENT E, GRAY MA. Novel regulation of cystic fibrosis transmembrane conductance regulator (CFTR) channel gating by external chloride. J Biol Chem. 2004;279:41658–41663. doi: 10.1074/jbc.M405517200. [DOI] [PubMed] [Google Scholar]
- 39.GONÇALVES AC, MARSON FA, MENDONÇA RM, RIBEIRO JD, RIBEIRO AF, PASCHOAL IA, LEVY CE. Saliva as a potential tool for cystic fibrosis diagnosis. Diagn Pathol. 2013;8:46–52. doi: 10.1186/1746-1596-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.BLOMFIELD J, WARTON KL, BROWN JM. Flow rate and inorganic componnets of submandibular saliva in cystic fibrosis. Arch Disease Childhood. 1973;48:267–274. doi: 10.1136/adc.48.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.CATALÁN MA, 1, NAKAMOTO T, GONZALEZ-BEGNE M, CAMDEN JM, WALL SM, CLARKE LL, MELVIN JE. Cftr and ENaC ion channels mediate NaCl absorption in the mouse submandibular gland. J Physiol. 2010;588:713–724. doi: 10.1113/jphysiol.2009.183541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.FERRAZZANO GF, SANGIANANTONI G, CANTILE T, AMATO I, ORLANDO S, INGENITO A. Dental enamel defects in Italian children with cystic fibrosis: an observational study. Community Dent Health. 2012;29:106–109. [PubMed] [Google Scholar]