Abstract
We have previously reported that adoptive transfer of tumor-draining lymph node (TDLN) B cells confers tumor regression in a spontaneous pulmonary metastasis mouse model of breast cancer. In this study, we identified IL-10-producing cells within these B cells, and found that IL-10 removal, either by using IL-10−/− TDLN B cells or by systemic neutralization of IL-10, significantly augmented the therapeutic efficacy of adoptively transferred TDLN B cells. Depletion of IL-10 in B-cell adoptive transfers significantly increased cytotoxic lymphocytes (CTLs) and B-cell activity of peripheral blood mononuclear cells (PBMCs) and splenic cells in the recipient. Activated TDLN B cells express Fas ligand, which was further enhanced by co-culture of these TDLN B cells with 4T1 tumor cells. Effector B cells killed tumor cells directly in vitro in an antigen-specific and Fas ligand-dependent manner. Trafficking of TDLN B cells in vivo suggested that they were recruited to the tumor and lung as well as secondary lymphoid organs. These findings further define the biological function of antitumor effector B cells, which may offer alternative cellular therapies to cancer.
Keywords: B cells, IL-10, Adoptive Immunotherapy, Cytotoxicity, Tumor, Fas
Introduction
Immunotherapy has become a viable treatment alternative for a number of advanced hematological malignancies and solid tumors [1]. To date, immunotherapy has focused on the generation of effector T cells against tumor [2–6]. In contrast, B cells are often overlooked in tumor immunology, likely because of the common notion that humoral and cytolytic responses work in opposition. In previous studies, B-cell function in host immune responses was mainly focused on antigen presentation and antibody production. Recent B-cell studies have demonstrated that B cells can act either as effector cells [7, 8] or as regulatory cells [9].
B cells are phenotypically and functionally heterogeneous [10, 11]. On one hand, in vivo primed and in vitro activated B cells have shown efficacy in adoptive immunotherapy of cancer [7, 8], and the effector B cells can directly kill tumor cells [8]. On the other hand, resting B cells can promote the development or progression of cancer [12–15]. One of the most significant findings in recent B-cell studies has been the identification of regulatory B cells or Breg cells [16–26], which can suppress inflammatory responses in experimental autoimmune encephalomyelitis (EAE), collagen induced arthritis (CIA), and intestinal inflammation [16–18]. In the majority of these studies, the function of regulatory B cells is dependent on IL-10 production, but other mechanisms, including expression of TNF family death-inducing ligands, have been described [27]. It has been found that differentiated B cells expressing IL-10 can repress antitumor immunity [19, 20].
We have previously published that about 40% of the tumor-draining lymph node (TDLN) cells are CD19+ B cells [7, 8]. Using a murine 4T1 pulmonary metastatic model, we found that adoptive transfer of LPS/anti-CD40-activated 4T1 TDLN B cells significantly inhibited the development of spontaneous 4T1 pulmonary metastasis in tumor-bearing mice [8]. In the current study, we sought to examine the mechanisms involved in the B-cell-mediated tumor repression, and the role of IL-10-producing B cells in regulating the antitumor efficacy of B effector cells given in adoptive immunotherapy.
Results
IL-10−/− B cells are more potent antitumor effector cells than WT B cells
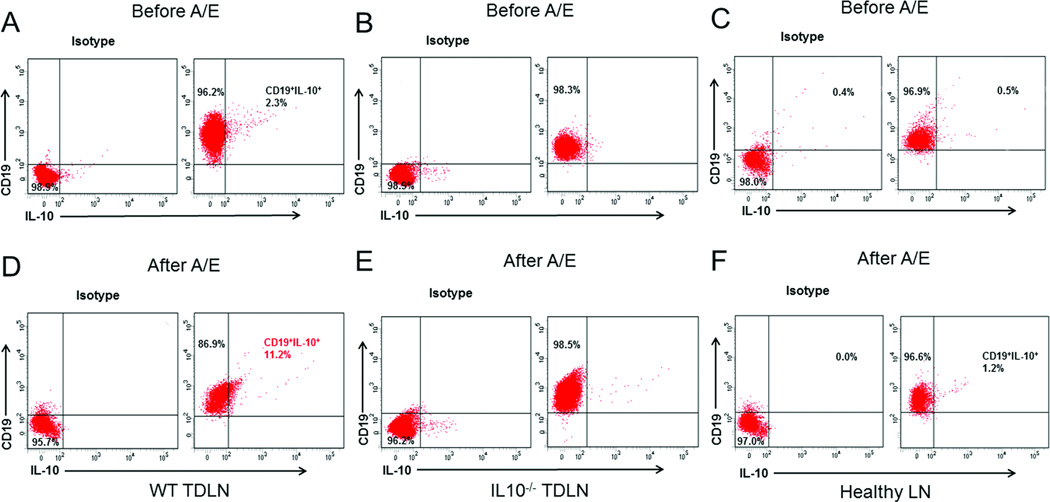
Breg cells have been found to be immunosuppressive [16–26]. To detect IL-10-producing cells in 4T1 TDLN B cells, we purified CD19+ B cells from WT and IL-10−/− 4T1 TDLN cells, respectively. WT 4T1 TDLNs were induced as previously described [8], and the IL-10−/− 4T1 TDLNs were induced by s.c. injection of 4T1 cells into the IL-10−/− BALB/c mice. The CD19+ and CD19+IL-10+ B-cell populations were assessed by flow cytometry. Among these freshly purified B cells, 2–3% of the WT B cells were CD19+IL-10+ (Figure 1A), but these cells were not detectable in the IL-10−/− B cells as expected (Figure 1B). After in vitro activation and expansion (A/E) with LPS plus anti-CD40, CD19+IL-10+ cells in WT TDLN B cells increased to 11% (Figure 1D), while CD19+IL-10+ cells in the IL-10−/− B cells remained undetectable (Figure 1E). There were almost no IL-10-producing B cells in healthy LN (<1% before A/E, Figure 1C; <2% after A/E, Figure 1F).
Figure 1.
Phenotype of 4T1 TDLN B cells and healthy B cells. B cells purified from WT 4T1 TDLNs, IL-10−/− 4T1 TDLNs and healthy LNs were activated and expanded (A/E) with LPS (5 µg/ml) and anti-CD40 mAb in vitro. Detection of IL-10-producing cells in (A, D) WT and (B, E) IL-10−/− CD19+ B cells purified from 4T1 TDLNs. B cells were stained with anti-CD19 on the cell surface and intracellularly stained with anti-IL-10 or isotype control antibodies before and after A/E. (C, F) Detection of IL-10-producing cells in CD19+ B cells purified from WT healthy LNs. B cells were stained with anti-CD19 and anti-IL-10 antibodies before and after A/E, and at least 10,000 cells were analyzed by flow cytometry. Cells were initially gated on forward and side scatter to remove debris and calculated by quadrant dot plot analysis. Representative plots from single experiments out of three independent experiments are shown.
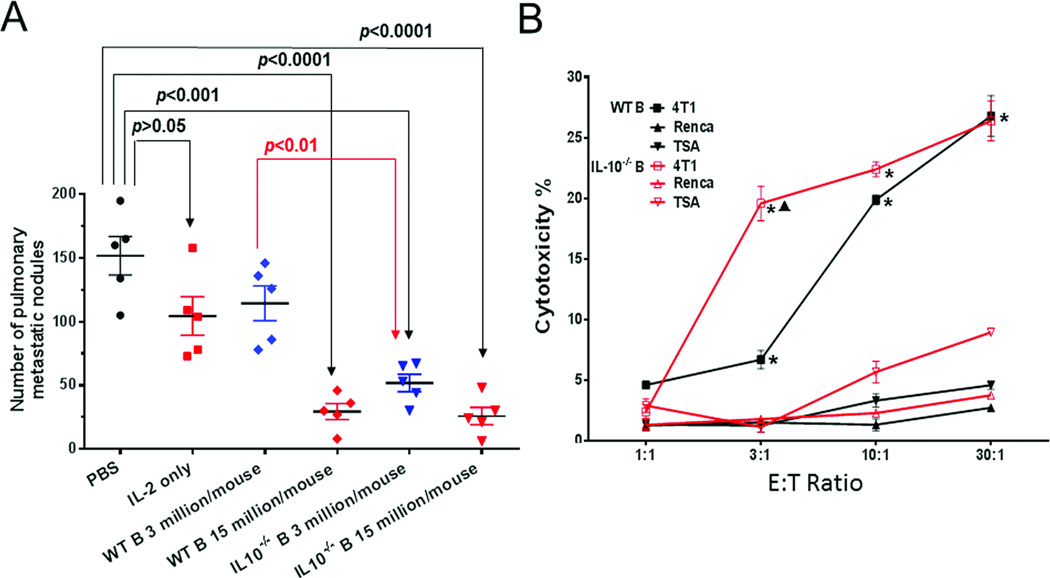
To investigate the role of IL-10-producing B cells in adoptive immunotherapy of cancer, we compared the therapeutic efficacy of IL-10−/− to WT TDLN B cells. Two weeks after 4T1 tumor cell injection into the mammary fat pad, tumor-bearing WT BALB/c mice were treated with activated WT or IL-10−/− 4T1 TDLN B cells. Two weeks later, mice lungs were collected to enumerate pulmonary metastases. As shown in Figure 2A, IL-2 alone or WT 4T1 TDLN B cells at a sub-optimal low dose (3 million/mouse) had a modest, but not significant reduction in pulmonary metastases compared with PBS-treated controls. However, adoptively transferred WT 4T1 TDLN B cells at a higher dose (15 million/mouse) significantly inhibited the metastasis of 4T1 tumor cells from the injection site (mammary fat pad) to the lung, which was consistent with our previous findings [8]. In comparison, the higher dose (15 million/mouse) of IL-10−/− 4T1 TDLN B cells demonstrated a similar antitumor activity as the higher dose of WT 4T1 TDLN B cells (p=0.7). Importantly, IL-10−/− B cells at the low dose (3 million/mouse) inhibited metastases significantly more effectively than WT B cells at the same low dose (p<0.01). These results indicated that IL-10−/− 4T1 TDLN B cells were more potent than WT 4T1 TDLN B cells on a per cell basis in adoptive immunotherapy.
Figure 2.
IL-10−/− 4T1 TDLN B cells are more effective than WT 4T1 TDLN B cells in vitro and in vivo. (A) Number of pulmonary metastatic nodules after adoptive transfer of WT vs. IL-10−/− B cells. Groups of mice (n = 5 mice per group) received adoptively transferred B cells plus IL-2, 2 weeks after intramammary fat pad injection of 4T1 tumor cells. Control groups received PBS or IL-2. Each dot represents the number of pulmonary metastasis in a single mouse, the mean (±SEM) lung nodules are indicated by the horizontal bars. This experiment is representative of 2 completed independently. (B) Cytotoxicity of 4T1 tumor cells by activated WT vs. IL-10−/− 4T1 TDLN B cells as measured in an LDH release assay. Cytotoxicity was plotted against the ratio of effector B cells: tumor target cells (E:T ratio) which were plated in triplicate wells. Renca and TSA, both syngeneic to BALB/c mice, were used as specificity controls. Results are shown as mean ± SEM of triplicate wells from a single experiment representative of two experiments performed. *p<0.05, WT or IL-10−/− B cells + 4T1 vs. + Renca or + TSA; ▲p<0.05, IL-10−/− B cells + 4T1 vs. WT B cells + 4T1. P-values are indicated and were determined by Student’s t-test
To determine the efficacy of activated IL-10−/− B cells to mediate 4T1 tumor cell lysis, IL-10−/− and WT TDLN B cells were prepared as in Figure 2A, and were incubated in vitro with 4T1 tumor cells and cytotoxicity was analyzed using the LDH release assay. Two other BALB/c tumors, Renca and TSA, were used for specificity controls. While neither IL-10−/− nor WT 4T1 TDLN B cells killed Renca and TSA notably, WT 4T1 TDLN B cells killed 4T1 tumor cells in a dose-dependent manner (Figure 2B). Importantly, while at the higher E:T ratios (10:1 and 30:1), there was no significant difference in 4T1 cell killing between the IL-10−/− and WT 4T1 TDLN B cells, IL-10−/− 4T1 TDLN B cells mediated 4T1 cell lysis much more effectively (p<0.05) than WT 4T1 TDLN B cells at a low E:T ratio (3:1). These data indicate that TDLN B cells could kill the tumor cells directly in a tumor antigen-specific manner, and that the IL-10−/− TDLN B cells are more potent than WT TDLN B cells in such direct killing in vitro. These data are supportive of our observation in vivo (Figure 2A) that IL-10−/− B cells are more potent antitumor effector cells than WT B cells.
IL-10 neutralization verifies that TDLN B cells are more effective in the absence of IL-10
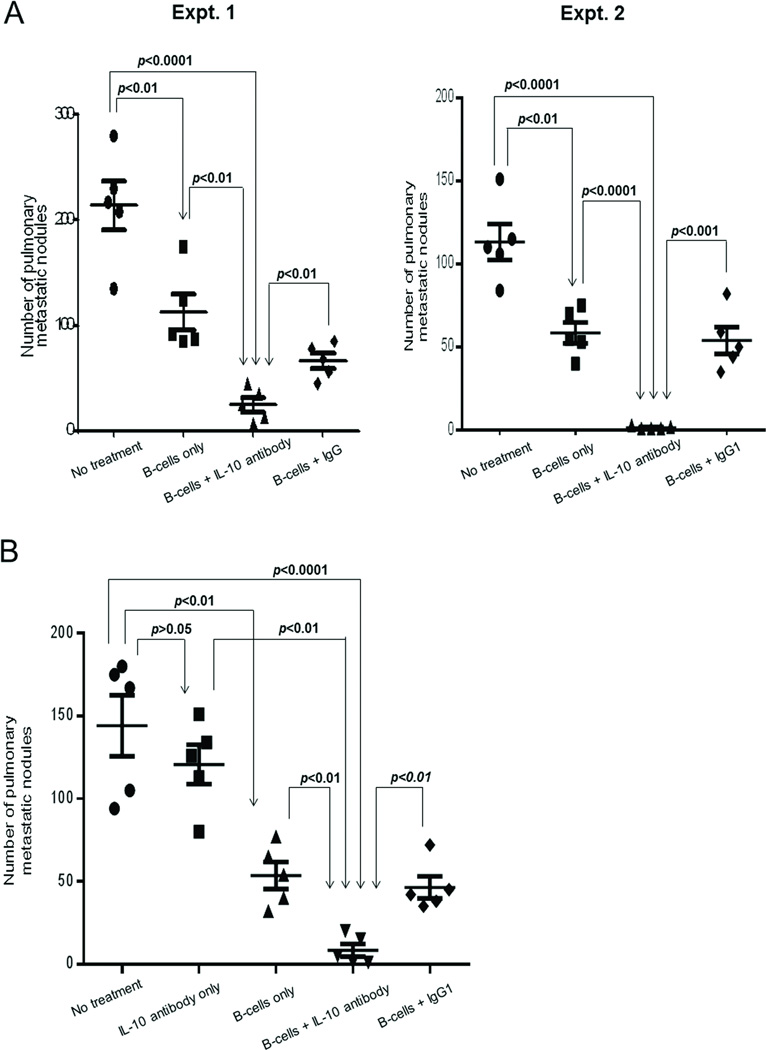
We used IL-10 antibody to neutralize IL-10 during the adoptive immunotherapy of cancer using effector TDLN B cells. As in Figure 2A, healthy BALB/c mice were inoculated with 4T1 cells in the mammary fat pad to induce spontaneous pulmonary metastasis and were treated 14 days later by adoptive transfer of activated WT 4T1 TDLN B cells i.v accompanied with IL-10 or isotype control antibody administration. As revealed in Figure 3A, with the injection of IL-10 antibody, infusion of 10 × 106 activated B cells resulted in significantly (p<0.01) reduced spontaneous 4T1 metastases compared with the results using same number of B cells without anti-IL-10 administration (B cells only) or with IgG (Expt.1) or IgG1 (Expt.2) administration. In additional experiments, we found that IL-10 antibody injection alone did not demonstrate significant antitumor effect compared with no treatment controls (Figure 3B). Together, these studies verify that adoptive immunotherapy using effector TDLN B cells is more effective in the absence of IL-10.
Figure 3.
Effect of IL-10 neutralization on the antitumor reactivity of adoptively transferred WT 4T1 TDLN B cells. (A) B cells were adoptively transferred with or without IL-10 antibody administration in mice with intramammary fat pad tumors. After 2 weeks, the number of pulmonary metastases per mouse was enumerated. Isotype control IgG and IgG1 were used in Expt. 1 and 2, respectively. (B) Antitumor reactivity of IL-10 antibody alone. Each symbol represents an individual mouse, two independent experiments are shown. Data are shown as mean ± SEM. P-values are indicated and determined by Student’s t-test.
Depletion of IL-10 significantly enhances systemic antitumor immunity
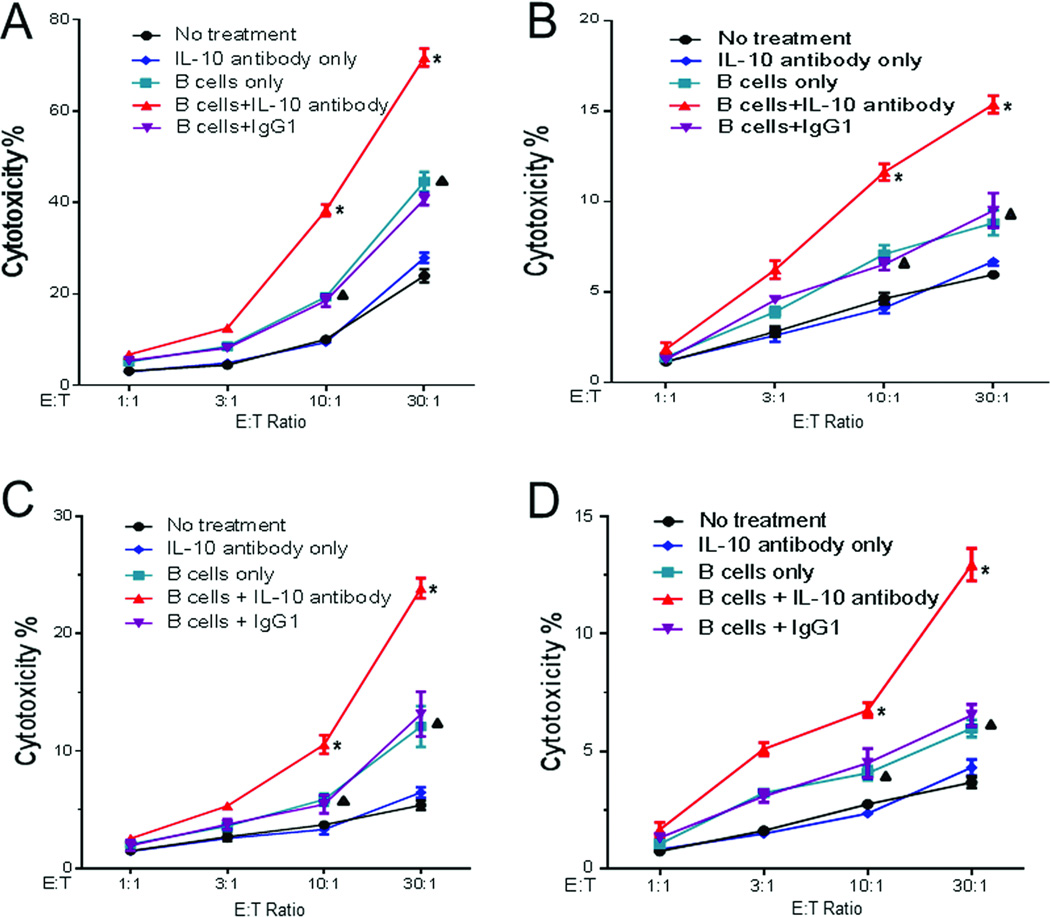
We purified PBMCs from the 4T1 tumoRbearing host subjected to WT TDLN B-cell adoptive immunotherapy with or without systemic IL-10 neutralization. T cells and B cells were purified from these PBMCs and were cultured as previously described [7, 8]. These cells were then analyzed for their lysis of the 4T1 cells. T cells (Figure 4A) and B cells (Figure 4B) generated from the PBMCs harvested from the hosts subjected to 4T1 TDLN B-cell + IL-10 antibody treatment killed 4T1 tumor cells significantly (p<0.05) more efficiently than all the controls.
Figure 4.
Effect of IL-10 neutralization on the cytotoxicity of 4T1 tumor cells. Effector cells were obtained from the 5 experimental groups shown in Figure 3B: (A) purified and activated T cells, (B) B cells from PBMCs; or (C) T cells, (D) B cells from spleens. Cytotoxicity was plotted against the ratio of effector B cells: tumor target cells (E:T ratio) which were plated in triplicate wells. Results are shown as mean ± SEM of triplicate wells from a single experiment representative of three experiments performed. Differences between groups were examined for statistical significance by Student’s t-test. *p<0.05 B cells+IL-10 antibody vs. all other groups at the ratios of 30:1 and 10:1; p<0.05 B cells only or B cells + IgG1 vs. No treatment or IL-10 antibody only at the ratios of 30:1 and 10:1.
We also purified splenic T- and B cells from all of the 5 experimental groups. Both splenic T- (Figure 4C) and splenic B cells (Figure 4D) harvested from the hosts subjected to 4T1 TDLN B-cell + IL-10 antibody treatment lysed 4T1 tumor cells significantly (p<0.05) more efficiently than T- and B cells prepared from the hosts subjected to 4T1 TDLN B-cell alone, 4T1 TDLN B-cell + IgG1, IL-10 antibody only, or no treatment. Together, these data indicate that depletion of IL-10 in B-cell adoptive transfer significantly enhanced host systemic antitumor immunity.
Direct killing of 4T1 cells by 4T1 TDLN B cells involves the Fas/FasL pathway
In our observed direct killing of 4T1 tumor cells by B cells, either by effector 4T1 TDLN B cells used for adoptive transfer (Figure 2B), or by PBMCs and splenic B cells harvested from the host subjected to B-cell + IL-10 antibody treatment (Figure 4B and D), there was no complement or other effector cells (e.g. NK cells, macrophages or neutrophils) added in the assay. Therefore, such direct killing was distinct from the traditional complement-dependent cytotoxicity (CDC) or antibody-dependent cell cytotoxicity (ADCC).
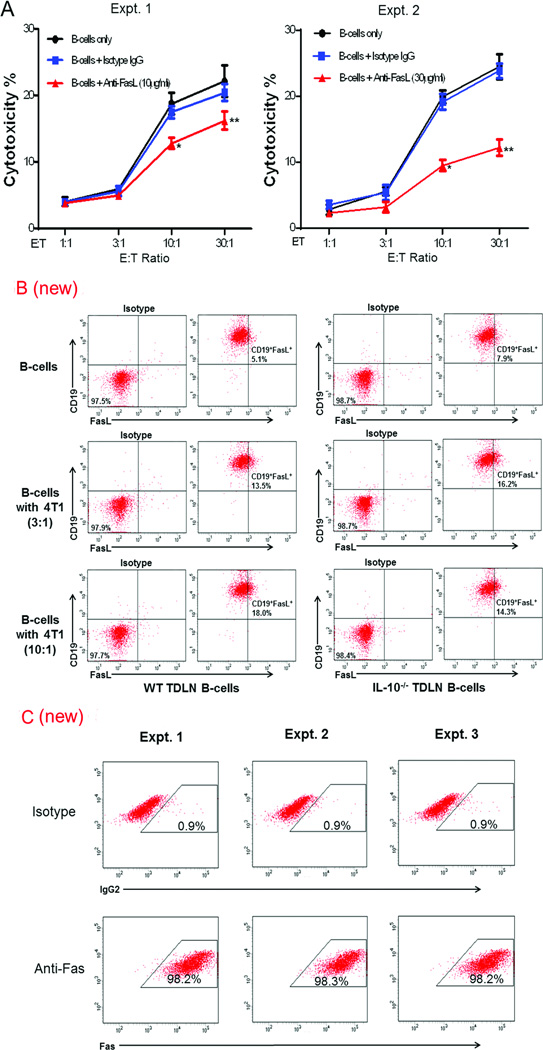
B cells have been shown to express FasL which can bind to the target cells expressing Fas resulting in target cell death [11, 28, 29]. To examine whether 4T1 TDLN B-cell-mediated direct killing of 4T1 cells involved the Fas/FasL pathway, we used anti-FasL antibody to block FasL during the LDH release assay. When added in culture, anti-FasL antibody significantly and dose-dependently decreased the killing efficacy of TDLN B cells on 4T1 tumor cells at the E:T ratios of 10:1 and 30:1 (Figure 5A, Expt. 1, anti-FasL = 10µg/ml; Expt. 2, anti-FasL = 30µg/ml).
Figure 5.
Effect of Anti-FasL blockade on the cytotoxicity of 4T1 TDLN B cells against 4T1 tumor cells. (A) B cells were co-cultured with 4T1 tumor cells with or without the addition of anti-FasL mAb at 10 or 30 µg/ml. Cytotoxicity was plotted against the ratio of effector B cells: tumor target cells (E:T ratio) which were plated in triplicate wells. Results are shown as mean ± SEM of triplicate wells from a single experiment representative of three experiments performed. Differences between groups were examined for statistical significance by Student’s t-test. *p<0.02 or p<0.001, B cells + anti-FasL vs. B cells only or vs. B cells + isotype IgG at 10:1 in Expt. 1 and 2 respectively; **p<0.005 or p<0.0005, B cells + anti-FasL vs. B cells only or vs. B cells + isotype IgG at 30:1 in Expt. 1 and 2, respectively. (B) Detection of FasL in B cells purified from WT and IL-10−/− 4T1 TDLNs after A/E and co-cultured with 4T1 tumor cells. After A/E, B cells were co-cultured with 4T1 at the ratios of 3:1 and 10:1 (B cells : 4T1) for 12 h. B cells were then stained with anti-CD19 and anti-FasL antibodies. Representative plots out of two independent experiments are shown. (C) Detection of Fas on 4T1 tumor cells. 4T1 cells were stained with anti-Fas antibody. Cells were initially gated on forward and side scatter to remove debris and calculated by quadrant dot plot and at least 10,000 cells were analyzed by flow cytometry. Results from three independent experiments are shown.
We used TDLN B cells isolated from IL-10−/− knockout mice and compared their FasL expression with WT TDLN B cells. As shown in Figure 5B, approximately 5% of the purified and anti-CD40/LPS activated/expanded (A/E) WT TDLN B cells expressed FasL. These are the effector cells we used for adoptive transfer, in vitro killing assays, and anti-FasL blockade throughout the study. As also observed in Figure 5B, there is a similar percentage (~8%) of the IL-10−/− TDLN B cells expressing FasL, showing no significant difference between these two types of B cells. Interestingly, we found that when TDLN B cells were co-cultured overnight with 4T1 tumor cells, the FasL expression was increased on the B cells. At WT TDLN B-cell:4T1 ratios of 3:1 and 10:1 the FasL expression on the B cells increased from 5.1% to 13.5% and 18.0%, respectively. We observed a similar increase of FasL expression on the IL-10−/− TDLN B cells after their co-culturing with 4T1 tumor cells in Figure 5B. Fas expression in target 4T1 tumor cells was very high (Fig. 5C), correlating with their sensitivity to be targeted by FasL+ activated TDLN B cells.
Adoptively transferred B cells traffic to the tumor and lung in vivo
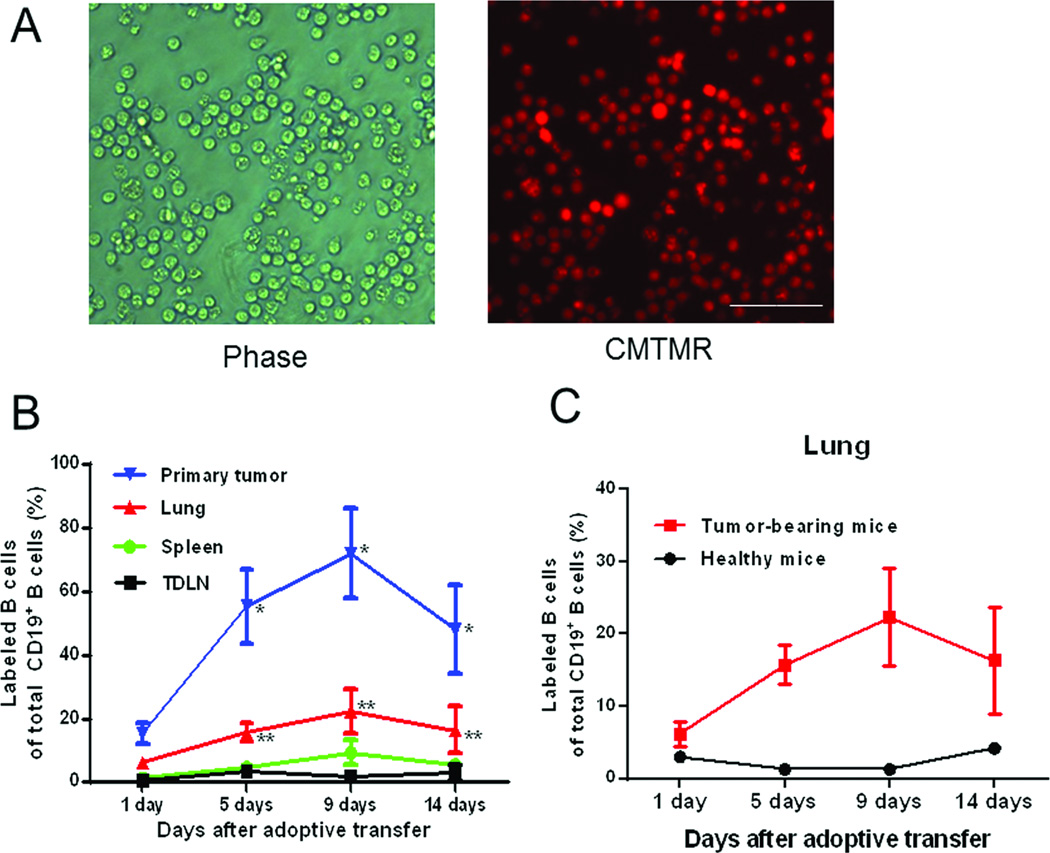
To address homing of the transferred B cells we pre-labeled the purified and activated/expanded TDLN B cells with 10 µM Cell Tracker™ Orange CMTMR (Figure 6A). After adoptive transfer, spleens, TDLNs, lungs and primary tumors were harvested at specified time points to detect the labeled live B cells in these tissues. As shown in Figure 6B, adoptively transferred B cells represented a high percentage of the total CD19+ B cells in the tumor site and lung peaking at 9 days post-transfer. Transferred B cells remained high in the tumor and lung on day 14 when lung metastases were examined. The results showed that transferred TDLN B cells represented a smaller fraction of the B cells found in the spleen and TDLNs but that the total numbers of transferred B cells in the spleen and TDLN were higher in comparison to those in the tumor and lung (Table 1). These data suggest that transferred B cells did not preferentially home to the tumor sites, but that they were more prone to entering sites of tumor than were endogenous B cells. This suggests that the ex vivo activation of TDLN B cells enhanced their trafficking to tumor sites which may have enhanced their ability to prevent lung metastasis.
Figure 6.
Trafficking of activated TDLN B cells in tumor-bearing and healthy mice. Activated 4T1 TDLN B cells were labeled with CMTMR and adoptively transferred into hosts with 4T1 intramammary fat pad tumor, or healthy hosts. (A) Phase contrast and fluorescence microscopy of CMTMR-stained B cells are shown. Original magnification × 200; white bar indicates 5 µm. (B) Percentages of transferred CMTMR-labelled TDLN B cells in TDLNs, spleen, lung and tumor in vivo. Labeled B cells were detected by flow cytometry. (C) Comparison of detected labeled B cells in lungs of tumor-bearing mice and healthy mice. Results are expressed as the mean percentages ± SEM of CD19+ labeled B cells (n= 5 mice). Results are shown from a single experiment representative of two experiments performed.
Table 1.
Numbers of transferred B cells detected in tumor-bearing BALB/c mice on days after B cell adoptive transfer as indicated (Mean ± SE)
| Primary tumor (× 104) |
Lung (× 104) |
Spleen (× 104) |
TDLN (× 104) |
|
|---|---|---|---|---|
| 1 day | 3.67 ± 0.38 | 1.97 ± 0.34 | 7.44 ±0.97 | 5.33 ± 1.04a |
| 5 days | 14.23 ± 1.39 | 5.87 ± 0.77 | 24.15 ± 5.24 | 35.90 ± 2.33b |
| 9 days | 17.34 ± 0.58 | 13.41 ± 0.95 | 43.34 ± 4.34 | 28.37 ± 3.03c |
| 14 days | 16.18 ± 2.16 | 14.71 ± 1.91 | 42.06 ± 9.55 | 26.87 ± 5.56 |
p<0.05, TDLN vs. Lung.
p<0.05, TDLN vs. Primary tumor or vs. Lung.
p<0.05, TDLN vs. Primary tumor or vs. Lung or vs. Spleen.
In the above experiments, we also used tumor-free mice as recipients of labeled TDLN B cells and found very few transferred B cells in the lung (Figure 6C) or in the spleen and LN of the healthy mice (data not shown). These results imply that localization and/or survival of the adoptively transferred TDLN B cells is critically dependent on interaction with the 4T1 tumor cells in vivo.
DISCUSSION
Breg cells, a subset of B cells analogous to regulatory T (Treg) cells, have been identified in experimental models of autoimmunity, infections, and cancer [16–26, 30]. Breg suppression appears to be directly mediated by the secretion of IL-10 and by B-cell interaction with pathogenic T cells to suppress immune responses [9]. IL-10-producing B cells play an important role in controlling encephalomyelitis, arthritis, and other inflammatory reactions [16–18, 23, 31]. Breg cells have demonstrated a negative effect in antitumor immunity, and promote the development of Treg cells. However, previous studies in our laboratory have shown that B cells isolated from tumor draining lymph nodes can induce anti-tumor T-cell responses and tumor regression [7, 8]. Further investigations were warranted to understand the opposing roles played by Breg cells and effector B cells in tumor immunity.
In this study, we found that approximately 2–3% of freshly isolated WT TDLN B cells and 10–12% of LPS/anti-CD40–activated WT TDLN B cells produce IL-10. In order to characterize the roles of these IL-10-producing B cells in adoptive immunotherapy, we generated IL-10−/− TDLN B cells and compared their therapeutic efficacy with equal number of WT TDLN B cells. We found that IL-10−/− B cells are significantly more potent antitumor effector cells than WT B cells in adoptive immunotherapy of cancer. In parallel, we observed that LPS/anti-CD40–activated IL-10−/− TDLN B cells mediated direct in vitro killing of cancer cells more potently than activated WT TDLN B cells. These data suggest that IL-10-producing TDLN B cells can suppress the antitumor function of the effector TDLN B cells. Our current data do not distinguish whether IL-10 production by B cells are from the same cells that express FasL and mediate tumor killing. We postulate that removal of IL-10-producing B cells may represent an effective strategy to enhance the therapeutic efficacy of adoptive cellular therapies.
We previously demonstrated that the adoptive transfer of purified effector B cells was highly potent in mediating tumor regression of established subcutaneous tumors in hosts that had been preconditioned with total body irradiation (500 cGy) which eliminated host T cells [7]. This clearly indicated that transferred B cells can act independently of T cells in causing tumor destruction in vivo. In addition, adoptively transferred effector B cells can also induce host T-cell anti-tumor activity [8]. We have previously reported that in the 3-day established pulmonary metastatic model the intravenous administration of neutralizing IL-10 mAb does not impact on the number of pulmonary metastases compared to untreated mice [32]. This indicates that endogenous host T- and B cells are not sufficient to mediate tumor regression when IL-10 is neutralized. In that same study, we found that adoptive transfer of activated T cells mediated tumor regression that was enhanced by IL-10 neutralization. This latter study plus our current data indicate that the ex vivo activation and adoptive transfer of either T or B effector cells is necessary and sufficient to see an effect of IL-10 neutralization.
To determine the mechanism by which the transferred B cells were controlling 4T1 tumor metastases, we studied the potential role of FasL expression by B cells in our model [11]. The Fas/FasL axis has shown a key role in regulating T cells during autoimmunity, infection, and cancer [11]. LPS-activated splenic B cells and B cells from healthy lymphoid tissues express FasL [28, 29, 33, 34]. Klinker et al reported that IL-5 and CD40L-activated B cells expressed FasL and induced T-cell apoptosis via the Fas/FasL axis [35]. In the current study we found that in vitro LPS/anti-CD40–activated TDLN B cells expressed FasL and directly killed 4T1 tumor cells. The tumor cells expressed high levels of Fas and the interaction of TDLN B cells with 4T1 led to increased levels of FasL expression on the B-cell surface. Blockade of FasL using anti-FasL antibody significantly reduced TDLN B-cell-mediated direct killing of 4T1 tumor cells, and the effect was anti-FasL dose-dependent. In terms of IL-10 involvement in this process, we observed that IL-10−/− B effector cells mediated less tumor killing, yet expressed FasL in a similar fashion to WT B cells. We did not investigate what mechanism may be involved in this phenomenon.
We have described that in vivo primed and in vitro activated effector B cells can mediate tumor regression after adoptive transfer [7, 8]. In an earlier report, Harada et al [36] demonstrated that LPS-activated B cells bound to anti-CD3 mAb enhanced the antitumor T-cell response. Unique to our model is the source of our in vivo primed B cells which are from tumor-draining LNs. Importantly, these LNs harbor tumor specific T- and B cells secondary to DC sensitization. Messina et al [37] reported that unique lymph node-like structures found in metastatic human melanomas correlated with improved overall survival. These structures contained an abundance of follicles with B- and T cells, suggesting the presence of an immune response associated with improved survival. We postulate that both a T- and B-cell response to tumor is important in developing effective immunotherapies.
It has been reported that T cells or TDLN cells can directly migrate to tumor and LN, and function in metastasis [38, 39]. In our model, transferred effector B cells trafficked to tumor, lung, spleen and TDLN, and the adoptively transferred B-cell numbers increased in all organs tested as time went on. The transferred B cells represented a high proportion of the B cells located in the lung and tumor sites, where we postulate they directly killed metastatic cells and/or decreased tumor cells migrating to lung. In contrast, transferred TDLN B cells were absent in tumor-free mice. The mechanisms by which the transferred effector B cells were recruited to the tumor and lung are of great interest and are the subject of continuing studies.
In summary, we have identified IL-10-producing cells within the purified TDLN B cells we have used in our previous reports for adoptive immunotherapy of cancer. Removal of IL-10, either by using the IL-10−/− TDLN B cells or by systemic neutralization of IL-10 using anti-IL-10 antibody, significantly augmented the therapeutic efficacy of adoptively transferred TDLN B cells. Depletion of IL-10 significantly affected systemic antitumor immunity, which was evident by enhanced killing activities of PBMCs and splenic CTLs and B cells isolated from recipient mice after B-cell adoptive transfer with anti-IL-10 antibody administration. Purified TDLN B effector cells kill tumor cells directly and such killing involves the Fas/FasL pathway. After adoptive transfer, TDLN B cells traffic into tumor, lung, spleen and TDLN. These findings further define the potential of B cells as an important component of adoptive immunotherapy for metastatic cancer.
Materials and Methods
Mice
Female WT and IL-10 KO (Il10tm1Cgn, IL-10−/−) BALB/c mice were purchased from the Jackson Laboratories (Bar Harbor, ME). IL-10 KO mice on BALB/c background are homozygous for a mutation in the IL-10 gene achieved by vectors designed to replace codons 5–55 of exon 1 with a 24 bp linker (providing a termination codon) and a neo expression cassette, to introduce a termination codon into exon 3. Mice were maintained in a pathogen-free environment and used at age 7 weeks or older. Principles of laboratory animal care (NIH publication No. 85-23, revised 1985) were followed. The animal protocols were approved by the University of Michigan Unit of Laboratory of Animal Medicine.
Murine tumor cells
The 4T1 cell line is a mammary carcinoma syngeneic to BALB/c mice (kindly provided by Dr. M. Sabel, University of Michigan). Inoculating 4T1 cells into the mammary fat pad induces the development of spontaneous pulmonary metastases. Renca is a kidney cancer cell line and TSA is a highly aggressive mammary adenocarcinoma, and these cell lines are all syngeneic to BALB/c mice and used as tumor antigen-specific controls. Renca and TSA were purchased from ATCC (American Type Culture Collection, Rockville, MD). All cell lines were maintained in vitro in complete medium (CM).
Tumor draining lymph nodes (TDLNs)
To induce TDLNs, 1 × 106 4T1 tumor cells in 0.1 ml PBS were injected subcutaneously (s.c.) into the lower flanks of WT or IL-10−/− syngeneic mice. Nine days after 4T1 cell inoculation, the draining inguinal lymph nodes were collected and processed using mechanical dissociation, filtered through nylon mesh and washed in HBSS. Multiple inguinal TDLNs were pooled from each group of mice for cell preparation.
T cell and B cell activation and expansion
CD19+ B cells were purified from the TDLN cells, healthy LN, peripheral blood mononuclear cells (PBMCs) or splenocytes using anti-CD19-coupled MACS beads (MiltenyiBiotec, Auburn, CA). CD3+ T cells were purified from PBMCs or splenocytes using anti-CD3-coupled MACS beads. B cells were activated with lipopolysaccharide (LPS, Sigma-Aldrich, Atlanta, GA) plus anti-CD40 (FGK45) mAb ascites in complete medium (CM) at 37°C with 5% CO2 for 3–4 days [7, 8]. FGK45 hybridoma cells (ATCC) were cultured and ascites were generated by the Hybridoma Core at the University of Michigan. T cells were activated with immobilized anti-CD3 and anti-CD28 mAbs in CM containing IL-2 (Prometheus Laboratories, San Diego, CA) [7, 8].
Flow cytometry
Cell expression of CD19, Fas, FasL, CD25 and IL-10 were analyzed. All conjugated antibodies (FITC or allophycocyanin anti-CD19, PE anti-Fas, PE anti-CD25, PE anti- IL-10 and PE anti-FasL) and isotype controls were purchased from BD Biosciences (San Jose, CA). For intracellular IL-10 expression, 1 million TDLN B cells were incubated with 2 µl/ml Leukocyte Activation Cocktail and 0.67 µl/ml Golgistop (BD Biosciences) in 6-well plate for 4–6 hours at 37°C with 5% CO2, and then stained with anti-IL-10. For FasL expression, purified and A/E TDLN B cells were cultured with or without 4T1 at 37°C with 5% CO2 overnight. After being fixed and permeabilized with Fixation/Perm Buffer (eBioscience, San Diego, CA), the cells were stained with anti-FasL. Isotype control staining was used to define the gates for positive and negative cells. BD FACSDiva software (version 7.0) was used for all flow cytometry analysis.
Adoptive TDLN B-cell therapy
Healthy BALB/c mice were inoculated with 5×104 4T1 cells into the mammary fat pad. Fourteen days after tumor inoculation, the tumor-bearing mice were treated with tail vein injection of activated WT or IL-10−/− 4T1 TDLN B cells. Commencing on the day of the effector B-cell transfer, intraperitoneal (i.p.) injections of IL-2 (40,000 IU) were administered in 0.5 ml of PBS and continued twice daily for 8 doses. Two weeks after B-cell transfer, all mice were sacrificed and lungs were harvested for enumeration of spontaneous pulmonary metastatic nodules. For IL-10 neutralization, the 4T1 tumor-bearing mice were prepared as above and starting on the same day as B-cell transfer mice were injected i.p. with IL-10 antibody (200 µg/mouse) or isotype control antibody (Bio X Cell, West Lebanon, NH) daily for 4 days. Two weeks after B-cell transfer, mice were sacrificed and pulmonary metastatic nodules were counted. At the same time, peripheral blood and spleens were collected for purification of PBMCs and splenic T- and B cells as described above.
Tracking adoptively transferred B cells in vivo
Activated TDLN B cells were labeled with 10 µM Cell Tracker™ Orange CMTMR (5-(and-6)-(((4-chloromethyl) benzoyl) amino) tetramethyl rhodamine) (Life Technology, Grand Island, NY) at 37°C for 45 minutes in the dark. The labeled B cells were visualized by Nikon eclipse-TE 300 and Q-capture Pro 7 software. At day 1/5/9/14 after adoptive transfer, the tumor-bearing mice and healthy mice were sacrificed and their spleens, TDLNs, lungs and tumors (healthy mice had no tumor) were collected and the labeled B cells of all B cells in these tissues were detected by flow cytometry.
LDH cytotoxicity assay
Cell cytotoxicity was assessed by measuring the release of cytoplasmic lactate dehydrogenase (LDH) using CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega, Madison, WI). Effector B cells were generated from WT or IL-10−/− TDLN B cells using LPS/anti-CD40 as described above. Target 4T1 cells were plated in triplicate in a 96-well U-bottom tissue culture plate (5000 cells/well) and co-incubated with TDLN B cells at effector to target cell ratios of 1:1, 3:1, 10:1 and 30:1. After 12 h of incubation, cells were centrifuged and 50 µl supernatant from each well was transferred to a fresh 96-well plate, 50 µl of the substrate mix was added and incubated at room temperature in the dark for 15 to 30 min. Before LDH measurement, 50 µl of stop solution was added to each well. Maximal release of LDH was performed by incubating the target cells with Lysis Solution (Promega). Target cells without effector cells were used as spontaneous release control. Absorbance was measured at 490 nm using a 96-well plate reader. For assays of PBMCs or spleen cell cytotoxicity, effector T- or B cells were generated by anti-CD3/anti-CD28 plus IL-2 or LPS/anti-CD40 activation, respectively. The T-cell killing assay was incubated for 4–6 hours instead of the 12 h used for B-cell-mediated cytotoxicity. For blocking of B-cell cytotoxicity the cultures were performed as described above in the presence or absence of 10 or 30 µg/mL anti-FasL antibody (Biolegend, San Diego, CA).
Statistical analysis
GraphPad Prism 6.0 software was used for statistical analysis. The significance of differences in numbers of metastatic nodules and cell lysis was determined using Student’s t-test. p<0.05 was considered statistically significant between the experimental groups.
Acknowledgments
This work was supported in part by NIH grant CA82529 and the Gillson Longenbaugh Foundation. It was also supported in part by National Science Fund of China (30971112), and National Outstanding Youth Foundation of China (81025008), National Natural Science Foundation of China (31221061), and National Natural Science Foundation of China (31270176).
Abbreviations
- TDLN
tumor-draining lymph node
- CM
complete medium
- s.c.
subcutaneous
- i.v.
intravenously
- i.p.
intraperitoneal
- A/E
activation/expansion
Footnotes
Conflict of Interest:
The authors declare no financial or commercial conflict of interest.
References
- 1.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward BA, Shu S, Chou T, Perry-Lalley D, Chang AE. Cellular basis of immunologic interactions in adoptive T cell therapy of established metastases from a syngeneic murine sarcoma. J Immunol. 1988;141:1047–1053. [PubMed] [Google Scholar]
- 3.Chang AE, Perry-Lalley DM, Shu S. Distinct immunologic specificity of tumor regression mediated by effector cells isolated from immunized and tumor-bearing mice. Cell Immunol. 1989;120:419–429. doi: 10.1016/0008-8749(89)90209-8. [DOI] [PubMed] [Google Scholar]
- 4.Geiger JD, Wagner PD, Cameron MJ, Shu S, Chang AE. Generation of T cells reactive to the poorly immunogenic B16-BL6 melanoma with efficacy in the treatment of spontaneous metastases. J Immunother Emphasis Tumor Immunol. 1993;13:153–165. doi: 10.1097/00002371-199304000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Li Q, Grover AC, Donald EJ, Carr A, Yu J, Whitfield J, Nelson M, et al. Simultaneous targeting of CD3 on T cells and CD40 on B or dendritic cells augments the antitumor reactivity of tumor-primed lymph node cells. J Immunol. 2005;175:1424–1432. doi: 10.4049/jimmunol.175.3.1424. [DOI] [PubMed] [Google Scholar]
- 6.Iuchi T, Teitz-Tennenbaum S, Huang J, Redman BG, Hughes SD, Li M, Jiang G, et al. Interleukin-21 augments the efficacy of T-cell therapy by eliciting concurrent cellular and humoral responses. Cancer Res. 2008;68:4431–4441. doi: 10.1158/0008-5472.CAN-07-5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Q, Teitz-Tennenbaum S, Donald EJ, Li M, Chang AE. In vivo sensitized and in vitro activated B cells mediate tumor regression in cancer adoptive immunotherapy. J Immunol. 2009;183:3195–3203. doi: 10.4049/jimmunol.0803773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q, Lao X, Pan Q, Ning N, Yet J, Xu Y, Li S, Chang AE. Adoptive transfer of tumor reactive B cells confers host T-cell immunity and tumor regression. Clin Cancer Res. 2011;17:4987–4995. doi: 10.1158/1078-0432.CCR-11-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mauri C, Ehrenstein MR. The 'short' history of regulatory B cells. Trends Immunol. 2008;29:34–40. doi: 10.1016/j.it.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Lapointe R, Bellemare-Pelletier A, Housseau F, Thibodeau J, Hwu P. CD40-stimulated B lymphocytes pulsed with tumor antigens are effective antigen-presenting cells that can generate specific T cells. Cancer Res. 2003;63:2836–2843. [PubMed] [Google Scholar]
- 11.Lundy SK. Killer B lymphocytes: the evidence and the potential. Inflamm Res. 2009;58:345–357. doi: 10.1007/s00011-009-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perricone MA, Smith KA, Claussen KA, Plog MS, Hempel DM, Roberts BL, St George JA, Kaplan JM. Enhanced efficacy of melanoma vaccines in the absence of B lymphocytes. J Immunother. 2004;27:273–281. doi: 10.1097/00002371-200407000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Qin Z, Richter G, Schuler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nat Med. 1998;4:627–630. doi: 10.1038/nm0598-627. [DOI] [PubMed] [Google Scholar]
- 14.Shah S, Divekar AA, Hilchey SP, Cho HM, Newman CL, Shin SU, Nechustan H, et al. Increased rejection of primary tumors in mice lacking B cells: inhibition of anti-tumor CTL and TH1 cytokine responses by B cells. Int J Cancer. 2005;117:574–586. doi: 10.1002/ijc.21177. [DOI] [PubMed] [Google Scholar]
- 15.Evans DE, Munks MW, Purkerson JM, Parker DC. Resting B lymphocytes as APC for naive T lymphocytes: dependence on CD40 ligand/CD40. J Immunol. 2000;164:688–697. doi: 10.4049/jimmunol.164.2.688. [DOI] [PubMed] [Google Scholar]
- 16.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 17.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 18.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inoue S, Leitner WW, Golding B, Scott D. Inhibitory effects of B cells on antitumor immunity. Cancer Res. 2006;66:7741–7747. doi: 10.1158/0008-5472.CAN-05-3766. [DOI] [PubMed] [Google Scholar]
- 20.Schioppa T, Moore R, Thompson RG, Rosser EC, Kulbe H, Nedospasov S, Mauri C, et al. B regulatory cells and the tumor-promoting actions of TNF-alpha during squamous carcinogenesis. Proc Natl Acad Sci U S A. 2011;108:10662–10667. doi: 10.1073/pnas.1100994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182:7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci. 2010;1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- 23.Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, Mauri C. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178:7868–7878. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- 24.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koni PA, Bolduc A, Takezaki M, Ametani Y, Huang L, Lee JR, Nutt SL, et al. Constitutively CD40-activated B cells regulate CD8 T cell inflammatory response by IL-10 induction. J Immunol. 2013;190:3189–3196. doi: 10.4049/jimmunol.1203364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klinker MW, Lundy SK. Multiple mechanisms of immune suppression by B lymphocytes. Mol Med. 2012;18:123–137. doi: 10.2119/molmed.2011.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hahne M, Renno T, Schroeter M, Irmler M, French L, Bornard T, MacDonald HR, Tschopp J. Activated B cells express functional Fas ligand. Eur J Immunol. 1996;26:721–724. doi: 10.1002/eji.1830260332. [DOI] [PubMed] [Google Scholar]
- 29.Strater J, Mariani SM, Walczak H, Rucker FG, Leithauser F, Krammer PH, Moller P. CD95 ligand (CD95L) in normal human lymphoid tissues: a subset of plasma cells are prominent producers of CD95L. Am J Pathol. 1999;154:193–201. doi: 10.1016/S0002-9440(10)65265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blair PA, Chavez-Rueda KA, Evans JG, Shlomchik MJ, Eddaoudi A, Isenberg DA, Ehrenstein MR, Mauri C. Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J Immunol. 2009;182:3492–3502. doi: 10.4049/jimmunol.0803052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maseda D, Candando KM, Smith SH, Kalampokis I, Weaver CT, Plevy SE, Poe JC, Tedder TF. Peritoneal Cavity Regulatory B Cells (B10 Cells) Modulate IFN-gamma+CD4+ T Cell Numbers during Colitis Development in Mice. J Immunol. 2013;191:2780–2795. doi: 10.4049/jimmunol.1300649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aruga A, Aruga E, Tanigawa K, Bishop DK, Sondak VK, Chang AE. Type 1 versus type 2 cytokine release by Vbeta T cell subpopulations determines in vivo antitumor reactivity: IL-10 mediates a suppressive role. J Immunol. 1997;159:664–673. [PubMed] [Google Scholar]
- 33.Nilsson N, Ingvarsson S, Borrebaeck CA. Immature B cells in bone marrow express Fas/FasL. Scand J Immunol. 2000;51:279–284. doi: 10.1046/j.1365-3083.2000.00701.x. [DOI] [PubMed] [Google Scholar]
- 34.Tian J, Zekzer D, Hanssen L, Lu Y, Olcott A, Kaufman DL. Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J Immunol. 2001;167:1081–1089. doi: 10.4049/jimmunol.167.2.1081. [DOI] [PubMed] [Google Scholar]
- 35.Klinker MW, Reed TJ, Fox DA, Lundy SK. Interleukin-5 Supports the Expansion of Fas Ligand-Expressing Killer B Cells that Induce Antigen-Specific Apoptosis of CD4(+) T Cells and Secrete Interleukin-10. PLoS One. 2013;8:e70131. doi: 10.1371/journal.pone.0070131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harada M, Okamoto T, Kurosawa S, Shinomiya Y, Ito O, Takenoyama M, Terao H, et al. The antitumor activity induced by the in vivo administration of activated B cells bound to anti-CD3 monoclonal antibody. Cell Immunol. 1995;161:132–137. doi: 10.1006/cimm.1995.1017. [DOI] [PubMed] [Google Scholar]
- 37.Messina JL, Fenstermacher DA, Eschrich S, Qu X, Berglund AE, Lloyd MC, Schell MJ, et al. 12-Chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy? Sci Rep. 2012;2:765. doi: 10.1038/srep00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brennen WN, Drake CG, Isaacs JT. Enhancement of the T-cell armamentarium as a cell-based therapy for prostate cancer. Cancer Res. 2014;74:3390–3395. doi: 10.1158/0008-5472.CAN-14-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skitzki J, Craig RA, Okuyama R, Knibbs RN, McDonagh K, Chang AE, Stoolman LM. Donor cell cycling, trafficking, and accumulation during adoptive immunotherapy for murine lung metastases. Cancer Res. 2004;64:2183–2191. doi: 10.1158/0008-5472.can-03-2799. [DOI] [PubMed] [Google Scholar]