Abstract
PURPOSE
To describe and characterize the surface topography and cleanliness of CAD/CAM manufactured zirconia abutments after steaming and ultrasonic cleaning.
MATERIALS AND METHODS
A total of 12 ceramic CAD/CAM implant abutments of various manufacturers were produced and randomly divided into two groups of six samples each (control and test group). Four two-piece hybrid abutments and two one-piece abutments made of zirconium-dioxide were assessed per each group. In the control group, cleaning by steam was performed. The test group underwent an ultrasonic cleaning procedure with acetone, ethyl alcohol and antibacterial solution. Groups were subjected to scanning electron microscope (SEM) analysis and Energy-dispersive X-ray spectroscopy (EDX) to verify and characterize contaminant chemical characterization non-quantitatively.
RESULTS
All zirconia CAD/CAM abutments in the present study displayed production-induced wear particles, debris as well as organic and inorganic contaminants. The abutments of the test group showed reduction of surface contamination after undergoing an ultrasonic cleaning procedure. However, an absolute removal of pollutants could not be achieved.
CONCLUSION
The presence of debris on the transmucosal surface of CAD/CAM zirconia abutments of various manufacturers was confirmed. Within the limits of the study design, the results suggest that a defined ultrasonic cleaning process can be advantageously employed to reduce such debris, thus, supposedly enhancing soft tissue healing. Although the adverse long-term influence of abutment contamination on the biological stability of peri-implant tissues has been evidenced, a standardized and validated polishing and cleaning protocol still has to be implemented.
Keywords: CAD/CAM zirconia abutments, Hybrid abutments, Abutment surface, Spectral-Electron-Microscopy (SEM), Energy Dyspersive X-ray spectroscopy (EDX), Surface topography, Surface contamination, Peri-implant soft tissue response, Ultrasonic cleaning
INTRODUCTION
There is considerable evidence to support the view that the long-term success of an implant rehabilitation significantly depends upon a proper peri-implant soft tissue integration, serving as a protective barrier between the oral environment and the underlying peri-implant bone.1,2,3 Peri-implant mucosa is generally recognized as a hypovascular and hypocellular scar tissue. It is immunologically highly inferior to the periodontal tissues around teeth, since it exhibits an impaired resistance to bacterial colonization.4,5 Consequently, recurrently challenging hazards that could have adverse effects on the attachment of peri-implant soft tissues, such as bacterial accumulation, mechanical overloading and prosthetic manipulation, should be avoided.1,6,7 Implant abutments, as part of the implant superstructure, are in direct contact with the surrounding tissues, influencing the soft tissue health and profile. Thus, the material, surface topography and cleanliness of an implant abutment seem to be of decisive importance for the quality of the attachment that forms between the mucosa and the abutment itself. Contaminants have been found on the external and internal surfaces of customized implant abutments after laboratory procedures, even after commonly applied cleaning procedures (i.e. vapour steaming).8,9 Such debris, present at the critical implant-tissue interface, could detrimentally influence the inflammatory response of peri-implant tissues. Plaque formation and bacteria colonization influenced by surface properties of the abutment and implant collar are considered to play a key role in the pathogenesis of infections. 10,11,12,13,14,15,16,17 At the same time, microbiological contamination due to different laboratory steps and auxiliary staff management has been documented on customized prosthetic components.8 The absence of micro-contaminants, on the other hand, could reduce the soft and hard tissue reaction to abutment insertion, reducing external bacterial adhesion and osteoclast activity.18,19 Different approaches are used to clean abutments. A steaming process was so far supposed to offer sufficent decontamination of abutment surfaces after technical stages of customization in the laboratory. Although surface pollutions can be substantially decreased after steaming, it does not allow for a complete surface cleaning. In addition, ultrasonic treatment has been recommended to clean dental and implant restorations before clinical use.20,21 High-frequency sound waves are applied to mechanically remove contaminants in an aqueous or organic medium. However, a lack of evidence is present regarding the quality of finishing on the abutment surface purity. Effective abutment decontamination protocols before packaging and minimal standards have not been determined. Stock titanium abutments were demonstrated to present contaminants and debris on the surface and at the connection level before and after technical laboratory procedures, such as milling, polishing and steam-cleaning.9 It has been shown that there are differences between brands regarding titanium stock abutment cleaning, surface morphology and composition, following the manufacturing and packaging processes.22 A recently randomized clinical trial demonstrated statistically less peri-implant bone resorption in a group of patients whose commercially available and customized titanium abutments were cleand with argon plasma versus a group with 5-second steam-cleaning.23,24
To date, there is scant information on the surface topography and cleanliness of computer-aided designed and manufactured (CAD/CAM) custom made zirconia abutments. Due to their tooth-like colour, superior fracture strength and possible biologic advantages zirconia abutments are increasingly used to achieve optimal mucogingival esthetics.25,26 In addition to the variety of implant-abutment connections available, it is possible to use prefabricated zirconia stock abutments (SZ) or computer-aided designed (CAD), computer-aided manufactured (CAM) zirconia abutments. Preventing soft tissue discolouration in cases of a thin gingival biotype, CAD/CAM custom zirconia abutments can be predictably designed to re-create the desired supporting crown orientation and morphology, facilitating the formation of anatomical mucosal topography and coronal contours for prosthetic replacement.27,28,29 One-piece (OP) and two-piece (TP) CAD/CAM zircona abutments are available today. OP abutments are completely manufactured in a central production process by CAD/CAM technology, including the implant abutment connection. TP abutments consist of a pre-fabricated insert-base of titanium on which a customized CAD/CAM zirconia coping is cemented in the laboratory (hybrid abutments).30 Significantly less wear at the implant-abutment interface and higher bending was achieved for CAD/CAM zirconia abutments with internal-hex connections via a secondary titanium insert (TP) than for OP and SZ abutments.31,32,33 To optimally take advantage of CAD/CAM zirconia abutments their surface morphology should enhance a soft tissue attachment on the one hand but, on the other hand, not favour mechanical plaque retention in order to prevent inflammatory processes. An Ra value of 0.2 µm was therefore suggested as a threshold surface roughness, below which bacterial adhesion cannot be reduced further.15,34 It has been reported that different abutment materials promote selective adherence during early plaque formation. The potential advantages of zirconia compared to titanium, with respect to biofilm formation in the oral cavity, has been demonstrated in various studies.28,35,36,37
Considering perio-prosthetic, functional and hygienic aspects, high quality pre-requisites have to be fulfilled by the surface of implant abutments during and after manufacturing processes. This applies both to the local manufacturing of CAD/CAM abutments in the laboratory as well as to its central milling by the CAD/CAM industry. The influence of conventional cleaning protocols on the surface property of zirconia-ceramic implant abutments has not yet been investigated and reliable, qualitative or quantitative surface evaluations are missing. The aim of the study was therefore to non-quantitaively describe and characterize contaminants at the transmucosal region of CAD/CAM zirconia abutments after steaming (control) and ultrasonic cleaning (test), using both SEM and chemical microanalysis. The working hypothesis was that no qualitative differences exist in abutment surface pollution among the two different experimental groups.
MATERIALS AND METHODS
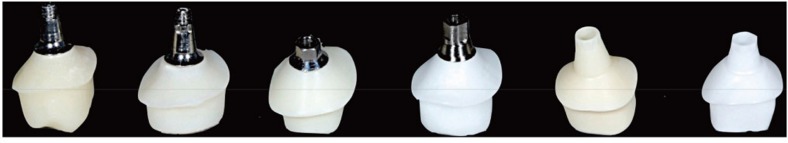
A total of 12 ceramic CAD/CAM implant abutments were produced for the present in vitro study and randomly divided into two identical groups (control and test group) of six samples each. Four two-piece hybrid abutments and two one-piece abutments made of zirconium oxide were assessed per each group. They included the following CAD/CAM systems and abutment pieces (subsequently referred to as samples 1-6) (Fig. 1):
Fig. 1. The six one- and two-piece ceramic CAD/CAM abutments examined in the test and control group (left to right): Sample 1: Compartis (Dentsply Degudent, Hanau, Germany) CAD/CAM zirconia coping on titanium insert with implant-abutment connection for Ankylos C implant; Sample 2: Custom milled lithium-disilicate coping (IPS e.max, Ivoclar Vivadent GmbH, Liechtenstein) on titanium insert with implant-abutment connection for Ankylos C/X implant; Sample 3: Bego CADAbut (Bego Medical, Bremen, Germany): Zirconia coping on titanium insert with implant-abutment connection for Semados implant; Sample 4: MedentiCAD (Medentika Implant GmbH, Hügelsheim, Germany): Zirconia coping on titanium insert with implant-abutment connection for Straumann Bone Level implant; Sample 5: Atlantis (Dentsply Implants, Mannheim, Germany): Zirconia abutment including the implant-abutment connection for Astra OsseoSpeed implant; Sample 6: Procera (Nobel Biocare, Zürich, Switzerland): Zirconia abutment including the implant-abutment connection for Nobel Active implant.
Two-piece abutments:
Sample 1. Compartis (Dentsply Degudent, Hanau, Germany): CAD/CAM zirconia coping on titanium insert with implant-abutment connection for Ankylos C implant.
Sample 2. Custom-milled lithium-disilicate coping (IPS e.max, Ivoclar Vivadent GmbH, Liechtenstein) on titanium insert with implant-abutment connection for Ankylos C/X implant.
Sample 3. Bego CADAbut (Bego Medical, Bremen, Germany): Zirconia coping on titanium insert with implant-abutment connection for Semados implant.
Sample 4. MedentiCAD (Medentika Implant GmbH, Hügelsheim, Germany): Zirconia coping on titanium insert with implant-abutment connection for Straumann Bone Level implant.
Two-piece abutments:
Sample 5. Atlantis (Dentsply Implants, Mannheim, Germany): Zirconia abutment including the implant-abutment connection for Astra OsseoSpeed implant.
Sample 6. Procera (Nobel Biocare, Zurich, Switzerland): Zirconia abutment including the implant-abutment connection for Nobel Active implant.
The master cast of a clinical case in which the right maxillary first molar had been replaced by an implant restoration served as model of origin. The emergence profile of the peri-implant mucosa had been pre-conditioned by means of a temporary implant-supported single crown. Six replica of the master cast were fabricated from dental stone and adjusted by a parallelometer in order to align the planned implant analogs of the various manufacturers in the same vertical and horizontal position. This ensured identical fabrication conditions for the abutments, despite the different implant-abutment geometries of the implant analogs. After a central drilling of the implant position planned, the corresponding implant analog of the respective manufacturer was positioned and plaster-embedded. As a result, six master casts were fabricated with an identical implant-shoulder-to-emergence-profile ratio. A standardised wax-up of the abutments was fabricated from try-in acrylic (with six different implant-abutment geometries) to ensure the comparability of the abutment samples of the various CAM-systems on the different implant types (Fig. 2). The sample-design for the one and two-piece CAD/CAM abutments were identical in their outer geometry and designed to allow placement of the crown margin slightly below the mucosa, following its scalopped anatomy. In case of the two-piece hybrid abutments, the bonding surfaces of the titanium inserts and zircona sleeves were blasted (aluminium oxide particles 50 µm; 2 bar/0.25 MPa; 20 seconds; distance 10 mm) and cleansed with alcohol. Subsequently, the titanium inserts were wetted with a metal-primer solution (GC MetalPrimer II, GC EUROPE N.V, Leuven, Belgium), whereas a bonding material was applied on the basal sections of the CAD/CAM zirconia sleeves (Monobond Plus, Ivoclar Vivadent, Schaan, Liechtenstein). All hybrid abutments were luted with a resin cement (Multilink Implant, Ivoclar Vivadent GmbH) following the manufacturer's specifications. Finally, removal of the bonding excess was performed as well as polishing of the bonding joint with silicone polishers and polishing paste according to a previously documented protocol.30
Fig. 2. Occlusal view of abutment samples on the respective master cast reveals differently pronounced emergence profiles and variously shaped abutment shoulders, despite mandatory abutment design (standardised wax-up of the abutment from try-in acrylic).
The delivered CAD/CAM abutment samples of the control group were solely steam-cleaned for 30s (VAP 1; Zhermark, Cologne, Germany) and subsequently examined for surface pollution by confocal microscopy. The particles detected on the surfaces were qualitatively analysed using a combination of spectral electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDX). The high-electron beam produced by the electronic microscope is known to impinge the sample surface and to stimulate the emission of characteristic X-rays from the contaminants. The emitted x-rays detected by EDX allow to obtain chemical profiles of the different element found on the abutment surface. Prior to microscopic analysis all ceramic abutment samples were sputter coated with a thin film of gold alloy. This conductive coating is needed to prevent charging of a specimen with an electron beam in high vacuum. 22 The abutments of the test group underwent a standardized ultrasonic cleaning procedure according to Canullo et al. prior to analysis.38 The samples were cleansed three times in an ultrasonic bath at 60℃ for 10 min each. The first bath contained pure acetone, the second pure ethylalcohol and the third one an antibacterial cleansing solution (Cl 4%, Fa. Soltec, Milano, Italy). After each solution, samples were immersed in demineralized water for 5 min at 60℃. Finally, the abutments were sprayed with nitrogen and subdued to SEM and EDX analysis.
RESULTS
Control group (Steam-cleaned abutments): SEM analysis and EDX characterisation of the abutment surfaces
After steam-cleaning, the various implant abutments presented differences with regard to their surface quality and cleanliness. Each of the CAD/CAM abutments were examined and displayed surface contamination of various degrees. On- and/or intra-layered particles or roughness resulting from the mechanical milling during the CAM process (Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9, A-D) were detected; (References to the tables in the captions to the figures: 0-values mean that the element was detected; blank spaces indicate that the element was not found. All values in atom %).
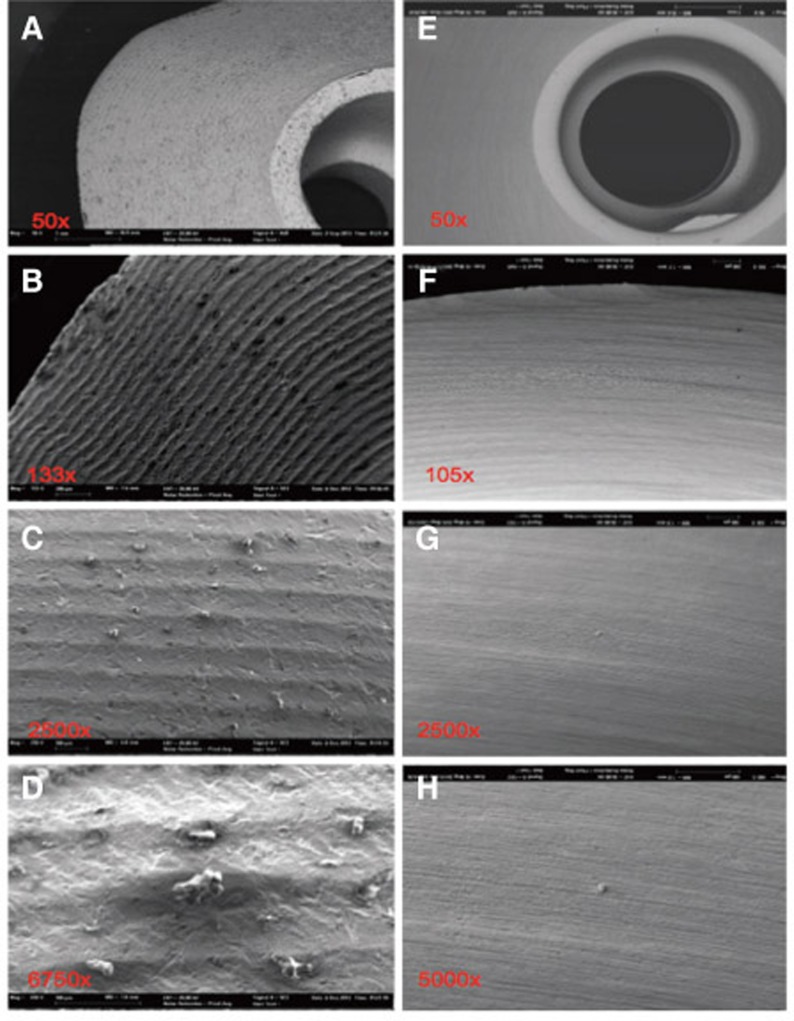
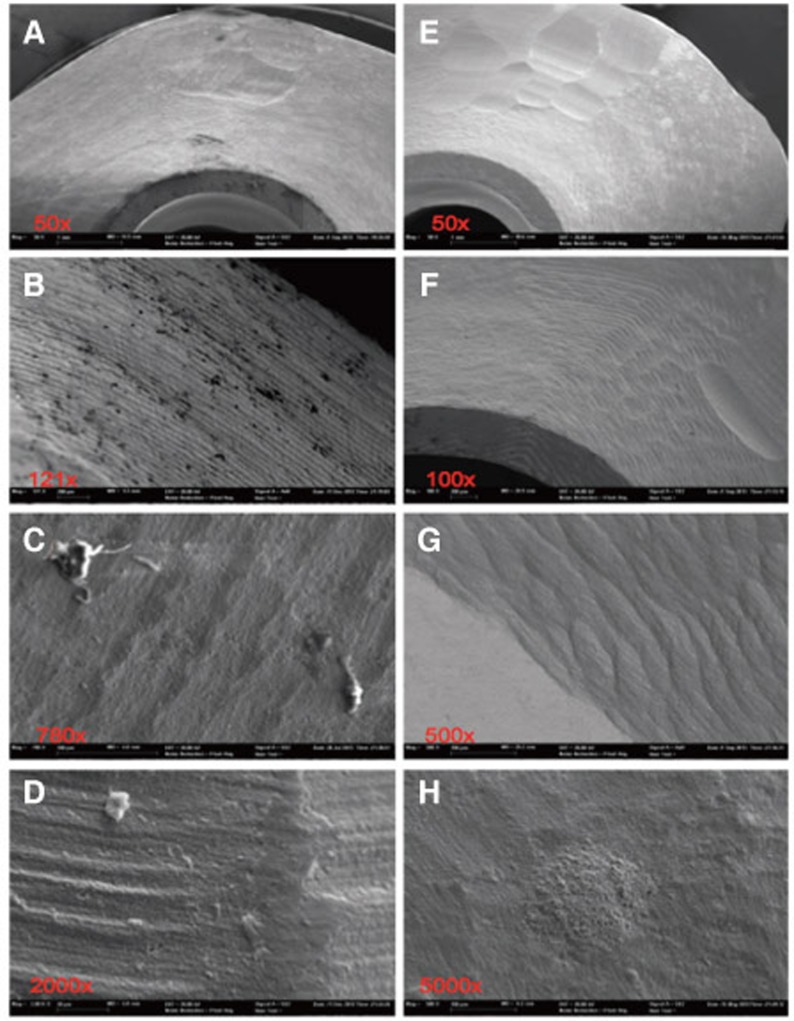
Fig. 4. Severely contaminated surface, distinctive and square-edge groove milling at steam-cleaned status (A-D) of sample 1; after ultra-soniccleaning (E-H) clear particle reduction, but residual machining traces.
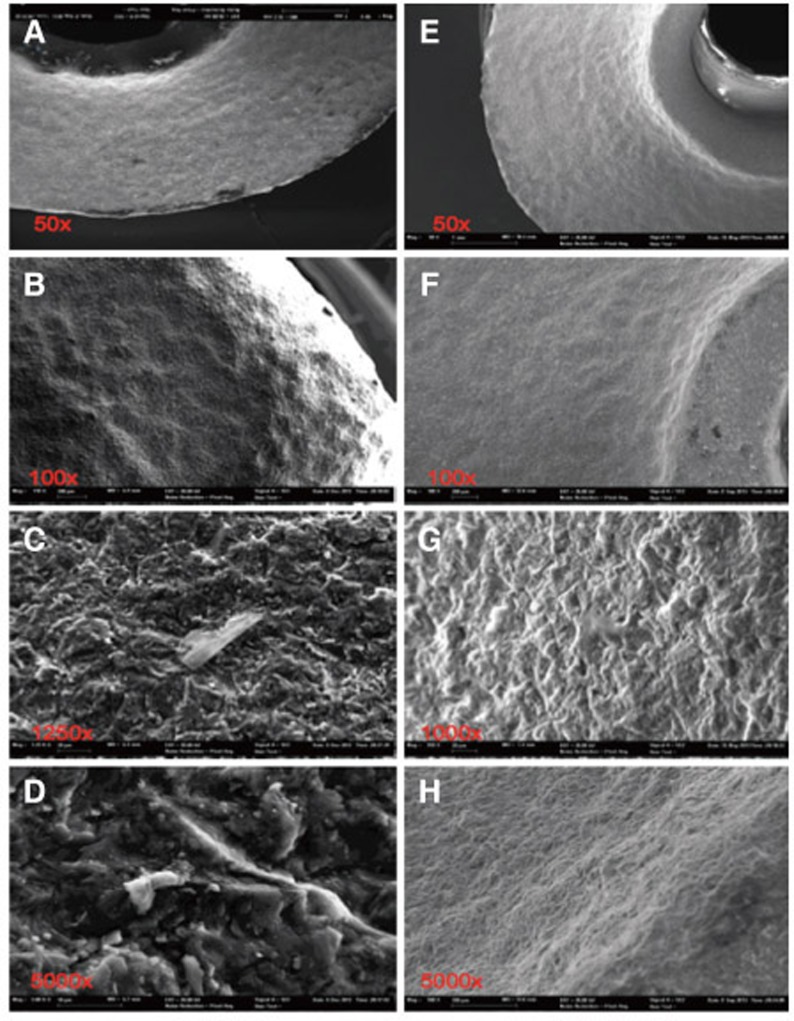
Fig. 5. Pictures A-D of steam-cleaned sample 2 show clearly visible contamination and a rippled, rounded edge profile. After ultra-sonic cleaning (E-H) the surface is still rough, but almost free of particles.
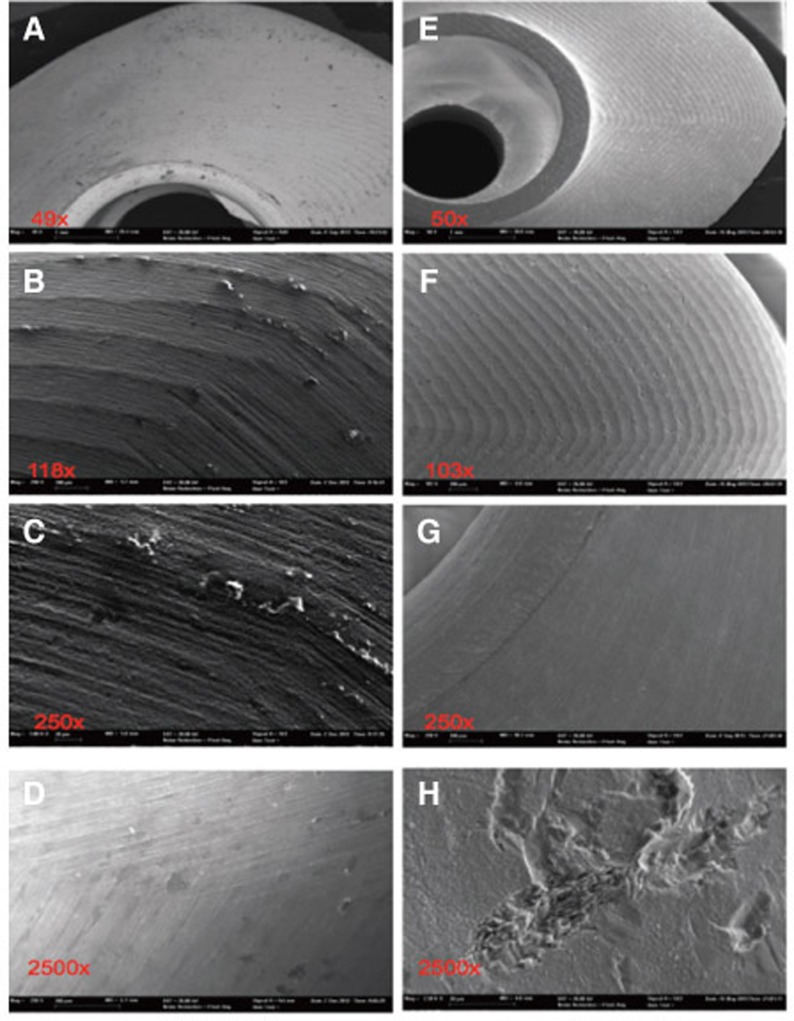
Fig. 6. Substantial particle and milling traces on the steam-cleaned sample 3 (A-D); particle-free surface after ultra-sonic cleaning (E-H), but severe roughening and impact traces resulting from CAD/CAM machining.
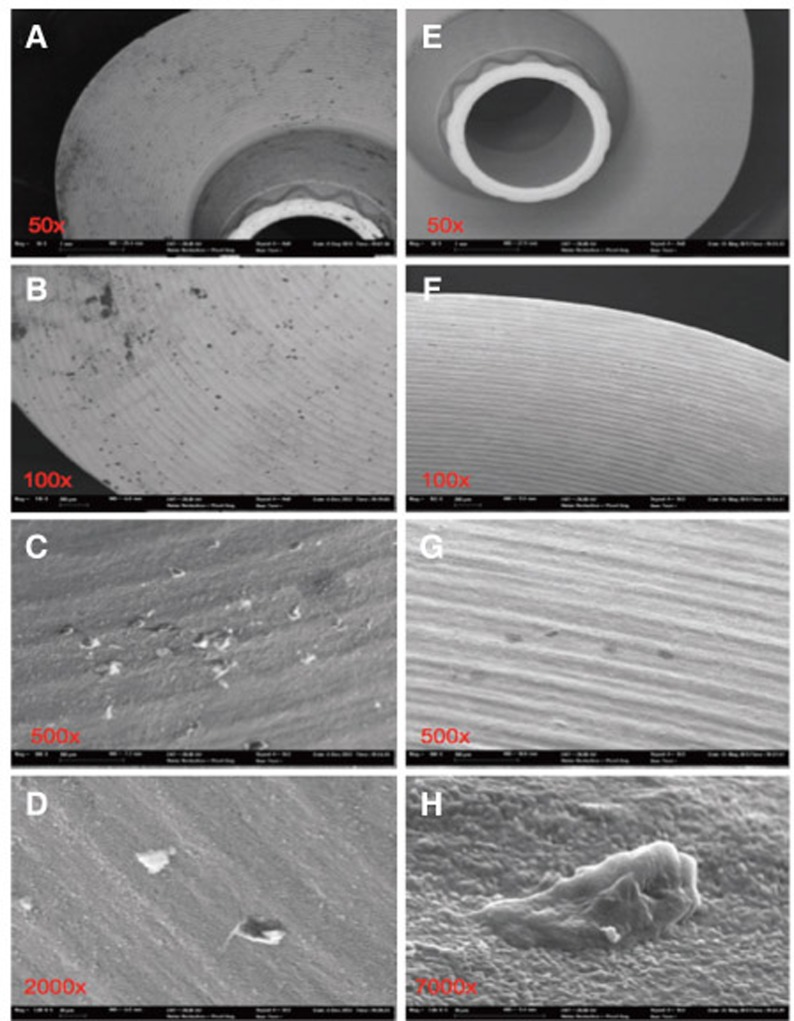
Fig. 7. Sample 4 before steam-cleaning (A-D) with substantial debris on the cone and the contact surface as well as severely roughened surface and isolated defects. Particles could be removed after the ultra-sonic cleaning process; CAD/CAM milling roughnesses and defects remained (E-H).
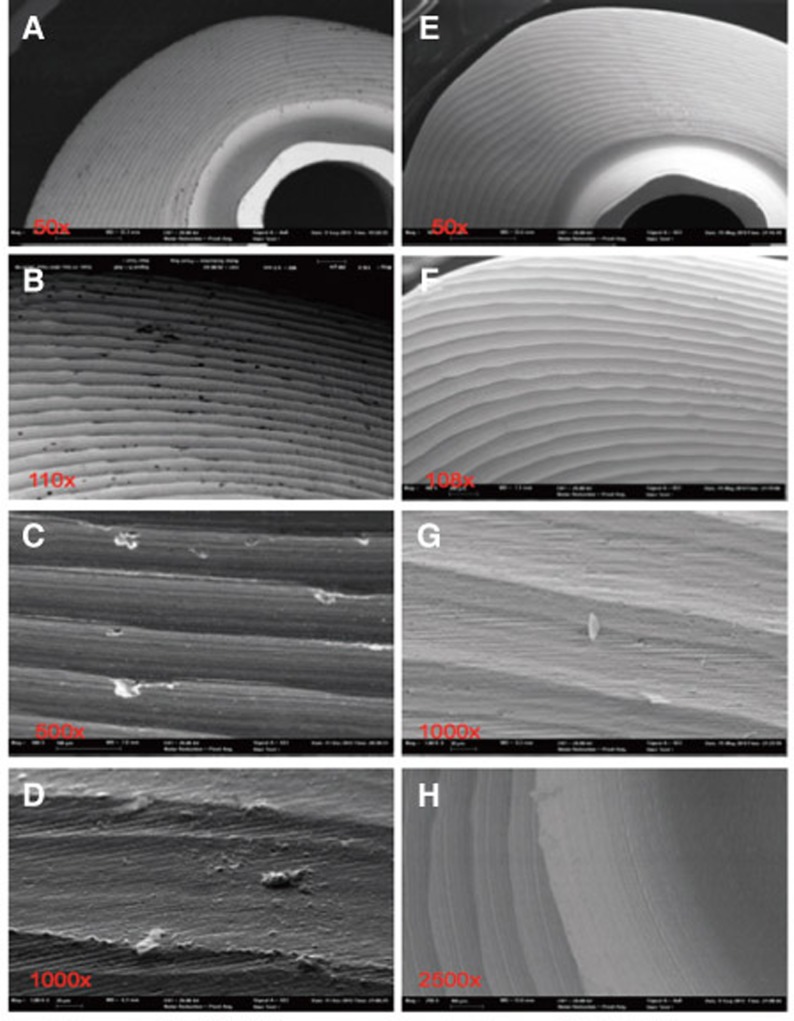
Fig. 8. Pictures A-D show steam-cleaned surface status with clearly visible debris and milling grooves. Pictures E-H reveal the reduction of surface contamination after ultrasonic cleaning procedure, with remaining pollutants (Sample 5).
Fig. 9. Sample 6 in steam-cleaned status (A-D) with significant particle debris and distinctive groove milling; after ultra-sonic cleaning (E-H) relatively isotropic surface roughness (picture g: remains of glass after cleaning, probably from internal glass vial used for transport).
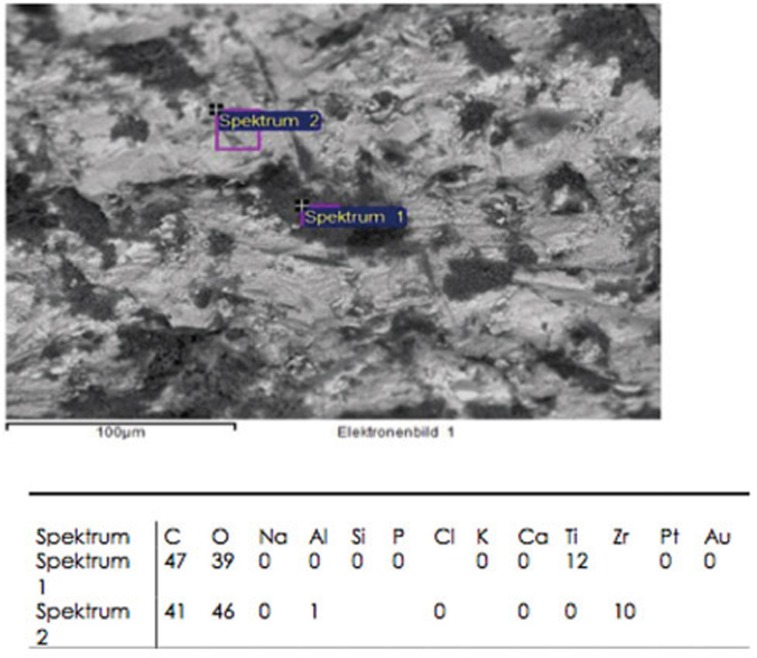
The chemical elements identified on the steam-cleaned abutment samples, both on the residual particles and/or roughnesses as well as on the smooth surfaces were registered. The elements primarily included aluminium (Al), boron (B), carbon (C), oxygen (O) and silicium (Si) in higher atomic percentages (up to more than 80 atom %) (Example Fig. 3). They occurred together with elements in single-digit or lower atomic percentages as e.g. with bromine (Br), chlorine (Cl), iron (Fe), potassium (Ka), calcium (Ca), copper (Cu), magnesium (Mg), sulphur (S), silicium (Si) or titanium (Ti). In addition, astatine (At), a radioactive element, chrome (Cr), phosphorus (P), platinum (Pt) and zink (Zn) were also detected. The element hafnium (Hf) often occurred in combination with the element zirconium and therefore cannot necessarily be regarded as contamination. The same is valid for the element yttrium which is used for stabilising the tetragonal status of zirconium oxide (Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9, A-D). The zirconia particles themselves were detected due to the roughening procedure during the manufacturing process. Aluminium and hafnium are also ingredients of polishing paste and could have, consequently, originated from polishing procedures. While traces of sulphur seem to residue from cleaning processes during the main production and cleansing procedure of the CAD/CAM abutments, traces of chlorine indicate an insufficiently removed cleansing solution.
Fig. 3. Example of spectra table of sample 1 (in atom percent): Among others contamination with titanium.
Test group (Ultrasonic cleaned abutments): SEM analysis and EDX characterisation of the abutment surfaces
Due to the ultrasonic cleaning process (acetone, ethyl alcohol, antibacterial cleaning solution for 10 min at 60° each), the pollutants on all abutment samples could be substantially reduced, but not completely removed (Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9, E-F). The EDX analysis revealed quantitatively small amounts of residual on- and inlay particles of zirconia, carbon and oxygen as well as, individually, aluminium and hafnium. Gold was detected in all cases which was linked to the prior gold sputtering of the ceramic abutments needed for examination by scanning electron microscopy. On sample 6 elements of silicium (Si), oxygen (O), sodium (Na) and aluminium (Al) was traced after the cleansing process. Typical components of glass could be detected. Considering the clear shape associated to a glass splinter and the brand-new glass vial used as transport container, it can be assumed that the particle identified was in fact a glass splinter (Fig. 9; G).
DISCUSSION
It has been documented that the properties of the abutment material placed in contact with the surrounding soft tissues have a decisive impact on the quality of the mucosal attachment.1 The present study revealed clear production-specific differences of the surface properties and purity of industrially and lab-fabricated CAD/CAM zirconia abutments. There was no abutment sample lacking significant contamination at delivery and steam-cleaning. The abutment surfaces displayed onlay and/or inlay contaminants which could be effectively reduced, but not completely removed after undergoing an ultrasonic cleaning for three times (acetone, ethyl alcohol, antibacterial solution). Direct and indirect biological but also biomechanical reactions have been discussed in the literature related to the presence of micro-contaminations on the transmucosal abutment area and basal implant-abutment connection. Contaminated surfaces can influence the process of intial soft tissue healing and attachment negatively, but also provoke an inflammatory hard tissue reaction with increased osteoclast activity. 18,19 From a mechanical point of view, debris and microresidues at the basal platform of an abutment could negatively impair the stability of the implant-abutment connection and the size of the micro-gap.39
The results of the present in vitro examination have been sustained by recent investigations. Canullo et al. demonstrated micro-contamination of various origins in lab studies on prefabricated and customized CAD/CAM milled titanium abutments.8,9,38 In the present study, these microcontaminants could be significantly reduced due to the cleaning procedure applied. In two randomized prospective clinical trials on periodontally healthy patients and patients with a history of periodontal disease, the same group of authors has proven superior bone level maintanance arround single implant-supported restorations with titanium abutments cleaned with argon plasma.23,24 Better hard tissue response around cleaned abutments suggests that cleaning procedures aimed at removing contamination could be strongly recommended. Further studies are needed to examine whether a plasma cleaning procedure has similarly positive clinical effects on zirconia abutments. Some of the abutment samples of the present lab study showed mechanically induced surface defects in the transmucosal region which manifested as roughnesses produced by irregularities during the CAM milling process. (Fig. 7; a & e). These production defects should be minimized from a biological and material processing point of view. Considering the use of an identical and standardized abutment wax-up for the inividual design and milling of the respective abutment sample, it was surprising to discover differently pronounced emergence profiles and variously shaped abutment shoulders among the zirconia abutments produced. No substantiated statements can be made on the reasons for these differences and their clinical relevance. However, the differing designs of the abutments based on a standardized model are thought-provoking with regard to the praised precision of CAD/CAM technology.
Due to their clinical significance, validated polishing and cleaning protocols for the processing of CAD/CAMgenerated abutments should be introduced with recommendations regarding the surface treatment. However, it should be considered that a subsequent excessive conditioning of the surface, especially inappropriate grinding, can have a negative effect on the resistance to fracture of the zirconia ceramic. Demands not to recondition CAD/CAM abutments after central production can no longer be sustained considering the results available. Diamond-coated polishing tools of decreasing grit-size seem to be suitable for polishing transmucosal abutment surfaces (colour-coding: blue, red, grey for high lustre).30 In this case the surface in the transmucosal region must neither be polished too smoothly to prevent initial attachment loss, nor be left too rough to abet an increased plaque adhesion. It has been proven that a reduction of the surface roughness below the threshold Sa-value of 0.2 µm will result in a retardation of the supra-and submucosal plaque maturation. On the other hand, a too smooth abutment surface might interfere with the stability of the soft tissue attachment. Therefore, a surface roughness of Sa = 0.2 µm for the transmucosal area of implant abutments has been proposed as a balance between both aspects (bacterial adhesion and soft tissue sealing).34 Clinically a tolerance Sa-value between 0.15 - 0.25 µm seems to be appropriate. With reference to Albrektsson and Wennerberg,40 a classification into non-tolerable, tolerable and optimal abutment-surface-roughnesses (ASR) could be suggested for daily practice. Based on the literature, the authors recommend the following Sa-values that still have to be validated by future investigations. However, it is mandatory to consider that the values recommended can be applied only for the submucosal contact region and not for the retention surfaces of the correspondig crown:
Classification of abutment-surface-roughnesses (ASR)
Sa < 0.10 µm
non-tolerable ASR (too smooth, imminent attachment loss)
Sa = 0.10 - 0.15 µm
tolerable ASR (not too smooth)
Sa = 0.15 - 0.25 µm
optimal ASR
Sa = 0.25 - 0.35 µm
tolerable ASR (not too rough)
Sa > 0.35 µm
non-tolerable ASR (too rough, imminant microbial contamination)
CONCLUSION
The presence of debris on the transmucosal surface of CAD/CAM zirconia abutments of various manufacturers was confirmed. Within the limits of the present in vitro study, the results confirm a substantial difference in the surface contamination between the control group (Zirconia CAD/CAM abutments solely treated by steam-cleaning) and the test group (Zirconia CAD/CAM abutments cleaned by ultrasound). A defined ultrasonic cleaning process can be advantageously employed to reduce such debris, thus, supposedly enhancing soft tissue healing. Although the adverse long-term influence of abutment contamination on the biological stability of peri-implant tissues has been evidenced, a standardised and validated polishing and cleaning protocol still has to be implemented.
ACKNOWLEDGEMENTS
The authors express their sincere gratitude to Dr. Matthias Barczewski for his specialist councel at performing and documenting the study design. We gratefully acknowledge Mrs. Lolita Reder for her assistance during the review process.
References
- 1.Abrahamsson I, Berglundh T, Lindhe J. The mucosal barrier following abutment dis/reconnection. An experimental study in dogs. J Clin Periodontol. 1997;24:568–572. doi: 10.1111/j.1600-051x.1997.tb00230.x. [DOI] [PubMed] [Google Scholar]
- 2.Lindhe J, Berglundh T. The interface between the mucosa and the implant. Periodontol 2000. 1998;17:47–54. doi: 10.1111/j.1600-0757.1998.tb00122.x. [DOI] [PubMed] [Google Scholar]
- 3.Welander M, Abrahamsson I, Berglundh T. The mucosal barrier at implant abutments of different materials. Clin Oral Implants Res. 2008;19:635–641. doi: 10.1111/j.1600-0501.2008.01543.x. [DOI] [PubMed] [Google Scholar]
- 4.Buser D, Weber HP, Donath K, Fiorellini JP, Paquette DW, Williams RC. Soft tissue reactions to non-submerged unloaded titanium implants in beagle dogs. J Periodontol. 1992;63:225–235. doi: 10.1902/jop.1992.63.3.225. [DOI] [PubMed] [Google Scholar]
- 5.Berglundh T, Lindhe J, Jonsson K, Ericsson I. The topography of the vascular systems in the periodontal and peri-implant tissues in the dog. J Clin Periodontol. 1994;21:189–193. doi: 10.1111/j.1600-051x.1994.tb00302.x. [DOI] [PubMed] [Google Scholar]
- 6.Barboza EP, Caúla AL, Carvalho WR. Crestal bone loss around submerged and exposed unloaded dental implants: a radiographic and microbiological descriptive study. Implant Dent. 2002;11:162–169. doi: 10.1097/00008505-200204000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Misch CE, Dietsh-Misch F, Hoar J, Beck G, Hazen R, Misch CM. A bone quality-based implant system: first year of prosthetic loading. J Oral Implantol. 1999;25:185–197. doi: 10.1563/1548-1336(1999)025<0185:ABQISF>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Canullo L, Micarelli C, Lembo-Fazio L, Iannello G, Clementini M. Microscopical and microbiologic characterization of customized titanium abutments after different cleaning procedures. Clin Oral Implants Res. 2014;25:328–336. doi: 10.1111/clr.12089. [DOI] [PubMed] [Google Scholar]
- 9.Canullo L, Micarelli C, Iannello G. Microscopical and chemical surface characterization of the gingival portion and connection of an internal hexagon abutment before and after different technical stages of preparation. Clin Oral Implants Res. 2013;24:606–611. doi: 10.1111/j.1600-0501.2012.02499.x. [DOI] [PubMed] [Google Scholar]
- 10.Meyle J, Gültig K, Nisch W. Variation in contact guidance by human cells on a microstructured surface. J Biomed Mater Res. 1995;29:81–88. doi: 10.1002/jbm.820290112. [DOI] [PubMed] [Google Scholar]
- 11.Grössner-Schreiber B, Herzog M, Hedderich J, Dück A, Hannig M, Griepentrog M. Focal adhesion contact formation by fibroblasts cultured on surface-modified dental implants: an in vitro study. Clin Oral Implants Res. 2006;17:736–745. doi: 10.1111/j.1600-0501.2006.01277.x. [DOI] [PubMed] [Google Scholar]
- 12.Teughels W, Van Assche N, Sliepen I, Quirynen M. Effect of material characteristics and/or surface topography on biofilm development. Clin Oral Implants Res. 2006;17:68–81. doi: 10.1111/j.1600-0501.2006.01353.x. [DOI] [PubMed] [Google Scholar]
- 13.van Winkelhoff AJ, Goené RJ, Benschop C, Folmer T. Early colonization of dental implants by putative periodontal pathogens in partially edentulous patients. Clin Oral Implants Res. 2000;11:511–520. doi: 10.1034/j.1600-0501.2000.011006511.x. [DOI] [PubMed] [Google Scholar]
- 14.Quirynen M, Bollen CM. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. J Clin Periodontol. 1995;22:1–14. doi: 10.1111/j.1600-051x.1995.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 15.Quirynen M, Bollen CM, Papaioannou W, Van Eldere J, van Steenberghe D. The influence of titanium abutment surface roughness on plaque accumulation and gingivitis: short-term observations. Int J Oral Maxillofac Implants. 1996;11:169–178. [PubMed] [Google Scholar]
- 16.Fürst MM, Salvi GE, Lang NP, Persson GR. Bacterial colonization immediately after installation on oral titanium implants. Clin Oral Implants Res. 2007;18:501–508. doi: 10.1111/j.1600-0501.2007.01381.x. [DOI] [PubMed] [Google Scholar]
- 17.Salvi GE, Fürst MM, Lang NP, Persson GR. One-year bacterial colonization patterns of Staphylococcus aureus and other bacteria at implants and adjacent teeth. Clin Oral Implants Res. 2008;19:242–248. doi: 10.1111/j.1600-0501.2007.01470.x. [DOI] [PubMed] [Google Scholar]
- 18.Mishra PK, Wu W, Rozo C, Hallab NJ, Benevenia J, Gause WC. Micrometer-sized titanium particles can induce potent Th2-type responses through TLR4-independent pathways. J Immunol. 2011;187:6491–6498. doi: 10.4049/jimmunol.1101392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piattelli A, Pontes AE, Degidi M, Iezzi G. Histologic studies on osseointegration: soft tissues response to implant surfaces and components. A review. Dent Mater. 2011;27:53–60. doi: 10.1016/j.dental.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Bentley EM. The value of ultrasonic cleaners in dental practice. Br Dent J. 1994;177:53–56. doi: 10.1038/sj.bdj.4808506. [DOI] [PubMed] [Google Scholar]
- 21.Jatzwauk L, Schöne H, Pietsch H. How to improve instrument disinfection by ultrasound. J Hosp Infect. 2001;48:S80–S83. doi: 10.1016/s0195-6701(01)90019-2. [DOI] [PubMed] [Google Scholar]
- 22.Sawase T, Wennerberg A, Hallgren C, Albrektsson T, Baba K. Chemical and topographical surface analysis of five different implant abutments. Clin Oral Implants Res. 2000;11:44–50. doi: 10.1034/j.1600-0501.2000.011001044.x. [DOI] [PubMed] [Google Scholar]
- 23.Canullo L, Penarrocha D, Micarelli C, Massidda O, Bazzoli M. Hard tissue response to argon plasma cleaning/sterilisation of customised titanium abutments versus 5-second steam cleaning: results of a 2-year post-loading follow-up from an explanatory randomised controlled trial in periodontally healthy patients. Eur J Oral Implantol. 2013;6:251–260. [PubMed] [Google Scholar]
- 24.Canullo L, Peñarrocha D, Clementini M, Iannello G, Micarelli C. Impact of plasma of argon cleaning treatment on implant abutments in patients with a history of periodontal disease and thin biotype: radiographic results at 24-month follow-up of a RCT. Clin Oral Implants Res. 2015;26:8–14. doi: 10.1111/clr.12290. [DOI] [PubMed] [Google Scholar]
- 25.Linkevicius T, Apse P. Influence of abutment material on stability of peri-implant tissues: a systematic review. Int J Oral Maxillofac Implants. 2008;23:449–456. [PubMed] [Google Scholar]
- 26.Nakamura K, Kanno T, Milleding P, Ortengren U. Zirconia as a dental implant abutment material: a systematic review. Int J Prosthodont. 2010;23:299–309. [PubMed] [Google Scholar]
- 27.Zembic A, Sailer I, Jung RE, Hämmerle CH. Randomizedcontrolled clinical trial of customized zirconia and titanium implant abutments for single-tooth implants in canine and posterior regions: 3-year results. Clin Oral Implants Res. 2009;20:802–808. doi: 10.1111/j.1600-0501.2009.01717.x. [DOI] [PubMed] [Google Scholar]
- 28.Sailer I, Philipp A, Zembic A, Pjetursson BE, Hämmerle CH, Zwahlen M. A systematic review of the performance of ceramic and metal implant abutments supporting fixed implant reconstructions. Clin Oral Implants Res. 2009;20:4–31. doi: 10.1111/j.1600-0501.2009.01787.x. [DOI] [PubMed] [Google Scholar]
- 29.Garbelotto LG, Maziero Volpato CA, Rocha Md, Maranghello CA, Calasans A, Ozcan M. Laboratory and clinical considerations on prosthetic zirconia infrastructures for implants. Implant Dent. 2013;22:578–583. doi: 10.1097/ID.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 30.Gehrke P, Alius J, Fischer C, Erdelt KJ, Beuer F. Retentive strength of two-piece CAD/CAM zirconia implant abutments. Clin Implant Dent Relat Res. 2014;16:920–925. doi: 10.1111/cid.12060. [DOI] [PubMed] [Google Scholar]
- 31.Sailer I, Sailer T, Stawarczyk B, Jung RE, Hämmerle CH. In vitro study of the influence of the type of connection on the fracture load of zirconia abutments with internal and external implant-abutment connections. Int J Oral Maxillofac Implants. 2009;24:850–858. [PubMed] [Google Scholar]
- 32.Stimmelmayr M, Edelhoff D, Güth JF, Erdelt K, Happe A, Beuer F. Wear at the titanium-titanium and the titanium-zirconia implant-abutment interface: a comparative in vitro study. Dent Mater. 2012;28:1215–1220. doi: 10.1016/j.dental.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Truninger TC, Stawarczyk B, Leutert CR, Sailer TR, Hämmerle CH, Sailer I. Bending moments of zirconia and titanium abutments with internal and external implant-abutment connections after aging and chewing simulation. Clin Oral Implants Res. 2012;23:12–18. doi: 10.1111/j.1600-0501.2010.02141.x. [DOI] [PubMed] [Google Scholar]
- 34.Bollen CM, Papaioanno W, Van Eldere J, Schepers E, Quirynen M, van Steenberghe D. The influence of abutment surface roughness on plaque accumulation and peri-implant mucositis. Clin Oral Implants Res. 1996;7:201–211. doi: 10.1034/j.1600-0501.1996.070302.x. [DOI] [PubMed] [Google Scholar]
- 35.Butz F, Heydecke G, Okutan M, Strub JR. Survival rate, fracture strength and failure mode of ceramic implant abutments after chewing simulation. J Oral Rehabil. 2005;32:838–843. doi: 10.1111/j.1365-2842.2005.01515.x. [DOI] [PubMed] [Google Scholar]
- 36.Degidi M, Artese L, Scarano A, Perrotti V, Gehrke P, Piattelli A. Inflammatory infiltrate, microvessel density, nitric oxide synthase expression, vascular endothelial growth factor expression, and proliferative activity in peri-implant soft tissues around titanium and zirconium oxide healing caps. J Periodontol. 2006;77:73–80. doi: 10.1902/jop.2006.77.1.73. [DOI] [PubMed] [Google Scholar]
- 37.Glauser R, Sailer I, Wohlwend A, Studer S, Schibli M, Schärer P. Experimental zirconia abutments for implant-supported single-tooth restorations in esthetically demanding regions: 4-year results of a prospective clinical study. Int J Prosthodont. 2004;17:285–290. [PubMed] [Google Scholar]
- 38.Canullo L, Micarelli C, Lembo-Fazio L, Iannello G, Clementini M. Microscopical and microbiologic characterization of customized titanium abutments after different cleaning procedures. Clin Oral Implants Res. 2014;25:328–336. doi: 10.1111/clr.12089. [DOI] [PubMed] [Google Scholar]
- 39.Micarelli C, Canullo L, Baldissara P, Clementini M. Implant abutment screw reverse torque values before and after plasma cleaning. Int J Prosthodont. 2013;26:331–333. doi: 10.11607/ijp.3396. [DOI] [PubMed] [Google Scholar]
- 40.Albrektsson T, Wennerberg A. Oral implant surfaces: Part 1--review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int J Prosthodont. 2004;17:536–543. [PubMed] [Google Scholar]