Abstract
PURPOSE
The aim of this study was to evaluate antibacterial activity and osteoblast-like cell viability according to the ratio of titanium nitride and zirconium nitride coating on commercially pure titanium using an arc ion plating system.
MATERIALS AND METHODS
Polished titanium surfaces were used as controls. Surface topography was observed by scanning electron microscopy, and surface roughness was measured using a two-dimensional contact stylus profilometer. Antibacterial activity was evaluated against Streptococcus mutans and Porphyromonas gingivalis with the colony-forming unit assay. Cell compatibility, mRNA expression, and morphology related to human osteoblast-like cells (MG-63) on the coated specimens were determined by the XTT assay and reverse transcriptase-polymerase chain reaction.
RESULTS
The number of S. mutans colonies on the TiN, ZrN and (Ti1-xZrx)N coated surface decreased significantly compared to those on the non-coated titanium surface (P<0.05).
CONCLUSION
The number of P. gingivalis colonies on all surfaces showed no significant differences. TiN, ZrN and (Ti1-xZrx)N coated titanium showed antibacterial activity against S. mutans related to initial biofilm formation but not P. gingivalis associated with advanced periimplantitis, and did not influence osteoblast-like cell viability.
Keywords: Periimplantitis, Antibacterial activity, Titanium nitride (TiN), Zirconium nitride (ZrN), Streptococcus mutans, Porphyromonas gingivalis
INTRODUCTION
Titanium is widely used as an implant material in the dental and orthopedic fields due to excellent mechanical properties, biocompatibility, and osseointegration.1 The long-term success of dental implants depends on integrating biomaterials with the surrounding bone.2 However, a titanium implant cannot integrate chemically to surrounding bone.3,4 Furthermore, titanium itself has no antibacterial activity. Therefore, there is a probable risk of plaque formation on titanium implants.5 Periimplantitis is a destructive inflammatory process affecting the soft and hard tissues around osseointegrated implants, leading to loss of supporting bone. It occurs in 28-56% of patients (12-40% of sites) with an implant installed.6 Thus, periimplantitis has become a big challenge in dental implant maintenance.
One of the methods to achieve antibacterial activity without affecting biocompatibility of the titanium implant is a surface coating. A TiN (titanium nitride) and ZrN (zirconium nitride) coating, which have excellent strength and wear resistance, have been introduced as a biomedical coating with excellent biocompatibility.7,8,9,10 Grössner et al. reported that physically modifying the titanium implant surfaces such as sputter coating with TiN or ZrN may reduce Streptococcus mutans adherence and could enhance adherence and growth of fibroblasts.2,11
The coating process is classified into physical vapor deposition (PVD) and chemical vapor deposition (CVD). Hard coatings, such as TiN or ZrN are generally deposited by PVD. The PVD coating processes are evaporation, sputtering, and ion plating. The PVD can operate at significantly lower temperatures than for CVD. But this leads to non-uniform of film thickness and large residual stress.12 Thus, AIP system using plasma energy was developed in order to improve these disadvantages. This system has high deposition rate and can produce the excellent adhesion between the coating layer.13
Streptococcus is the dominant pioneer species among over several hundred species of oral bacteria.14 These initial colonizers prepare a favorable condition for subsequent colonizers and provide a binding region preferred by bacterial pathogens associated with periimplantitis such as Porphyromonas species.15 Although reducing the number of initially attached bacteria is an important characteristic of a dental implant surface, antibacterial activity against Porphyromonas species causing periimplantitis by producing toxins stimulating bone and soft tissue inflammation should be considered as well.
The purpose of this study was to evaluate antibacterial activity against not only S. mutans but also P. gingivalis and osteoblast-like cell viability according to the ratio of TiN and ZrN coating on titanium using an arc ion plating system.
MATERIALS AND METHODS
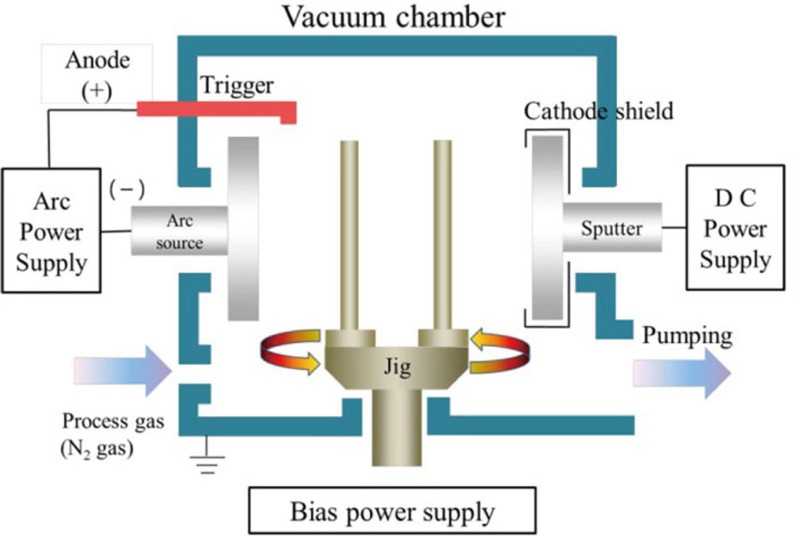
Commercially pure titanium (ASTM Grade II, Kobe Steel Co., Kobe, Japan) were used as the substrate. Specimen discs size is 15 mm in diameter and 3 mm in thickness. Discs surface were ground down to 1200 grit SiC paper under water, then polished using 0.3 µm alumina suspension. Discs were then cleaned with acetone, ethyl alcohol and distilled water for 10min in an ultrasonic cleaner. TiN, ZrN and (Ti1-xZrx)N coating was performed with the arc ion plating system (ATS-MC-STD-300; ATECH System, Seoul, Korea) for 60 min, -100V, with N2 at a flow rate of 110 sccm (standard cubic centimeters per minute) (Fig. 1). Experimental groups were TiN, ZrN, (Ti75Zr25)N, (Ti50Zr50) N and (Ti25Zr75)N. Control group was polished-Ti. All specimens (n=346) were ultrasonically cleaned with acetone, ethyl alcohol and distilled water and were sterilized with ethylene oxide gas.
Fig. 1. Schematic diagram of AIP system.
Surface topography was observed using scanning electron microscopy (FE-SEM S-4700, Hitachi; Horiba, Tokyo, Japan) to analyze the surface characteristics of the each group (n=3). A two dimensional-contact stylus profilometer (DIAVITE DH-7, Asmeto AG, Basel, Switzerland) was used to measure surface roughness (Ra). Five specimens from each group were performed for the measurement. Three measurements on a specimen were measured to calculate the mean roughness (×500 magnification). Two specimens of each group were embedded in the acrylic resin (Caulk/Dentsply, Milford, DE, USA) to measure thickness of coating. The embedded specimens were cut in the middle using linear precision saw (ISOMET®5000, Buehler Ltd., Lake Bluff, IL, USA). Thickness of coating was measured using scanning electron microscopy (FE-SEM S-4700, Hitachi; Horiba, Tokyo, Japan).
Antibacterial activity of the TiN, ZrN and (Ti1-xZrx)N coating specimens was demonstrated against S. mutans strain ATCC® 25175™ and P. gingivalis 381. S. mutans was maintained in brain heart infusion (BHI) broth (Becton, Dickinson and Co. Sparks, MD, USA) and P. gingivalis was maintained anaerobically in tryptic soy broth (TSB) (Becton, Dickinson) supplemented with hemin (10 µg/mL; EMD Chemicals, Inc., San Diego, CA, USA) and menadione (5 µg/mL; Sigma Chemical Co., St. Louis, MO, USA). Five specimens from each group were placed in a 24-well plate and incubated with 1 mL of a 1.5 × 107 cells/mL bacterial suspension for 24 hr at 37℃. After the incubation, the bacterial suspensions were transferred to 900 µL of phosphate-buffered saline (PBS) and diluted to 10-6. A 100 µL aliquot of diluted solution was plated on BHI and TSB agar plates, spread evenly with a spreader, and cultured at 37℃ for 2 and 7 days, respectively. At the end of the incubation period, the number of colonies (colony-forming units) was counted. The results were determined from three independent experiments performed in duplicate.
Human Ostoeoblast like cell line MG-63 was used for biocompatibility test. Three specimens from each group were incubated at a cell density of 4 × 104 cells/mL in a culture plate. Cell viability was examined by the XTT assay after culturing the cells for 24 hours. RNA transcripts in the MG-63 cells grown on the specimens were evaluated using reverse transcription polymerase chain reaction (RT-PCR). RNA was extracted from the cells on the specimens. Each specimen was reverse transcribed into cDNA using a Quantitect Reverse Transcription kit (Qiagen, Valencia, CA, USA). The oligonucleotide primers corresponded to osteonectin, integrin-β1, and β-actin. All oligonucleotide primers were synthesized by Invitrogen (Carlsbad, CA, USA). β-actin mRNA served as an internal control for specimen loading and mRNA integrity. The results were determined from three independent experiments performed in duplicate.
IBM SPSS Statistics 20 (IBM SPSS Inc., Chicago, IL, USA) was used for statistical analyses. The Kruskal-Wallis and post-hoc Tukey's HSD tests were used to make comparisons between the different surfaces. P-values <.05 were considered significant.
RESULTS
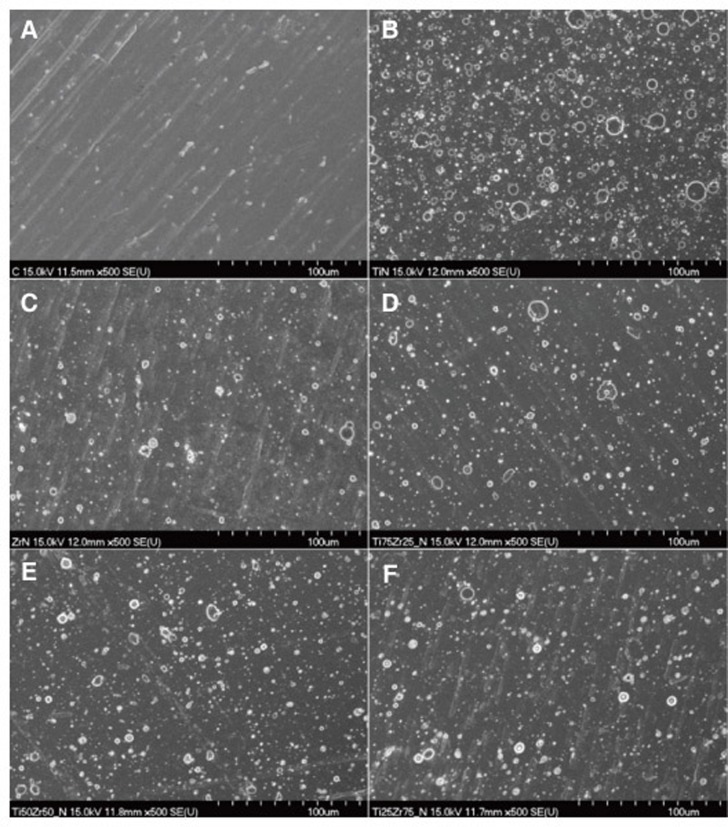
The surface topography of the TiN, ZrN and (Ti1-xZrx)N coated specimens are shown in Fig. 2. Various droplets were observed on the TiN, ZrN and (Ti1-xZrx)N coated surfaces. No differences were observed among the TiN, ZrN and (Ti1-xZrx)N coated specimens. And, the Ra values of the TiN, ZrN and (Ti1-xZrx)N coated surface were 0.26-0.30 µm. The thickness of the TiN, ZrN and (Ti1-xZrx)N coating were 1.52-2.39 µm.
Fig. 2. Scanning electron microscopy images (×500 magnification) of the titanium specimen surface. (A) polished-Ti, (B) TiN, (C) ZrN, (D) (Ti75Zr25)N, (E) (Ti50Zr50)N, (F) (Ti25Zr75)N.
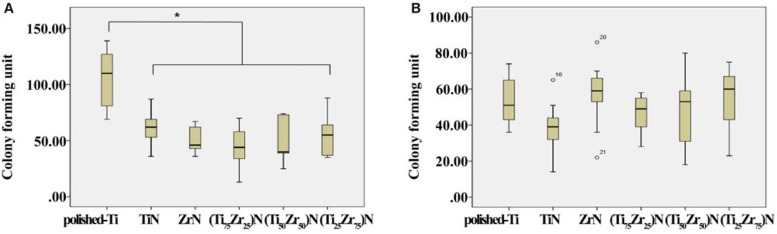
As a result of the antibacterial activity test, the number of S. mutans colonies on the TiN, ZrN and (Ti1-xZrx)N coated surface decreased significantly compared to those on the polished-Ti surface (P<.05). No significant differences were observed among the TiN, ZrN and (Ti1-xZrx)N coated specimens regardless of ratio (Fig. 3A). However, the number of P. gingivalis colonies on all surfaces showed no significant differences (Fig. 3B).
Fig. 3. Numbers of Streptococcus mutans (A) and Porphyromonas gingivalis (B) colonies from the different groups.
*Significant difference with polished-Ti (P<.05). Outliers are denoted by circles.
Data are mean and standard deviation values of three independent experiments performed in duplicate.
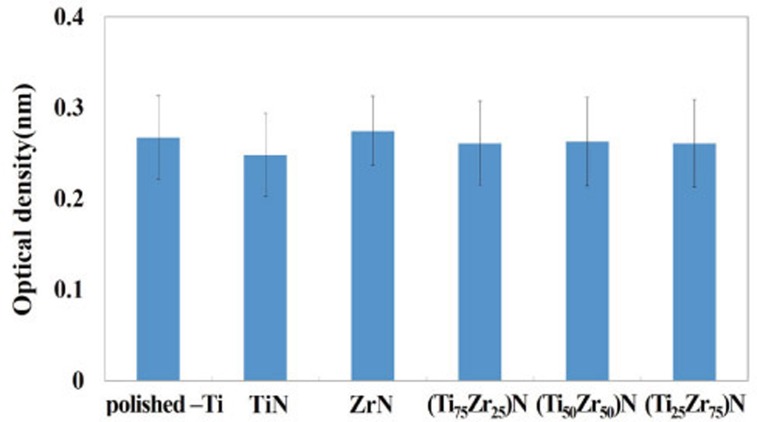
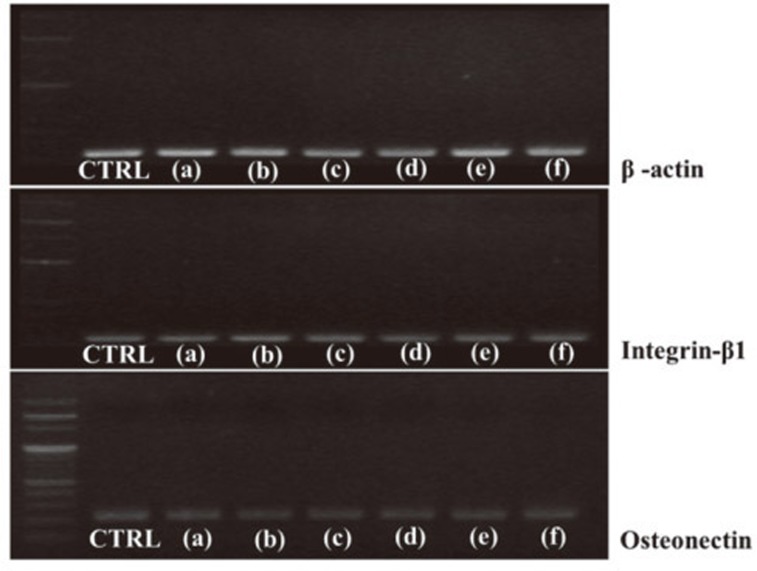
The osteoblast-like cell viability values were slightly lower or higher for the TiN, ZrN and (Ti1-xZrx)N coated specimens than those for polished-Ti. However, no significant difference was observed among the six groups (Fig. 4). Osteonectin, integrin-β1, and β-actin mRNA expression is shown in Fig. 5. As a result of the RT-PCR measurement, no significant differences were observed between the levels of osteonectin, integrin-β1, or β-actin mRNA in cells on all specimens.
Fig. 4. Evaluation of MG-63 cell viability on specimens after 24 hr by the XTT assay.
Fig. 5. Reverse transcription-polymerase chain reaction measurements of osteonectin, integrin β1, and β-actin mRNA levels from MG-63 cells. (a) polished-Ti, (b) TiN, (c) ZrN, (d) (Ti75Zr25)N (e) (Ti50Zr50)N (f) (Ti25Zr75)N.
DISCUSSION
This study evaluated the antibacterial activity against not only S. mutans but also P. gingivalis and osteoblast-like cell viability according to the ratio of TiN and ZrN coating on titanium using an arc ion plating system. The arc ion plating system has the advantage that TiN, ZrN and (Ti1-xZrx)N coating can be applied variously on the substrates. However, it is difficult to uniformly deposit metal ions from a target onto the substrate, as the formation of droplets worsens the mechanical properties of the coating layer. The droplet creation process generated during arc ion plating is complex and depends on the surface roughness of the substrate.16 Quirynen et al. insisted that a surface roughness < 0.2 µm does not have an effect on bacterial adhesion because most bacteria are > 0.2 µm.17 Therefore, the polished group with surface roughness less than 0.2 µm was used as a control group. Increased surface roughness leads to enhanced bacterial adhesion by providing shelter.18,19 In this study, the Ra values of the TiN, ZrN and (Ti1-xZrx)N coated surface were 0.26-0.30 µm.
This study used the AIP coating system, which is different from the sputter coating system used by Größner et al.2 Though the AIP system has a high deposition rate and can produce excellent adhesion between the coating layer and the substrate, this results in a thicker and more irregular coating layer than sputter coating. Therefore, we expected that the thicker and irregular TiN and ZrN coating layer would have antibacterial activity against not only Streptococcus mutans but also Porphyromonas gingivalis associated with periimplantitis. Furthermore, we confirmed the effect of the thicker and irregular TiN and ZrN coating layer on osteoblast-like cell activity.
S. mutans is the predominant bacteria in the human oral cavity and is associated with initial adhesion. P. gingivalis in the subgingival area can attach to an implant and is associated with periimplantitis as it produces toxins stimulating bone and soft tissue inflammation. As a result of the antibacterial activity test, the number of S. mutans colonies on the TiN, ZrN and (Ti1-xZrx)N coated surface decreased significantly compared to those on the polished-Ti surface. Despite the TiN, ZrN and (Ti1-xZrx)N coated surface roughness > 0.2 µm, the S. mutans colonies decreased significantly. This result indicates that S. mutans was not influenced by surface roughness possibly because its size is > 0.3 µm.20 We considered that the TiN, ZrN and (Ti1-xZrx)N coated surface inhibited initial adhesion of S. mutans. The results also coincide that hard coatings such as TiN or ZrN seemed to reduce accumulation of plaque by masking the underlying more reactive titanium surface.2 However, this study indicated that TiN, ZrN and (Ti1-xZrx)N coating on titanium using an arc ion plating system showed no antibacterial activity against P. gingivalis.
According to Jeyachandran et al., bacterial adhesion on titanium films were minimized by tailoring the surface chemical stoichiometry of the films using a sputtering system.21 They showed titanium films with various surface compositions, such as oxide and nitride combinations, resulted in nearly nil bacterial adhesion. Nevertheless, adhesion of P. gingivalis was reported to increase on TiN coating which has 123 nm of thickness by sputter coating.22 According to Yoshinari et al., even though TiN coating thickness was 3 µm, TiN coating did not inhibit adhesion of P. gingivalis.23 In this study, the thickness of the TiN, ZrN and (Ti1-xZrx)N coating were 1.52-2.39 µm. Therefore, it seemed that TiN coating on titanium showed no antibacterial activity against P. gingivalis without reference to the thickness of coating.
Interestingly, TiN, ZrN and (Ti1-xZrx)N coated titanium showed antibacterial activity against S. mutans but not P. gingivalis. The responses to TiN, ZrN and (Ti1-xZrx)N coating seemed to be different according to bacteria species, and specific chemical composition and thickness of coating is considered to be essential in order to suppress certain bacterial activity. Since many bacterial species cause periimplantitis, further studies should be performed with different or mixed bacterial species associated with periimplantitis.24
Surface characteristics of biomaterials, such as surface topography and surface roughness, play an essential part in osteoblast adhesion on biomaterials.25 After implantation, a complex biofilm of proteins and cells forms on materials in contact with the blood within seconds.26 The biofilm is composed of cell adhesion promoting proteins and cell-activating ligands. RT-PCR measurement was performed for molecular biological evaluation of osteoblast-like cell adhesion. Integrin β1 is the major integrin subunit involved in osteoblast adhesion to biomaterials27 and it plays a role in initial cell adhesion by mediating adhesion proteins. The extracellular matrix (ECM) protein osteonectin is an essential component of the noncollagenous matrix in bone. It is associated with binding Ca2+ ions and hydroxyapatite during mineralization.28,29 Thus, production of osteonectin regulates the degree of cell adhesion to biomaterials. No significant differences were observed between the levels of osteonectin, integrin-β1, or β-actin mRNA in cells on all specimens. This result indicates that TiN, ZrN and (Ti1-xZrx)N coated surfaces did not influence osteoblast-like cell activity.
This study evaluated cell viability for only 24 hours and more comprehensive results would likely be obtained with longer term evaluation. Long term evaluation of adhesion, proliferation and differentiation of cells should be performed as well. RT-PCR measurements of adhesion associated proteins other than osteonectin and integrin β1 should also be performed.
A limitation of this study is that the evaluation of antibacterial activity and osteoblast-like cell viability was only performed in vitro. Further studies are needed to demonstrate the antibacterial activities of TiN, ZrN and (Ti1-xZrx)N coated surfaces in vivo. The development of coating methods that produce surfaces suitable for preventing bacterial adhesion is required for further studies.
CONCLUSION
This study conclude that the TiN, ZrN and (Ti1-xZrx)N coating on titanium using an arc ion plating system show antibacterial activity against S. mutans related to initial biofilm formation but not P. gingivalis associated with advanced periimplantitis. The TiN, ZrN and (Ti1-xZrx)N coating on titanium did not influence osteoblast-like cell viability.
Footnotes
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2013R1A1A1010115).
References
- 1.Niinomi M. Metallic biomaterials. J Artif Organs. 2008;11:105–110. doi: 10.1007/s10047-008-0422-7. [DOI] [PubMed] [Google Scholar]
- 2.Grössner-Schreiber B, Griepentrog M, Haustein I, Müller WD, Lange KP, Briedigkeit H, Göbel UB. Plaque formation on surface modified dental implants. An in vitro study. Clin Oral Implants Res. 2001;12:543–551. doi: 10.1034/j.1600-0501.2001.120601.x. [DOI] [PubMed] [Google Scholar]
- 3.Watzak G, Zechner W, Ulm C, Tangl S, Tepper G, Watzek G. Histologic and histomorphometric analysis of three types of dental implants following 18 months of occlusal loading: a preliminary study in baboons. Clin Oral Implants Res. 2005;16:408–416. doi: 10.1111/j.1600-0501.2005.01155.x. [DOI] [PubMed] [Google Scholar]
- 4.Isidor F. Influence of forces on peri-implant bone. Clin Oral Implants Res. 2006;17:8–18. doi: 10.1111/j.1600-0501.2006.01360.x. [DOI] [PubMed] [Google Scholar]
- 5.Becker W, Becker BE, Newman MG, Nyman S. Clinical and microbiologic findings that may contribute to dental implant failure. Int J Oral Maxillofac Implants. 1990;5:31–38. [PubMed] [Google Scholar]
- 6.Jung CW. Peri-implant disease and GBR. 1st ed. Seoul: Narae publishing; 2001. pp. 2–7. [Google Scholar]
- 7.Elias CN, Figueira DC, Rios PR. Influence of the coating material on the loosing of dental implant abutment screw joints. Mater Sci Eng C. 2006;26:1361–1366. [Google Scholar]
- 8.Chou WJ, Yu GP, Huang JH. Corrosion resistance of ZrN films on AISI 304 stainless steel substrate. Surf Coat Technol. 2003;167:59–67. [Google Scholar]
- 9.Moon BH, Choe HC, Brantley WA. Surface characteristics of TiN/ZrN coated nanotubular structure on the Ti-35Ta-xHf alloy for bio-implant applications. Appl Surf Sci. 2012;258:2088–2092. [Google Scholar]
- 10.Damaschek R, Strydom IL, Bergmann H. Improved adhesion of TiN deposited on prenitrided steels. Surf Eng. 1997;13:128–132. [Google Scholar]
- 11.Groessner-Schreiber B, Neubert A, Müller WD, Hopp M, Griepentrog M, Lange KP. Fibroblast growth on surfacemodified dental implants: an in vitro study. J Biomed Mater Res A. 2003;64:591–599. doi: 10.1002/jbm.a.10417. [DOI] [PubMed] [Google Scholar]
- 12.Chollet L, Perry AJ. The stress in ion-plated HfN and TiN coatings. Thin Solid Films. 1985;123:223–234. [Google Scholar]
- 13.Jeong YH, Kwag DM, Chung CH, Kim WG, Choe HC. Corrosion characteristics and surface morphologies of TiN and ZrN film on the abutment screw by Arc-ion coating(2) Corrosion Sci Technol. 2011;10:212–217. [Google Scholar]
- 14.Nakazato G, Tsuchiya H, Sato M, Yamauchi M. In vivo plaque formation on implant materials. Int J Oral Maxillofac Implants. 1989;4:321–326. [PubMed] [Google Scholar]
- 15.Mombelli A, Lang NP. Microbial aspects of implant dentistry. Periodontol 2000. 1994;4:74–80. doi: 10.1111/j.1600-0757.1994.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 16.Tai CN, Koh ES, Akari K. Macroparticles on TiN films prepared by the arc ion plating process. Surf Coat Technol. 1990;43/44:324–335. [Google Scholar]
- 17.Quirynen M, van der Mei HC, Bollen CM, Schotte A, Marechal M, Doornbusch GI, Naert I, Busscher HJ, van Steenberghe D. An in vivo study of the influence of the surface roughness of implants on the microbiology of supraand subgingival plaque. J Dent Res. 1993;72:1304–1309. doi: 10.1177/00220345930720090801. [DOI] [PubMed] [Google Scholar]
- 18.Lin NM, Huang XB, Zou JJ, Zhang XY, Qin L, Fan AL, Tang B. Effects of plasma nitriding and multiple arc ion plating TiN coating on bacterial adhesion of commercial pure titanium via in vitro investigations. Surf Coat Technol. 2012;209:212–215. [Google Scholar]
- 19.Bollen CM, Lambrechts P, Quirynen M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: a review of the literature. Dent Mater. 1997;13:258–269. doi: 10.1016/s0109-5641(97)80038-3. [DOI] [PubMed] [Google Scholar]
- 20.Ryan V, Hart TR, Schiller R. Size determination of Streptococcus mutans 10499 by laser light scattering. Biophys J. 1980;31:313–324. doi: 10.1016/S0006-3495(80)85061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeyachandran YL, Narayandass SK, Mangalaraj D, Bao CY, Martin PJ. The effect of surface composition of titanium films on bacterial adhesion. Biomed Mater. 2006;1:L1–L5. doi: 10.1088/1748-6041/1/1/L01. [DOI] [PubMed] [Google Scholar]
- 22.Jeyachandran YL, Venkatachalam S, Karunagaran B, Narayandass SK, Mangalaraj D, Bao CY, Zhang CL. Bacterial adhesion studies on titanium, titanium nitride and modified hydroxyapatite thin films. Mater Sci Eng C. 2007;27:35–41. [Google Scholar]
- 23.Yoshinari M, Oda Y, Kato T, Okuda K. Influence of surface modifications to titanium on antibacterial activity in vitro. Biomaterials. 2001;22:2043–2048. doi: 10.1016/s0142-9612(00)00392-6. [DOI] [PubMed] [Google Scholar]
- 24.Ata-Ali J, Candel-Marti ME, Flichy-Fernández AJ, Peñarrocha-Oltra D, Balaguer-Martinez JF, Peñarrocha Diago M. Peri-implantitis: associated microbiota and treatment. Med Oral Patol Oral Cir Bucal. 2011;16:e937–e943. doi: 10.4317/medoral.17227. [DOI] [PubMed] [Google Scholar]
- 25.Anselme K. Osteoblast adhesion on biomaterials. Biomaterials. 2000;21:667–681. doi: 10.1016/s0142-9612(99)00242-2. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz Z, Lohmann CH, Vocke AK, Sylvia VL, Cochran DL, Dean DD, Boyan BD. Osteoblast response to titanium surface roughness and 1alpha,25-(OH)(2)D(3) is mediated through the mitogen-activated protein kinase (MAPK) pathway. J Biomed Mater Res. 2001;56:417–426. doi: 10.1002/1097-4636(20010905)56:3<417::aid-jbm1111>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 27.Anselme K. Osteoblast adhesion on biomaterials. Biomaterials. 2000;21:667–681. doi: 10.1016/s0142-9612(99)00242-2. [DOI] [PubMed] [Google Scholar]
- 28.Roach HI. Why does bone matrix contain non-collagenous proteins? The possible roles of osteocalcin, osteonectin, osteopontin and bone sialoprotein in bone mineralisation and resorption. Cell Biol Int. 1994;18:617–628. doi: 10.1006/cbir.1994.1088. [DOI] [PubMed] [Google Scholar]
- 29.zur Nieden NI, Kempka G, Ahr HJ. In vitro differentiation of embryonic stem cells into mineralized osteoblasts. Differentiation. 2003;71:18–27. doi: 10.1046/j.1432-0436.2003.700602.x. [DOI] [PubMed] [Google Scholar]